Abstract
Background
The effectiveness of vector control on malaria transmission by long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) depends on the vectors entering houses to blood feed and rest when people are inside houses. In the Solomon Islands, significant reductions in malaria have been achieved in the past 20 years with insecticide-treated bed nets, IRS, improved diagnosis and treatment with artemisinin combination therapies; despite the preference of the primary vector, Anopheles farauti, to feed outdoors and early in the evening and thereby avoid potential exposure to insecticides. Rational development of tools to complement LLINs and IRS by attacking vectors outdoor requires detailed knowledge of the biology and behaviours of the target species.
Methods
Malaria transmission in Central Province, Solomon Islands was estimated by measuring the components comprising the entomological inoculation rate (EIR) as well as the vectorial capacity of An. farauti. In addition, the daily and seasonal biting behaviour of An. farauti, was examined and the duration of the feeding cycle was estimated with a mark-release-recapture experiment.
Results
Anopheles farauti was highly exophagic with 72 % captured by human landing catches (HLC) outside of houses. Three-quarters (76 %) of blood feeding on humans was estimated to occur before 21.00 h. When the hourly location of humans was considered, the proportion of exposure to mosquito bites on humans occurring indoors (πi) was only 0.130 ± 0.129. Peak densities of host seeking An. farauti occurred between October and January. The annual EIR was estimated to be 2.5 for 2012 and 33.2 for 2013. The length of the feeding cycle was 2.1 days.
Conclusions
The short duration of the feeding cycle by this species offers an explanation for the substantial control of malaria that has been achieved in the Solomon Islands by LLINs and IRS. Anopheles farauti is primarily exophagic and early biting, with 13 % of mosquitoes entering houses to feed late at night during each feeding cycle. The two-day feeding cycle of An. farauti requires females to take 5–6 blood meals before the extrinsic incubation period (EIP) is completed; and this could translate into substantial population-level mortality by LLINs or IRS before females would be infectious to humans with Plasmodium falciparum and Plasmodium vivax. Although An. farauti is primarily exophagic, the indoor vector control tools recommended by the World Health Organization (LLINs and IRS) can still provide an important level of control. Nonetheless, elimination will likely require vector control tools that target other bionomic vulnerabilities to suppress transmission outdoors and that complement the control provided by LLINs and IRS.
Keywords: Anopheles farauti, Solomon Islands, Bionomics, Mark-release-recapture, Feeding cycle, Seasonality, Biting profile
Background
The basic reproductive number (R0) [1] is determined by the intensity of malaria transmission which depends largely on the parameters comprising vectorial capacity [2, 3] (the human biting density, proportion of blood-meals on humans and the mosquito life expectancy). The vector life expectancy, in turn, is a function of its survivorship per feeding cycle and the length of the feeding or gonotrophic cycle. The effectiveness of vector control depends on where and when a vector seeks human blood meals (which is determined, in part, by the location of humans). These parameters vary by species, both geographically and temporally, and will determine the effectiveness of vector control strategies implemented across different seasons and locations.
The Solomon Islands is currently undertaking country-wide intensified malaria control with the goal of malaria elimination in targeted provinces. Malaria transmission is predominantly by Anopheles farauti. The vector control strategies are those recommended by the World Health Organization’s Malaria Policy Advisory Committee (WHO MPAC)—universal distribution of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) in limited areas [4]. Exposure of vectors to the insecticides occurs when mosquitoes enter houses late at night while seeking a blood meal (LLINs) or when resting after blood feeding (IRS) [5, 6]. Although An. farauti varies in its degree of anthropophagy across Melanesia, it is highly anthropophagic in the Solomon Islands [7]. In the Solomon Islands, An. farauti displays behavioural resistance to insecticides by feeding mostly outdoors and early in the evening [8]. Anopheles farauti first shifted its behaviour to feed early in the evening when people were outdoors in response to the DDT spray campaigns in the 1970s, thus avoiding the insecticide [9, 10]. This behavioural shift was one reason that the original Malaria Eradication Programme (MEP) of the early 1970s failed [11] and, malaria cases surged throughout the Solomon Islands until insecticide treated nets (ITNs) and LLINs were introduced in 1992–1993, and 2005, respectively [12]. This insecticide avoidance behaviour appears to be maintained by the widespread use of LLINs, as recent surveys show that this early outdoor biting behaviour still persists in at least three other An. farauti populations in the Solomon Islands [13–15].
Despite the challenge of behavioural resistance in An. farauti, there have been significant reductions in malaria achieved in the Solomon Islands in the past 20 years with ITNs, IRS and improved anti-malarials. However, malaria elimination remains, perhaps, an insurmountable challenge with these available intervention tools. New vector control interventions are needed to complement the indoor killing of LLINs and IRS by attacking outdoor feeding or other behavioural vulnerabilities of An. farauti [16, 17]. Rational development of such tools requires detailed knowledge about the biology and behaviours of vectors. The isolated island populations of An. farauti display variability in their night biting profile, blood feeding patterns and the degree of endophily, likely the result of restricted gene flow among island populations [18]. In this paper, a number of key vector parameters were measured for An. farauti, in Central Province, Solomon Islands to determine potential behavioural vulnerabilities for vector control. These parameters were the daily and seasonal biting behaviour, the time and location (indoors or outdoors) of blood feeding and the length of the feeding cycle.
