Abstract
Cancer heterogeneity, which enables clonal survival and treatment resistance, is shaped by active immune responses. Antigen-specific T cells can control cancer, as revealed clinically by immunotherapeutics such as adoptive T–cell transfer and checkpoint blockade. The host immune system is thus a powerful tool that if better harnessed could significantly enhance the efficacy of chemotherapeutics and improve outcomes for cancer sufferers. To realize this vision, however, a number of research frontiers must be tackled. These include developing strategies for neutralizing tumor-promoting inflammation, broadening T cell repertoires (via vaccination), and elucidating the mechanisms by which immune cells organize tumor microenvironments to regulate T cell activity. Such efforts will pave the way for identifying new targets for combination therapies that overcome resistance to current treatments and promote long-term cancer control.
Keywords: cancer, inflammation, immunogenic cell death, leukocytes, dendritic cells, vaccine
Introduction
Cancer is an insidious disease traditionally classified by cell and tissue type of origin. Cancer has historically been treated according to a “one size fits all” approach based on broad pathologic criteria and involving various regimens of cytotoxic therapy. With the advent of modern sequencing methodologies, however, we now appreciate that significant genomic, transcriptomic, and epigenetic heterogeneity exists within individual tumor types; this recognition has enabled subclassification of tumors of common origin. This in turn has led to improved outcomes for some cancer types, as response rates to targeted and cytotoxic therapies increase when patients are stratified based on the molecular characteristics of their tumors. Examples include imatinib in chronic myelogenous leukemia (Druker et al., 2006), HER2-targeted therapies for HER2-positive breast cancer (Shepard et al., 1991), and estrogen antagonists for estrogen-receptor-positive breast cancers (Heiser et al., 2012). These molecular advances helped usher in a new era of precision medicine that is reshaping clinical treatment across the cancer spectrum. However, there remain significant fractions of patients that do not respond to “designer” therapies even when their tumors are classified based on molecular and pathologic criteria. Additional tumor or systemic characteristic(s) are thus unaccounted for that not only impact neoplastic growth and dissemination, but also impact response to therapy.
Recent seminal in vivo studies revealed that neoplastic cells rely on the diversity of normal resident and recruited accessory cells to support their evolution (Hanahan and Coussens, 2012). Accessory cells are now recognized as “neoplastic cell-extrinsic hallmarks of cancer” and include those forming the hematogenous and lymphatic vasculature, tissue-specific mesenchymal support cells and myeloid and lymphoid-lineage immune cells. Accessory cells integrate with the dynamic soluble and insoluble matrices constituting the “tumor stroma”; collectively, they fuel neoplastic evolution (Hanahan and Coussens, 2012). In other words, reciprocal interactions between accessory cells, their mediators, structural components of the extracellular matrix (ECM) and genetically altered neoplastic cells regulate all aspects of tumorigenicity. This realization fueled the development of anti-cancer agents targeting the vasculature (Kerbel, 2011). However, it is now clear that some aspects of the immune response accompanying tumor development, such as those that neutralize tumor-promoting chronic inflammation and/or embolden or unleash the cytotoxic activities of antigen-specific T cells, also represent tractable targets for anti-cancer therapy (Coussens et al., 2013; Pardoll, 2012).
Indeed, cancer is visible to the immune system, i.e., immunogenic, during early neoplasia. Classic studies from Schreiber and colleagues in mice with carcinogen-initiated sarcomas revealed that the immune system could recognize and reject cancerous cells (Dunn et al., 2004). The elimination can be explained by cytotoxicity by antigen-specific T cells responding to relatively high mutational burdens induced by carcinogens and thus providing neo-antigens for T cell priming; these findings established the principles of elimination, equilibrium and eventually escape when neoplastic cells become invisible to the immune system (Dunn et al., 2004). Neoplastic cells can also escape when tumor arises out of chronically inflamed tissues – there, chronic infiltration of tissue by leukocytes (e.g., type 2 cytokine-activated myeloid cells and immune suppressive B, T and myeloid subsets) subvert T cell-directed elimination and thus aid tissue-based programs, e.g., angiogenesis, lymphangiogenesis, matrix remodeling, etc., supporting neoplastic progression (Coussens et al., 2013).
Mounting observations in humans support the concept that cancer initiation and progression is significantly impacted by altered or misled immune responses (Figure 1). Individuals suffering from chronic inflammatory conditions are at increased risk for developing cancer (Thun et al., 2004). Incidence of viral (DNA tumor virus) and carcinogen-associated cancers is increased in immune-compromised individuals, even as the relative risk of cancer types lacking viral or carcinogen etiology is diminished (reviewed in: (de Visser et al., 2006)). Age-related immunosenescence likely plays a role in increased incidence of malignancy in aged individuals (Campisi et al., 2011). The advent of some biologic therapies impacting how tissues activate and resolve inflammation, e.g., tumor necrosis factor (TNF) blockade (Bongartz et al., 2006), also skews cancer incidence metrics. However, the role(s) that immune pathways play in driving malignancy remains to be clarified. How does the immune system recognize tissue-specific mediators triggering and maintaining chronic inflammatory responses? What oncogenic events and altered metabolic states lead to the generation of neo-antigens that in turn induce T cell responses? What physiological mechanisms regulate immune homeostasis such that (acute) inflammation can be resolved as rapidly as it is activated (a critical control program to thwart autoimmunity)? What is the role of the host microbiota in regulating systemic immune responses to neoplasia? How do neoplastic cells survive immune attack by T cells?
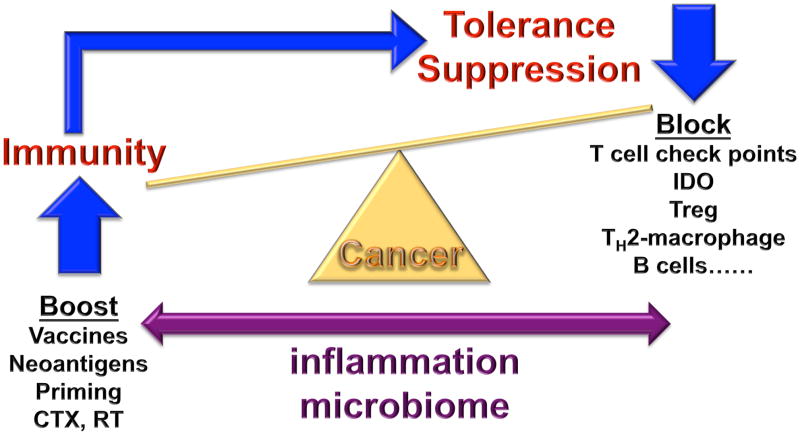
Figure 1. The makings of tumor immunity.
The communication between cancer and the immune system is a dynamic process, reminiscent of a balance. When immunity to cancer is ‘up’ and the suppressive processes are ‘down’, cancer is under control. However, a strong anti-tumor immune response will trigger largely physiological processes designed to dampen effector T cells to prevent tissue damage and maintain tissue homeostasis. Given that the immunity might have evolved mainly to maintain self, to establish coexistence with environment and to occasionally protect self from external threats, the suppression prevails. Multiple pathways of suppression are at play in tumor microenvironments including cells such as TH2-polarized macrophages, immature and suppressive monocytes, regulatory B cells and regulatory T cells, as well as molecules such as check points that control T cell differentiation (for example CTLA-4 and IDO) and effector function (such as PD-1). Pharmacological blockade of these inhibitory pathways can tip the balance towards anti-cancer effector T cells. The latter ones can be primed or boosted by antigen presenting cells (DCs) and/or by co-stimulatory signals (for example CD137 ligands). Recent studies demonstrate that thymus-independent neo-antigens generated in adult life by somatic mutation or post-translational regulation (for example phosphorylation) might be more immunogenic (or perhaps linked with less suppression) than shared tumor antigens. Neo-antigens can occur as random results of somatic mutation, as well as a by-product of anticancer treatments, e.g., chemotherapy (CTX) or radiation therapy (RT), or by targeting epigenetic control mechanisms or drugs intervening with DNA repair pathways. They can be presented to T cells in exogenous vaccines, as well as endogenously via DCs that captured dying neoplastic cells. When T cells specific to defined antigens kill neoplastic cells, such process can enable generation of responses to other antigens, so called epitope spreading. A critical factor in the balance between immunogenicity and suppression is inflammation (which in turn is impacted by the microbiome); indeed, the type of inflammation (tumor destructing TH1 or tumor promoting TH2 and TH17) should become a part of TNM grading, along with pathology, microbiome phenotype, and immune infiltrate assessment.
