Abstract
Rationale
Endovascular interventions performed for atherosclerotic lesions trigger excessive vascular smooth muscle cell (SMC) proliferation leading to intimal hyperplasia. Our previous studies show that following endovascular injury, elevated TGF-β/Smad3 promotes SMC proliferation and intimal hyperplasia. Furthermore in cultured SMCs, elevated TGF-β/Smad3 increases the expression of several Wnt genes. Here we investigate a crosstalk between TGF-β/Smad3 and Wnt/β-catenin signaling and its role in SMC proliferation.
Methods and Results
To mimic TGF-β/Smad3 up-regulation in vivo, rat aortic SMCs were treated with Smad3-expressing adenovirus (AdSmad3) or AdGFP control followed by stimulation with TGF-β1 (or solvent). AdSmad3/TGF-β treatment up-regulated Wnt2b, Wnt4, Wnt5a, Wnt9a, and Wnt11 (confirmed by qRT-PCR and ELISA), and also increased β-catenin protein as detected by Western blotting. Blocking Wnt signaling using a Frizzled receptor inhibitor (Niclosamide) abolished TGF-β/Smad3-induced β-catenin stabilization. Increasing β-catenin through degradation inhibition (using SKL2001) or by adenoviral expression enhanced SMC proliferation. Furthermore, application of recombinant Wnt2b, Wnt4, Wnt5a, or Wnt9a, but not Wnt11, stabilized β-catenin and stimulated SMC proliferation as well. In addition, increased β-catenin was found in the neointima of injured rat carotid artery where TGF-β and Smad3 are known to be up-regulated.
Conclusions
These results suggest a novel mechanism whereby elevated TGF-β/Smad3 stimulates the secretion of canonical Wnts which in turn enhances SMC proliferation through β-catenin stabilization. This crosstalk between TGF-β/Smad3 and Wnt/β-catenin canonical pathways provides new insights into the pathophysiology of vascular SMCs linked to intimal hyperplasia.
Keywords: TGFβ/Smad3, Wnt/β-Catenin, vascular smooth muscle cells, proliferation
1. Introduction
Atherosclerosis continues to be the leading cause of morbidity and mortality in the Western world, with thousands of Americans undergoing endovascular procedures or surgical revascularizations each year[1]. These interventions cause endothelial denudation and Smooth Muscle Cell (SMC) injury, transforming SMCs from a quiescent to a synthetic and proliferative state[2]. Uncontrolled proliferation of SMCs leads to the formation of highly cellular neointima (a process termed intimal hyperplasia or IH), leading to renarrowing of the lumen (restenosis)[3]. In spite of advances in anti-proliferative endovascular interventions, a significant number of these eventually fail due to IH[4]. Thus, it is imperative to understand the molecular underpinning of IH.
Our group has uncovered that canonical TGF-β/Smad3 signaling is enhanced after vascular injury in humans and in an animal model as well[5, 6]. Previous investigators have reported that TGF-β1 inhibits SMC proliferation in vitro[7]. This effect is reversed in the presence of elevated levels of Smad3 and leading to increases in SMC proliferation, migration and de-differentiation while inhibiting apoptosis[8-11]. Upon TGF-β activation, the TGF-β receptor phosphorylates Smad3 which, in a complex with Smad2 and Smad4, translocates to the nucleus to regulate gene expression. Thus our previous studies suggest that elevated expression of Smad3 following arterial injury transforms TGF-β1 into a stimulant of SMC proliferation. Despite these advances, the mechanisms responsible for TGF-β/Smad3-stimulated IH remain poorly understood. To this end, we recently conducted an Affymetrix array and discovered that multiple genes associated development including Wnt genes are upregulated in SMCs following treatment with TGF-β/Smad3[9].
The Wnt family consists of 19 conserved genes that encode for secreted glycoprotein ligands for Frizzled receptors[12]. Wnt family proteins have diverse roles in embryogenesis including stimulation of proliferation[13]. Wnt signaling is mediated through canonical or non-canonical pathways based on whether β-catenin is utilized as a downstream effector. The specific pathway activated depends on the Wnt ligand, cell surface receptor and intracellular context[14]. The canonical Wnt signaling pathway is dynamically regulated through the balance of β-catenin expression and degradation by a complex composed of APC, Axin2, GSK-3β, and CK1. Canonical Wnt binding to the Frizzled receptor triggers phosphorylation of the degradation complex leading to the cytoplasmic accumulation of β-catenin, which can eventually translocate to the nucleus to act as a regulator of gene expression. Wnt signaling is critically important in the development of blood vessels; however, the role of Wnt signaling in vascular pathophysiology is not well understood[15].
