Abstract
Background:
Axial dynamization of fractures can promote healing, and overly stiff fixation can suppress healing. A novel technology, termed active plating, provides controlled axial dynamization by the elastic suspension of locking holes within the plate. This prospective, controlled animal study evaluated the effect of active plates on fracture-healing in an established ovine osteotomy model. We hypothesized that symmetric axial dynamization with active plates stimulates circumferential callus and delivers faster and stronger healing relative to standard locking plates.
Methods:
Twelve sheep were randomly assigned to receive a standard locking plate or an active locking plate for stabilization of a 3-mm tibial osteotomy gap. The only difference between plates was that locking holes of active plates were elastically suspended, allowing up to 1.5 mm of axial motion at the fracture. Fracture-healing was analyzed weekly on radiographs. After sacrifice at nine weeks postoperatively, callus volume and distribution were assessed by computed tomography. Finally, to determine their strength, healed tibiae and contralateral tibiae were tested in torsion until failure.
Results:
At each follow-up, the active locking plate group had more callus (p < 0.001) than the standard locking plate group. At postoperative week 6, all active locking plate group specimens had bridging callus at the three visible cortices. In standard locking plate group specimens, only 50% of these cortices had bridged. Computed tomography demonstrated that all active locking plate group specimens and one of the six standard locking plate group specimens had developed circumferential callus. Torsion tests after plate removal demonstrated that active locking plate group specimens recovered 81% of their native strength and were 399% stronger than standard locking plate group specimens (p < 0.001), which had recovered only 17% of their native strength. All active locking plate group specimens failed by spiral fracture outside the callus zone, but standard locking plate group specimens fractured through the osteotomy gap.
Conclusions:
Symmetric axial dynamization with active locking plates stimulates circumferential callus and yields faster and stronger healing than standard locking plates.
Clinical Relevance:
The stimulatory effect of controlled motion on fracture-healing by active locking plates has the potential to reduce healing complications and to shorten the time to return to function.
Research over the past fifty years has consistently shown that controlled axial dynamization promotes callus formation and improves the speed and strength of fracture-healing1-8. For example, Goodship and Kenwright demonstrated that 1-mm axial dynamization delivered more than three times stronger healing and two times faster healing compared with rigid fixation4. Conversely, deficient fracture motion caused by overly stiff fixation constructs can suppress secondary fracture-healing, contributing to delayed union, nonunion, osteolysis, and fixation failure9-13. Consequently, there have been persistent efforts to develop plating technologies for controlled axial dynamization1,3,4,8,14,15.
Before the advent of locked plating, these efforts did not yield clinically viable solutions, as plates needed to be firmly compressed onto the bone surface to obtain stable fixation. For example, Longfellow15 was granted a patent in 1949 for a sliding plate “to permit axial movement for growth of callous [sic],” reasoning that “holding fragments in fixed positions forestalls natural movement between fragments necessary for growth of callous [sic].” A subsequent study demonstrated that sliding plates led to faster healing by allowing axial loading at the fracture site6. However, concerns about implant wear and fracture stability detracted from the clinical feasibility of the sliding plate concept. Foux et al. explored an axially flexible plating concept using elastic sleeves between screw heads and plate holes3. They demonstrated superior healing compared with rigid plating in a canine model. Their strategy relied on plate sliding on the bone surface to achieve fracture motion, which precluded screws from being fully tightened. Hence, their concept enabled motion at the cost of construct stability. To overcome this lack of stability, Uhthoff et al. replaced elastic sleeves with resorbable sleeves, allowing for delayed motion after sleeve resorption8. However, delayed motion is less effective than early motion to stimulate callus formation16.
The advent of locked plating enabled novel strategies for dynamization, because locked constructs with fixed-angle locking screws do not require plate compression onto the bone surface. For example, far cortical locking enables controlled axial dynamization through flexion of screws that lock into the plate and the far cortex, but retain a motion envelope at the near cortex. Far cortical locking enables dynamization without sacrificing construct stability17. It delivered symmetric callus bridging and yielded 157% stronger healing compared with standard locked plating in an ovine study1. Clinically, distal femoral fractures stabilized with far cortical locking constructs healed at a mean time of sixteen weeks with a 3% nonunion rate18. Far cortical locking has been implemented in commercial implants7,19-22 and has been simulated using standard locking screws by means of overdrilling14 or slotting23 of the near cortex.
The present study investigated an alternative strategy for axial dynamization of locking plates, termed active plating. Active plates utilize standard locking screws that lock into the threaded hole of elastically suspended sliding elements embedded inside the plate. A biomechanical study demonstrated that active plates provide axial dynamization without decreasing construct strength24. This prospective, controlled animal study evaluated healing of fractures stabilized with active plates in comparison with standard locking plates in an ovine osteotomy model. We hypothesized that symmetric axial dynamization with active plates will yield circumferential callus formation and will provide faster and stronger healing compared with standard locking plates.
Materials and Methods
Using an established, large-animal, fracture-healing model25, twelve sheep were randomly assigned to receive a standard locking plate or an active locking plate for stabilization of a 3-mm transverse osteotomy gap. This gap model correlates clinically with bridge plating of a comminuted fracture, in which all load is initially transmitted through the plate. Fracture-healing was monitored weekly on radiographs. After sacrifice at nine weeks postoperatively, the callus volume and distribution were assessed by quantitative computed tomography (QCT). Finally, to determine their strength, healed tibiae and contralateral tibiae were tested in torsion until failure.