A common feature of all cancers, regardless of origin, is prominent presence of diverse assemblages of immune cells (Coussens et al., 2013). The consequences of such infiltrates on the fate of cancerous cells are diverse. For example, under continual immune pressure, i.e., antigen presentation to T cells, neoplastic cells become “immune-edited” to escape immune surveillance (Dunn et al., 2004), and instead co-opt immune cells to favor their sustained proliferation (Balkwill et al., 2005). Nonetheless, recent studies demonstrate that the presence of lymphoid aggregates is linked with improved responses to cancer therapies, for example standard cytotoxic therapies, vaccine-based treatments or immune checkpoint blockade (Topalian et al., 2015). Such “hot” tumors are thus more amenable to control than “cold” tumors, i.e., tumors with diminished T cell infiltrates, thus driving modern cancer medicine to investigate how to reprogram the tumor microenvironment (TME) to attract the right type of immune infiltrate. This topic, along with other open questions in the field of oncoimmunology is discussed hereunder.
The makings of the immune response to cancer
Tumors are organized tissues with numerous reciprocal local and systemic connections with immune cell populations of both the myeloid and lymphoid lineages. Here, we summarize the key myeloid and lymphoid populations regulating the immune response to cancer, and how the fundamental physiological processes they govern are harnessed for neoplastic progression and tumor formation.
The myeloid compartment
Myeloid cells have multiple homeostatic functions that are coopted by evolving neoplasms; these can be roughly summarized as i) antigen capture for degradation (macrophages) or presentation (dendritic cells (DCs)); ii) tissue repair (macrophages), and iii) effector functions (mast cells, monocytes and granulocytes). Neoplastic cells can alter the steady-state activity of all myeloid cells present in the TME, including tissue-resident and blood-derived cells, by secreting factors such as interleukin (IL)-6 or granulocyte-macrophage colony-stimulating factor (GM-CSF), that increase recruitment and proliferation of immature myeloid cells atypical under physiological conditions (Gabrilovich et al., 2012).
An important feature of myeloid cells is their functional plasticity in response to environmental signals. This property can dictate such opposite outcomes as antigen degradation or antigen presentation when macrophages acquire DC capabilities (Banchereau et al., 2000); tissue repair rather than inflammation when macrophages are polarized towards type 2 states and protective or non-protective T cell immunity when programmed by cancer-derived factors (Balkwill et al., 2005). Thus, plasticity and communication within the myeloid compartment, and between myeloid and other immune cells and stromal components, is critical for tumor formation.
Cancer antigen presentation and dendritic cells
Cancer antigens are presented to T cells either at tumor sites or in draining lymph nodes by DCs. Cancer antigens, soluble and cell-borne, are transported to lymph nodes via lymphatic vessels. Soluble antigen is captured by lymph-node-resident DCs while tissue-resident DCs capture antigen at tumor sites; either population can present antigen locally or migrate through lymphatic vessels to present in lymph nodes (Steinman, 2011). DCs display protein antigens in the context of classical major histocompatibility (MHC) class I and MHC class II molecules, or lipid antigens in the context of non-classical CD1 molecules that allow selection of rare antigen-specific T lymphocytes including CD8+ T cells, CD4+ T cells, and NK T cells. Compared with other antigen-presenting cells (APCs), DCs are extremely efficient in their ability to induce antigen-specific T cell responses, justifying their name “professional APCs” (Lanzavecchia and Sallusto, 2001). Naïve CD8+ T cells differentiate into cytotoxic T lymphocytes (CTLs) in lymphoid organs upon encounter with DCs presenting tumor-derived peptides in the context of co-stimulation through CD80, CD70 and 4-1BB, as well as through DC-derived cytokines such as IL-12, type I interferon and IL-15 (Steinman, 2012). The priming of new T cell repertoires during tumorigenesis may be critical for clinical success of therapeutic agents aiming to unleash antigen-specific CTL activities. Naive CD4+ T cells can give rise to helper cells with distinct cytokine profiles, or to Fox-P3+ regulatory T cells (Treg) whose role is to dampen CTL activity and avoid autoimmune responses (Zhu and Paul, 2008).
DCs express numerous pattern recognition receptors, including lectins, Toll-like receptors (TLRs), NOD-like receptors (NLRs) and helicases, through which they can sense microbes and tissue damage (cancer) such as increased pericellular nucleic acids (Pulendran, 2015). If DCs do not receive maturation signals, such as when exposed to high levels of IL-10 (Ruffell et al., 2014), they remain immature and antigen presentation instead leads to T cell suppression. DC plasticity in response to extrinsic signals, together with the existence of discrete subsets with unique functions, empowers DCs as key initiators and regulators of the immune response (Pulendran, 2015). We will illustrate this point briefly; mouse and human DC subset biology was recently reviewed elsewhere (Merad et al., 2013).
The diversity of human DC subsets was revealed by studies of blood and skin DCs. Three main cell-surface markers distinguished human-blood-circulating DC subsets: CD303 (BDCA-2) on plasmacytoid DCs (pDCs), CD1c (or BDCA-1) expressed on the majority of circulating DCs, and CD141/BDCA-3 expressed on a small fraction (Merad et al., 2013). Human CD141+CD1c− DCs uniquely express TLR3, produce IL-12 and efficiently cross-prime CD8+ T cells when activated with poly I:C (Joffre et al., 2012). However other human DCs, such as epidermal Langerhans cells and CD1c+ DCs, also cross-present antigens to CD8+ T cells. Indeed, our studies have unraveled the basic principles by which human DC subsets differentially regulate CD8+ T cells (Klechevsky et al., 2008). Thus, human Langerhans cells are highly efficient at priming cytotoxic CD8+ T cells while CD14+ dermal DCs prime type 2 cytokine-secreting CD8+ T cells (Klechevsky et al., 2008). Blood and tissue-resident CD1c+ DCs, but not CD141+ DCs, exposed to live-attenuated influenza virus promote CD103 (αE integrin) expression on CD8+ T cells and their accumulation in epithelia (Yu et al., 2013).
The lymphoid compartment in tumors includes natural killer (NK) cells, γδ T cells, NK T cells, CD4+ T cells, CD8+ T cells, and B cells. Their functional activity depends upon expression of restriction elements, including peptide-MHC complexes (pMHC; for T cells), the MHC class I molecule (for NK cells), or surface proteins (for B cell products, i.e., antibodies) that can be recognized in a specific manner. In addition, lymphoid cells can be induced to secrete different types of cytokines based on effector functions. For example, following an activating stimulus, TH1-polarized CD4+ T cells secrete IL-2, TNFα, and IFN-γ; in conjunction with cytotoxic CD8+ T cells, they promote macrophage cytotoxic activity (Stout and Bottomly, 1989) and can induce up-regulation of antigen processing and expression of MHCI and II molecules in professional APCs (i.e., macrophages and DCs). In contrast, expression of IL-4, -5, -6, -10 and -13 by TH2-polarized CD4+ T cells can induce T cell anergy and loss of T cell-mediated cytotoxicity, enhance humoral immunity and regulate the tumor-promoting activities of macrophages (DeNardo et al., 2009).
CD8+ T cells are considered the major anti-cancer effector cells as they can give rise to CTLs that kill cancer cells presenting a specific pMHC complex (Appay et al., 2008). CTLs can be generated through either the priming of naive T cells or reprogramming of memory T cells. Naive CD8+ T cells differentiate into CTLs in lymphoid organs upon encounter with APCs presenting tumor-derived peptides in the context of appropriate co-stimulation and cytokine help. The ideal properties of anti-cancer CD8+ T cells include: high affinity for pMHC on tumor cells; high levels of cytotoxic mediators, e.g., granzymes A and B and perforin; expression of surface molecules allowing trafficking into the tumor; and extended longevity and memory, thus enabling CTL generation upon antigen re-exposure (Appay et al., 2008).
Memory T cells have long been described as two circulating populations: central memory T cells that migrate between the secondary lymphoid organs and are capable of mounting proliferative responses on pathogen re-encounter; and effector memory T cells that traffic between blood and extralymphoid compartments for peripheral immune surveillance (Mueller et al., 2012). Tissue-resident memory T cells are a third and phenotypically distinct category. Studies in mice and humans have shown this latter population can be superior to circulating central memory T cells at providing rapid long-term protection against re-infection (Sheridan and Lefrancois, 2011). Therefore, an active mechanism of peripheral T cell retention likely exists not only to facilitate clearance of infected cells, but also to promote accumulation at sites having cleared an infectious virus. CD103/β7 integrin endows peripheral CD8+ T cells with a unique capacity to access epithelial compartments. Expression of CD103 on CTLs mediates adherence to E-cadherin and appears to be important in the final stages of neoplastic cell lysis and rejection (Le Floc’h et al., 2007). Indeed, for mucosal cancer vaccines, homing to and retention of CD8+ T cells in mucosa is critical for efficacy (Sandoval et al., 2013).