Methods
Study site
The study was conducted in Haleta village on Ngella Sule Island in Central Province (−9°5′56″S, 160°6′56″E; Fig. 1) where malaria transmission is hypoendemic [19]. This rural coastal village is bounded by the ocean to the south, with high ground of ≈360 m elevation on the north. This community of 470 people live in 107 houses constructed predominantly of bamboo walls or woven palm fronds with thatched roofs and open eaves (Bed net census, 2010, Solomon Islands Ministry of Health, Unpublished data). Domestic animals consist of pigs (predominantly housed in pens), chickens, dogs and cats. The climate of the region is continuous hot/wet with a median annual rainfall of 2837 mm (based on 43 years of data collected at the provincial capital Tulagi approximately 10 km from Haleta village) [20]. While rain falls throughout the year, there is higher precipitation from January to March (mean monthly rainfall of 344 mm), with relatively less rain between April and December (mean monthly rainfall of 200 mm). The mean daily coastal temperature ranges between 24 and 30 °C with an annual mean of 26 °C.
Fig. 1.
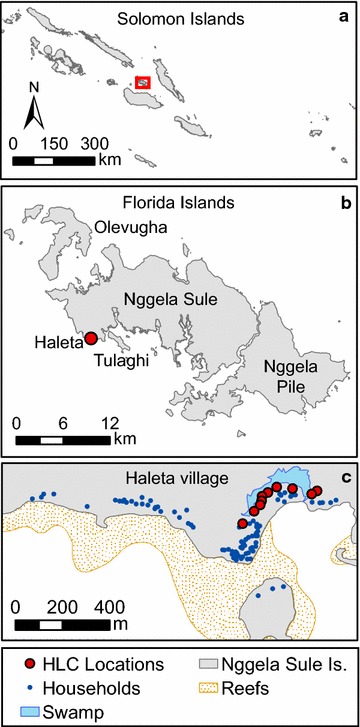
Map of the Solomon Islands (a) showing Haleta village on Nggela Sule Island in Central Province (b −9°5′56″S, 160°6′56″E) as well as the layout of the village (c)
Mosquito sampling and processing
Anopheline mosquitoes were sampled by human landing catches (HLC) [21]. Village collectors captured mosquitoes that landed on their exposed legs and feet with a mouth aspirator at designated collection stations in Haleta village. Mosquitoes were held in individual waxed paper cups by hour and location of collection (geographic location within the village or indoors/outdoors). Numbers of An. farauti caught per hour and location per collector were recorded based on morphological examination [22] prior to dissecting subsamples for parity determination and spermatheca insemination [23]. Parity was assessed by dissecting the ovaries, drying on a glass microscope slide and examining under 100–200 times magnification for the presence or absence of skeins at the end of the tracheoles [23]. The insemination status of female An. farauti was assessed by dissecting and rupturing the spermatheca under a cover slip and examining under 400 times magnification for spermatozoa [23]. All specimens were preserved in 100 % ethanol and a subsample subjected to subsequent species identification analysis using the Internal Transcribed Spacer Region II of the ribosomal DNA (ITS2) [24] and detection of Plasmodium DNA in heads and thoraces by nested PCR [25].
All night biting profile
The indoor and outdoor biting profile of An. farauti was estimated from five households from 18.00 to 06.00 h from the 24th to the 28th of July 2012 by HLC. Mosquitoes were collected by hour and the households were separated by a distance of ≥20 m with collectors working in pairs, one indoors the other outdoors 10 m away. The collectors were systematically rotated between working the early and late shifts on each night.
The biting behaviour of An. farauti was analysed to estimate endophagy, nocturnal biting and human contact indoors (πi). Endophagy or the propensity to bite indoors was defined as the total number of An. farauti collected indoors divided by the total of indoor plus outdoor An. farauti collected. The ability to obtain a blood meal on humans indoors (nocturnal activity) was calculated as the total number of bites indoors plus outdoors during sleeping hours (21.00–05.00 h) divided by the total during the entire night. The analysis was extended to calculate the proportion of human contact with mosquito bites occurring indoors (πi) (see [26] for detailed formulas). To determine this, the number of people outdoors in the HLC area was counted hourly from 18.00 to 06.00 h each night for 14 consecutive nights beginning on 23rd Nov 2011. The number of people indoors for each hour was calculated as the difference between the hourly outdoor count and the mean number of occupants seen outdoors at 18.00 h.
Seasonality of Anopheles farauti
Biting densities of An. farauti were estimated by HLC between August 2011 and February 2014 from 18.00 to 00.00 h for a minimum of five nights each month by 10 village collectors working outdoors distributed along the village (Fig. 1). As the majority of biting occurred from 18.00 to 00.00 h, this time period was selected as a time and cost effective measure for estimating anopheline density and seasonality. Samples of An. farauti were dissected to determine parity (six occasions: November 2011, February, May, July, August, and November 2012) and insemination status (three occasions: November 2011, February and May 2012). PCR assays were used to identify a subset of specimens species [24] and identify infections of Plasmodium sporozoites [25].