Locking Plate Constructs
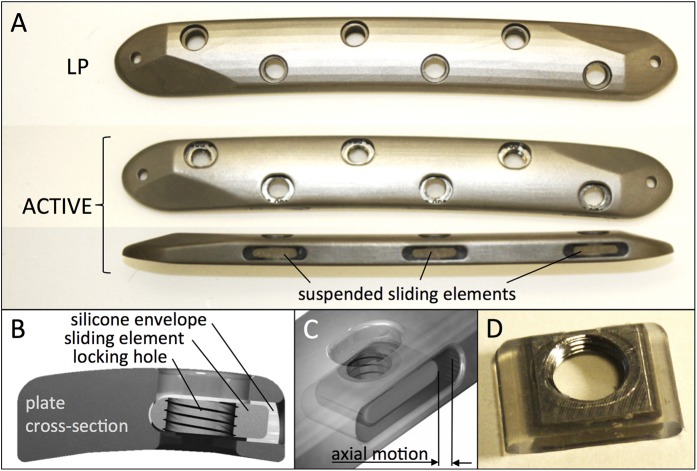
Standard locking plates and active locking plates had an identical cross-sectional geometry, representative of typical 4.5-mm large-fragment plates. Plates had six holes; were 127 mm long, 16 mm wide, and 5.6 mm thick; and were made of Ti6Al4V ELI (extra low interstitial) titanium alloy. Plates only differed in that locking holes of active locking plates were integrated in individual sliding elements that were elastically suspended in a silicone envelope inside lateral plate pockets (Fig. 1). The silicone suspension consisted of long-term implantable medical-grade silicone elastomer (HCRA 4750; Applied Silicone) that was molded onto the sliding elements. Lateral pockets were arranged in an alternating pattern from both plate sides, resulting in a staggered locking hole configuration. The pocket geometry combined with the silicone suspension allowed controlled axial translation, which enabled up to 1.5 mm of axial motion across the fracture gap while providing stable fixation in response to bending and torsional loading24.
Fig. 1.
Figs. 1-A through 1-D Standard and active locking plates. Fig. 1-A The standard locking plate (LP) and active locking plate (ACTIVE) were identical, with the exception of elastic suspension of locking holes in ACTIVE plates. Figs. 1-B and 1-C Cross-section and semitransparent illustration of an ACTIVE plate. Fig. 1-D Sliding element with locking hole, embedded in silicone envelope.
The stiffness of standard locking plate and active locking plate constructs was characterized by bench testing of three plates per group, applied to bridge 3-mm gap osteotomies in cadaveric ovine tibiae. Axial compression was applied through a sphere proximally to permit physiological bending under axial loading17,24,26. Constructs were stepwise loaded in 50-N increments up to 1000 N. The resulting motion at the medial and lateral aspects of the osteotomy was measured with calipers for calculation of construct stiffness.
Animal Model
The ovine tibia osteotomy model was employed as it represents the most prevalent large-animal model for evaluation of fracture-healing25,27. The study protocol and sample size were approved by the pertinent animal care committee and were consistent with a prior study on locking plate dynamization to facilitate result comparability1. Twelve skeletally mature female Swiss Alpine sheep, with a mean value (and standard deviation) of 2.0 ± 0.1 years for age and 68 ± 7 kg for weight, were randomized into the standard locking plate group or the active locking plate group. Under general anesthesia, an approximately 8-cm-long medial incision was made over the tibia of one hind leg, with the surgical procedure being randomized between the right and left hind legs. All six screw holes were drilled from medial to lateral in the intact tibia with a custom template. A transverse osteotomy was performed with a 0.6-mm-thick saw blade under constant irrigation. Osteotomies were stabilized with plates applied to the medial tibial shaft in a periosteum-sparing biological fixation technique to preserve periosteal perfusion28, using six 4.5-mm bicortical locking screws. The resulting osteotomy gap had a controlled width of 3 mm, formed by the distance between the central screw holes in plates, which was 2.4 mm greater than that in the drill template, and by the osteotomy cut of 0.6 mm. For medication, the standard protocol of the ovine fracture model was followed7,27. Antibiotic prophylaxis with benzylpenicillin and gentamicin and analgesia with carprofen (4 mg/kg of body weight) and buprenorphine were initiated preoperatively and were continued for four days postoperatively.
Postoperatively, a cylindrical cast was applied over a soft padding layer proximal to the hoof and extending to the stifle joint. In the ovine osteotomy model, this routine prophylactic measure is essential to prevent tibial fracture caused by bending loads while allowing axial load-bearing and walking immediately postoperatively1,7. Each sheep was housed in a single 2.5-m2 pen and walked on the first postoperative day. After two weeks, sheep were housed in pairs in 5-m2 pens until sacrifice at postoperative week 9.
Radiography
Radiographs were made immediately postoperatively and at weekly intervals, starting at postoperative week 3. To make radiographs without cast interference, casts were removed and were reapplied weekly through postoperative week 9. At each time point, an anteroposterior radiograph and two lateral oblique radiographs (+10° and −10° from straight lateral) were made to visualize the anterior, posterior, and lateral cortices without obstruction by the medially applied plate. Projected callus areas were measured using validated custom software developed to objectively quantify periosteal callus size29.