Upon arrival in tumor beds, CD8+ T cells must confront numerous barriers including intrinsic checkpoint regulators, such as CD28-CTLA-4, PD1-PD-L1, and ILTs (Pardoll, 2012); extrinsic checkpoint regulators, such as Treg cells (Fehervari and Sakaguchi, 2004) or myeloid cells (Gabrilovich et al., 2012); a corrupted TME with protumor inflammation (Coussens et al., 2013); antigen loss and immune evasion of tumor targets (Klebanoff et al., 2011); and tissue-specific alterations, such as fatty cells in breast cancer or desmofibrosis in pancreatic cancer stroma. Defining strategies for bypassing these obstacles and improving the clinical efficacy of T cell therapies is the object of intense study.
An important concept recently proposed by Mellman and colleagues is the cancer-immunity cycle (Chen and Mellman, 2013). It becomes apparent that any effective immune response against cancer will generate resistance via physiological pathways that evolved to protect tissue homeostasis. Hereunder, we discuss how this cycle is altered in cancer pathogenesis and how it can be harnessed therapeutically. Clearly, combination therapies that intervene at several distinct pathways within the cancer-immunity cycle are needed to achieve cancer control.
Chronic inflammation and alterations of leukocyte compartments in cancer
Basic principles
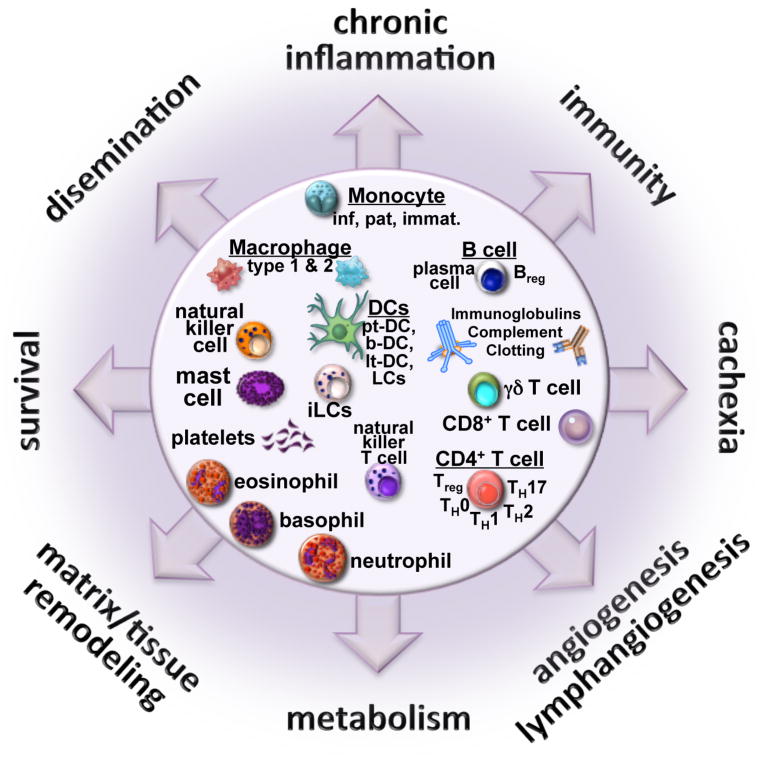
Unabated inflammation is a hallmark of cancer and is mediated by immune cells attracted to or residing at sites of neoplastic transformation (Balkwill et al., 2005). Indeed, immune cells are selectively recruited into early neoplastic tissues, likely in response to hardwired pathways utilized by all tissues to resist/repair damage caused by bacterial, viral or other pathogenic assaults. When successful, “initiated” pre-neoplastic cells are purged by the immune system (Dunn et al., 2004). When the immune system fails, neoplastic cells are retained in “damaged” TMEs and provided a survival advantage resulting from abundant bioavailable mediators liberated as a function of tissue remodeling (Hanahan and Coussens, 2012). Ensuing neoplastic progression requires sustained presence of select immune subtypes that, combined with ongoing host-derived programs (angiogenesis, matrix and tissue remodeling, etc.), contribute to cancer progression (Hanahan and Coussens, 2012) (Figure 2).
Figure 2. Immune-mediated landscape.
The yin and yang implications of tumor-immune system communications form the basis for disease pathophysiology, and at the same time, targets for therapeutic intervention. The disease landscape emerging from these multi-factorial interactions is orchestrated by the three compartments, i.e., the cancer, the immune system and the host. The outputs are numerous and dramatically opposite, as well as both local and systemic, and include: immunity that might control cancer; chronic inflammation which can be linked with tissue remodeling processes and metabolic changes that support neoplastic cell survival and primary tumor development; angiogenesis and lymphangiogenesis that can also support metastatic dissemination; as well as systemic consequences for the host including cachexia. Clearly, therapy going forward will require a well-timed and orchestrated combination of therapies, targeting multiple modes of communication and effect, to combat this multi-factorial disease taking into account the patients’ steady-state commensal bacteria complexity and load, and how that is impacted by therapy.
The classic view that immune cells merely facilitate tumor rejection has been supplanted by a more complex view of leukocytes having both tumor-promoting and tumor-inhibiting properties (Coussens et al., 2013). This is best explained by the existence of (at least) two types of inflammation with opposing effects on tumors: chronic inflammation, which promotes neoplastic cell survival, angiogenesis, tissue remodeling and metastasis, and acute inflammation that triggers neoplastic cell destruction. While chronic inflammation is often linked with the presence of TH2 responses, acute inflammation associated with cancer destruction is linked to TH1 responses.
As neoplastic cells escape elimination, some become less immunogenic by down-regulating MHC molecules; however, most if not all also activate intrinsic gene-expression programs that are inherently T cell-suppressive and myelo-stimulatory, e.g., TH2 responses. Cytokines implicated in these scenarios include transforming growth factor (TGF)β; IL-4, -13, -8, and -10; thymic stromal lymphopoietin (TSLP); and indoleamine 2,3-dioxygenase (IDO) (Coussens et al., 2013). This enables recruitment of FoxP3+CD4+ Treg cells, TH2-CD4+ T cells, TH2-polarized macrophages and monocytes, and B regulatory cells (Bregs). In response to TH2-mediated activation, myeloid cells commonly increase synthesis of angiogenic (e.g., VEGF), growth and/or survival (e.g., EGF, TNFα) factors that directly regulate epithelial cell proliferation, as well as tissue-remodeling enzymes (e.g., metallo-, cysteine, and serine proteases). These activities are remarkably pro-tumorigenic in that they nurture a TME favoring neoplastic cell survival and sustained proliferation (Balkwill et al., 2005). Simultaneous TH2 activation of macrophages and monocytes also increases expression of molecules, e.g., inducible nitric oxide synthase or Arginase 1, that directly and indirectly suppress CD8+ T cell proliferation, and cytokines such as IL-10 that inhibit DC maturation and antigen cross-presentation to T cells (Ruffell et al., 2014). Thus, TH2-type immune microenvironments are both tumor-promoting and immune-suppressive. Notably, in the colon, tumor-promoting immunity via IL-17 (TH17)-mediated activation of myeloid and lymphoid cells has been reported (Wang et al., 2009; Wu et al., 2009).
Tumor-promoting activities of the myeloid compartment
Owing to their established role in wound healing, we investigated the ability of myeloid cells infiltrating early benign tissues to foster malignancy. In mice prone to squamous carcinogenesis, mast cells and macrophages activate pro-neoplastic angiogenic and tissue-remodeling programs (Coussens et al., 1999). In other studies of mice bearing mammary carcinomas, macrophages could regulate neoplastic cell dissemination and metastasis via EGF-mediated paracrine interactions with neoplastic epithelial cells (Lin et al., 2001). In human cancers, multiple studies have reported that the presence of macrophages in stroma correlates with aggressive disease and outcome (Komohara et al., 2014). Macrophages are recruited into tumors following activation of colony stimulating factor-1 receptor (CSF1R) by either CSF1 or IL-34, two high-affinity ligands for CSF1R; the chemokine CCL2 may also facilitate macrophage recruitment (Qian et al., 2011). A CSF1-response gene-expression signature has been identified in 17–25% of breast cancers associated with decreased estrogen receptor and progesterone receptor expression (Beck et al., 2009); serum concentrations of CSF-1 correlate positively with breast tumor size and predict poor survival (Aharinejad et al., 2013). In addition, in two independent breast cancer cohorts, intratumoral macrophage presence was correlated with potentially prognostic tumor features (high grade, hormone receptor-negativity, basal-like subtype, and increased risk of death) (Komohara et al., 2014). Macrophages therefore serve as promising targets for novel therapeutic interventions, particularly for patients with high-risk disease. Conversely, favorable prognosis has been associated with some tumor types exhibiting increased macrophage infiltration, e.g., non-small-cell lung cancer, prostate, colorectal and gastric cancers (Komohara et al., 2014). Whether these distinctions reflect true differences in macrophage biology and function or arise due to discordant detection methodologies is unclear.