For 2012 and 2013, the annual entomological inoculation rate (EIR) was calculated using the equation: EIR = S × B × 365 [27, 28]. Where, S is the sporozoite rate (defined as the number of mosquitoes with malaria specific DNA detected in the head and thorax/no. of mosquitoes tested), and B is the annual human biting rate (mean number of mosquitoes collected per collector per night/calibration factor). As mosquitoes were only collected from 18.00 to 00.00 h the estimated biting rate was adjusted to account for the proportion of An. farauti that fed after midnight; using a calibration factor of 0.93 to estimate the all night biting rate (see Results).
Duration of the gonotrophic cycle
Freshly blood-fed An. farauti from HLC were placed individually into 70 ml specimen jars with damp cotton-wool covered with filter paper as an oviposition substrate. The top of each jar was covered with netting and damp cotton-wool to maintain high humidity. The containers were held at ambient temperature and exposed to normal day/night light regimes. Hourly examination of the containers for eggs commenced at dusk (18.00 h) 43 h after blood-feeding. The hour in which eggs were laid was recorded.
Duration of the feeding cycle by mark-release-recapture experiment
The length of the feeding cycle (defined as the period between two consecutive blood-meals) for An. farauti was estimated from a mark-release-recapture experiment using mosquitoes captured by HLC from 29th November to 9th December 2012. Mosquitoes were collected from 18.00 to 00.00 at 16 outdoor HLC stations positioned throughout the village (Fig. 1). Blood fed mosquitoes were placed into plastic 250 ml cups covered with netting, each cup containing a maximum of 100 mosquitoes. A small amount of fluorescent powder (BioQuip Products, Inc. California, USA and Glow Paint Industries, Queensland, Australia) was sifted through the netting into the cup; a fine tipped transfer pipette was use to aerosolise the powder which coated the mosquitoes. The effectiveness of this procedure was checked by examining the mosquitoes in each cup with a LED UV torch (400 nm wavelength) to ensure that they were adequately marked with the powder. The mosquitoes were released between 00.00 h and 01.00 h on the night of collection from a single outdoor location. The distance from the release site to the most distant HLC collection station was 190 m. Mosquitoes were marked on nights 1, 2 and 3 using a different colour (blue, pink, and yellow) fluorescent powder each night. On nights 2 through 11, all captured An. farauti were visually checked for fluorescent dust using a UV torch. Recaptured marked mosquitoes and unmarked mosquitoes which were not released were stored for species identification.
The mean length of the feeding cycle (U) was estimated as:
where R represents the number of mosquitoes recaptured on day i after release [29, 30].
Statistical analysis
The data was compiled in a series of tables which detailed the results of: (1) mosquito collections, (2) dissections, (3) molecular analyses, (4) mark-release-recapture releases, and (5) oviposition [31]. Statistical differences in endophagy (indoor versus outdoor biting) and nocturnal biting (sleeping hours were 21.00–05.00 h) were compared with generalized linear models (GLMs) with a negative binomial distribution. The temporal change in the biting rate and the proportion parous were analysed with GLMs with a negative binomial and binomial distributions, respectively. All analyses were conducted using the R package V3.1.2 [32].
Ethics
Ethical approval for the study was obtained from the National Health Research and Ethics Committee, Solomon Islands (02-05-2011), the James Cook University Human Research Ethics Committee, Australia (H4122) and the University Hospitals Case Medical Centre Institutional Review Board for Human Investigation, USA (05-11-11). Collectors were selected and trained from residents of Haleta after obtaining informed consent. Only village adults who likely have some immunity to malaria were asked to participate in the landing catches and were instructed to capture the mosquitoes before they bite and all took malaria prophylaxis. To estimate the duration of the feeding cycle by mark-release-recapture, mosquitoes were offered a human blood meal from one of the listed authors who was taking malaria prophylaxis prior to release.
Results
In Haleta village, 21,619 female anophelines were collected by HLC. All specimens were morphologically An. farauti s.l. A subset of the specimens (n = 1315) were confirmed as An. farauti s.s. by molecular analysis (with samples selected across the longitudinal dataset).
All night biting profile of Anopheles farauti
Anopheles farauti was highly exophagic (β = 0.953, se = 0.197, p < 0.0001), with the proportion of endophagy estimated as 0.28 ± 0.03 (mean proportion indoors ± se; Fig. 2a). The nocturnal biting activity (proportion biting during sleeping hours [21.00–05.00 h]) of An. farauti was 0.239 ± 0.025 (proportion nocturnal ± se). Significantly more biting occurred outside of sleeping hours (β = 1.625, se = 0.187, p < 0.0001) with 76 % of the overall biting occurring before 21.00 h and 93 % before midnight (Fig. 2b). After adjusting for human behaviour (location of people indoors or outdoors over the night), the estimated biting rate for an unprotected person (Bu) was 6.8 bites per night and the proportion exposed to mosquito bites indoors (πi) was only 0.130 ± 0.129 (Fig. 2c).