It has been reported that Wnt4 is up-regulated in the injured arterial wall, and promotes SMC proliferation and IH via stabilization of β-catenin[16]. However, the mechanism behind Wnt4 up-regulation remains unclear; whether other Wnt ligands also contribute to SMC proliferation is not known. Furthermore, the involvement of other Wnts in vascular pathology has not been investigated. We were intrigued by our previous findings of TGF-β/Smad3 induced proliferation, which eventually lead to our Affymetrix Array as previously described[9]. Our gene array data indicated that elevated TGF-β/Smad3 in SMCs increased the expression of several Wnts including Wnt4, implicating a crosstalk between the TGF-β/Smad3 pathway and Wnt/β-catenin signaling. While a crosstalk between these two pathways has been reported in other cell types, its role in the pathophysiology of vascular SMCs is not known[17]. In this study, we tested the functional role and signaling mechanisms of several Wnt proteins that were up-regulated by TGF-β/Smad3. We demonstrated that Wnts 2b, 4, 5a, and 9a were capable of stabilizing β-catenin and increasing SMC proliferation. Our results suggest that TGF-β in the background of elevated Smad3 enhances canonical Wnt/β-catenin signaling which promotes SMC proliferation.
2. Materials & Methods
2.1 Reagents
Dulbecco's modified Eagle's medium (DMEM) and other cell culture materials were purchased from Thermo Scientific (Rockford, IL). Recombinant human TGF-β1 and Wnts, as well as rat PDGF-bb were from R&D Systems (Minneapolis, MN). Niclosamide and SIS3 were from Sigma Aldrich (St. Louis, MO). SKL2001 was from Merck Millipore (Darmstadt, Germany).
2.2 Vascular Smooth Muscle Cell Culture & Treatments
Vascular smooth muscle cells (SMCs) were isolated from the thoracoabdominal aorta of adult male Sprague-Dawley rats as described by Clowers et al[18]. SMCs were cultured in low glucose DMEM supplemented with 10% Fetal Bovine Serum (FBS), Penicillin-Streptomycin (Invitrogen, Carlsbad CA) and incubated at 37°C with 5% CO2. Passage 4-6 rat SMCs were used for all experiments. When utilized for experiments, cells were starved in 0.2% FBS overnight and then treated with control solvent (4mM HCl + 0.2% BSA), TGF-β1 (5 ng/mL), or recombinant Wnts (500 ng/mL) dissolved in DMEM supplemented with 0.2% FBS. When inhibitors were utilized, they were allowed to incubate for 1 hr prior to stimulation.
2.3 Viral Infection
Adenovirus expressing GFP (AdGFP) and Smad3 (AdSmad3) were constructed as previously described[19]. The two constructs are identical except the Smad3 gene in AdSmad3. Adenovirus expressing β-catenin was purchased from ViGene Biosciences (Rockville, MD), and its AdNull control was purchased from Agilent Technologies (Santa Clara, CA). SMCs at 70-80% confluence were transduced with adenovirus in DMEM with 2% FBS for 4 h and then allowed to recover in DMEM with 10% FBS overnight. Cells were then starved with 0.2% FBS for 24 h and treated with TGF-β1 (5 ng/ml) or control solvent for 24 to 48 h. Adenovirus infection did not markedly alter SMC phenotype as evidenced by only a minor change of the SMC marker smooth muscle actin (α-SMA) in response to AdGFP infection (Figure S1A). Overexpression of Smad3 via AdSmad3 was confirmed by Western blotting (Figure S1B).
2.4 CellTiter-Glo Cell Viability Assay
Rat vascular smooth muscle cells (2000/per well in a 96 well plate) were starved in DMEM media supplemented with 0.2% FBS for 24 h. Cells were then stimulated with Wnts recombinant proteins or TGF-β1 in DMEM media with 0.2% FBS. For adenoviral proliferation assays, transductions were carried out as previously described. 96 h after treatment, 50 uL of CellTiter-Glo (Promega, Madison WI) reagent was added to each well. The plate was then analyzed using the Flexstation 3 plate reader (Molecular Devices, Sunnyville CA).
