Abstract
Toll-like receptors (TLRs), key initiators of innate immune responses, recognize antigens and are essential in linking innate and adaptive immune responses. Misrecognition and over-stimulation/expression of TLRs may contribute to the development of chronic inflammatory diseases and autoimmune diseases. However, appropriate and mature TLR responses are associated with the establishment of resistance against some infectious diseases. In this study, we assessed the mRNA expression profile of TLRs 1-10 in splenic and ileal mononuclear cells (MNCs) and dendritic cells (DCs) of germ-free (GF) and conventional pigs at different ages. We found that the TLR mRNA expression profiles were distinct between GF and conventional pigs. The expression profiles were also significantly different between splenic and ileal MNCs/DCs. Comparison of the TLR expression profiles in GF and conventional newborn and young pigs demonstrated that exposure to commensal microbiota may play a more important role than age in TLR mRNA expression profiles. To our knowledge, this is the first report that systematically assesses porcine TLRs 1-10 mRNA expression profiles in MNCs and DCs from GF and conventional pigs at different ages. These results further highlighted that the commensal microbiota of neonates play a critical role through TLR signaling in the development of systemic and mucosal immune systems.
Keywords: mRNA expression, Toll-like receptor, microbiota
Introduction
Toll-like receptors (TLRs) are a type of pattern recognition receptor (PRR) that interact with microbe-associated molecular patterns (MAMPs) and activate signaling pathways to induce innate immune response and also to initiate specific adaptive immune responses (Kawai and Akira, 2009; Werling and Jungi, 2003). There are 11 TLR family members in humans, among them, TLR11 is a pseudogene (Chuang and Ulevitch, 2001; Ishii et al., 2008). Human TLRs 1 to 10 are divided into two subpopulations according to their cellular localization (Chuang and Ulevitch, 2001; Kawai and Akira, 2009). TLRs 1, 2, 4, 5, 6 and 10 are localized to the cell surface, while TLRs 3, 7, 8, 9 are primarily localized in intracellular vesicles such as the endosome and the endoplasmic reticulum (Kawai and Akira, 2009; Rich et al., 2012; Takeda and Akira, 2015). To date, MAMPs for all human TLRs, with the exception of TLR 10, have been identified (Lee et al., 2012; Takeda and Akira, 2015). The MAMPs for human TLRs 1-9 are triacyl lipopeptides, peptidoglycan, double-stranded RNA, lipopolysaccharide, flagellin, diacyl lipopeptides, single-stranded RNA (TLR7/8), CpG DNA, respectively (Takeda and Akira, 2015). After recognizing specific MAMPs, signaling pathways downstream of the TLRs are triggered and type I interferon (IFN) and inflammatory cytokines are produced (Kawai and Akira, 2011; Werling and Jungi, 2003). Moreover, TLRs recognize numerous synthetic (imiquimod) and endogenous ligands [danger-associated molecular patterns and products of damaged tissue (heat shock proteins, endoplasmin)] and play a crucial role in shaping intestinal immune function and maintaining gut homeostasis (Abreu, 2010; Frosali et al., 2015).
TLRs are mainly found in tissues and cells involved in immune function, such as mesenteric lymph nodes (MLNs) and the spleen, as well as those exposed to the exterior environment such as mucosa (including epithelial cells and subepithelial components) in the intestine and the lung (Zarember and Godowski, 2002). The expression profiles of TLRs differ among tissues and cell types (Flo et al., 2001; Muzio et al., 2000; Zarember and Godowski, 2002). For example, the TLR4 mRNA expression was higher in murine CD11c+ splenic DCs than in CD11c+ lamina propria DCs, whereas the TLR5 mRNA expression showed the opposite trend (Uematsu et al., 2006). Among these immune cells, dendritic cells (DCs) are critical mediators to achieve TLR signaling functions (Hemmi and Akira, 2005; Reis e Sousa, 2004). Additionally, DCs in different anatomical locations have varying functions, which may be associated with the different TLR mRNA expression profiles (Iwasaki and Kelsall, 1999; Uematsu et al., 2006). For instance, murine naïve CD4+ T cells activated by DCs from the Peyer's patches (PP) produced higher levels of interleukin 4 (IL-4) and IL-10 than those activated by splenic DCs (Iwasaki and Kelsall, 1999); mucosal DCs promoted the differentiation of Th17 cells and contributed to IgA B cell class switching (Denning et al., 2007; Sato et al., 2003). Therefore, it is necessary to assess and compare the TLR expression in DCs from different anatomical sites.
Although TLRs are important for host defense, recognition of self-molecules by TLRs and loss of negative balancing of TLR signals are associated with pathological (chronic) inflammation and autoimmune disease (Kawai and Akira, 2010; Marshak-Rothstein, 2006; Marshak-Rothstein and Rifkin, 2007). This has led to an increase in the study of TLRs as therapeutic targets for immune disorders (Keogh and Parker, 2011; Li et al., 2013). Currently, most models tested are in vitro or murine models in vivo (Li et al., 2013). However, as demonstrated by the different expression patterns of TLR4 in monocytes and macrophages after LPS treatment, the expression and regulation of TLR function differs between mice and humans (Bryant and Monie, 2012; Rehli, 2002; Vaure and Liu, 2014). This suggests that the murine model may not be adequate for studies of human TLRs and highlights the need for alternative animal models. Pigs are being increasingly recognized and used as a relevant model for studies of infectious disease and human immunity (Fairbairn et al., 2011; Gonzalez et al., 2010; Yang et al., 2014; Yang and Yuan, 2014; Yuan et al., 1996; Q. Zhang et al., 2013); however, knowledge of the porcine TLR expression and function is limited in comparison to mice and humans. Existing evidence suggests that the pig TLR system may be closer to that of humans than the murine system is (Jungi et al., 2011; Vaure and Liu, 2014). Although, porcine, human and murine TLR4 promoter sequences were similar, murine TLR4 promoter exhibited significant differences in the regulation of gene expression; whereas porcine TLR4 promoter shared more common features with the human TLR4 promoter (Roger et al., 2005; Thomas et al., 2006). More studies of porcine TLR expression and function are needed to evaluate if the pig model can effectively mimic and predict human conditions and outcomes.
The immune system of the neonate is less developed than that of the adult and this may extend to TLR expression (Bailey et al., 2005; Lee and Mazmanian, 2010; Pott et al., 2012). There are two important periods during the development of the immune system – immediately after birth and after weaning. In the former period, neonates are exposed to non-sterile environments, and in the later period, the organism undergoes extensive exposure to new antigens due to the introduction of solid food and non-milk based diets (Bailey et al., 2005). Therefore, in addition to adulthood, birth and weaning were chosen as two important time points for examination in this study. Additionally, this study used germ-free (GF) animals to provide a comparative control to define how the microbiota/diet affects the developing immune system (Falk et al., 1998; Lee and Mazmanian, 2010; Macpherson and Harris, 2004).
We assessed the TLR1-10 mRNA expression profiles in mononuclear cells (MNCs) and DCs from spleen, ileum and MLNs in GF and conventional pigs at newborn, weaning and adult stages to compare the difference of TLR mRNA expression in tissue-specific and age-dependent manner.