Fig. 2.
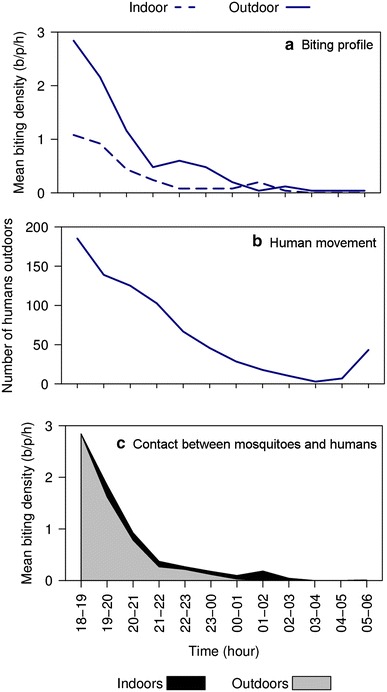
The hourly indoor and outdoor profile of Anopheles farauti biting (a) and the number of humans outside of houses throughout the night (b) in Haleta village, Central Province, Solomon Islands. The stacked line graph (c) is the estimated contact between humans and mosquitoes, which considers the movement pattern of people by weighting the indoor and outdoor biting rates throughout the night by the proportion of humans that are typically indoors or outdoors at each time period [41, 42]. Note b/p/h = bites/person/hour
Seasonality of Anopheles farauti
The density of host seeking An. farauti varied temporally (β = 0.078, se = 0.002, p < 0.0001) with the highest densities occurring between October and January, reaching ≈40 bites per person from 18.00 to 00.00 h in October 2012 and January 2014 (Fig. 3). The average human biting rate of An. farauti was 14.81 bites/person/night (b/p/n). The sporozoite rate in An. farauti was 0.0047 based on the analyses of 4707 An. farauti heads and thoraxes for Plasmodium DNA. The overall EIR was estimated to be 25.3 infective bites/person/year (ib/p/y; Table 1). The EIR varied annually from 2.5 ib/p/y in 2012 to 35.7 ib/p/y in 2013. The overall parity rate of An. farauti was estimated to be 0.59 parous (439/739, for the period November 2011 to December 2012). Parity was temporally influenced (β = −0.338, se = 0.044, p < 0.0001) with monthly parity estimates ranging between 0.41 and 0.76; with parity rates higher in February (0.73; n = 184), May (0.57; n = 118) and July (0.62; n = 43) and lowest in the latter half of the year in August (0.44; n = 133) and November (0.41; n = 131). The majority, 96 %, of host-seeking An. farauti were inseminated (n = 344/358).
Fig. 3.
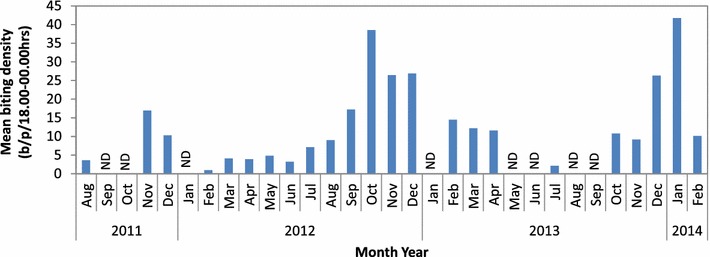
Monthly biting rate for Anopheles farauti in Haleta village, Central Province, Solomon Islands, estimated using human landing catch from 18.00 to 00.00 h. Note ND no data
Table 1.
The estimated malaria transmission intensity attributable to Anopheles farauti in Haleta village, Central Province, The Solomon Islands
| Time | Sporozoite rate (n positive) | All night biting rate (b/p/18.00–06.00 h)a | Daily EIR (ib/p/d)b | Annual EIR (ib/p/y)c | |||
|---|---|---|---|---|---|---|---|
| Total tested | P. falciparum | P. vivax | Overall | ||||
| Nov 2011 | 207 | 0.0000 (0) | 0.0000 (0) | 0.0000 (0) | 15.16 | 0.000 | |
| 2012 | 2062 | 0.0000 (0) | 0.0005 (1) | 0.0005 (1) | 13.98 | 0.007 | 2.5 |
| 2013 | 1907 | 0.0026 (5) | 0.0052 (10) | 0.0073 (14)d | 13.33 | 0.098 | 35.7 |
| Jan–Feb 2014 | 531 | 0.0038 (2) | 0.0113 (6) | 0.0132 (7)d | 27.90 | 0.368 | |
| Overall | 4707 | 0.0015 (6) | 0.0036 (17) | 0.0047 (22)d | 14.81 | 0.069 | 25.3 |
aAll night biting rate was calculated with a calibration factor of 93 % biting before midnight. This figure was calculated from the biting profile presented in the first section of this paper
bDaily EIR [infective bites per person per day (ib/p/d)] = sporozoite rate × biting rate (18.00–06.00 h)
cAnnual EIR [infective bites per person per year (ib/p/y)] = sporozoite rate × biting rate (18.00–06.00 h) × 365
dThese sample periods include mixed P. falciparum and P. vivax infections (one from 2013, one from Jan–Feb 2014 and thus two mixed infections overall)
Duration of the gonotrophic cycle
The time from blood engorgement to oviposition was recorded for 145 An. farauti. Of these, 44.1 % (n = 64) of An. farauti laid eggs on the second night after blood-feeding, 46.2 % (n = 67) laid eggs on the third night and the remainder (9.7 %; n = 14) laid on the forth night. The average interval from blood feeding to oviposition was 61.2 ± 1.1 h (n = 111) or 2.6 days. The length of the gonotrophic cycle ranged from 43 to 83 h.