For volumetric callus assessment at postoperative week 9, QCT scans of the excised tibiae were obtained in accordance with an established protocol30 after implant removal to prevent metal interference. Callus volume was rendered (Amira, FEI) in an automated, non-blinded approach, using consistent Hounsfield unit (HU) thresholds of 600 HU to differentiate callus from soft tissue and 1600 HU to differentiate callus from cortical bone. To quantify callus distribution, the total callus volume was divided into four quadrants, and the volumes of the anterior, posterior, and lateral callus were extracted.
Mechanical Testing
The proximal and distal ends of the tibiae were cemented in mounting fixtures that were separated by 170 mm and were aligned with the tibial shaft axis. To ensure unconstrained torsion of the tibial shaft in a materials testing system (Instron 8874), the distal fixture was mounted on an x-y table that enabled translation but prevented rotation of the distal fixture around the diaphyseal axis. After implant removal, rotation was applied proximally at 10° per minute until specimen failure in torsion. Failure was defined as the instant at which rotational displacement caused a decrease in torsional moment due to specimen fracture or shear movement at the osteotomy. The failure mode was identified on QCT reconstructions obtained after the torsion test. The strength of healed tibiae was quantified by their energy to failure, calculated by integrating the area under the torsion versus rotation curve up to the peak torque at which failure occurred1.
Statistical Analysis
All data are reported as the mean and the standard deviation. Statistical differences between groups were tested using two-tailed, unpaired Student t tests at a level of significance of α = 0.05. Paired tests were performed for comparison of medial and lateral cortex motion within the same groups.
Results
Eleven of twelve sheep had an uneventful experimental procedure and recovery. One sheep developed a painful hoof infection of a foreleg, unrelated to the experimental procedure. This infection prevented weight-bearing and required intensive treatment with nonsteroidal anti-inflammatory drugs known to suppress fracture-healing31. This sheep was replaced to retain the original sample size.
Construct Stiffness
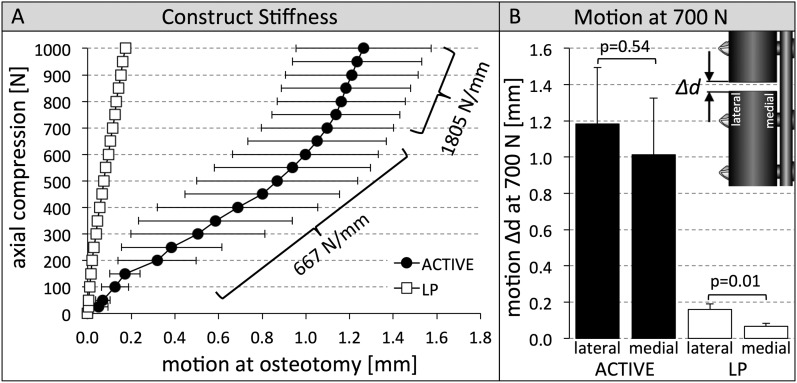
Bench-top tests demonstrated that the initial axial stiffness of active locking plate constructs (667 ± 161 N/mm) was 89.3% lower (p < 0.001) compared with standard locking plate constructs (6239 ± 740 N/mm) (Fig. 2-A). The axial stiffness of active locking plate constructs increased for loads of >700 N to 1805 ± 116 N/mm because of progressive compression of the silicone suspension, but remained 64% lower compared with standard locking plate constructs (p < 0.001). In active locking plate constructs, 700-N loading induced motion of 1.2 ± 0.3 mm at the lateral cortex and 1.0 ± 0.3 mm at the medial cortex (Fig. 2-B). In standard locking plate constructs, motion in response to 700-N loading remained below the 0.2-mm threshold required for stimulation of callus formation2,4,32, and the medial cortex motion (0.07 ± 0.02 mm) adjacent to the plate was significantly lower (p = 0.01) than lateral cortex motion (0.16 ± 0.03 mm).
Fig. 2.
Figs. 2-A and 2-B Construct stiffness and interfragmentary motion at 700 N for both locking plate constructs. Fig. 2-A Active locking plate (ACTIVE) constructs were up to 89% less stiff than the standard locking plate (LP) constructs. Fig. 2-B ACTIVE constructs induced symmetric motion of 1.0 to 1.2 mm at 700-N compression, and LP constructs induced asymmetric motion of <0.2 mm. The error bars indicate the standard deviation.
Radiographic Assessment
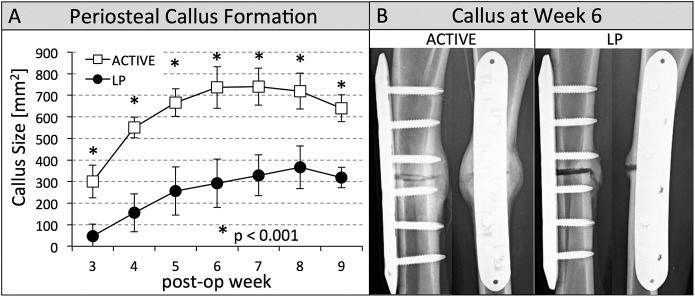
At each time point, the active locking plate group had significantly more callus (p < 0.001) than the standard locking plate group (Fig. 3-A). At the earliest time point (postoperative week 3), callus in the active locking plate group (296 ± 84 mm2) was more than six times greater (p < 0.001) than it was in the standard locking plate group (47 ± 55 mm2). At postoperative week 6, all tibiae of the active locking plate group had bridging callus at the lateral, anterior, and posterior cortices visible on radiographs (Fig. 3-B). In the standard locking plate group, only 50% of these cortices had bridging callus.