2.5 Western Blot
Following treatment for the indicated time, SMCs were lysed in RIPA buffer (25 mM Tris-HCL, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) (Thermo Scientific, Rockford, IL) with PMSF (Sigma Aldrich, St Louis MO), Protease Inhibitor Cocktail B (EMD Millipore, Darmstadt, Germany), and Phosphatase Inhibitor Cocktail III (Sigma Aldrich, St. Louis, MO). Protein quantification was performed utilizing the Bio-Rad DC Protein Assay Reagents (Hercules, CA). Fifteen micrograms of protein from each sample were separated on 10% SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules CA). Membranes were blocked with 5% BSA and then incubated with a primary antibody at 4 °C overnight, washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. The following primary antibodies and dilution ratios were used: anti-β-catenin (1:4000, Abcam); anti-p-LRP6 (1:1000, Cell Signaling CST2568); anti-Smad3 (1:1000, Santa Cruz sc8332); anti-alpha-SMA (1:3000, Sigma-Aldrich); anti-GAPDH (1:1000, Thermo-Fisher). PVDF membranes were washed and bound antibody was detected by Thermo Fisher West Pico Chemiluminescent Substrate (Waltham, MA). The Chemiluminescent signal was acquired with LAS-4000 mini (GE, Piscataway NJ) and quantified with ImageJ. For re-blotting, PVDF membranes were stripped with Restore Western Blot Stripper (Thermo Scientific, Rockford IL) and then blocked in 5% BSA solution for 1 h before incubation with antibody. β-actin (Sigma Aldrich, St. Louis MO) was utilized as a loading control.
2.6 Reverse Transcription Polymerase Chain Reaction Quantitative PCR (qRT-PCR)
RNA was isolated from rat smooth muscle cells using the E.Z.N.A RNA Kit 1 (Omega Bio-Tek, Norcross, GA) and quantified with Nanodrop 1000 (Thermo Fisher, Wilmington DE). cDNA was synthesized from 500 ng total RNA with iScript cDNA Synthesis Kit (Bio-Rad, Hercules CA) as instructed. Primers were synthesized in Biotechnology Center at the University of Wisconsin-Madison. Quantitative RT-PCR was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad CA) using the SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad CA).
2.7 Rat Carotid Artery Balloon Angioplasty Model
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Protocols for surgery (#M02273) were approved by the Institutional Animal Care and Use Committee of the University of Madison-Wisconsin. Surgeries were performed under isoflurane anesthesia, and all efforts were made to minimize suffering. Male Sprague-Dawley rats were anesthetized with inhaled isoflurane, and underwent balloon angioplasty of the common carotid artery. The external carotid artery was then ligated to reestablish blood flow through the common carotid into the internal carotid artery. In control animals, the carotid artery was exposed and manipulated similar to the injury model except angioplasty. Rats were euthanized fourteen days after surgery and the carotid arteries were fixed in 4% paraformaldehyde for paraffin embedding and sectioning.
2.8 Immunohistochemistry
The 5 μm artery sections were deparaffinized, and blocked in PBS with 5% BSA and 5% Donkey Serum after antigen retrieval using Dako Target Retrieval Solution 10x (Agilent Technologies, Santa Clara, CA). Sections were incubated with β-catenin antibody overnight at 4°C, followed by incubation with conjugated secondary antibody Alexa-488 after washing. The fluorescent signal was visualized on a Nikon Eclipse TE2000-inverted microscope. Digital images were acquired with a CoolSNAPcfMonochrome camera (Photometrics) and analyzed with ImageJ. Four animals from each group were used for this study, with 5 sections selected from each animal, and 2 regions eventually selected from each section for quantification. The raw fluorescence intensities were calculated for the intima and media compared to the control. Ratios were then generated and averaged for the mean and SEM for each group.