Duration of the feeding cycle
During this experiment, 3891 anophelines were captured by HLC and identified morphologically as An. farauti s.l., with 100 % of a subset being molecularly identified as An. farauti s.s. (n = 189). To estimate the length of the feeding cycle, 1751 blood-fed female An. farauti of unknown chronological age were marked with fluorescent dust (a different colour on each night) and released (282 on night 1, 266 on night 2 and 203 on night 3). Subsequently, 105 marked An. farauti were recaptured (a recapture rate of 14 %). The interval between release and recapture (the length of the feeding cycle) was 2.1 days (Fig. 4). Three feeding cycles of two days duration are clearly evident after the mosquitoes were released. The majority, 82 %, of mosquitoes sought blood meals two nights after their previous blood meal, with 11 and 7 % seeking blood meals at 1 and 3 night intervals, respectively.
Fig. 4.
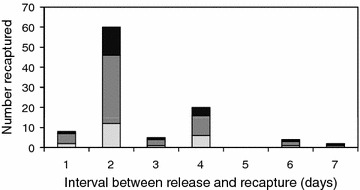
The feeding cycle length of Anopheles farauti examined by a mark-release-recapture experiment, expressed as a frequency histogram of the interval of time between release and recapture for each individual mosquito. Note: the different colours represent the information from each of the three events when mosquitoes were released
Discussion
The effectiveness of vector control is a function of both mosquito and human behaviours. For LLINs and IRS, the degree to which the vector feeds or rests indoors (i.e., how endophagic or endophilic) as well as the frequency at which the vector blood feeds will largely determine the proportion that survive for the duration of the extrinsic incubation period. Indoor feeding and resting are determined, in large part by the location of humans (indoors or outdoors) when mosquitoes are seeking blood meals (e.g., mosquitoes seeking human blood meals earlier in the evening are more likely to feed on humans outdoors when few people are inside houses). The duration of peak mosquito density is important for the selection and timing of the application of insecticides used in IRS (as different insecticides and formulations vary in their effective half-life).
Most populations of An. farauti in the Solomon Islands bite outdoors and early in the evening. Previously reported πi values (the proportion of feeds on humans taking place indoors) were 0.314 for Guadalcanal Province in 2007-08 [15]1 to 0.368–0.570 for Temotu Province in 2008–2010 [13] with the highest value recorded in Isabel Province in 2009 (0.546) [14]. The lowest proportion of bites on humans indoors for An. farauti was found in this study in Haleta village on Ngella Sule, Central Province, with only 13 % of human feeds indoors. This island was designated as a “problem area” during the original malaria eradication programme [10], which is understandable as the early outdoor feeding of An. farauti found in this study would minimize exposure to the insecticides used in IRS and ITNs and limit the effectiveness of the interventions.
The terms gonotrophic and feeding cycle are often used interchangeably despite the fact that they are, in fact, describing slightly different time intervals (i.e., the period between successive oviposition and blood feeding events, respectively). Mark-release-recapture experiments using HLC estimated the feeding cycle length whereas the gonotrophic cycle length was estimated by measuring the duration between blood feeding and oviposition of mosquitoes held under field laboratory conditions. Feeding cycle length estimates from mark-release-recapture, for all anopheline species range from 2 to 4 days [33, 34]. The feeding cycle length for An. farauti in Central Province is one of the shortest recorded at 2.1 days, but is comparable with previous estimates for this species from Guadalcanal Province, Solomon Islands [35] and Madang Province, Papua New Guinea [29, 30, 36] which ranged between 2 and 3 days. The feeding cycles among malaria vectors in different villages in Madang, Papua New Guinea were 2.7–3.7 days for Anophelespunctulatus, 2.4–3.2 days for Anopheles koliensis and 2.1–3.0 days for An. farauti [30]. The local environment was found to exert a greater influence on the duration of the feeding cycle than the species of mosquito, with permanent pool breeders having a shorter cycle then temporary pool breeders. If extensible to the Solomon Islands, the environmental conditions in the coastal villages where An. farauti is found would have been predicted to have a short gonotrophic cycle, since the vector is laying its eggs in a permanent breeding sites (coastal lagoons and swamps) located in close proximity to villages and thus the human host.
The estimated length of the gonotrophic cycle (2.6 days) was longer than the estimate of the feeding cycle (2.1 days) calculated from the mark-release-recapture experiment. It is possible that the laboratory conditions (e.g., sugar deprivation, limited space, temperature, etc.) in which the gonotrophic cycle was estimated from egg development were sufficiently different from the field conditions in which the feeding cycle was measured to explain the difference between the estimates of the gonotrophic and feeding cycles. A similar study for Anopheles albitarsis in Brazil [37], also found a longer gonotrophic cycle (calculated from oviposition observations) than the feeding cycle (from mark-release-recapture experiments).