Materials and Methods
Animals and experimental design
In this study, five groups of pigs (newborn GF, newborn conventional, young GF, young conventional, adult conventional) were used. Four pigs were included in each group as replications. GF pigs (Landrace × Yorkshire × Duroc) were hysterectomy-derived, fed with cow milk and maintained in sterile isolation units as described previously (Saif et al., 1996; Yuan and Saif, 2002). Specific pathogen-free conventional pigs (Landrace × Yorkshire × Duroc) were naturally derived from Landrace × Yorkshire sows bred to Duroc boars, nursed on the sows until 3 weeks then switched to plant based solid diet. One- to 4-day-old and 4-week-old GF and conventional piglets were euthanized and ileum, spleen and MLNs were collected. Additionally ileum, spleen and MLNs were collected from conventional adult pigs (Landrace × Yorkshire) (at the average age of 10 – 11 months). As no GF adult pigs were available due to facility limitations (inability to maintain adult pigs in GF isolators); there was no GF adult pig group.
Isolation of MNCs and DCs from spleen, ileum and MLNs
MNCs were isolated from spleen, ileum and MLNs as described previously (Yuan et al., 1996). DCs were isolated from MNCs of different tissue origin of individual pigs. MNC numbers were too low to yield adequate amounts of DCs from newborn pigs for further study. Cell separation buffer (MACS buffer) consisted of PBS, 2 mM EDTA and 0.5% BSA, filtered sterilized and stored at 4°C. MNCs were counted and centrifuged at 300 × g for 10 min at 4°C. The pellet was resuspended in 1 mL MACS buffer per unit (107 MNCs/unit), centrifuged at 300 × g for 10 min at 4°C. The cell pellet was resuspended in 80 μL MACS buffer per unit, followed by the addition of 10 μL of mouse anti- porcine CD3 antibody (Ab) (IgG1) (SouthernBiotec, Birmingham, Alabama, USA), 10 μL of mouse anti-porcine CD21 Ab (IgG1) (SouthernBiotec, Birmingham, Alabama, USA), 2.5 μL of mouse anti-porcine SWC1 Ab (IgG2b) (AbD Serotec, Raleigh, NC, USA), and 2.5 μL of mouse anti-porcine SWC9 Ab (IgG1) (AbD Serotec, Raleigh, NC, USA), that binding with the surface marker of T cell, B cell, granulocyte/monocyte and macrophage respectively, then mixed gently and incubated at 4°C for 20 min. The cells were then washed with 1 mL MACS buffer per unit of MNCs and centrifuged at 300 × g for 10 min at 4°C. The cell pellet was resuspended in 80 μL of MACS buffer, followed by the addition of 20 μL of anti-mouse IgG MicroBeads (Miltenyi Biotec, San Diego, CA, USA) per unit of MNCs, and gently mixed and incubated at 4°C for 20 min. The cells were washed with 1 mL MACS buffer per unit of MNCs and centrifuged at 300 × g for 10 min at 4°C, followed by resuspension in 500 μL of MACS buffer. LD columns (Miltenyi Biotec, San Diego, CA, USA) were placed on QuadroMACS™ separator (Miltenyi Biotec, San Diego, CA, USA) followed by adding the MNCs for DC negative selection, following the manufacturer's recommendations.
Examination of isolated DC purity by flow cytometry
As described previously, 105 cells were stained with mouse anti-porcine CD3e-FITC (SouthernBiotec, Birmingham, Alabama, USA), mouse anti-porcine CD21-FITC (SouthernBiotec, Birmingham, Alabama, USA), mouse anti-porcine SWC1-FITC (AbD Serotec, Raleigh, NC, USA) and mouse anti-porcine SWC9-FITC (AbD Serotec, Raleigh, NC, USA) monoclonal antibodies (mAb) to characterize the frequencies of T cell, B cell, granulocyte/monocyte and macrophage and to determine the purity of isolated DCs by flow cytometry (Vlasova et al., 2013). Acquisition of 50,000 events was conducted using MACSQuant® Analyzer flow cytometer (Miltenyi Biotec, San Diego, CA, USA). Analyses were performed using MACSQuantify™ software (Miltenyi Biotec, San Diego, CA, USA).
RNA extraction and cDNA synthesis
RNA extraction was conducted on isolated MNCs or DCs from each tissue sample using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer's recommendations. RNase-Free DNase Set (Qiagen, Valencia, CA, USA) was used to digest DNA during RNA extraction following the manufacturer's recommendations. Concentration and purity of RNA were assessed by NanoDrop 2000c spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The ratio of absorbance at 260 nm and 280 nm was examined to analyze the RNA purity. A ratio of ~2.0 was accepted as “pure” of RNA. RNA integrity was analyzed via running denaturing agarose gel. The clear 18S and 28S ribosomal RNA bands indicated the high RNA integrity. RNA was stored at −80°C until used. SuperScript® III First-Strand synthesis kit (Life Technologies, Carlsbad, CA, USA) was used to synthesize cDNA from the sample RNA. 30 ng total RNA was used in 20 μL of the reaction system following the manufacturer's recommendations. The synthesized cDNA was stored at −20°C until used.
TLR mRNA expression detection by real-time PCR
Construction of plasmid vector pUC57 with target mRNA sequences of porcine TLR1-10 and β-actin was conducted by GenScript (Piscataway, NJ, USA). Six 10-fold serial dilutions of plasmid template were used to establish the standard curves of TLRs and β-actin depending on known concentrations of plasmid at the PCR cycle and the relative Ct value. The correlation coefficient of the established standard curve was from 0.993 to 0.998. The amplification efficiency of the primers was 90.42-97.87%. The mRNA expression level of β-actin was detected to determine a consistent mRNA expression. Real time PCR was conducted to detect the porcine TLR mRNA expression in different tissue origin MNCs/DCs using LightCycler® 480 SYBR Green I Master (Roche, Indianapolis, IN, USA). 1 μL of synthesized cDNA was used in every real-time PCR reaction. The primers used in this experiment are listed in Table 1. The amplification efficiency of the primers was 64.01% - 85.27%. For the real-time PCR, the following conditions were applied: 95°C for 15 min for initial denaturation, followed by 40 PCR cycles at 94°C for 10 sec, 57°C for 15 sec, and 70°C for 20 sec. A melting curve analysis was then performed. RNA-free water was used as a negative control. All samples were tested in duplicate.
Table 1.
Primer sequences for porcine TLRs 1-10 genes
| Gene | Genebank accession number | Primer sequence (5’ → 3’) | Reference |
|---|---|---|---|
| TLR1 | NM_001031775.1 |
F:
AGATTTCGTGCCACCCTATG R: CCTGGGGGATAAACAATGTG |
(Uddin et al., 2013) |
| TLR2 | NM_213761.1 |
F:
TGCTATGACGCTTTCGTGTC R: CGATGGAGTCGATGATGTTG |
|
| TLR3 | NM_001097444.1 |
F:
GAGCAGGAGIIIGCCTTGTC R: GGAGGTCATCGGGTATTTGA |
|
| TLR4 | NM_001113039.2 |
F:
TCATCCAGGAAGGTTTCCAC R: TGTCCTCCCACTCCAGGTAG |
|
| TLR5 | NM_001123202.1 |
F:
GGTCCCTGCCTCAGTATCAA R: TGTTGAGAAACCAGCTGACG |
|
| TLR6 | NM_213760.1 |
F:
TCAAGCATTTGGACCTCTCA R: TTCCAAATCCAGAAGGATGC |
|
| TLR7 | NM_001097434.1 |
F:
TCTGCCCTGTGATGTCAGTC R: GCTGGTTTCCATCCAGGTAA |
|
| TLR8 | NM_214187.1 |
F:
CTGGGATGCTTGGTTCATCT R: CATGAGGTTGTCGATGATGG |
|
| TLR9 | NM_213958.1 |
F:
AGGGAGACCTCTATCTCCGC R: AAGTCCAGGGTTTCCAGCTT |
|
| TLR10 | NM_00103534.1 |
F:
GTCTCCCAATTTCGTCCAGA R: TGAGAGCTTTCAGTGCAGGA |
Author |
| γ-actin | U07786 |
F:
CAGGTCATCACCATCGGCAACG R: GACAGCACCGTGTTGGCGTAGAGGT |
(Collado-Romero et al., 2010) |
Statistical analysis
All quantitative real-time PCR data were transferred from the mean Ct value of replicated samples to copy number according to the established standard curve. Differences in mean values between groups were analyzed by two-tailed t test and were defined as significant at p < 0.05. Statistical analyses were performed by GraphPad Prism 6.0c software (GraphPad Software, La Jolla, CA, USA).