Neutrophils, on the other hand, are typically less abundant than macrophages in solid tumors, but their presence correlates with reduced survival in head and neck and breast cancers, and similar to macrophages, neutrophils develop polarized phenotypes that either favor or restrict tumor progression (Fridlender and Albelda, 2012). Recent studies identified granule products that suppress T cell function (Sippel et al., 2011). Neutrophil expansion in mammary carcinomas of mice bearing mutant p53 alleles is driven by T cell-derived IL-17; this results in systemic granulocyte colony-stimulating-factor (G-CSF)-dependent expansion and polarization towards a T cell-suppressive phenotype that facilitates metastatic dissemination and colonization (Coffelt et al., 2015). In contrast, neutrophils create a tumor-restrictive microenvironment in the lung that resists neoplastic progression and metastatic dissemination (Fridlender and Albelda, 2012).
Eosinophils, like other myeloid lineage cells, can exert cytotoxic immune-effector activities. Tumor-associated tissue eosinophilia (TATE) is associated with improved prognosis for a number of malignancies, including gastrointestinal, bladder and prostate cancers; in contrast, TATE is associated with poor outcome in Hodgkin’s lymphoma, cervical carcinoma, and oral squamous-cell carcinoma (Davis and Rothenberg, 2014). Eosinophils have been associated with degranulation and release of cytotoxic proteins that mediate tumor rejection; recent results also reveal their role in normalizing the vasculature to improve CD8+ T cell trafficking associated with tumor regression (Carretero et al., 2015).
Monocytes, once in tissues, can differentiate into macrophages and DCs. Two circulating monocyte populations have been identified: classical inflammatory monocytes that are CCR2HIGH, and non-classical patrolling monocytes that are CX3CR1HIGH (Geissmann et al., 2003). Recruitment of inflammatory monocytes into tissues is normally guided by the CCR2-CCL2 axis in response to parasitic or bacterial infections; in tumors, when CCR2HIGH monocytes are recruited, they can promote neoplastic cell survival and extravasation through VEGF and CSF1 production (Qian et al., 2011). CCR2HIGH monocytes promote survival of metastatic cells through a CCL3-dependent mechanism (Kitamura et al., 2015). CX3CR1HIGH monocytes instead patrol capillaries in response to the CXC3R1-CX3CL1 axis; in these locales, they are positioned to scavenge particles and debris, and thus are more likely to be found in wounds when inflammation is resolving. At sites of metastasis, CX3CR1HIGH monocytes recruit NK cells that in turn kill metastatic cells, thereby providing a potent survival advantage (Hanna et al., 2015). In pancreatic adenocarcinomas, activation of the Ras oncogene leads to increased expression of GM-CSF and recruitment of immature monocytes that subsequently suppress CD8+ T cell proliferation to enhance tumor progression (Pylayeva-Gupta et al., 2012), analogous to other tumor systems (Gabrilovich et al., 2012).
Mast cells, present in all vascularized tissues, respond to diverse stimuli by either secreting or releasing (via degranulation) biologically active compounds, e.g., proteolytic enzymes, heparin, histamine, prostaglandins, cytokines and chemokines. Mast cells are key for maintaining tissue homeostasis and best known for their effector functions following IgE-stimulated allergic responses and anaphylaxis (Metz et al., 2007). Mast cells have been implicated in the vascularization of a multitude of solid human tumor types, likely owing to their proteolytic products and high VEGF expression following activation (Coussens et al., 1999; Marichal et al., 2013) following CCL2-mediated recruitment where their bioactive mediators promote neoplastic progression.
Tissue-specificity of myeloid programing
While it is conceptually unclear how some myeloid cells adopt a TH2-or protumorigenic state to support neoplastic progression, some clues have emerged in recent genetic studies. Several groups revealed that lymphocytes drive initial myeloid cell programming to foster chronic inflammation in a tissue-specific manner. For example, during mammary branching morphogenesis and ductal carcinogenesis, cytokines derived from TH2-CD4+ T cells, e.g., IL-4 and -13, activate macrophages and monocytes infiltrating mammary tissue (DeNardo et al., 2009; Plaks et al., 2015). In neoplastic scenarios, signaling downstream of IL-4 receptors on monocytes and macrophages triggers protumorigenic TH2 gene-expression programs that activate tissue-remodeling cascades, via expression and activation of cathepsin proteases, and immune-suppressive programs, via upregulation of IL-10 and immune-checkpoint molecules (DeNardo et al., 2009; Gocheva et al., 2010; Mitchem et al., 2013; Ruffell et al., 2014). Mast cells and macrophages (as well as other myeloid cell types) are TH2-programmed in early squamous and pancreatic carcinomas by a diversity of pathways, which also include activation of immunoglobulin receptors (FcγRs) by immune complexes (ICs) (Affara et al., 2014; Andreu et al., 2010). ICs are composed of antigen-specific antibodies and complement proteins that variably activate FcR and complement receptors depending on composition of IC and status of the myeloid cell being activated (Karsten and Kohl, 2012). While these humoral immune-regulated paracrine programs were known to shape outcomes in chronic inflammatory diseases, recognition of their significance in solid tumors was paradigm shifting, and highlighted the significance of hard-wired tissue-specific programs shaping host response to disease. These data illustrate the diversity of pathways utilized by innate immune cells to propel cancer by directly enhancing tissue-based programs favoring survival of neoplastic cells, in concert with direct and indirect activation of programs to extinguish cytotoxic immune responses aiding immune escape (Figure 2).
TH2-based targets for anti-cancer therapy
The collective evidence supports a protumorigenic role for chronic inflammation in cancer but also suggests this inflammation is malleable, akin to the healing of acute wounds during which immune cells toggle between TH1 and TH2 states. Thus, the hypothesis that TH2-driven myeloid cells could be re-programmed, or at least neutralized, to reduce the presence or immunosuppressive status of macrophages, trigger anti-tumor immunity, and/or suppress tumor growth has been tested in several tissue-specific cancer models. We and others have evaluated CSF1-neutralizing monoclonal antibodies (αCSF1 mAB) and small-molecule CSF1R inhibitors for their ability to suppress macrophage survival and/or presence in tumors, in combination with chemotherapy (CTX) or radiation therapy (RT) (DeNardo et al., 2011; Ruffell et al., 2014; Shiao et al., 2015). These studies reveal increased chemo-and radiation sensitivity associated with anti-tumor immune responses directed by CD8+ T cell infiltration of tumors, culminating in reduced primary tumor growth and metastasis with increased survival. Other preclinical studies revealed that CSF1/CSF1R-blockade, as monotherapy or combined with CTX/RT, improved outcomes for glioma, prostate and pancreatic adenocarcinoma, and melanoma, where CSF1R-blockade improved antitumor efficacy of immune checkpoint blockade and adoptive T cell therapy (reviewed in: (Ruffell and Coussens, 2015)). Importantly, administration of RG7155, a CSF1R-activation-blocking mAb, in patients with diffuse-type giant cell tumors reduced CSF1R+CD163+ macrophage levels; this translated into objective clinical responses (Ries et al., 2014). Treatment of tenosynovial giant-cell tumors with a small-molecule inhibitor of CSF1R kinase increased progression-free survival and improved outcomes as a monotherapy (Tap et al., 2015). The macrophage presence in tumors has also been therapeutically manipulated by targeting the macrophage signaling protein acting through its transmembrane receptor kinase RON, wherein activation of RON in macrophages favors conversion of micrometastatic lesions to overt metastases by suppressing antitumor immune responses. Functional RON blockade in preclinical models potentiates tumor-specific CD8+ T cell responses, indicating that RON inhibitors may also improve outcomes for cancer patients (Eyob et al., 2013).
Bruton’s tyrosine kinase (BTK) is an attractive target as BTK is activated downstream of the B-cell receptor and FcγR and PI3Kγ in some myeloid subsets (Smith et al., 2001). In vitro, tumor cell challenge via co-culture with splenic cells from B-cell-deficient versus B-cell-proficient mice revealed that IFNγ release from CD8+ and NK cells is increased when B cells are absent, whereas presence of B cells or B-cell-derived IL-10 was associated with reduced IFNγ (Inoue et al., 2006). Though these in vitro studies indicate that B cells can direct T cell responses, the role of myeloid cells as mediators of these responses is now clear and indicate that therapies targeting common pathways in B cells and/or myeloid cells, such as SYK, BTK, PI3Kγ, may be efficacious in solid tumors, analogous to efficacy observed for BTK and PI3Kδ inhibitors in B-cell malignancies (Hendriks et al., 2014). This concept was recently validated preclinically, whereby BTK inhibition enhanced survival of mice bearing pancreatic adenocarcinoma (Gunderson et al., 2016; Masso-Valles et al., 2015), neuroendocrine cancers (Soucek et al., 2011), and other subcutaneous tumors (Sagiv-Barfi et al., 2015) where a common feature was reduced inflammation and inflammatory desmoplasia with evidence of macrophage repolarization.