Fig. 3.
Figs. 3-A and 3-B Periosteal callus formation and callus at postoperative week 6 for both groups. Fig. 3-A Progression of periosteal callus formation. The active locking plate (ACTIVE) group developed significantly more callus (p < 0.001), indicated by asterisks, than the standard locking plate (LP) group at each time point from postoperative weeks 3 through 9. The error bars indicate the standard deviation. Fig. 3-B By week 6, all visible cortices had bridged in the ACTIVE group, but only 50% of these cortices had bridged in the LP group, as shown in two representative specimens.
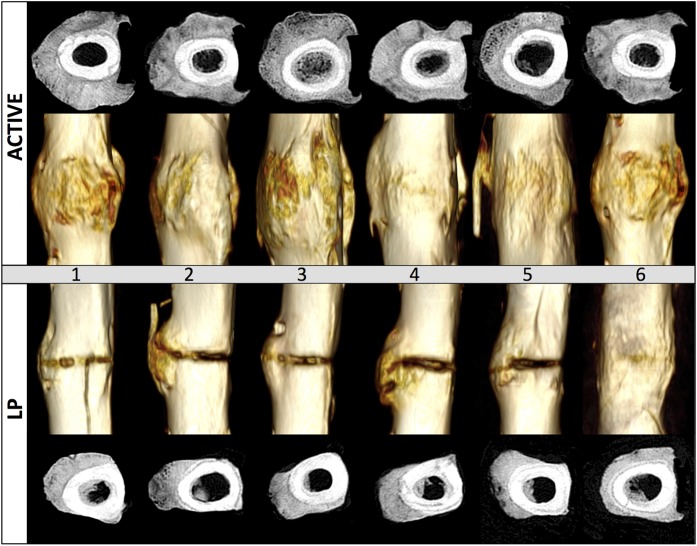
The total callus volume assessed from QCT at postoperative week 9 was 117% greater in the active locking plate group compared with the standard locking plate group (p < 0.001) (Table I). Transverse QCT slices extracted adjacent to the osteotomy gap demonstrated that each active locking plate group specimen developed circumferential callus that circumscribed the lateral, anterior, and posterior cortices up to the medial plate (Fig. 4). In contrast, only one of the six standard locking plate constructs developed circumferential callus. In standard locking plate constructs, the mean callus volume in the anterior quadrant (1.3 ± 0.9 cm3) and posterior quadrant (1.0 ± 0.6 cm3) was more than two times smaller than that in the lateral quadrant opposite the plate (3.0 ± 0.5 cm3).
TABLE I.
Comparison of Outcome Parameters Between the Standard Locking Plate Group and the Active Locking Plate Group
| Parameters | Standard Locking Plate Group* | Active Locking Plate Group* | Difference | P Value |
| Sheep characteristics | ||||
| Weight (kg) | 71 ± 9 | 65 ± 5 | −8% | 0.21 |
| Age (mo) | 24 ± 1 | 25 ± 2 | 1% | 0.58 |
| Construct stiffness (N/mm) | ||||
| In compression ≤700 N | 6239 ± 740 | 667 ± 161 | −89% | <0.001 |
| In compression >700 N | 5023 ± 420 | 1805 ± 116 | −64% | <0.001 |
| Callus volume from tomography scans (cm3) | ||||
| Total | 6.0 ± 1.8 | 13.0 ± 2.7 | 117% | <0.001 |
| Anterior | 1.3 ± 0.9 | 4.5 ± 2.0 | 252% | 0.006 |
| Posterior | 1.0 ± 0.6 | 2.5 ± 0.8 | 147% | 0.004 |
| Lateral | 3.0 ± 0.5 | 4.4 ± 0.5 | 46% | <0.001 |
| Mechanical properties, absolute | ||||
| Peak torsion (Nm) | 28 ± 25 | 72 ± 9 | 157% | 0.006 |
| Rotation to failure (deg) | 7 ± 2 | 16 ± 1 | 131% | <0.001 |
| Energy to failure† (Nm × deg) | 134 ± 152 | 669 ± 105 | 399% | <0.001 |
| Mechanical properties, normalized‡ | ||||
| Peak torsion (Nm) | 34 ± 30 | 87 ± 11 | 156% | 0.003 |
| Rotation to failure (deg) | 42 ± 12 | 92 ± 8 | 119% | <0.001 |
| Energy to failure† (Nm × deg) | 17 ± 17 | 81 ± 9 | 371% | <0.001 |
| Mechanical properties, contralateral§ | ||||
| Peak torsion (Nm) | 80 ± 12 | 83 ± 11 | 4% | 0.62 |
| Rotation to failure (deg) | 16 ± 2 | 17 ± 1 | 8% | 0.21 |
| Energy to failure† (Nm × deg) | 738 ± 156 | 825 ± 108 | 12% | 0.30 |
The values are given as the mean and the standard deviation.