Results
The purity of DCs in negative selected cells was high
The percentages of T cells, B cells, monocytes/granulocytes and macrophages in the negatively selected cells were assessed using CD3, CD21, SWC1 and SWC9 mAbs, respectively. The percentage of T cells in the negatively selected cells was 0.78%-3.06%. B cells presented 1.46%-4.21% in the selected cells. There were 0.15%-0.75% and 0.36%-1.43% of monocytes/granulocytes and macrophages in the selected cells. The low amount of T cells, B cells, monocytes/granulocytes and macrophages in the negative selected MNCs demonstrated the high purity of isolated DCs.
TLR mRNA expression profiles differed between GF and conventional pigs
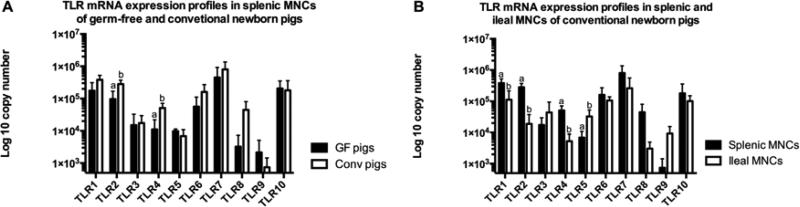
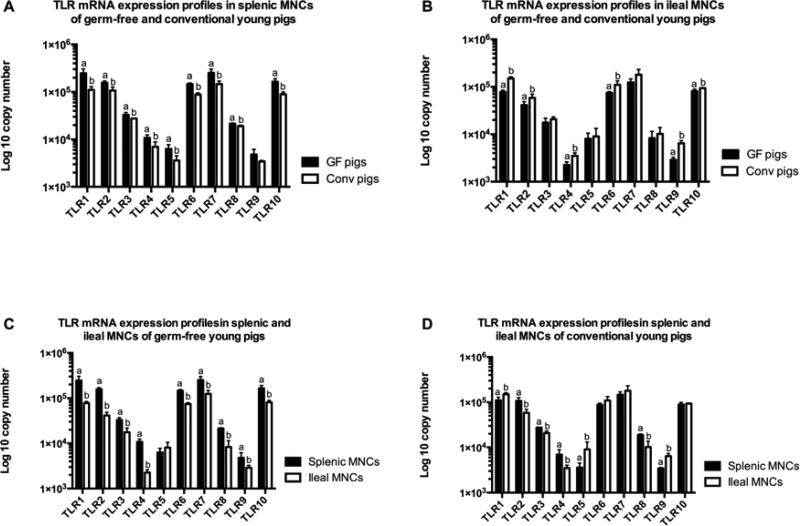
The mRNA expression levels of TLRs 1-10 in splenic MNCs were generally higher in newborn conventional piglets as compared to newborn GF piglets, whereas, the expression levels were greater in young GF pigs than young conventional pgs. In the newborn (1-4 day-old) pigs, TLRs 1, 2, 4, 6, 7, and 8 mRNA expression levels in splenic MNCs were higher in conventional pigs compared to GF pigs, particularly TLR2 and TLR4 which showed statistically significant differences between the two groups (Figure 1A). In contrast, TLRs 3, 5, 9 and 10 had equal or lower expression levels in the conventional pigs compared to GF pigs (Figure 1A). However, the expression levels in ileal MNCs did not show any significant differences between newborn conventional and GF pigs (data not shown). In young (4-week-old) GF and conventional (weaned at 3-week-old) pigs, splenic MNCs of GF pigs had significantly higher TLR mRNA expression levels, with the exception of TLR9 which, while still higher in GF pigs, showed no statistically significant difference between the two groups (Figure 2A). Interestingly, the TLR mRNA expression profile in ileal MNCs of young pigs displayed the opposite trend to what was observed in splenic MNCs. Ileal MNCs from conventional young pigs generally had higher (TLRs 3, 5, 7 and 8) and significantly higher (TLRs 1, 2, 4, 6, 9 and 10) TLR mRNA expression levels than in GF young pigs (Figure 2B).
Figure 1. TLRs 1-10 mRNA expression profiles in newborn pigs.
(A) The expression profiles in splenic MNCs of newborn pigs; (B) The expression profile in splenic and ileal MNCs of conventional newborn pigs. Bars represent SD, different letters indicate significant difference (p < 0.05). GF: germ-free; Conv: conventional. (n=4)
Figure 2. TLRs 1-10 mRNA expression profiles in germ-free and conventional young pigs.
(A) The expression profiles in splenic MNCs of young pigs; (B) The expression profiles in ileal MNCs of young pigs; (C) The expression profiles in splenic and ileal MNCs of young germ-free pigs; (D) The expression profiles in splenic and ileal MNCs of young conventional pigs. Bars represent SD, different letters indicate significant difference (p < 0.05). Gn: germ-free; Conv: conventional. (n=4)
Most TLR mRNA expression levels were higher in splenic MNCs than in ileal MNCs of conventional newborn pigs, GF young pigs and conventional adult pigs
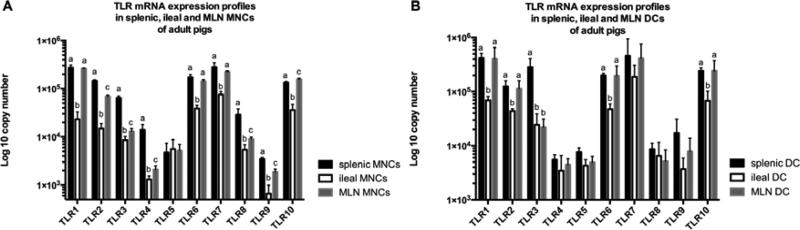
In conventional newborn pigs, most of the TLR mRNA expression levels were higher in splenic MNCs than in ileal MNCs, particularly those of TLR 1, 2 and 4, which had significantly higher expression levels in splenic MNCs; whereas, the expression of TLRs 3, 5 and 9 was greater in ileal MNCs (Figure 1B). However, there was no significant difference in the expression levels between splenic MNCs and ileal MNCs in GF newborn pigs (data not shown). In young GF and conventional pigs, there were significant differences of TLRs mRNA expression profile between splenic and ileal MNCs. In GF young pigs, TLR mRNA expression levels were statistically higher in splenic MNCs than ileal MNCs, with the exception of TLR5 (Figure 2C). In conventional young pigs, mRNA expression levels of TLRs 1, 5 and 9 were significantly higher in ileal MNCs than in splenic MNCs, whereas, TLRs 2, 3, 4 and 8 mRNA expression levels were lower in ileal MNCs than in splenic MNCs (Figure 2D). In conventional adult pigs, ileal MNCs, except TLR5, showed the lowest TLR mRNA expression levels among three different tissue-derived MNCs (spleen, ileum and MLNs) (Figure 3A). There was no statistical difference of TLR5 mRNA expression level among splenic, ileal and MLN MNCs (Figure 3A). In addition, splenic MNCs had significantly higher mRNA expression levels of TLRs 2, 3, 4, 8 and 9 than MLN MNCs in adult conventional pigs (Figure 3A).