If these preclinical findings are any indication, immune therapies targeting macrophages and/or other protumorigenic immune cells could alter the human tumor immune microenvironment in a way that fosters the cytotoxic properties of CD8+ T cells. As immune-checkpoint inhibitors of pathways regulating T cell activity are proving efficacious for subsets of cancer patients, we predict that combining these two immune-based approaches represents a compelling clinical opportunity. However, it is likely that not all tumors will respond; thus, identifying predictive biomarkers and correlates of therapeutic response is a top priority. Based on preclinical data evaluating macrophage antagonists and checkpoint inhibitors, we predict that biomarkers of response will also be reflected by changes in peripheral blood. Such biomarkers will form the basis for simple, non-invasive diagnostic and/or prognostic screens aiding early detection in susceptible populations (Figure 4).
Figure 4. Multi-modal biomarker-based approach for optimal immune-mediated tumor control.
Cancer medicine is evolving. Going forward, individuals with cancer will be evaluated for biomarkers enabling stratification to determine most optimal combinations for therapy based on tumor-based and systemic biomarkers. Eventually, all patients with cancer will be treated with checkpoint inhibitors, either directly or after interventions targeting inflammation (for example with TH2-blockade therapies, radiation therapy or epigenetic modulation), or vaccination via DCs to boost T cell repertoires, or adoptive T cell transfer. Based on the known tissue-embedded programs empowered to control auto-immunity, it is reasonable to anticipate that a majority of patients will develop acquired resistance followed by immune escape; this will lead to the next cycle of treatments incorporating multi-modal biomarkers (e.g., based on microbiome phenotype, circulating free DNA (cfDNA), circulating cytokine levels) and perhaps NK cells recognizing loss of MHC class I by neoplastic cells, thus rendering them invisible to T cells. Cytotoxic treatments such as with NK cells or standard cytotoxic therapy (CTX or RT), or oncolytic viruses will release neo-antigens that can be used for generation of the next round of effector T cells. Whole exome sequencing (WES) of tumor samples as well as cfDNA will yield information on mutational load that can in turn be used as one class of neoantigens for vaccination and priming of new T cell repertoires. T cell receptors (TCR) can be assessed using genomic approaches enabling sequencing of TCRβ chains to assess repertoire diversity. Given the importance of T cell specificity for relevant antigens, strategies enabling paired sequencing of α and β TCR chains will be invaluable as well as high-throughput tetramer analysis. In addition, RNAseq and epigenetic analysis of tumors and their infiltrates will enable assessment of the type and flavor of inflammation. Future studies will incorporate metabolomics to this biomarker portfolio.
In preclinical models, regardless of tumor or approach, TH1 immunity emerges when dominant TH2-driver pathways are attenuated; when concomitant with cytotoxic therapy, tumor growth stalls or regresses by CD8+ T cell-dependent mechanisms. These findings highlight the importance of neutralizing pro-tumor inflammation as a therapeutic strategy, and indicate that tumor-infiltrating CTLs can be mobilized in tumors with low mutational burdens. These data also highlight the clinical need for biomarkers that identity tissue-specific programs driving TH2 immune responses; such data is needed to inform precision medicine strategies employing TH2-blockade, in concert with other immune, targeted or cytotoxic approaches (Figure 4).
Immune-targeted therapies focused on T cells
Basic principles
Cancer immunotherapy historically relied on two principal mechanisms of action: i) “passive” immunotherapy via provision of anti-tumor antibodies, e.g., Trastuzumab (αHER2 mAB) or Rituximab (αCD20 mAB), or adoptive transfer of cytotoxic T and NK cells; and ii) “active” immunotherapy that mobilizes the patient’s immune cells via checkpoint blockade, i.e., administration of antibodies directed against immune-regulatory checkpoint molecules expressed on T cells, or via vaccines that expand antigen-specific T cells. In all circumstances, T cells are the drug—and we are learning that T cells have the ability to clinically control some cancers (Postow et al., 2015). T cells can be targeted in three major ways: 1) by being liberated by checkpoint inhibitors; 2) through adoptive transfer when T cells are missing, as validated by the clinical success of genetically engineered T cell therapies: and 3) through induction in vivo by vaccination or endogenous mechanisms subsequent to other anti-cancer therapies (e.g., CTX, targeted therapies or anticancer antibodies) (Palucka and Banchereau, 2013).
Immune checkpoint blockade (ICB) can unleash the power of naturally occurring T cells by eliminating negative signals that block T cell function (Pardoll, 2012). ICB has produced durable clinical responses and improved survival across a variety of cancers (Topalian et al., 2015). CTL expansion and function is carefully regulated by Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), Programmed cell death protein-1 (PD-1) and other molecules, so as to maintain a delicate balance between the resolution of infection, elimination of infected cells, protection of tissue homeostasis and prevention of autoimmune attack. CTLA-4 is a cell-surface receptor expressed by activated T cells with homology to the T cell costimulatory molecule CD28. Although CD28 and CTLA-4 are both ligands for B7-1 (CD80) and B7-2 (CD86), they serve opposing roles in regulating T cell activation. CD28 provides costimulatory signals required for T cell activation, whereas CTLA-4 negatively modulates T cell responses by raising the activation threshold for T cell priming; thus, CTLA-4 is probably most important during priming. PD-1 binds programmed-death-ligand 1 (PD-L1; a.k.a., B7-H1 or CD274) expressed by neoplastic cells, various immune cells, mesenchymal support cells and vascular cells; this interaction negatively regulates T cell activation when engaged with an APC and/or effector function when engaged with other PD-L1-positive cells. Indeed, binding of PD-L1 to its receptors suppresses T cell migration, proliferation and restricts cancer cell killing (Topalian et al., 2015); thus, PD-1 is probably most important in regulating effector functions after CD8+ T cells are activated. PD-1 and CTLA-4 regulate distinct phases of T cell differentiation and function and their inhibition might need to be optimally phased for maximum efficacy. This concept needs to be incorporated in the next generation of clinical trials of combination therapy regimens, especially when combined with vaccines or agents that reprogram myeloid cells to foster a TH1-type activation state.
Indeed, combination therapies targeting the two checkpoints, i.e., CTLA-4 and PD-1, further increase progression-free survival in patients with metastatic melanoma (Larkin et al., 2015); however, in other cancers these responses are present in fewer patients. Resolving the natural and acquired resistance to checkpoint inhibition therapy represents the next frontier in basic research and clinical development (Figure 4). As the effector arm of checkpoint inhibition, T cells could underpin the major resistance mechanisms for checkpoint blockade. Thus, non-responding patients might actually lack naturally occurring T cells with specificity against neoplasias, and/or their T cells could be held hostage and rendered dysfunctional in TMEs via pathways other than checkpoints, such as immune-suppressive microenvironments directed by TH2-activated myeloid, Treg or Breg cells (Coussens et al., 2013). Links between treatment resistance and T cell shortage are supported by recent findings that tumor-specific mutations generate neo-antigens that in turn may drive anti-tumor responses. Indeed, whole-exome sequencing of malignant melanomas from patients treated with CTLA-4 blockers demonstrated an association between mutational load and degree of clinical benefit (Snyder et al., 2015), however in other melanoma cohorts, recurrent neo-antigen peptide sequences were not found to predict responder populations (Van Allen et al., 2015). In non-small-cell lung cancers treated with αPD-1 mAb, higher mutation burden in tumors was associated with durable clinical benefit and progression-free survival (Rizvi et al., 2015). Neo-antigens arising as products of somatic mutations are not presented in the thymus; thus, they can be recognized by the immune system as foreign antigens, similar to viral antigens or organ transplants, because the T cells have not been eliminated or tolerized. These concepts were put forward early (Srivastava, 2000), but validated only recently (Schumacher and Schreiber, 2015), thanks to the availability of massively parallel sequencing.
Cancer Vaccines
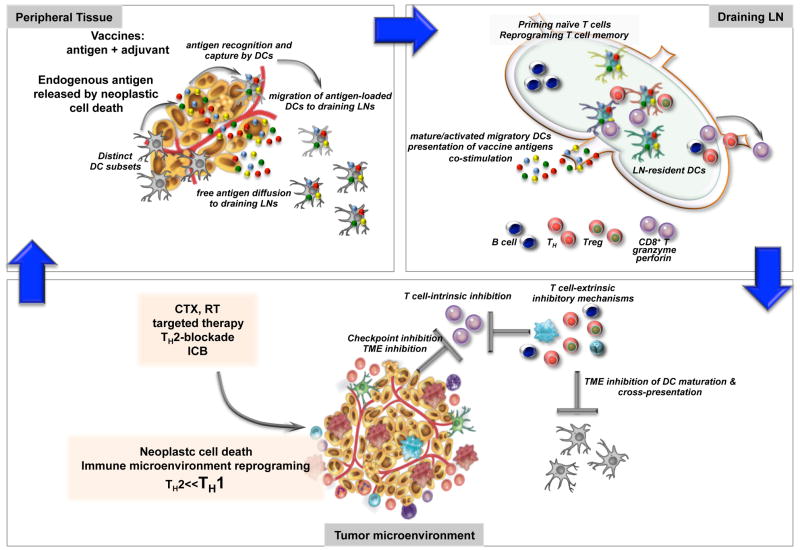
Patients may fail or resist checkpoint therapy owing to a lack of pre-existing T cell infiltrates. Therefore, vaccination and adoptive transfer strategies to first induce and expand the breadth of endogenous T cell responses could prove useful. Vaccines are composed of antigens and adjuvants. Responses to vaccination and adjuvants involve DCs that capture and present vaccine antigens, thereby facilitating differentiation of lymphocytes and subsequent immunity (Figure 3). DCs also integrate the adjuvant signals and determine the quality of induced immune responses.