These results represent the area under the torsion-rotation curve.
These values were the percentage of the contralateral tibiae.
These values were for the contralateral tibiae of the standard locking plate and active locking plate groups, provided for reference only.
Fig. 4.
Transverse cross-sections adjacent to the osteotomy and volumetric renderings depict circumferential and abundant callus in the active locking plate (ACTIVE) group, but not the standard locking plate (LP) group. Images depict the medial (plated) cortex to the right.
Mechanical Testing
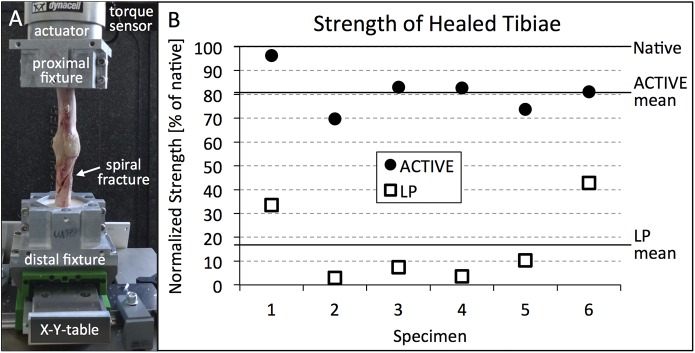
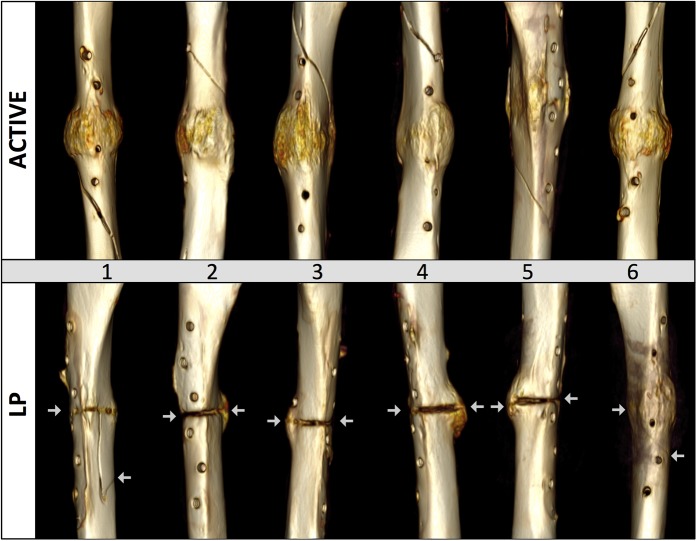
After implant removal, active locking plate group specimens sustained a 157% greater torque until failure (p = 0.006) than standard locking plate group specimens. The strength of active locking plate group specimens, expressed in terms of the energy to induce failure, was 399% greater than in standard locking plate group specimens (p < 0.001). Compared with contralateral intact tibiae, active locking plate group specimens had recovered 81% (range, 70% to 96%) of their native strength (Fig. 5). Standard locking plate group specimens had recovered 17% (range, 3% to 43%) of their native strength. All active locking plate group specimens failed by spiral fracture through a screw hole outside the callus zone (Fig. 6). In contrast, all standard locking plate group specimens fractured through either completely or partially through the osteotomy gap.
Fig. 5.
Figs. 5-A and 5-B Photograph of the active locking plate (ACTIVE) specimen and scatterplot showing the strength of the healed tibiae in the ACTIVE group and the standard locking plate (LP) group. Fig. 5-A Torsion test setup with ACTIVE specimen. Fig. 5-B Strength of healing in terms of energy to failure in torsion, normalized to the native strength of contralateral tibiae (100%).
Fig. 6.
Failure mode: all active locking plate (ACTIVE) specimens failed by spiral fracture proximal or distal to the osteotomy gap, but standard locking plate (LP) specimens fractured through either completely or partially through the osteotomy gap. The arrows indicate the ends of the fracture.
Discussion
Results of this investigation validated the hypothesis that axial dynamization with active locking plates yields circumferential callus formation and provides faster and stronger healing compared with standard locking plates in a controlled ovine fracture-healing model.
Circumferential callus formation was likely a consequence of the symmetric axial motion provided by active plating constructs. In contrast, deficient and asymmetric motion in standard locking plate group constructs prevented circumferential callus formation and suppressed cortical bridging at the plate side where motion is minimal9,33. This healing suppression adjacent to the plate can persist for a prolonged time. A recent study documented that, twenty-one months after locked plating of high tibial osteotomies, 65% of patients displayed incomplete consolidation of the osteotomy underneath the locking plate11. Asymmetric motion can also be exacerbated by two alternative strategies for stiffness reduction of locked plating constructs: the use of flexible titanium plates9, and increasing the bridge span over the fracture26,34. Both of these strategies enhance plate flexion, which increases motion at the cortex opposite the plate, but not adjacent to the plate33. In contrast, active plates deliver symmetric motion without requiring a long bridge span, as demonstrated in the present study by locking screws placed in proximity to the osteotomy gap.