Figure 3. TLRs 1-10 mRNA expression profiles in MNCs and DCs of conventional adult pigs.
(A) The expression profiles in MNCs of conventional adult pigs; (B) The expression profiles in DCs of conventional adult pigs. Bars represent SD, different letters indicate significant difference (p < 0.05). (n=4)
TLR mRNA expression levels were increased in splenic MNCs, but decreased in ileal MNCs of young compared to adult conventional pigs
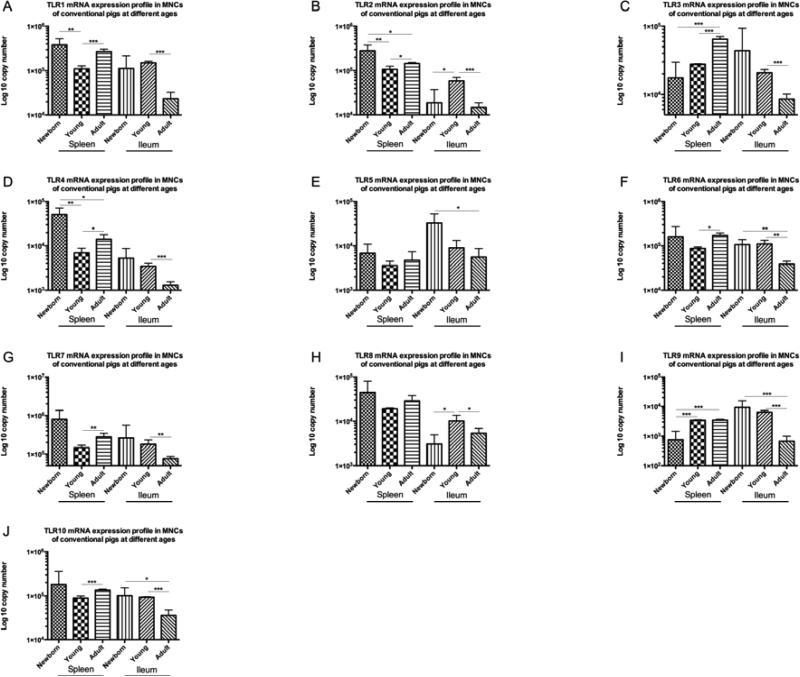
TLR mRNA expression levels in splenic MNCs of conventional newborn pigs showed inconsistent trends when compared to the expression levels in conventional young pigs and adult pigs. The mRNA expression levels of TLR2 and TLR4 in splenic MNCs of newborn pigs were statistically greater than in young and adult pigs (Figures 4B and 4D). However, TLR9 expression levels in splenic MNCs of newborn pigs were significantly lower than the levels in young and adult pigs (Figure 4I). TLRs 6, 7 and 10 mRNA expression levels in conventional newborn pigs did not show statistical differences with either conventional young or adult pigs (Figures 4F, 4G and 4J). TLRs 1, 2, 3, 4, 6, 7 and 10 mRNA expression levels in splenic MNCs of young pigs were statistically lower than the levels in adult pigs (Figures 4A-4D, 4F, 4G and 4J); whereas no significant differences were found in TLRs 5 and 8 (4E and 4H). Overall, the TLR mRNA expression levels in splenic MNCs showed an increasing trend from conventional young pigs to adult pigs.
Figure 4. TLRs 1-10 mRNA expression profiles in splenic and ileal MNCs of conventional pigs at different ages.
(A) TLR1; (B) TLR2; (C) TLR3; (D) TLR4; (E) TLR5; (F) TLR6; (G) TLR7; (H) TLR8; (I) TLR9; (J) TLR10 expression profiles in conventional pigs at different ages. Bars represent SD. * p < 0.05; ** p < 0.01; *** p < 0.001. (n=4)
Interestingly, in ileal MNCs, the overall TLRs mRNA expression levels presented a decreasing trend from conventional young pigs to adult pigs. Except for TLR5, the mRNA expression levels of TLRs in ileal MNCs of young pigs were significantly higher than the levels in adult pigs (Figure 4). The expression levels in ileal MNCs of conventional newborn pigs also showed variable trends (Figure 4).
In GF pigs, only the expression levels of TLRs 5, 6 and 8 in splenic MNCs and TLR 8 in ileal MNCs differed significantly between newborn and young pig groups (Table 2).
Table 2.
Differentiation mRNA expression of porcine TLRs 1-10 in splenic and ileal MNCs of GF newborn and young pigs
| TLR1 | TLR2 | TLR3 | TLR4 | TLR5 | TLR6 | TLR7 | TLR8 | TLR9 | TLR10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spleen | Newborn | 1.75E+05 ± 1.34E+05 | 9.67E+04 ± 7.21E+04 | 1.50E+04 ± 1.77E+04 | 1.11E+04 ± 1.10E+04 | 9.83E+03 ± 1.54E+03 | 5.63E+04 ± 5.48E+04 | 4.51E+05 ± 4.70E+05 | 3.23E+03 ± 4.06E+03 | 2.15E+03 ± 2.97E+03 | 2.06E+05 ± 1.46E+05 |
| Young | 2.46E+05 ± 5.92E+04 | 1.57E+05 ± 1.36E+04 | 3.29E+04 ± 3.90E+03 | 1.08E+04 ± 1.58E+03 | 6.30E+03 ± 1.42E+03 | 1.46E+05 ± 8.85E+03 | 2.52E+05 ± 4.92E+04 | 2.14E+04 ± 6.80E+02 | 4.80E+03 ± 1.37E+03 | 1.64E+05 ± 2.53E+04 | |
| P value | 0.376 | 0.154 | 0.096 | 0.965 | 0.025 | 0.018 | 0.434 | <0.001 | 0.168 | 0.596 | |
| Ileum | Newborn | 9.85E+04 ± 6.61E+04 | 8.77E+04 ± 9.34E+04 | 5.87E+04 ± 6.15E+04 | 1.40E+03 ± 1.07E+03 | 8.45E+04 ± 9.56E+04 | 1.05E+05 ± 1.35E+05 | 2.79E+05 ± 2.35E+05 | 2.70E+03 ± 2.49E+03 | 2.84E+03 ± 2.19E+03 | 7.39E+04 ± 3.70E+04 |
| Young | 7.80E+04 ± 6.37E+03 | 4.13E+04 ± 7.26E+03 | 1.76E+04 ± 4.15E+03 | 2.26E+03 ± 3.56E+02 | 8.08E+03 ± 2.44E+03 | 7.44E+04 ± 4.88E+03 | 1.24E+05 ± 2.37E+04 | 8.31E+03 ± 3.17E+03 | 2.89E+03 ± 3.83E+02 | 8.10E+04 ± 8.46E+03 | |
| P value | 0.562 | 0.361 | 0.230 | 0.172 | 0.159 | 0.670 | 0.235 | 0.020 | 0.966 | 0.722 |
The mean of copy number with standard deviation for each group of TLR mRNA in splenic and ileal MNCs of GF pigs was shown in the table. P value < 0.05 was defined as significant difference. (n=4)
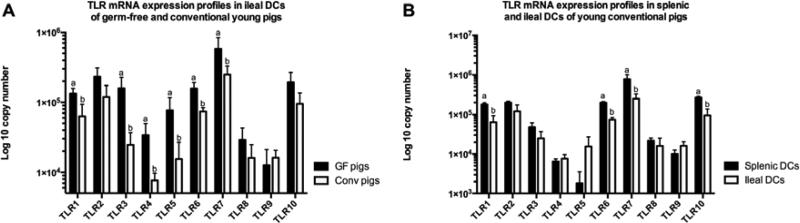
Ileal DCs of young GF and conventional pigs had different mRNA expression profiles, whereas, splenic DCs had similar expression profiles in these two groups
As initial MNC numbers were too low to yield adequate amounts of DCs from newborn pigs for further study, the TLR mRNA expression profile in DCs of newborn pigs could not be examined. Furthermore, as no GF adult pigs were available due to facility limitations (inability to maintain adult pigs in GF isolators), the TLR mRNA expression profiles of DCs of GF adult pigs were not studied. Generally, the mRNA expression levels of the TLRs in ileal DCs were greater in GF young pigs than in the conventional young pigs with TLRs 1, 3, 4, 5, 6 and 7 mRNA expression levels in ileal DCs being significantly higher (Figure 5A). However, there was no difference in splenic DCs between the young GF pigs and conventional pigs (data not shown).