2.9 ELISA
Smooth Muscle Cells were infected with AdGFP or AdSmad3 and treated as previously described. At 24 hrs, conditioned media was collected and incubated overnight in a 96 well plate. Positive controls of β-catenin Ab and HRP-Rabbit Ab were utilized for positive controls. The plate was blocked in 2% BSA/PBS for 1 hr, and then incubated with primary antibody overnight at 4 °C. Antibodies to Wnts 5a (1:500, Sigma Aldrich, St Louis MO), Wnt9a (1:1000, Santa Cruz, Dallas TX), and Wnt11 (1:1000, Thermo Scientific, Rockford IL) were utilized. Plates were washed and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 4 h at room temperature. Afterwards, plates were washed and bound antibody was detected by Thermo Fisher West Pico Chemiluminescent Substrate (Waltham, MA) and analyzed using the Flexstation 3 plate reader (Molecular Devices, Sunnyville CA).
2.10 Statistical Analysis
Datasets were analyzed using independent-samples t test or ANOVA. Post hoc tests were performed when appropriate. Statistical significance was regarded as p<0.05.
3. Results
3.1 TGF-β/Smad3 up-regulates Wnt gene expression in rat aortic SMCs
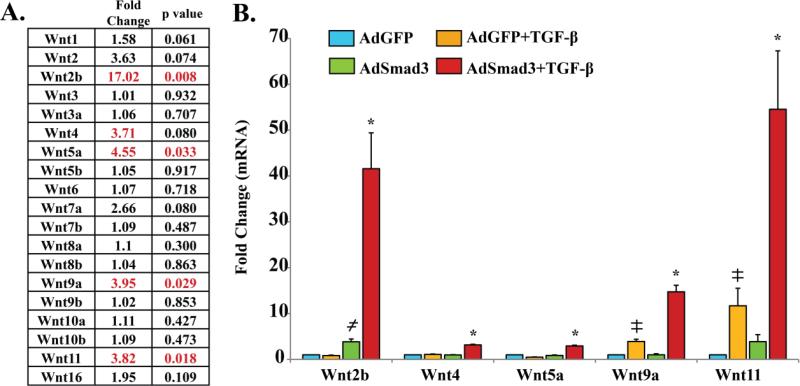
In our recent report, Affymetrix array analysis indicated that treatment of rat primary aortic SMCs with AdSmad3 and TGF-β1 up-regulated the expression of multiple development-related genes [9] including several Wnt genes (Figure 1A). We then selected the significantly up-regulated Wnts, i.e. Wnt2b, Wnt5a, Wnt9a, and Wnt11, using the criteria of more than 2 fold change with a P value <0.05 for further investigation. Although its gene expression change was not statistically significant (P = 0.08), Wnt4 was also selected, as previous studies have demonstrated an important role of Wnt4 in regulating SMC functions after vascular injury[16]. To confirm the gene array results, RNA was isolated from AdGFP or TGF-β1/Smad3 treated SMCs and quantitative RT-PCR was then performed to evaluate the expression of the aforementioned Wnt genes (Figure 1B). While Smad3 overexpression or recombinant TGF-β1 alone could significantly increase the expression of some of the Wnts (Wnt2b, Wnt9a, Wnt11), Smad3 overexpression followed by TGF-β1 stimulation substantially up-regulated all the tested Wnts. In addition, confirming the Smad3-specific regulation, a Smad3-specific inhibitor (SIS3) abrogated TGF-β1/Smad3-stimulated Wnt up-regulation, as exemplified by the Wnt2b data (Figure S2). TGF-β/Smad3-stimulated up-regulation of Wnts at protein levels was confirmed by ELISA assays of conditioned SMC media using Wnt5, Wnt9a, and Wnt11 as representatives (Figure S3). Up-regulation of the 5 Wnts appeared to be TGF-β/Smad3-specific since treatment of SMCs with PDGF-BB did not produce significant changes in their expression (Figure S4).
Figure 1. TGF-β/Smad3 stimulate the expression of several Wnt family genes in cultured rat aortic smooth muscle cells (SMCs).
Rat SMCs were infected with AdGFP (control) or AdSmad3 followed by treatment with solvent or TGF-β (5 ng/mL) for 24 h.
A. Affymetrix Array data[9] showing fold changes and p values (n=3) of Wnt family gene expression after TGF-β/Smad3 treatment versus AdGFP control.