Faster healing with active locking plate constructs compared with that with standard locking plate constructs is supported by the findings that active locking plate specimens had more than six times more periosteal callus by postoperative week 3 and had bridging callus at all visible cortices by postoperative week 6. Clinical benefits of faster healing include an earlier return to function and a lower risk of fixation failure due to a reduced load-sharing duration of the fixation construct.
Stronger healing with active locking plate constructs compared with that with standard locking plate constructs is supported by the findings that active locking plate group specimens survived a 131% greater torsional displacement and a 157% higher torsional load and required 399% more energy to induce failure. Energy to failure is a clinically relevant strength measure, as it provides a summary index accounting for the load and the torsional displacement to failure. Active locking plate group specimens consistently recovered between 70% and 96% of the strength of the non-involved contralateral tibiae. Moreover, the callus strength in active locking plate group specimens was likely greater than the reported strength results, as failure occurred by spiral fracture through screw holes outside the callus zone. Extrapolating from a cadaveric study on the effects of screw holes on diaphyseal strength35, a 4.5-mm screw hole will reduce the strength of the ovine tibia by 15% to 30%, which would fully account for the observed strength loss in active locking plate group specimens compared with contralateral tibiae. In contrast, the finding that half of all standard locking plate group specimens recovered <10% of their native strength, and none recovered more than 43% of their native strength, provides further evidence that excessively stiff fixation suppresses healing.
Silicone elastomer has been used for more than forty years for finger joint replacements36 and continues to serve in a range of dental37, spinal38, and arthroplasty36,39 implants to provide elastic fixation, motion, and impact damping40. Its use for osteosynthesis implants is novel and active plates have only recently been cleared by the United States Food and Drug Administration. Long-term implantable silicone elastomer is highly biocompatible and complications are rare and are limited to arthroplasty implants embedded inside a joint capsule41. For active plating, no sign of adverse reactions was found during dissection of soft tissues adjacent to the plate. Given its long clinical history, the use of silicone elastomer constitutes a novel yet conservative strategy to integrate controlled dynamization in modern locking plates.
Results of the present study can be directly correlated with those of two studies that employed the ovine osteotomy model to investigate locking plate dynamization with far cortical locking screws1 and Dynamic Locking Screws (DLS) (DePuy Synthes)7. Standard locked constructs in all three studies caused deficient, asymmetric callus formation, whereby healed tibiae of the locked control groups failed at torsional loads of 28 ± 12 Nm1, 27 ± 15 Nm7, and 28 ± 25 Nm in the present study. Dynamic stabilization increased the mean torsional load to failure by 54% with far cortical locking screws, 106% with DLS, and 156% with active locking plates. This comparison suggests that active plates provide a highly effective alternative for axial dynamization of locking plates.
The present study had several limitations. Results were specific to the ovine 3-mm gap osteotomy model and therefore require careful interpretation before extrapolation to clinical practice. The ovine model was selected as it represents the most established fracture-healing model in large animals25,27. Load transmission in the ovine tibia corresponds in magnitude to lower-extremity loading in humans42. Also, fracture-healing in sheep is similar to that in humans25. Nevertheless, further studies are required to establish the clinical efficacy of active plates and to explore their effects on the healing of simple, well-reduced fractures. As a further limitation, strength assessment was limited to torsion because of the destructive nature of strength tests. Torsion was chosen over bending because bending strength is highly affected by rotational orientation of the tibia and torsional strength is not.
Furthermore, findings are specific to fracture fixation in strong, non-osteoporotic bone, whereby early and controlled dynamization consistently improved the speed and strength of fracture-healing compared with that of standard locked plating. Additionally, given that locked and nonlocked plating constructs have a comparable stiffness in strong bone43, it seems unlikely that nonlocked constructs will provide sufficient dynamization to stimulate fracture-healing comparable with that for active locking plates. For fracture fixation in osteoporotic bone, active locking plates combine the strength benefits of fixed angle locking screws with accelerated fracture healing to attain fracture-healing prior to fatigue of the osteosynthesis.
In conclusion, the results of this study confirmed that overly rigid locked plating constructs suppress callus formation and healing. By providing symmetric axial dynamization, active locking plates delivered faster callus formation, consistent and circumferential bridging, and stronger healing compared with standard locking plates. Although caution is advised in applying these results to clinical practice, it would seem wise to use standard locking plates in diaphyseal fracture locations only with careful consideration of the risk of excessive stiffness of these implants.
Footnotes
Investigation performed at the Portland Biomechanics Laboratory, Legacy Research Institute, Portland, Oregon; Musculoskeletal Research Unit, Vetsuisse Faculty, University of Zürich, Zürich, Switzerland; and Institute of Biomechanics, Trauma Center Murnau, Murnau, Germany
A commentary by Erika Jasmin Mitchell, MD, is linked to the online version of this article at jbjs.org.
Disclosure: The National Institute of Health (NIH/NIAMS grant R41AP061201) and the Zimmer Corporation supported this study. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author (or the author’s institution) had a relevant financial relationship in the biomedical arena outside the submitted work and “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work.