Figure 5. TLRs 1-10 mRNA expression profiles in DCs of young pigs.
(A) The expression profiles in ileal DCs of young pigs; (B) The expression profiles in splenic and ileal DCs of conventional young pigs. Bars with same letter in each TLR are not significant difference (p > 0.05). Bars represent SD and bars with different letters in each TLR are significant difference (p < 0.05). (n=4)
TLRs 1, 2, 6 and 10 mRNA expression levels in ileal DCs of adult pigs were significantly lower than those in splenic and MLN DCs
Only TLR5 mRNA expression levels showed a statistically difference between splenic and ileal DCs in young GF pigs, while the expression levels of other TLRs were similar in the two groups (data not shown). However, in conventional young pigs, several TLRs showed higher mRNA expression levels in splenic DCs than ileal DCs, with TLRs 1, 6, 7 and 10 significantly so (Figure 5B). In conventional adult pigs, the mRNA expression levels of TLRs 1, 2, 3, 6 and 10 in ileal DCs were statistically lower than the levels in splenic DCs (Figure 3B). Additionally, TLR 1, 2, 6 and 10 mRNA expression levels in ileal DCs were significantly lower compared with the levels in MLN DCs (Figure 3B). Interestingly, TLR3 mRNA expression level in MLN DCs was similar to ileal DCs and was also significantly lower than in splenic DCs (Figure 3B).
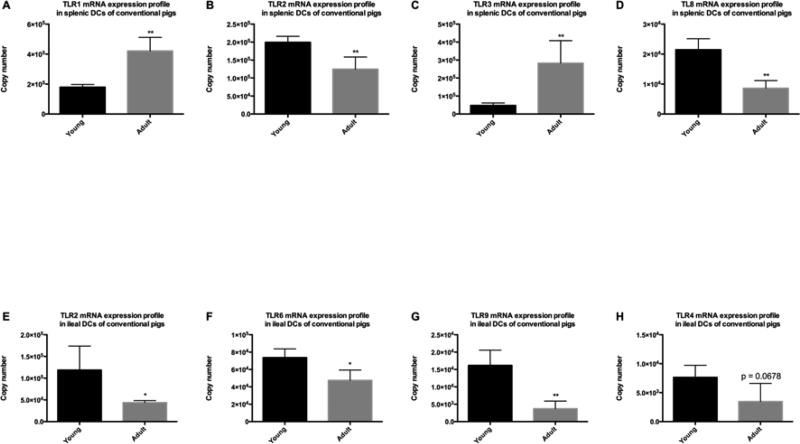
Most of the TLR mRNA expression levels in ileal DCs were higher in conventional young pigs than adult pigs; while the TLR expression profiles in splenic DCs from these groups showed variable trends
In splenic DCs, TLR1 and TLR3 mRNA expression levels were significantly lower in conventional young pigs than adult pigs (Figure 6A and 6C); whereas, the expression levels of TLR 2 and 8 were significantly higher in young pigs than adult pigs (Figure 6B and 6D). In ileal DCs, the majority of TLR mRNA expression levels in conventional young pigs were similar to those of adult pigs (data not shown). TLRs 2, 6 and 9 in ileal DCs had significantly greater expression levels in conventional young pigs than adult pigs (Figure 6E-6G), while the expression levels of TLR4 exhibited a higher trend in young pigs (p = 0.0678) (Figure 6H).
Figure 6. TLRs mRNA expression profiles in splenic and ileal DCs of conventional pigs at different ages.
(A) TLR1 expression profile in splenic DCs; (B) TLR2 expression profile in splenic DCs; (C) TLR3 expression profile in splenic DCs; (D) TLR8 expression profile in splenic DCs; (E) TLR2 expression profile in ileal DCs; (F) TLR 6 expression profile in ileal DCs; (G) TLR9 expression profile in ileal DCs; (H) TLR4 expression profile in ileal DCs. Bars represent SD. The significant differences between two groups were indicated with star(s). * p < 0.05; ** p < 0.01. (n=4)
Discussion
Birth and weaning are two key developmental milestones of the immune system of mammals (Cebra, 1999; Holsapple et al., 2003). During these two periods of time, animals experience antigen exposure as the result of contact with non-sterile environment and a new diet (Bailey et al., 2005). In interacting with microorganisms and food antigens, TLRs play a key role shaping the immune system (Abreu, 2010; Hua and Hou, 2013; Takeda and Akira, 2005). To investigate how microbiota/diet affect the development of the immune system, GF animals were used to provide a comparative control for conventional animals (Lee and Mazmanian, 2010; Macpherson and Harris, 2004). To understand how microbiota/diet impacts TLR expression, we compared the porcine TLRs mRNA expression profiles in GF and conventional pigs at birth (1-4 days of age) and 1-week post weaning (4 weeks of age) in this study.
Others have compared TLR expression profiles in intestinal epithelial cells of GF and conventional pigs. One study examined the mRNA expression of TLRs 2, 4 and 9 using real-time RT-PCR in whole caudal small intestinal tissues and enterocytes at 14 days of age between GF and GF pigs conventionalized with sow feces and then kept in GF conditions (Willing and Van Kessel, 2007). They found that the expression of TLRs 2, 4 and 9 was increased in conventional pigs compared to GF pigs in both enterocytes and whole intestinal tissue, particularly TLR2. However, another study using similar methods failed to find a difference in TLRs 4 and 9 mRNA expression between GF and conventionalized pigs (Chowdhury et al., 2007). In a recent study, the TLRs 2, 4, 5 and 9 mRNA expression levels in caudal ileal tissue did not differ significantly between GF and conventional pigs at 5 weeks of age (George et al., 2012). In our study, we did not find statistically significant differences of TLRs 1-10 mRNA expression in ileal MNCs between newborn (1-4 days of age) GF and conventional pigs (data not shown), suggesting that priming of intestinal TLR responses with commensal microbiota requires some time. However, mRNA expression levels of TLRs 1, 2, 4, 6, 9 and 10 in ileal MNCs were significantly higher in conventional young (4 weeks of age) pigs than the levels in GF young (4 weeks of age) pigs (Figure 2B). The discordant results between our and other studies may due to the different types of cells (small intestinal epithelial cells vs. ileal MNCs) and time points (14 days of age vs. 5 weeks of age vs. 4 weeks of age) used in the studies. Furthermore, the conventionalized pigs used in the other studies were kept in GF conditions and did not receive sow colostrum and milk, which may have affected the experimental results. There is increasing evidence demonstrate that breast milk play an crucial role in shaping mucosal immunity (Parigi et al., 2015).