The An. farauti population in this area exhibited a single peak biting season between October and January. In Haleta the parity data followed a seasonal trend with higher parity rates occurring during peak adult densities and declining from February with lowest rates in August and November 2012 when An. farauti densities would begin to increase (Fig. 3) with the emergence of nulliparous mosquitoes into the adult population. This should be considered when planning vector control, with the bulk of activities completed before commencement of the peak biting season. A very similar temporal pattern and similar genetic population of An. farauti [18] was found in Guadalcanal [15]. A supporting study of the larval populations in Guadalcanal [38] demonstrated that larval presence and density also varied seasonally and was primarily driven by rainfall.
Historical estimates of the sporozoite rates and EIR for An. farauti are not available for Central Province, but are available for Guadalcanal Province (the nearest province). During the early 1990s and in the absence of vector control, EIR values as high as 1022 ib/p/y were recorded in Guadalcanal [39]. The intensified vector control programme implemented by the Ministry of Health and Medical Services over the last decade has had a substantial impact on transmission as evidenced by the greatly diminished and now relatively low EIRs estimated here in 2012 (2.5 ib/p/y) and 2013 (35.7 ib/p/y).
Despite the early and outdoor biting habits of An. farauti, the frequency of blood feeding by this species offers an explanation for the substantial malaria control that has been achieved by LLINs and IRS in the Solomon Islands. With each successive feeding cycle there is a multiplicative effect that increases the proportion of the total vector population exposed to insecticides. In the Solomon Islands where the annual mean temperature is ≈26 °C, the length of the extrinsic incubation period (EIP) is estimated to be 12 and 9 days for Plasmodium falciparum and Plasmodium vivax, respectively [40]. With an estimated feeding cycle of two days, An. farauti, will have 6 and 5 opportunities to enter a house before completion of the P. falciparum and P. vivax EIP, respectively. Although only 13 % (πi) of An. farauti will be potentially exposed to insecticides by biting late and indoors during each feeding cycle, this will cumulate in significant mortality across the multiple feeding cycles required to complete the EIP. Assuming that LLINs have the potential to kill 80 % of those mosquitoes that enter and attempt to feed on sleeping humans, this could translate into 47 and 41 % population-level mortality before An. farauti would be infectious to humans with P. falciparum and P. vivax, respectively.2 This emphasizes the fact that although the population of An. farauti is primarily exophagic, indoor vector control tools still provide significant control [41]. This is an important consideration, as evidence has been emerging from other anopheline populations that the proportion of feeding indoors is diminishing, such as for An.funestus in Tanzania [42], Benin [43] and Senegal [44] as well as An.gambiae s.s. in Equatorial Guinea [45].
Conclusion
LLINs and IRS have had a significant impact on malaria transmission despite the outdoor and early biting habits of An. farauti, the primary malaria vector in the Solomon Islands. Here key bionomic parameters of the malaria vector, An. farauti, that determine the potential for transmission (i.e., vectorial capacity) and the vulnerability to control interventions were estimated. The protective effect against LLINs and IRS that An. farauti enjoys by virtue of biting outdoors is offset by its short feeding cycle which potentially exposes this vector 4–6 times during the course of an EIP to the insecticides in LLINs and IRS. Nonetheless, elimination will likely require vector control tools that target other bionomic vulnerabilities to suppress transmission outdoors and to complement the control provided by LLINs and IRS.
Availability of data and materials
The datasets supporting the conclusions of this article are available in the James Cook University Tropical Data Hub repository: http://dx.doi.org/10.4225/28/56C671268CF73.
Authors’ contributions
TLR supervised the overall field studies and drafted the manuscript. TLR, NFL, FHC and TRB contributed to the experimental designs. All authors participated in the field experiments. WKC, RDC and NWB conducted the molecular analyses. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by Grant No. 45114 from the Bill and Melinda Gates Foundation to the Malaria Transmission Consortium for the work in the Solomon Islands. In addition, the support of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health for the International Center of Excellence in Malaria Research in the Southwest Pacific (subaward to James Cook University; award number U19AI08986) is gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders or the Australian Defence Force and/or extant Defence Force Policy. Technical assistance when conducting the field collections was provided by John Lodo of the Vector Borne Disease Control Programme, Solomon Islands. Additional PCR analyses for species identification and sporozoite detection were conducted by Mr Luke Ambrose, Mr Andrew Maynard, Dr Tyrone Lavery and Mr Peter Moore at The University of Queensland. The authors thank the community of Haleta for their support as well as the support of Mr Albino Bobogare, Director of National Vector Borne Disease Control Programme, Solomon Islands and Prof James Kazura, Programme Director of the International Center of Excellence in Malaria Research in the Southwest Pacific.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- EIP
extrinsic incubation period
- EIR
entomological inoculation rate
- GLM
generalized linear model
- IRS
indoor residual spraying
- HLC
human landing catch
- LLINs
long-lasting insecticidal nets
- MEP
Malaria Eradication Program
- WHO MPAC
World Health Organization’s Malaria Policy Advisory Committee
- πi
the proportion of feeds on humans taking place indoors
Footnotes
The πi value for Guadalcanal was calculated using the raw An. farauti dataset from the publication and the human movement profile from Central Province presented in this paper.