References
- 1.Bottlang M, Lesser M, Koerber J, Doornink J, von Rechenberg B, Augat P, Fitzpatrick DC, Madey SM, Marsh JL. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010. July 7;92(7):1652-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, Margevicius KJ, Augat P. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. 1998. October;355(Suppl):S132-47. [DOI] [PubMed] [Google Scholar]
- 3.Foux A, Yeadon AJ, Uhthoff HK. Improved fracture healing with less rigid plates. A biomechanical study in dogs. Clin Orthop Relat Res. 1997. June;339:232-45. [DOI] [PubMed] [Google Scholar]
- 4.Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985. August;67(4):650-5. [DOI] [PubMed] [Google Scholar]
- 5.Kenwright J, Richardson JB, Cunningham JL, White SH, Goodship AE, Adams MA, Magnussen PA, Newman JH. Axial movement and tibial fractures. A controlled randomised trial of treatment. J Bone Joint Surg Br. 1991. July;73(4):654-9. [DOI] [PubMed] [Google Scholar]
- 6.Panagiotopoulos E, Fortis AP, Lambiris E, Kostopoulos V. Rigid or sliding plate. A mechanical evaluation of osteotomy fixation in sheep. Clin Orthop Relat Res. 1999. January;358:244-9. [PubMed] [Google Scholar]
- 7.Richter H, Plecko M, Andermatt D, Frigg R, Kronen PW, Klein K, Nuss K, Ferguson SJ, Stöckle U, von Rechenberg B. Dynamization at the near cortex in locking plate osteosynthesis by means of dynamic locking screws: an experimental study of transverse tibial osteotomies in sheep. J Bone Joint Surg Am. 2015. February 4;97(3):208-15. [DOI] [PubMed] [Google Scholar]
- 8.Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006. March;11(2):118-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lujan TJ, Henderson CE, Madey SM, Fitzpatrick DC, Marsh JL, Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010. March;24(3):156-62. [DOI] [PubMed] [Google Scholar]
- 10.Rahn BA, Gallinaro P, Baltensperger A, Perren SM. Primary bone healing. An experimental study in the rabbit. J Bone Joint Surg Am. 1971. June;53(4):783-6. [PubMed] [Google Scholar]
- 11.Röderer G, Gebhard F, Duerselen L, Ignatius A, Claes L. Delayed bone healing following high tibial osteotomy related to increased implant stiffness in locked plating. Injury. 2014. October;45(10):1648-52. Epub 2014 Apr 16. [DOI] [PubMed] [Google Scholar]
- 12.Strauss EJ, Schwarzkopf R, Kummer F, Egol KA. The current status of locked plating: the good, the bad, and the ugly. J Orthop Trauma. 2008. August;22(7):479-86. [DOI] [PubMed] [Google Scholar]
- 13.Woo SL, Lothringer KS, Akeson WH, Coutts RD, Woo YK, Simon BR, Gomez MA. Less rigid internal fixation plates: historical perspectives and new concepts. J Orthop Res. 1984;1(4):431-49. [DOI] [PubMed] [Google Scholar]
- 14.Linn MS, McAndrew CM, Prusaczyk B, Brimmo O, Ricci WM, Gardner MJ. Dynamic locked plating of distal femur fractures. J Orthop Trauma. 2015. October;29(10):447-50. [DOI] [PubMed] [Google Scholar]
- 15.Longfellow EE. Surgical appliance for bone fracture. US Patent and Trademark Office, patent number 2,486,303. 1949. [Google Scholar]
- 16.Goodship AE, Cunningham JL, Kenwright J. Strain rate and timing of stimulation in mechanical modulation of fracture healing. Clin Orthop Relat Res. 1998. October;355(Suppl):S105-15. [DOI] [PubMed] [Google Scholar]
- 17.Bottlang M, Doornink J, Fitzpatrick DC, Madey SM. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Joint Surg Am. 2009. August;91(8):1985-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottlang M, Fitzpatrick DC, Sheerin D, Kubiak E, Gellman R, Vande Zandschulp C, Doornink J, Earley K, Madey SM. Dynamic fixation of distal femur fractures using far cortical locking screws: a prospective observational study. J Orthop Trauma. 2014. April;28(4):181-8. [DOI] [PubMed] [Google Scholar]
- 19.Döbele S, Gardner M, Schröter S, Höntzsch D, Stöckle U, Freude T. DLS 5.0—the biomechanical effects of dynamic locking screws. PLoS One. 2014. April 10;9(4):e91933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doornink J, Fitzpatrick DC, Madey SM, Bottlang M. Far cortical locking enables flexible fixation with periarticular locking plates. J Orthop Trauma. 2011. February;25(Suppl 1):S29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freude T, Schroeter S, Plecko M, Bahrs C, Martetschlaeger F, Kraus TM, Stoeckle U, Doebele S. Dynamic-locking-screw (DLS)-leads to less secondary screw perforations in proximal humerus fractures. BMC Musculoskelet Disord. 2014;15:194 Epub 2014 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freude T, Schröter S, Gonser CE, Stöckle U, Acklin YP, Höntzsch D, Döbele S. Controlled dynamic stability as the next step in “biologic plate osteosynthesis” - a pilot prospective observational cohort study in 34 patients with distal tibia fractures. Patient Saf Surg. 2014. January 21;8(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner MJ, Nork SE, Huber P, Krieg JC. Less rigid stable fracture fixation in osteoporotic bone using locked plates with near cortical slots. Injury. 2010. June;41(6):652-6. Epub 2010 Mar 16. [DOI] [PubMed] [Google Scholar]