In this study, we demonstrated that the mRNA expression profiles of TLRs differed in MNCs/DCs from different tissues. Generally, expression levels of most TLRs were higher in splenic MNCs/DCs compared to ileal MNCs/DCs in conventional pigs, regardless of the age (newborn, young and adult). Our data in adult pigs is consistent with a recent study of TLR expression along the intestinal tract in 70-day-old pigs in that most of the TLR mRNA expression levels was higher in MLN than ileal PP (Gourbeyre et al., 2015). Using Taqman real-time PCR, mRNA expression of human TLRs 1-10 was assessed in human adult tissue cDNA pools (Clontech) and compared amongst the different tissues (Zarember and Godowski, 2002). This data showed that the TLR expression levels were higher in spleen than small intestine, except for TLR5, for which similar expression levels were observed. Another group of investigators found similar results in adult human tissues where the TLR mRNA expression levels were greater in spleen than small intestine, with the exception of TLR3 and TLR5 (Nishimura and Naito, 2005). In our study, we found a similar trend where TLR mRNA expression levels were greater in splenic MNCs than ileal MNCs, except TLR5. Similar results were also found in another study using a mouse model where most of the murine TLR mRNA expression was higher in splenic DCs than intestinal PP DCs after LPS stimulation in vitro (Davies et al., 2010). They suggested that the lower TLR mRNA expression in PP DCs might be one of the mechanisms exploited by PP DCs to regulate immune responses to commensal bacterial inducing tolerogenic state (Davies et al., 2010). The upregulated TLR expression in splenic MNCs of adult pigs than young pigs, in contrast may represent the more mature state of the immune system of the former group. In addition, studies showed that flagellin-induced activation of TLR5 on dendritic cells elicited production of the cytokine IL-22 to induce a protective gene expression program in intestinal epithelial cells (B. Zhang et al., 2014). This may indicate that TLR5 signaling is critical to induce antiviral effects in the gut and may contribute to the different expression profile of TLR5 compared to other TLRs. More studies are needed to understand the mechanisms of this phenomenon. Of all TLRs recognizing numerous microbial, synthetic and endogenous ligands, only TLR5 appears to be specific for a single protein moiety, flagellin of invasive bacteria (Steiner, 2007). This specificity may require the increased TLR5 expression in the gut mucosa versus spleen observed in our study and by others (Frosali et al., 2015; Uematsu et al., 2008). Further, exclusive association of TLR5 activation with pro-inflammatory stimuli/signaling (Steiner, 2007) may result in the decreased expression levels of TLR5 mRNA (compared to other TLRs) under homeostatic conditions as we observed in our study. However, as we were not able to collect relative data in neonatal or young animals, no comparison of our data for newborn and young pig data could be made with others.
As the intestine is continuously exposed to various antigens, such as food, commensal bacteria, and enteric pathogens, it is critical to avoid over stimulation and maintain the homeostasis in the intestine (Testro and Visvanathan, 2009). Increasing evidence suggests that over-stimulation of intestinal TLRs correlates with the development of certain gastrointestinal diseases, such as Crohn's disease, in which the TLR 2 and 4 expression (mRNA and protein) levels were up-regulated in both colon and immune cells of MLNs (Gomariz et al., 2005; Hausmann et al., 2002). Studies of systemic lupus erythematosus (SLE) in a mouse model indicated that the over-expression of systemic TLR7 may be associated with the development of this autoimmune disease (Fischer and Ehlers, 2008). Therefore, we assume that the lower mRNA expression levels of TLRs in ileal MNCs/DCs than splenic MNCs/DCs in conventional pigs may be effect of the microbiota-driven immunoregulatory/immunosuppressive mechanisms to avoid overt stimulation via controlling the expression (dampening) levels of TLRs. The microbiota directly interact with gut immune cells, which may exert regulatory effect on expression of TLRs where as such effect is minimal in spleen. Our data in GF pigs further supports this idea as there is no statistical difference in mRNA expression between splenic and ileal MNCs of GF newborn pigs or between splenic and ileal DCs of GF young pigs, except for TLR5 expression levels in DCs of GF young pigs. Furthermore, most of the TLR expression levels in MLN MNCs/DCs of adult pigs are also greater than in ileal MNCs/DCs. The different sets of data between conventional and GF pigs also demonstrate that colostrum and milk, as well as microbiota, have effects on the immune development in neonates (Bailey et al., 2005; Bauer et al., 2006; Field, 2005).
Many studies have been conducted to demonstrate the age-dependent expression of TLRs in humans and animals, such as cattle and pigs (Iram et al., 2012; Malmuthuge et al., 2012; Uddin et al., 2013). The comparison of TLR mRNA expression profiles in newborn, young and adult pigs in this study demonstrated that most of the TLR mRNA expression levels in ileal MNCs were significantly higher in young pigs than adult pigs, except TLR5 (Figure 4). This may be representative of different maturation stages of the immune system: with TLR responses activated during weaning (adaptation to a new diet and discontinued passive protection via sow milk) with a subsequent decrease in the adult animals to achieve/maintain immune homeostasis. A study of TLR mRNA expression in the gastrointestinal tract of dairy calves showed similar results to ours in that the expression of TLRs 2 and 4-10 in the ileum was significantly down-regulated with increasing age (3-week-old to 6-month-old), except TLRs 1 and 3, in which the expression was not associated with increasing age (Malmuthuge et al., 2012). However, another study using1-day-old, 2-month-old and 5-month old pigs reported different results where TLRs 1, 5, 7, 9 and 10 expression levels in ileum were highest in 2-month-old pigs but not statistically different (Uddin et al., 2013). Furthermore, they found the highest expression of TLR3 in ileum of 5-month-old pigs. The discordant results may be due to the different time points (4-week-old vs. 2-month-old). As the 4-week-old conventional pigs we used in this study were just weaned for one week, while the 2-month-old pigs were weaned for a month, it is very likely that the weaned pigs are still adapting to the introduction of new diet and have distinct TLR mRNA expression profiles compared to the 2-month-old pigs. Additionally, we failed to observe significant differences of TLR mRNA expression levels in ileal MNCs between GF newborn and young pigs, except TLR8 (Table 2). We concluded that most of the TLR mRNA expression in ileal MNCs was not age-dependent and may be explained by the exposure to non-sterile environment, removal of passive protection via maternal milk and new diet in the conventional vs. GF pigs. In addition, although TLR 7 and 8 recognize same major ligands, there are different populations of cells expressing TLR 7 and 8 and their signaling pathways do not overlap completely (Gorden et al., 2005), which might have contributed to the different mRNA expression profiles between TLR 7 and 8 observed in our study. The TLR expression levels in splenic MNCs were significantly higher in adult pigs than in young pigs, except TLRs 5, 8 and 9 (Figure 4). This result was opposite to what we observed in ileal MNCs. It may be due to minimal interaction between microbiota and spleen that results in exertion of less immunoregulatory/immunosuppressive effects on spleen than on gut. Studies indicated that the proportion of lymphocyte subsets were distinct in different human lymphoid tissues (Hsu et al., 1983; Junker et al., 2009). Furthermore, the TLR expression profiles were divergent in different types of lymphocytes, and even dissimilar among distinct subsets (Hubert et al., 2006; Muzio et al., 2000; Zarember and Godowski, 2002). Therefore, it is very likely that the expression profile in splenic MNCs contrasts to that of ileal MNCs. Additionally, we noted that TLRs 2 and 4 mRNA in splenic MNCs were expressed significantly higher in newborn pigs than young and adult pigs (Figure 4B and 4D). Studies using SPF mice with intraperitoneal injection of LPS for 14 days showed a decrease of TLRs 2 and 4 protein expression in spleen (Zhong et al., 2008). Combined with our data showing that the TLRs 2 and 4 mRNA expression in splenic MNCs were unchanged in GF newborn and young pigs (Table 2), we assumed that the decrease of TLRs 2 and 4 expression might be mediated by the microbiota exposure after birth and during the weaning period.