These basic calculations account for the maximum possible efficacy of indoor vector control in the absence of any other mortality factors. The population-level mortality across multiple feeding cycles was calculated as 1−(SF). Where S = the proportion surviving each feeding cycle calculated as 1 – (πi x 0.8); and F = the number of feeding cycles.
Contributor Information
Tanya L. Russell, Email: tanya.russell@jcu.edu.au
Nigel W. Beebe, Email: n.beebe@uq.edu.au
Hugo Bugoro, Email: hugo.bugoro@sig.gov.sb.
Allan Apairamo, Email: allan.apairmo@gmail.com.
Weng K. Chow, Email: Weng.Chow@defence.gov.au
Robert D. Cooper, Email: Bob.Cooper@defence.gov.au
Frank H. Collins, Email: frank@nd.edu
Neil F. Lobo, Email: nlobo@nd.edu
Thomas R. Burkot, Email: tom.burkot@jcu.edu.au
References
- 1.Garrett-Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquito’s vectorial capacity. Nature. 1964;204:1173–1175. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957. [Google Scholar]
- 3.Cohuet A, Harris C, Robert V, Fontenille D. Evolutionary forces on Anopheles: what makes a malaria vector? Trends Parasitol. 2010;26:130–136. doi: 10.1016/j.pt.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global Technical Strategy for Malaria 2016–2030. Geneva: World Health Organization; 2015.
- 5.Lengeler C. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657. doi:10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed]
- 7.Russell TL, Beebe NW, Bugoro H, Apairamo A, Cooper RD, Lobo NF, et al. Determinants of host feeding success by Anopheles farauti. Malar J. 2016. doi:10.1186/s12936-016-1168-y. [DOI] [PMC free article] [PubMed]
- 8.Russell TL, Beebe NW, Bugoro H, Apairamo A, Cooper RD, Lobo NF, et al. Anopheles farauti is a homogeneous population that blood feeds early and outdoors in the Solomon Islands. Malar J. 2016. doi:10.1186/s12936-016-1194-9. [DOI] [PMC free article] [PubMed]
- 9.Taylor B. Changes in the feeding behaviour of a malaria vector, Anopheles farauti Lav., following the use of DDT as a residual spray in houses in the British Solomon Islands Protectorate. Trans R Entomol Soc London. 1975;127:227–292. [Google Scholar]
- 10.Paik Y-H, Avery JG. Problem areas in the malaria eradication programme in the British Solomon Islands. P N G Med J. 1973;17:61–67. [PubMed] [Google Scholar]
- 11.Avery J. A review of the malaria eradication programme in the British Solomon Islands 1970–1972. P N G Med J. 1974;17:50–60. [PubMed] [Google Scholar]
- 12.Kere NK, Arabola A, Bakote’e B, Qalo O, Burkot TR, Weber RH, et al. Permethrin-impregnated bednets are more effective than DDT house-spraying to control malaria in Solomon Islands. Med Vet Entomol. 1996;10:145–148. doi: 10.1111/j.1365-2915.1996.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 13.Bugoro H, Cooper R, Butafa C, Iro’ofa C, Mackenzie D, Chen C-C, et al. Bionomics of the malaria vector Anopheles farauti in Temotu Province, Solomon Islands: issues for malaria elimination. Malar J. 2011;10:133. doi: 10.1186/1475-2875-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bugoro H, Iro’ofa C, Mackenzie D, Apairamo A, Hevalao W, Corcoran S, et al. Changes in vector species composition and current vector biology and behaviour will favour malaria elimination in Santa Isabel Province, Solomon Islands. Malar J. 2011;10:287. doi: 10.1186/1475-2875-10-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugoro H, Hii J, Butafa C, Iroofa C, Apairamo A, Cooper R, et al. The bionomics of the malaria vector Anopheles farauti in Northern Guadalcanal, Solomon Islands: issues for successful vector control. Malar J. 2014;13:56. doi: 10.1186/1475-2875-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56. doi: 10.1186/1475-2875-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govella NJ, Ferguson H. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol. 2012;3. doi:10.3389/fphys.2012.00199. [DOI] [PMC free article] [PubMed]
- 18.Ambrose L, Cooper RD, Russell TL, Burkot TR, Lobo NF, Collins FH, et al. Microsatellite and mitochondrial markers reveal strong gene flow barriers for Anopheles farauti in the Solomon Archipelago: implications for malaria vector control. Int J Parasitol. 2014;44:225–233. doi: 10.1016/j.ijpara.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waltmann A, Darcy AW, Harris I, Koepfli C, Lodo J, Vahi V, et al. High rates of asymptomatic, sub-microscopic Plasmodium vivax infection and disappearing Plasmodium falciparum malaria in an area of low transmission in Solomon Islands. PLoS Negl Trop Dis. 2015;9:e0003758. doi: 10.1371/journal.pntd.0003758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookfield HC, Hart D. Rainfall in the tropical southwest Pacific. Canberra: Department of Geography, Publ G/3, The Australian National University; 1966.