Figure 3. The priming of cancer immunity.
The cycle of anti-tumor immunity starts presumably with presentation of cancer antigens liberated in the process of cell turn-over; this same pathway can be followed for vaccination as illustrated herein. Antigens are sensed and captured either by tissue resident DCs or by DCs in draining lymph nodes (LNs). DCs initiate an immune response by presenting captured antigens, in the form of peptide–major histocompatibility complex (MHC) molecule complexes, to naive (that is, antigen inexperienced) T cells in lymphoid tissues. When compared with other APCs, such as macrophages, DCs are extremely efficient and can elicit very low numbers of T cells to respond. Naïve CD8+ T cells differentiate into CTLs in lymphoid organs upon encounter with DCs presenting tumor-derived peptides in the context of co-stimulation through CD8, CD70 and 4-1BB, as well as DC-derived cytokines such as IL-12 and IL-15. Naive CD4+ T cells can give rise to helper cells (e.g., TH) with distinct cytokine profiles, or to regulatory T cells (Treg) whose role is to dampen the immune response. T cells migrate through blood and lymphatics. Upon arrival in tumor beds, CD8+ T cells must confront numerous barriers including: i) intrinsic regulators, for example CD28-CTLA-4, PD1-PDL1, and ILTs, as well as extrinsic regulators cells such as Tregs, Bregs or myeloid cells; ii) a corrupted TME with pro-tumor inflammation; iii) impaired cross presentation due to TME-based DC inhibition; iv) antigen loss and immune evasion of tumor target; and v) tissue-specific alterations such as fatty cells in breast cancer or desmofibrosis in pancreatic cancer stroma. Killing of tumor cells either via T cells or by standard therapy can lead to endogenous antigen release and DC activation so called “endogenous vaccination” thereby closing the cycle. Inevitable to this is the induction of tissue resistance mechanisms, for example, expression of PD-L1 on neoplastic cells, as the result of powerful effector immunity including actions of IFNγ. Thus, future immunotherapy approaches will be based on combinations of different therapeutics targeting distinct components of this cycle, for example, via intratumoral delivery of activating agents able to reprogram the function of infiltrating leukocytes.
Several phase III clinical trials testing various cancer vaccine platforms, including DC-based and viral-vector-based vaccines, are ongoing. These exogenous vaccine platforms will need to be accompanied by high-throughput genomics to incorporate personalized cancer-specific mutations and candidate peptide antigens. Indeed, proof-of-concept trials in patients with advanced melanoma demonstrated that naturally occurring neoantigen-specific immunity was enhanced by vaccination with DCs loaded with patient-specific peptides (Carreno et al., 2015). Another concept is endogenous vaccination based on antigen released upon standard CTX/RT or oncolytic viruses (i.e., viruses that preferentially infect and kill cancer cells); this however requires effective antigen presentation to generate therapeutic T cell immunity (Palucka and Banchereau, 2013). DCs are skewed by tumors towards pro-tumor immunity; thus, reprogramming to foster TH1-skewed mature functionality in vivo is critical for success of endogenous vaccination. Our understanding of cancer genomics, the biology of antigen presentation and T cell biology has advanced impressively and continues to increase; this knowledge will feed into the development of the next-generation cancer vaccines that, combined with checkpoint inhibitors, hold promise for improving outcomes for cancer patients (Figure 4).
DC vaccines can be exploited as anti-cancer therapeutics through various strategies, including: non-targeted peptide/protein and nucleic-acids-based vaccines captured by DCs in vivo; vaccines composed of antigens directly coupled to anti-DC-antibodies; or vaccines composed of ex vivo–generated DCs loaded with tumor antigens (Palucka and Banchereau, 2013). DCs are also engaged in response to complex vaccine preparations such as GVAX®, a tumor-cell-based vaccine in which cancer cells genetically modified to express GM-CSF attract and activate DCs (Le et al., 2010). Other vaccine platforms are based on recombinant-attenuated Listeria monocytogenes (Lm), an intracellular bacterium that targets DCs in vivo and utilizes MHCI and II antigen-processing pathways (Le et al., 2012), as well as intratumoral delivery of oncolytic viruses; these can be modified to express GM-CSF to attract DCs and lymphocytes at the lysed tumor site (Russell et al., 2012). Finally, pioneering studies from Ralph Steinman and Michel Nussenzweig demonstrated the principle of targeting antigens to DCs in vivo through coupling of antigens to antibodies specific to DC surface receptors such as DEC205 or DCIR (Bonifaz et al., 2002; Soares et al., 2007). Importantly, in the absence of adjuvants, targeting antigens to DEC205+ DCs in vivo induces antigen-specific tolerance (Hawiger et al., 2001). Administration of these complex vaccines with DC activators such as TLR3, TLR7-8, or CD40 agonists enables maturation of DCs and consequent establishment of immunity rather than tolerance (Bonifaz et al., 2002; Soares et al., 2007). It remains to be seen which vaccine platform will be most effective at priming and boosting T cells in patients; this clearly represents the next frontier in research.
T cell-dependent nature of cytotoxic and targeted therapy
Cancer medicine evolved largely based on the principle that rapidly proliferating malignant cells can be eradicated by cytotoxic regimens (CTX or RT) or by targeted drugs attacking attributes of mutationally corrupted cells. As discussed above, the recent advent and remarkable efficacy of immune-checkpoint inhibitors revealed the clinical potential of harnessing endogenous mechanisms of anti-tumor immunity in tumors harboring significant mutational burdens. Upon reflection, however, it is appreciated that conventional cytotoxic approaches modulate the composition and functional bioactivity of intratumoral leukocytes, in addition to effects on neoplastic cells (Galluzzi et al., 2015). Furthermore, in some scenarios, the efficacy of neoadjuvant CTX correlates with increased presence of intratumoral immune-effector T cells (Galluzzi et al., 2015). These correlations are not limited to cytotoxic regimens—the tyrosine kinase inhibitor imatinib also leads to increased presence of CTLs and NK cells in gastrointestinal tumors in a manner that correlates with disease outcome (Kroemer et al., 2013), while efficacy against chronic myelogenous leukemia can be reversed by co-administration of type I IFN (Galluzzi et al., 2015). A recent study in breast cancer also revealed that efficacy of transtuzumab emtansine is linked to elicitation of anti-tumor immune responses (Muller et al., 2015).
Malignant cells can emit danger signals, albeit distinct from those of normal tissue that are sensed by immune cells and thus are antigenic. Increased antigenicity is linked to either mutational burden, where peptides from mutant proteins are presented by MHC molecules (Gubin et al., 2014), or to ectopic expression of cancer testis or oncofetal antigens typically only expressed during embryonic or fetal development (Whitehurst, 2014). The increased adjuvanticity of neoplastic cells is linked to metabolic stress caused by their sustained proliferation, and to their ability to adapt and survive in hypoxic TMEs (Krysko et al., 2012). Furthermore, preclinical data have emerged supporting the proposition that tumors treated with conventional CTX engage antigenic and adjuvant immune-mediated mechanisms. In murine tumor models, the anti-neoplastic effects of anthracyclines are significantly reduced when either γδ or CD8+ T cells are depleted, but not when B or NK cells are absent, DC infiltration is blocked or corrupted, immune-stimulatory type I IFNs or IL-17 are lacking, or DAMP-mediated recruitment and activation of effector cells is thwarted (Kroemer et al., 2013). Cyclophosphamide, oxaliplatin and bortezomib similarly rely on immune-mediated mechanisms for their efficacy; these commonly used cytotoxics elicit effector cell activity via plasma membrane exposure of calreticulin and release of the chromatin-binding protein high mobility group box 1 (HMGB1). This in turn fosters DC maturation and TLR4 and RAGE activation (Apetoh et al., 2007), thus increasing adjuvanticity of malignant cells.
Taxanes, broadly used microtubule inhibitors, and vinca alkaloids promote polyploidization due to mitotic interference, thus leading to endoplasmic reticulum stress responses favoring calreticulin exposure and immune-mediated elimination (Senovilla et al., 2012). Clinically, docataxel, vinorelbine and cisplatin all lead to increased abundance of circulating CTLs, and decreased presence of Treg cells and immature myeloid cells harboring T cell-suppressive activity, this latter effect is also shared by gemcitabine, a common CTX for pancreatic adenocarcinomas, and 5-fluoruracil. Interestingly, paclitaxel is also a TLR4 ligand and thus enhances T cell priming by DCs (Pfannenstiel et al., 2010).