To our knowledge, this is the first report that systematically assesses porcine TLRs 1-10 mRNA expression profiles in MNCs and DCs from GF and conventional pigs at different ages. Our study showed that porcine TLR gene expression differs significantly in MNCs/DCs from different tissues at different ages and according to microbiota/diet status. These data suggest that the uptake of colostrum and/or sow milk and the encounter with environmental commensal microbiota after birth and/or during weaning may be essential for the development of the innate immune system. Studies of the expression pattern of porcine TLR genes will help to understand the interaction between the commensal microbiota and the host systemic and mucosal immune system. Currently, there is a lack of specific anti-porcine TLR antibodies, thus, we were unable to examine the respective TLRs protein expression profiles. Therefore, studying the mRNA expression profile can provide insights as to the effects of microbiota and diet on immune system development.
Conclusions
The results obtained in this study suggest the importance of maternal immune factors/diet and commensal microbiota for the porcine TLR-mediated development of the innate immunity in the neonatal and weaning periods. Further studies are necessary to understand the factors and mechanisms that regulate the distinct expression profiles of mRNA of porcine TLRs and to associate those with respective TLR protein expression patterns.
Highlights.
Systematically assess porcine TLRs 1-10 mRNA expression profiles.
Splenic and ileal mononuclear cells have different TLR expression profiles.
Colostrum/breast milk and commensal microbiota affect innate immunity development.
Acknowledgements
We thank Dr. Juliette Hanson, Ronna Wood, Megan Strother, Dennis Hartzler and Jeffrey Ogg for animal care assistance. This work was supported by a grant from the NIH, NIAID # R01 A1099451.
Abbreviations
- DCs
dendritic cells
- GF
germ-free
- IFN
interferon
- IL-4
interleukin 4
- MAMPS
microbe-associated molecular patterns
- MLNs
mesenteric lymph nodes
- MNCs
mononuclear cells
- PRR
pattern recognition receptor
- PP
Peyer's patches
- SLE
systemic lupus erythematosus
- TLRs
Toll-like receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors disclose no potential conflicts of interest.
Reference
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. doi:10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Bailey M, Haverson K, Inman C, Harris C, Jones P, Corfield G, Miller B, Stokes C. The development of the mucosal immune system pre- and post-weaning: balancing regulatory and effector function. P Nutr Soc. 2005;64:451–457. doi: 10.1079/pns2005452. doi:10.1079/PNS2005452. [DOI] [PubMed] [Google Scholar]
- Bauer E, Williams BA, Smidt H, Verstegen MWA, Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intest Microbiol. 2006;7:35–51. [PubMed] [Google Scholar]
- Bryant CE, Monie TP. Mice, men and the relatives: cross-species studies underpin innate immunity. Open Biol. 2012;2:120015. doi: 10.1098/rsob.120015. doi:10.1098/rsob.120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- Chowdhury SR, King DE, Willing BP, Band MR, Beever JE, Lane AB, Loor JJ, Marini JC, Rund LA, Schook LB, Van Kessel AG, Gaskins HR. Transcriptome profiling of the small intestinal epithelium in germfree versus conventional piglets. BMC Genomics. 2007;8:215. doi: 10.1186/1471-2164-8-215. doi:10.1186/1471-2164-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang T, Ulevitch RJ. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001;1518:157–161. doi: 10.1016/s0167-4781(00)00289-x. [DOI] [PubMed] [Google Scholar]
- Collado-Romero M, Arce C, Ramírez-Boo M, Carvajal A, Garrido JJ. Quantitative analysis of the immune response upon Salmonella typhimurium infection along the porcine intestinal gut. Vet Res. 2009;41:23. doi: 10.1051/vetres/2009072. doi:10.1051/vetres/2009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JM, MacSharry J, Shanahan F. Differential regulation of Toll-like receptor signalling in spleen and Peyer's patch dendritic cells. Immunology. 2010;131:438–448. doi: 10.1111/j.1365-2567.2010.03317.x. doi:10.1111/j.1365-2567.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang Y-C, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. doi:10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Fairbairn L, Kapetanovic R, Sester DP, Hume DA. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J Leukocyte Biol. 2011;89:855–871. doi: 10.1189/jlb.1110607. doi:10.1189/jlb.1110607. [DOI] [PubMed] [Google Scholar]
- Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr. 2005;135:1–4. doi: 10.1093/jn/135.1.1. [DOI] [PubMed] [Google Scholar]
- Fischer M, Ehlers M. Toll-like receptors in autoimmunity. Ann NY Acad Sci. 2008;1143:21–34. doi: 10.1196/annals.1443.012. doi:10.1196/annals.1443.012. [DOI] [PubMed] [Google Scholar]
- Flo TH, Halaas O, Torp S, Ryan L, Lien E, Dybdahl B, Sundan A, Espevik T. Differential expression of Toll-like receptor 2 in human cells. J Leukocyte Biol. 2001;69:474–481. [PubMed] [Google Scholar]
- Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the intricate interaction among Toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res. 2015;2015:489821. doi: 10.1155/2015/489821. doi:10.1155/2015/489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Circle K, Lindblom S, Vilain S, Rosa AJM, Francis D, Brozel V, Kaushik RS. Assessment of Toll-like receptors in the ileum of weanling pigs-responses to feed antibiotic chlortetracycline and gnotobiotic conditions. J Clin Cell Immunol. 2012;03 doi:10.4172/2155-9899.1000125. [Google Scholar]
- Gomariz RP, Arranz A, Abad C, Torroba M, Martinez C, Rosignoli F, Garcia-Gómez M, Leceta J, Juarranz Y. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukocyte Biol. 2005;78:491–502. doi: 10.1189/jlb.1004564. doi:10.1189/jlb.1004564. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Azevedo MSP, Jung K, Vlasova A, Zhang W, Saif LJ. Innate immune responses to human rotavirus in the neonatal gnotobiotic piglet disease model. Immunology. 2010;131:242–256. doi: 10.1111/j.1365-2567.2010.03298.x. doi:10.1111/j.1365-2567.2010.03298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. doi:10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- Gourbeyre P, Berri M, Lippi Y, Meurens F, Vincent-Naulleau S, Laffitte J, Rogel-Gaillard C, Pinton P, Oswald IP. Pattern recognition receptors in the gut: analysis of their expression along the intestinal tract and the crypt/villus axis. Physiol Rep. 2015;3 doi: 10.14814/phy2.12225. doi:10.14814/phy2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W, Rogler G. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–135. doi: 10.1159/000086657. doi:10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, West LJ, Landreth KS. Species comparison of anatomical and functional immune system development. Birth Defects Res B Dev Reprod Toxicol. 2003;68:321–334. doi: 10.1002/bdrb.10035. doi:10.1002/bdrb.10035. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Cossman J, Jaffe ES. Lymphocyte subsets in normal human lymphoid tissues. Am J Clin Pathol. 1983;80:21–30. doi: 10.1093/ajcp/80.1.21. [DOI] [PubMed] [Google Scholar]
- Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol Immunol. 2013;10:103–106. doi: 10.1038/cmi.2012.61. doi:10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert FX, Voisine C, Louvet C, Heslan JM, Ouabed A, Heslan M, Josien R. Differential Pattern Recognition Receptor Expression but Stereotyped Responsiveness in Rat Spleen Dendritic Cell Subsets. J Immunol. 2006;177:1007–1016. doi: 10.4049/jimmunol.177.2.1007. doi:10.4049/jimmunol.177.2.1007. [DOI] [PubMed] [Google Scholar]