B. Quantitative RT-PCR confirming up-regulation of Wnts 2b, 4, 5a, 9a, and 11. ╪, ≠: P<0.05, each compared to AdGFP control; *: P<0.05, compared to the other 3 conditions; n=3 independent experiments.
3.2 TGF-β/Smad3 stabilizes β-Catenin protein in a Wnt-dependent manner
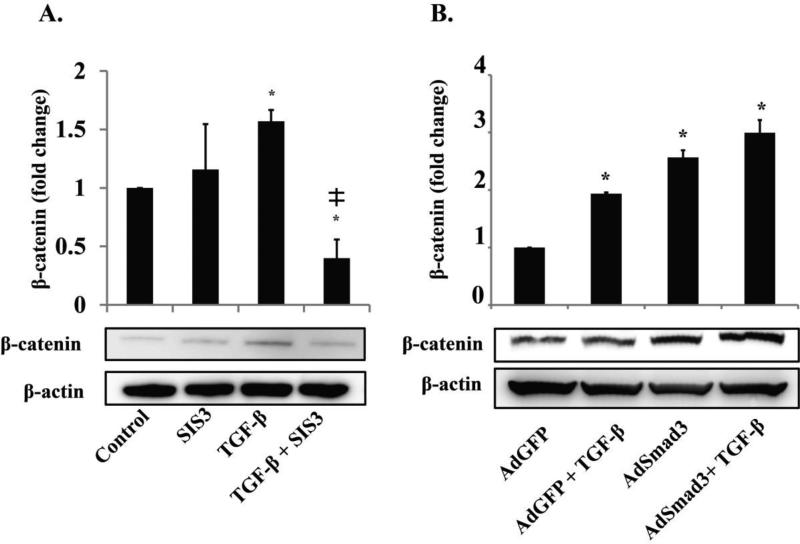
Because some of the up-regulated Wnts have been associated with the canonical β-catenin pathway in other cell types, we hypothesized that TGF-β/Smad3 could activate the canonical Wnt/β-catenin pathway in an autocrine manner via stimulation of Wnt production. Activation of canonical Wnt pathway inhibits β-catenin phosphorylation and subsequent degradation, thus an increase of β-catenin protein serves as an indicator of activated canonical Wnt signaling. Therefore, we determined the effect of TGF-β/Smad3 on β-catenin protein levels by Western blotting (Figure 2). After treating SMCs with TGF-β1 (5 ng/mL), we did not see a significant increase of β-catenin protein (at 6h and 24h, see Figure S5) until 48h (Figure 2B) compared to the cells that were not treated with TGF-β1 (P<0.05). We therefore performed 48h treatments to up-regulate β-catenin protein throughout this study. In order to determine whether the TGF-β stimulatory effect was mediated by Smad3, we repeated the experiment in SMCs with Smad3 activity blocked by a specific inhibitor, SIS3, or increased Smad3 expression using an adenovirus. The Western blot data show that application of SIS3 in the presence of TGF-β1 dramatically decreased β-catenin by over 1 fold (P<0.05) (Figure 2A), supporting an important role of Smad3 in TGF-β-induced β-catenin elevation. SIS3 did not have a significant effect on β-catenin levels in SMCs that were not stimulated with recombinant TGF-β1, probably because the endogenous Smad3 basal activity was low. In the gain-of-function experiments, we found that Smad3 overexpression substantially enhanced TGF-β's effect on β-catenin (Figure 2B). These results indicate that TGF-β stimulates increase of β-catenin protein via the canonical Smad3 signaling pathway. Given that TGF-β/Smad3 treatment did not increase β-catenin gene transcription, as indicated by both gene array and RT-PCR (Figure S6), we infer that an increase in β-catenin protein reflects its stabilization. Furthermore, we found that TGF-β/Smad3 treatment increased β-catenin in the nucleus (Figure S7). That β-catenin was increased rather than decreased in the cytosol suggests that increased nuclear abundance of β-catenin resulted from its stabilization not merely its re-distribution into the nucleus.
Figure 2. TGF-β/Smad3 treatment increases β-catenin protein levels in SMCs in a Smad3 dependent manner.