Cyclophosphamide also provokes relocalization of intestinal gram-positive bacteria to secondary lymphoid organs, resulting in generation of TH17 cells secreting IL-17 and IFNγ that promote anti-tumor immune responses (Viaud et al., 2013). In murine tumor models, therapies targeting TH2-based programs (e.g., CSF1R or RON antagonists, BTK or SYK inhibitors, B cell-depletion, αIL-4 or αIL-13 mAbs) enhance efficacy of either CTX or RT by T cell-dependent mechanisms (Ruffell and Coussens, 2015). Perhaps the most compelling evidence is that provided by recent data showing that immune-checkpoint blockade, when combined with CTX, improves overall survival in several cancer types beyond CTX alone (Topalian et al., 2015). The ability of these agents to activate adaptive stress-response pathways and send danger signals operative as immunologic adjuvants inherently increases the antigenicity of tumors even when mutational burden is low. These untoward effects can be capitalized upon to improve outcomes for individuals with cancer.
A role for the microbiome in regulating systemic cancer risk, and response to therapy
If the precision medicine equation wasn’t sufficiently complicated by neoplastic cell genomics, epigenomics, host immune responses and the TME, mounting evidence points to an additional consideration when attempting to stratify patients and predict therapy response: the host microbiome. The context and composition of common microorganisms living in the gut not only shapes local immune responses but also regulates systemic immunity, and thus impacts the risk of and progression to malignancy and the response to anti-cancer therapies. Intra-abdominal infections and use of antibiotics has long been associated with increased incidence of colorectal cancer (Wang et al., 2014). In mouse models, attenuating or selectively altering the composition of gut microorganisms influences both the incidence and progression of cancer (Zitvogel et al., 2015). Intestinal microorganisms not only impact local risk of tumorigenesis, but also influence neoplastic progression distally by altering inflammatory and metabolic circuitry. These experimental results correlate with epidemiologic data revealing increased incidence of breast cancer in women with significant history of antibiotic use (Zitvogel et al., 2015).
Gut microbiota composition is dramatically impacted by common anti-neoplastic drugs including RT, allogeneic stem-cell transplantation and select CTXs, notably 5-fluorouracil and irinotecan (Zitvogel et al., 2015). Along these lines, the gut microbiota affects the amenability of some tumor types to therapy by impacting regulatory aspects of the immune response. Examples include translocation of gut microbiota across the intestinal epithelium in response to lymphodepleting irradiation where DCs are inadvertently activated, leading to altered serum cytokines and improved responses to adoptively transferred CTLs; these beneficial effects are abated by antibiotics (Paulos et al., 2007). Similarly, cyclophosphamide alters composition of gut microbiota resulting in translocation of gram-positive bacteria into secondary lymphoid organs wherein pathogenic TH17 and memory TH1 cells are activated; tumors grown in germ-free mice or antibiotics tropic for gram-positive bacteria exhibit reduced TH17 responses and tumor-resistance to cyclophosphamide (Viaud et al., 2013). Antibiotic eradication of gram-positive bacteria also impairs the efficacy of CpG-oligonucleotide immunotherapy and platinum CTX by altering the myeloid cells within the TME (Iida et al., 2013). Bifidobacterium occupancy supports anti-tumor immunity against melanoma and improves the efficacy of αPD-L1 and αCTLA-4 mAb therapy by altering DC activity leading to improved antigen-specific CD8+ T cell function—these effects were reduced by ampicillin, colistin or streptomycin, but enhanced by vancomycin due to preferential enhancement of Bacteroidales colonization (Vetizou et al., 2015). These data underscore the impact of gut commensals on therapeutic responsiveness.
Could selectively manipulating the gut microbiota impact risk of developing cancer, limit incidence of select tumor types, and/or improve activity of some anti-cancer therapies? Zitvogel and colleagues have proposed four distinct approaches for manipulating the gut microbiota to boost cancer therapy: i) preferential use of antibiotics selective for untoward bacterial species; ii) increased use of probiotics; iii) increased use of prebiotics to stimulate healthy gut colonization; and iv) use of postbiotics, nonviable products of microbiota that exert biological activities in hosts (Zitvogel et al., 2015). Prospective stool analysis and monitoring in cancer patients receiving therapy will surely reveal biomarkers that, if harnessed, could improve patient stratification and/or support new microbiota-based strategies for boosting therapeutic responsiveness, e.g., fecal transplant of beneficial species.
Multi-modal tumor and systemic biomarkers for stratification and resistance monitoring
A major clinical goal is to understand the multi-modal tissue-based and systemic pathways regulating therapy responses so as to minimize resistance and maximize efficacy of cancer medicine (Figure 4). Whether therapies target tumor-intrinsic pathways, host pathways or commensal microbiota, it is clear that understanding non-genomic mechanisms of resistance from an integrated standpoint is critical.
Understanding which immune cell types are present in and around a tumor currently provides invaluable retrospective information regarding tumor ecology and/or tumor response to therapy. However, we must improve our ability to integrate information on not only the complexity of leukocytes in tumors, but also their geography in tumor nests and stroma. Immune cells are scattered in tumor core and within tumor stroma, in invasive margins and in organized lymphoid structures often distant from neoplastic cells. Investigating the mechanisms governing formation of tertiary lymphoid structures (TLSs) found in numerous cancers represents a new frontier for biomedical research. Such topology has been reported by Galon and colleagues to be clinically important in colorectal cancer, where a statistically significant correlation between immune cell density and patient outcome was revealed (Galon et al., 2006). Moreover, development of TLS in individuals with pancreatic adenocarcinoma treated with vaccines correlated with improved clinical outcomes (Le et al., 2015). Furthermore, compared with single-region analysis, combined analysis of the tumor core and invasive margins improved the accuracy of survival prediction in different patient groups (Galon et al., 2006). These early results form the basis for immune stratification of patients, or the so-called Immunoscore, and its coordinated assessment in the clinic (Ascierto et al., 2013). An international consortium has been initiated to validate and promote the Immunoscore in routine clinical settings (Ascierto et al., 2013); results of this international effort may lead to implementation of the Immunoscore as a new classification metric, designated TNM-I (TNM-Immune).
Will the Immunoscore provide enough additional information to prospectively predict response to therapy? Likely not. We predict that integrating the Immunoscore with additional metrics will be critical for guiding patient stratification and phasing of combinatorial therapies. Such metrics will include genomic and exomic features of neoplastic cells (through sequencing of neoplastic cells themselves or cell-free circulating tumor DNA (ctDNA)), tracking the expansion of tumor-specific CD4+ and CD8+ T cells, monitoring serum cytokine fluxes, and evaluating the composition and health of commensal bacteria (Figure 4). Serum cytokines have long been enigmatic due to their labile nature and the detection limitations of conventional methodologies. That said, serum biomarker signatures are now able to discern asymptomatic early stage pancreatic cancer from healthy controls with 96% accuracy (Ghatnekar et al., 2013), and can be used to monitor the pharmacodynamics of CSF1R-targeted therapies (Butowski et al., 2015). Moreover, transcriptional profiling of blood monocytes in renal cell carcinoma identifies biomarkers correlating with tumor staging (Chittezhath et al., 2014), and mRNA sequencing of tumor-educated blood platelets distinguishes cancer patients from healthy individuals with 96% accuracy (Best et al., 2015). Thus, multi-modal functional diagnostic strategies integrating the tumor, host and commensals will likely forge the advent of next-generation precision bioinformatics to match patients with appropriately combined and phased anti-cancer therapies.
OncoImmunology treatment paradigm
Future immunotherapies will be based on cycles of interventions designed to boost and modulate anti-cancer immunity (Figure 4). Indeed, as we re-discover and refine the fundamental principles of tumor immunology, it is increasingly clear that curing cancer might not be a realistic goal. Rather, aiming for a continuum of treatment cycles designed and based on mechanistic in vivo studies and in-depth analysis of each patient’s tumor will be necessary for optimizing outcomes. Clinical trials with checkpoint inhibitors teach us that in situ immune infiltration is critical for tumor regression; however, not all immune infiltrates are equal and, as discussed throughout this article, the quality of immune response is a critical factor for therapeutic success. This in turn is determined by underlying inflammation, which we assert must become a staging parameter, along with classical pathology-based schemas and the Immunescore. It will also need to be established to what extent inflammation, which clearly plays a role in epithelial tumors, impacts other tumor types, e.g., melanoma or sarcomas. Additional parameters pertain to the specificity of infiltrating T cells against cancer antigens, as again, the infiltrate with passenger T cells might not be therapeutically useful and should be tested. Eventually all patients will be treated with checkpoint inhibitors, either directly or after interventions targeting inflammation, by vaccination to boost T cell repertoires, or by adoptive T cell transfer. The majority of patients will subsequently develop acquired resistance followed by immune escape; this will lead to the next cycle of treatments incorporating multi-modal biomarkers (e.g., based on microbiome phenotype, ctDNA, circulating cytokine levels) and perhaps NK cells recognizing loss of MHC class I by neoplastic cells, thus rendering them invisible to T cells. Cytotoxic treatments, such as with NK cells, standard CTX/RT or oncolytic viruses, will release neo-antigens that can be used to generate the next round of effector T cells. To this latter point, we must fully understand the rules of T cell priming in vivo in humans, identify the most effective ways to utilize DCs for priming, and develop strategies for mobilizing the naïve T cell repertoire from the thymus in adults (Sportes et al., 2008). In later rounds of therapy, the scope of neo-antigens will likely be broadened as, in addition to somatic mutations, neo-antigens can be generated via epigenetic and post-translational regulation. Last but not least, the role of Tregs, so well established in murine cancer, will need to be redefined in humans. Resolving all of these challenges will surely keep us busy for a long while.