- 21.Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, et al. Incidence of malaria among mosquito collectors conducting human landing catches in Western Kenya. Am J Trop Med Hyg. 2013;88:301–308. doi: 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belkin JN. The mosquitoes of the South Pacific (Diptera, Culicidae) Berkeley and Los Angeles: University of California Press; 1962. [Google Scholar]
- 23.WHO. Manual on practical entomology in malaria. Part II. Methods and techniques. WHO Offset Publication No. 13. Geneva: World Health Organization, Division of Malaria and Other Parasitic Diseases; 1975.
- 24.Beebe NW, Saul A. Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction—restriction fragment length polymorphism analysis. Am J Trop Med Hyg. 1995;53:478–481. doi: 10.4269/ajtmh.1995.53.478. [DOI] [PubMed] [Google Scholar]
- 25.Snounou G, Viriyakosol S, Xin Ping Z, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 26.Killeen GF, Kihonda J, Lyimo E, Oketch FR, Kotas ME, Mathenge E, et al. Quantifying behavioural interactions between humans and mosquitoes: evaluating the protective efficacy of insecticidal nets against malaria transmission in rural Tanzania. BMC Infect Dis. 2006;6:161. doi: 10.1186/1471-2334-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 28.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birley MH, Charlwood JD. The effect of moonlight and other factors on the oviposition cycle of malaria vectors in Madang, Papua New Guinea. Ann Trop Med Hyg. 1989;83:415–422. doi: 10.1080/00034983.1989.11812366. [DOI] [PubMed] [Google Scholar]
- 30.Charlwood JD, Graves PM, Birley MH. Capture-recapture studies with mosquitos of the group of Anopheles punctulatus Dönitz (Diptera, Culicidae) from Papua New Guinea. Bull Entomol Res. 1986;76:211–227. doi: 10.1017/S000748530001470X. [DOI] [Google Scholar]
- 31.Russell TL, Beebe NW, Bugoro H, Apairamo A, Chow W, Cooper RD, et al. Dataset examining host feeding parameters of Anopheles farauti in Haleta village, Solomon Islands. James Cook University Tropical Data Hub; 2016. doi:10.4225/28/56C671268CF73.
- 32.R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013.
- 33.Silver JB. Mosquito ecology: field sampling methods. 3. New York: Springer; 2008. [Google Scholar]
- 34.Guerra C, Reiner R, Perkins T, Lindsay S, Midega J, Brady O, et al. A global assembly of adult female mosquito mark-release-recapture data to inform the control of mosquito-borne pathogens. Parasit Vectors. 2014;7:276. doi: 10.1186/1756-3305-7-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hii JLK. Antimalaria program. WHO assignment report. (WP)MAL/SOL/MAL/001-E. Honiara: WHO Regional Office for the Western Pacific; 1988.
- 36.Charlwood JD, Graves PM, Marshall TFC. Evidence for a ‘memorized’ home range in Anopheles farauti females from Papua New Guinea. Med Vet Entomol. 1988;2:101–108. doi: 10.1111/j.1365-2915.1988.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 37.Dos Santos RL, Forattini OP, Burattini MN. Laboratory and field observations on duration of gonotrophic cycle of Anopheles albitarsis s.l. (Diptera: Culicidae) in southeastern Brazil. J Med Entomol. 2002;39:926–930. doi: 10.1603/0022-2585-39.6.926. [DOI] [PubMed] [Google Scholar]
- 38.Bugoro H, Hii J, Russell T, Cooper R, Chan B, Iro’ofa C, et al. Influence of environmental factors on the abundance of Anopheles farauti larvae in large brackish water streams in Northern Guadalcanal, Solomon Islands. Malar J. 2011;10:262. doi: 10.1186/1475-2875-10-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hii JLK, Kanai L, Foligela A, Kan SKP, Burkot TR, Wirtz RA. Impact of permethrin-impregnated mosquito nets compared with DDT house spraying against malaria transmission by Anopheles farauti and An. punctulatus in the Solomon Islands. Med Vet Entomol. 1993;7:333–338. doi: 10.1111/j.1365-2915.1993.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald G. The analysis of the sporozoite rate. Trop Dis Bull. 1952;49(6):569–589. [PubMed] [Google Scholar]
- 41.Govella NJ, Okumu FO, Killeen GF. Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg. 2010;82:415–419. doi: 10.4269/ajtmh.2010.09-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;206:1622–1629. doi: 10.1093/infdis/jis565. [DOI] [PubMed] [Google Scholar]
- 44.Sougoufara S, Diedhiou S, Doucoure S, Diagne N, Sembene P, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy M, Overgaard H, Abaga S, Reddy V, Caccone A, Kiszewski A, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island. Equatorial Guinea. Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are available in the James Cook University Tropical Data Hub repository: http://dx.doi.org/10.4225/28/56C671268CF73.