A. Rat aortic SMCs were incubated with the Smad3 inhibitor SIS3 (10 μM) or DMSO (control) for 1h and then treated with TGF-β (5 ng/mL) or solvent for 48 h. ╪ P<0.05 compared to TGF-β alone, n=3 independent experiments.
B. Rat aortic SMCs were infected with AdGFP (control) or AdSmad3 followed by treatment with solvent or TGF-β (5 ng/mL) for 48 h. * p<0.05 compared to AdGFP, n=3 independent experiments.
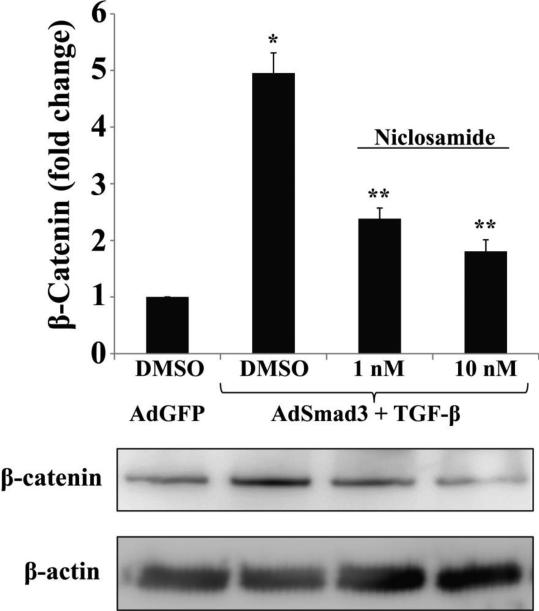
We next tested whether TGF-β/Smad3 enhanced β-catenin stabilization through Wnt signaling. We used Niclosamide, a small molecule inhibitor that abrogates Wnt signaling by promoting Frizzled receptor internalization[20, 21]. We observed a dose-dependent decrease in β-catenin levels after incubating TGF-β/Smad3-treated SMCs with Niclosamide (Figure 3). Moreover, while TGF-β/Smad3 increased LRP-6 phosphorylation, an indicator of activated canonical Wnt signaling [20], niclosamide abolished this effect (Figure S8). These results suggest TGF-β/Smad3 can stabilize β-catenin by stimulating the production of Wnts, which then act in an autocrine manner to activate the canonical Wnt/β-catenin pathway.
Figure 3. Wnt inhibitor Niclosamide abrogates TGF-β/Smad3 induced β-catenin elevation in SMCs.
Rat aortic SMCs were infected with AdSmad3 or AdGFP (control) followed by incubation with niclosamide (1 and 10 nM) or DMSO (Control) for 1h, and then treated with TGF-β (5 ng/mL) or solvent for 48 h. * p<0.05 compared to AdGFP, ** p<0.05 compared to AdSmad3 + TGF-β (DMSO), n=3 independent experiments.
3.3 Recombinant Wnt2b, Wnt4, Wnt5a or Wnt9a but not Wnt11 enhances β-catenin stabilization
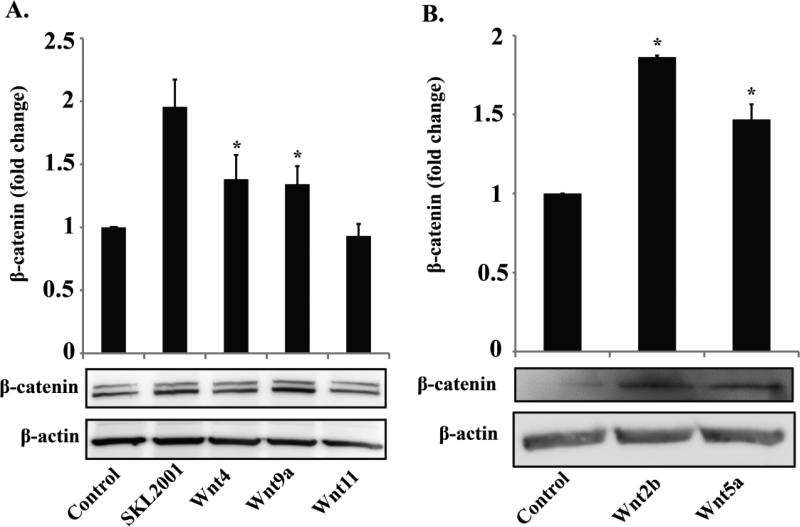
Wnt ligands can signal via the canonical pathway resulting in β-catenin stabilization, or alternatively, through non-canonical (non β-catenin) pathways[12]. In order to further determine which of the TGF-β/Smad3 up-regulated Wnts are responsible for β-catenin stabilization, we evaluated the effect of each Wnt on β-catenin stabilization using recombinant proteins. SKL2001, a small molecule inhibitor of β-catenin degradation was used as a positive control for β-catenin stabilization[22]. As shown in Figure 4, recombinant Wnt2b showed a potency in β-catenin stabilization similar to that of SKL2001. In addition, recombinant Wnt4, Wnt5a, and Wnt9a each increased β-catenin by ~30-50%. Interestingly, Wnt11 did not alter β-catenin protein levels, providing a “scrambled Wnt-like” control demonstrating the functional specificity of the other 4 Wnts. These results indicate that among the Wnts that are significantly up-regulated by TGF-β/Smad3, Wnt2b, Wnt4, Wnt5a, and Wnt9a are able to stabilize β-catenin, suggesting a canonical Wnt signaling pathway, whereas Wnt11 likely signals through a non-canonical pathway.