Acknowledgments
The authors thank members of the Palucka and Coussens laboratories for critical insight and discussions, Drs. Amanda Lund and Jeffrey Nolz for critical feedback, Dr. Anna Lisa Lucido for help with editing the manuscript and all authors contributing to studies discussed herein, but not mentioned due to space consideration. AKP thanks Dr. Jacques Banchereau, a long-time collaborator. The authors acknowledge support from the NIH/NCI and Susan B. Komen Foundation (AKP and LMC), DOD BCRP Era of Hope Scholar Expansion Award, a Stand Up To Cancer – Lustgarten Foundation Pancreatic Cancer Convergence Dream Team Translational Research Grant (SU2C-AACR-DT14-14), and Brenden-Colson Center for Pancreatic Care (to LMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
A. Karolina Palucka, Email: karolina.palucka@jax.org.
Lisa M Coussens, Email: coussenl@ohsu.edu.
References
- Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharinejad S, Salama M, Paulus P, Zins K, Berger A, Singer CF. Elevated CSF1 serum concentration predicts poor overall survival in women with early breast cancer. Endocrine-related cancer. 2013;20:777–783. doi: 10.1530/ERC-13-0198. [DOI] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. FcRgamma activation regulates inflammationassociated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunological reviews. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Ascierto PA, Capone M, Urba WJ, Bifulco CB, Botti G, Lugli A, Marincola FM, Ciliberto G, Galon J, Fox BA. The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:54. doi: 10.1186/1479-5876-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Beck AH, Espinosa I, Edris B, Li R, Montgomery K, Zhu S, Varma S, Marinelli RJ, van de Rijn M, West RB. The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin Cancer Res. 2009;15:778–787. doi: 10.1158/1078-0432.CCR-08-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, Schellen P, Verschueren H, Post E, Koster J, et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, Cloughesy TF, Marimuthu A, Haidar S, Perry A, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age-related degenerative disease? Semin Cancer Biol. 2011;21:354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigenspecific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J, et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41:815–829. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015 doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes & development. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2:1–8. doi: 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annual review of immunology. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Eyob H, Ekiz HA, Derose YS, Waltz SE, Williams MA, Welm AL. Inhibition of ron kinase blocks conversion of micrometastases to overt metastases by boosting antitumor immunity. Cancer discovery. 2013;3:751–760. doi: 10.1158/2159-8290.CD-12-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Ghatnekar O, Andersson R, Svensson M, Persson U, Ringdahl U, Zeilon P, Borrebaeck CA. Modelling the benefits of early diagnosis of pancreatic cancer using a biomarker signature. International journal of cancer Journal international du cancer. 2013;133:2392–2397. doi: 10.1002/ijc.28256. [DOI] [PubMed] [Google Scholar]
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes & development. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, et al. Bruton’s Tyrosine Kinase (BTK)- dependent immune cell crosstalk drives pancreas cancer Cancer Discov. 2016 doi: 10.1158/2159-8290.CD-15-0827. Published OnlineFirst December 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–990. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser LM, Sadanandam A, Kuo WL, Benz SC, Goldstein TC, Ng S, Gibb WJ, Wang NJ, Ziyad S, Tong F, et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci U S A. 2012;109:2724–2729. doi: 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14:219–232. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Karsten CM, Kohl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology. 2012;217:1067–1079. doi: 10.1016/j.imbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Kerbel R. Anti-angiogenesis in cancer; met and unmet goals - an interview with Robert Kerbel by Francesco Bertolini. The International journal of developmental biology. 2011;55:395–398. doi: 10.1387/ijdb.103217fb. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunological reviews. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer science. 2014;105:1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual review of immunology. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–298. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Dubenksy TW, Jr, Brockstedt DG. Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol. 2012;39:311–322. doi: 10.1053/j.seminoncol.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer journal. 2010;16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc’h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1:269–279. doi: 10.1158/2326-6066.CIR-13-0119. [DOI] [PubMed] [Google Scholar]
- Masso-Valles D, Jauset T, Serrano E, Sodir NM, Pedersen K, Affara NI, Whitfield JR, Beaulieu ME, Evan GI, Elias L, et al. Ibrutinib exerts potent antifibrotic and Palucka and Coussens Inflammation and Cancer 31 antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015;75:1675–1681. doi: 10.1158/0008-5472.CAN-14-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunological reviews. 2007;217:304–328. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Annu Rev Immunol. 2012 doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Muller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7:315ra188. doi: 10.1126/scitranslmed.aac4925. [DOI] [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaks V, Boldajipour B, Linnemann JR, Nguyen NH, Kersten K, Wolf Y, Casbon AJ, Kong N, van den Bijgaart RJ, Sheppard D, et al. Adaptive Immune Regulation of Mammary Postnatal Organogenesis. Dev Cell. 2015;34:493–504. doi: 10.1016/j.devcel.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B. The varieties of immunological experience: of pathogens, stress, and dendritic cells. Annu Rev Immunol. 2015;33:563–606. doi: 10.1146/annurev-immunol-020711-075049. [DOI] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras- Induced GM-CSF Production Promotes the Development of Pancreatic Neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breasttumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. Mucosal Imprinting of Vaccine-Induced CD8+ T Cells Is Crucial to Inhibit the Growth of Mucosal Tumors. Sci Transl Med. 2013;5:172ra120. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- Shepard HM, Lewis GD, Sarup JC, Fendly BM, Maneval D, Mordenti J, Figari I, Kotts CE, Palladino MA, Jr, Ullrich A, et al. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol. 1991;11:117–127. doi: 10.1007/BF00918679. [DOI] [PubMed] [Google Scholar]
- Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res. 2015;3:518–525. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, Waziri A. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res. 2011;17:6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays. 2001;23:436–446. doi: 10.1002/bies.1062. [DOI] [PubMed] [Google Scholar]
- Snyder A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA- 4 blockade. N Engl J Med. 2015;372:783. doi: 10.1056/NEJMc1415938. [DOI] [PubMed] [Google Scholar]
- Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-{gamma} by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007 doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L, Buggy JJ, Kortlever R, Adimoolam S, Monclus HA, Allende MT, Swigart LB, Evan GI. Modeling pharmacological inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia. 2011;13:1093–1100. doi: 10.1593/neo.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. Immunotherapy of human cancer: lessons from mice. Nat Immunol. 2000;1:363–366. doi: 10.1038/808795. [DOI] [PubMed] [Google Scholar]
- Steinman R. Cancer therapeutics: time to swim downstream? The oncologist. 2011;16:1479–1480. doi: 10.1634/theoncologist.2011-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annual review of immunology. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- Stout RD, Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989;142:760–765. [PubMed] [Google Scholar]
- Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, Chmielowski B, Staddon AP, Cohn AL, Shapiro GI, et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N Engl J Med. 2015;373:428–437. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Henley SJ, Gansler T. Inflammation and cancer: an epidemiological perspective. Novartis Found Symp. 2004;256:6–21. [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MH, Goldinger SM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Chang CH, Lin JW, Wu LC, Chuang LM, Lai MS. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with Type 2 diabetes mellitus. International journal of cancer Journal international du cancer. 2014;135:956–967. doi: 10.1002/ijc.28738. [DOI] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehurst AW. Cause and consequence of cancer/testis antigen activation in cancer. Annu Rev Pharmacol Toxicol. 2014;54:251–272. doi: 10.1146/annurev-pharmtox-011112-140326. [DOI] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, Anguiano E, Pourpe S, Goller K, Pascual V, et al. Human CD1c(+) Dendritic Cells Drive the Differentiation of CD103(+) CD8(+) Mucosal Effector T Cells via the Cytokine TGF-beta. Immunity. 2013;38:818–830. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Galluzzi L, Viaud S, Vetizou M, Daillere R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7:271ps271. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]