- 24.Tsai S, Fitzpatrick DC, Madey SM, Bottlang M. Dynamic locking plates provide symmetric axial dynamization to stimulate fracture healing. J Orthop Res. 2015. August;33(8):1218-25. Epub 2015 May 21. [DOI] [PubMed] [Google Scholar]
- 25.Nunamaker DM. Experimental models of fracture repair. Clin Orthop Relat Res. 1998. October;355(Suppl):S56-65. [DOI] [PubMed] [Google Scholar]
- 26.Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003. November;34(Suppl 2):B11-9. [DOI] [PubMed] [Google Scholar]
- 27.Auer JA, Goodship A, Arnoczky S, Pearce S, Price J, Claes L, von Rechenberg B, Hofmann-Amtenbrinck M, Schneider E, Müller-Terpitz R, Thiele F, Rippe KP, Grainger DW. Refining animal models in fracture research: seeking consensus in optimising both animal welfare and scientific validity for appropriate biomedical use. BMC Musculoskelet Disord. 2007. August 1;8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002. November;84(8):1093-110. [DOI] [PubMed] [Google Scholar]
- 29.Lujan TJ, Madey SM, Fitzpatrick DC, Byrd GD, Sanderson JM, Bottlang M. A computational technique to measure fracture callus in radiographs. J Biomech. 2010. March 3;43(4):792-5. Epub 2009 Nov 14. [DOI] [PubMed] [Google Scholar]
- 30.Augat P, Merk J, Genant HK, Claes L. Quantitative assessment of experimental fracture repair by peripheral computed tomography. Calcif Tissue Int. 1997. February;60(2):194-9. [DOI] [PubMed] [Google Scholar]
- 31.Kurmis AP, Kurmis TP, O’Brien JX, Dalén T. The effect of nonsteroidal anti-inflammatory drug administration on acute phase fracture-healing: a review. J Bone Joint Surg Am. 2012. May 2;94(9):815-23. [DOI] [PubMed] [Google Scholar]
- 32.Plecko M, Lagerpusch N, Andermatt D, Frigg R, Koch R, Sidler M, Kronen P, Klein K, Nuss K, Bürki A, Ferguson SJ, Stoeckle U, Auer JA, von Rechenberg B. The dynamisation of locking plate osteosynthesis by means of dynamic locking screws (DLS)-an experimental study in sheep. Injury. 2013. October;44(10):1346-57. Epub 2012 Nov 24. [DOI] [PubMed] [Google Scholar]
- 33.Claes L. Biomechanical principles and mechanobiologic aspects of flexible and locked plating. J Orthop Trauma. 2011. February;25(Suppl 1):S4-7. [DOI] [PubMed] [Google Scholar]
- 34.Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004. September;18(8):488-93. [DOI] [PubMed] [Google Scholar]
- 35.McBroom RJ, Cheal EJ, Hayes WC. Strength reductions from metastatic cortical defects in long bones. J Orthop Res. 1988;6(3):369-78. [DOI] [PubMed] [Google Scholar]
- 36.Escott BG, Ronald K, Judd MG, Bogoch ER. NeuFlex and Swanson metacarpophalangeal implants for rheumatoid arthritis: prospective randomized, controlled clinical trial. J Hand Surg Am. 2010. January;35(1):44-51. [DOI] [PubMed] [Google Scholar]
- 37.Gaggl A, Schultes G. Biomechanical properties in titanium implants with integrated maintenance free shock absorbing elements. Biomaterials. 2001. November;22(22):3061-6. [DOI] [PubMed] [Google Scholar]
- 38.Lazennec JY, Aaron A, Brusson A, Rakover JP, Rousseau MA. The LP-ESP(®) lumbar disc prosthesis with 6 degrees of freedom: development and 7 years of clinical experience. Eur J Orthop Surg Traumatol. 2013. February;23(2):131-43. Epub 2013 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Namdari S, Weiss AP. Anatomically neutral silicone small joint arthroplasty for osteoarthritis. J Hand Surg Am. 2009. February;34(2):292-300. [DOI] [PubMed] [Google Scholar]
- 40.Capanni F, Hansen K, Fitzpatrick DC, Madey SM, Bottlang M. Elastically suspending the screw holes of a locked osteosynthesis plate can dampen impact loads. J Appl Biomech. 2015. June;31(3):164-9. Epub 2015 Feb 2. [DOI] [PubMed] [Google Scholar]
- 41.Foliart DE. Synovitis and silicone joint implants: a summary of reported cases. Plast Reconstr Surg. 1997. January;99(1):245-52. [DOI] [PubMed] [Google Scholar]
- 42.Taylor WR, Ehrig RM, Heller MO, Schell H, Seebeck P, Duda GN. Tibio-femoral joint contact forces in sheep. J Biomech. 2006;39(5):791-8. [DOI] [PubMed] [Google Scholar]
- 43.Fitzpatrick DC, Doornink J, Madey SM, Bottlang M. Relative stability of locked plating fixation in a model of the osteoporotic femoral diaphysis. Clin Biomech (Bristol, Avon). 2009. February;24(2):203-9. Epub 2008 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]