- Iram N, Mildner M, Prior M, Petzelbauer P, Fiala C, Hacker S, Schoppl A, Tschachler E, Elbe-Burger A. Age-related changes in expression and function of Toll-like receptors in human skin. Development. 2012;139:4210–4219. doi: 10.1242/dev.083477. doi:10.1242/dev.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. doi:10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–240. doi: 10.1084/jem.190.2.229. doi:10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungi TW, Farhat K, Burgener IA, Werling D. Toll-like receptors in domestic animals. Cell Tissue Res. 2011;343:107–120. doi: 10.1007/s00441-010-1047-8. doi:10.1007/s00441-010-1047-8. [DOI] [PubMed] [Google Scholar]
- Junker Y, Bode H, Wahnschaffe U, Kroesen A, Loddenkemper C, Duchmann R, Zeitz M, Ullrich R. Comparative analysis of mononuclear cells isolated from mucosal lymphoid follicles of the human ileum and colon. Clin Exp Immunol. 2009;156:232–237. doi: 10.1111/j.1365-2249.2009.03883.x. doi:10.1111/j.1365-2249.2009.03883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. doi:10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. doi:10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. doi:10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh B, Parker AE. Toll-like receptors as targets for immune disorders. Trends Pharmacol Sci. 2011;32:435–442. doi: 10.1016/j.tips.2011.03.008. doi:10.1016/j.tips.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12:168–179. doi: 10.1038/nri3151. doi:10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. doi:10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang X, Zhang F, Yin H. Toll-like receptors as therapeutic targets for autoimmune connective tissue diseases. Pharmacol Ther. 2013;138:441–451. doi: 10.1016/j.pharmthera.2013.03.003. doi:10.1016/j.pharmthera.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. doi:10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Malmuthuge N, Li M, Fries P, Griebel PJ, Guan LL. Regional and age dependent changes in gene expression of Toll-like receptors and key antimicrobial defence molecules throughout the gastrointestinal tract of dairy calves. Vet Immunol Immunop. 2012;146:18–26. doi: 10.1016/j.vetimm.2012.01.010. doi:10.1016/j.vetimm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. doi:10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. doi:10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- Parigi SM, Eldh M, Larssen P, Gabrielsson S, Villablanca EJ. Breast Milk and Solid Food Shaping Intestinal Immunity. Front Immunol. 2015;6:415. doi: 10.3389/fimmu.2015.00415. doi:10.3389/fimmu.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott J, Stockinger S, Torow N, Smoczek A, Lindner C, McInerney G, Bäckhed F, Baumann U, Pabst O, Bleich A, Hornef MW. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog. 2012;8:e1002670. doi: 10.1371/journal.ppat.1002670. doi:10.1371/journal.ppat.1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehli M. Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 2002;23:375–378. doi: 10.1016/s1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. doi:10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Rich RR, Fleisher TA, Shearer WT, Schroeder H, Frew AJ, Weyand CM. Clinical immunology: principles and practice. 4 ed. Elsevier Saunders; Philadelphia: 2012. [Google Scholar]
- Roger T, Miconnet I, Schiesser A-L, Kai H, Miyake K, Calandra T. Critical role for Ets, AP-1 and GATA-like transcription factors in regulating mouse Toll-like receptor 4 (Tlr4) gene expression. Biochem J. 2005;387:355–365. doi: 10.1042/BJ20041243. doi:10.1042/BJ20041243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl. 1996;12:153–161. doi: 10.1007/978-3-7091-6553-9_17. [DOI] [PubMed] [Google Scholar]
- Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171:3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- Steiner TS. How flagellin and toll-like receptor 5 contribute to enteric infection. Infect Immun. 2007;75:545–552. doi: 10.1128/IAI.01506-06. doi:10.1128/IAI.01506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2015;109:14.12.1–14.12.10. doi: 10.1002/0471142735.im1412s109. doi:10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. doi:10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Testro AG, Visvanathan K. Toll-like receptors and their role in gastrointestinal disease. J Gastroenterol Hepatol. 2009;24:943–954. doi: 10.1111/j.1440-1746.2009.05854.x. doi:10.1111/j.1440-1746.2009.05854.x. [DOI] [PubMed] [Google Scholar]
- Thomas AV, Broers AD, Vandegaart HF, Desmecht DJ-M. Genomic structure, promoter analysis and expression of the porcine (Sus scrofa) TLR4 gene. Mol. Immunol. 2006;43:653–659. doi: 10.1016/j.molimm.2005.04.001. doi:10.1016/j.molimm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Uddin MJ, Kaewmala K, Tesfaye D, Tholen E, Looft C, Hoelker M, Schellander K, Cinar MU. Expression patterns of porcine Toll-like receptors family set of genes (TLR1-10) in gut-associated lymphoid tissues alter with age. Res Vet Sci. 2013;95:92–102. doi: 10.1016/j.rvsc.2013.01.027. doi:10.1016/j.rvsc.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang B-G, Jung Y-J, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. doi:10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. doi:10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. doi: 10.3389/fimmu.2014.00316. doi:10.3389/fimmu.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova AN, Chattha KS, Kandasamy S, Siegismund CS, Saif LJ. Prenatally acquired vitamin A deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J Immunol. 2013;190:4742–4753. doi: 10.4049/jimmunol.1203575. doi:10.4049/jimmunol.1203575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling D, Jungi TW. TOLL-like receptors linking innate and adaptive immune response. Vet Immunol Immunop. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- Willing BP, Van Kessel AG. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J. Anim. Sci. 2007;85:3256–3266. doi: 10.2527/jas.2007-0320. doi:10.2527/jas.2007-0320. [DOI] [PubMed] [Google Scholar]
- Yang X, Li G, Wen K, Bui T, Liu F, Kocher J, Jortner BS, Vonck M, Pelzer K, Deng J, Zhu R, Li Y, Qian Y, Yuan L. A neonatal gnotobiotic pig model of human enterovirus 71 infection and associated immune responses. Emerg Microbes Infect. 2014;3:e35. doi: 10.1038/emi.2014.35. doi:10.1038/emi.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yuan L. Neonatal gnotobiotic pig models for studying viral pathogenesis, immune responses, and for vaccine evaluation. Br J Virol. 2014;1:87–91. [Google Scholar]
- Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunop. 2002;87:147–160. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. doi:10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- Zhang B, Chassaing B, Shi Z, Uchiyama R, Zhang Z, Denning TL, Crawford SE, Pruijssers AJ, Iskarpatyoti JA, Estes MK, Dermody TS, Ouyang W, Williams IR, Vijay-Kumar M, Gewirtz AT. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science. 2014;346:861–865. doi: 10.1126/science.1256999. doi:10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Widmer G, Tzipori S. A pig model of the human gastrointestinal tract. Gut Microbes. 2013;4:193–200. doi: 10.4161/gmic.23867. doi:10.4161/gmic.23867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Ma HY, Yang Q, Gu FR, Yin GQ, Xia CM. Decrease in toll-like receptors 2 and 4 in the spleen of mouse with endotoxic tolerance. Inflamm Res. 2008;57:252–259. doi: 10.1007/s00011-007-7104-4. doi:10.1007/s00011-007-7104-4. [DOI] [PubMed] [Google Scholar]