Figure 4. Recombinant Wnt proteins increase β-catenin levels in rat SMCs.
Rat aortic SMCs were treated with β-catenin stabilizer SKL2001 (40 μM), recombinant Wnt4, Wnt9a, or Wnt11 (A), or with Wnt2b or Wnt5a (B) each at a concentration of 500 ng/ml for 48h. Experiments in A and B were performed separately at different times. The doublet β-catenin bands appeared in Experiment A likely because of the use of a different batch (lot) of the same antibody from Abcam. * p<0.05 compared to control, n=3 independent experiments.
3.4 Recombinant Wnt2b, Wnt4, Wnt5a or Wnt9a but not Wnt11 stimulates SMC proliferation
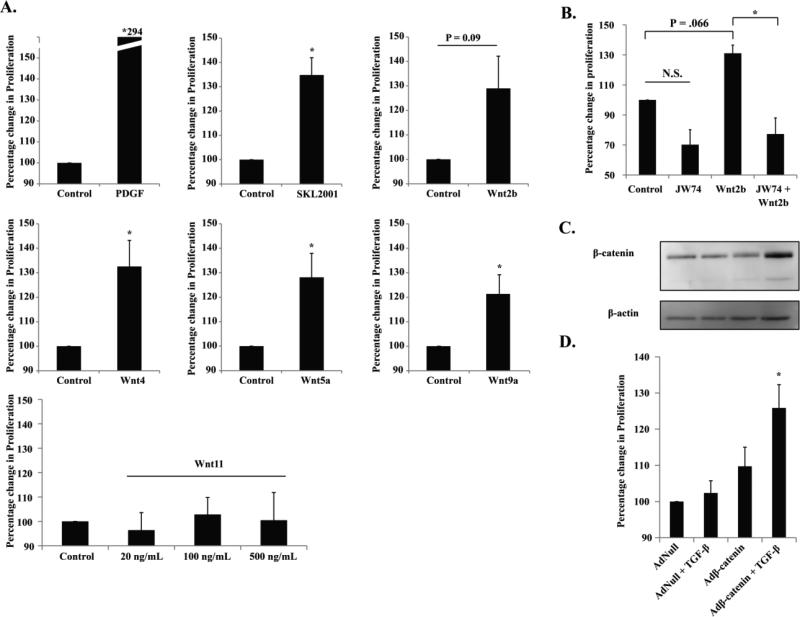
Up to this point, our results have demonstrated that elevated TGF-β/Smad3 signaling up-regulates Wnt2b, Wnt4, Wnt5a, and Wnt9a in rat aortic SMCs, allowing for β-catenin stabilization in canonical Wnt signaling. In light of our previous finding that elevated TGF-β/Smad3 signaling in rat aortic SMCs stimulates cell proliferation, we hypothesized that the crosstalk observed here between the canonical TGF-β/Smad3 pathway and the canonical Wnt/β-catenin pathway may have a functional effect on SMC proliferation. Therefore, we added each of the recombinant Wnts (2b, 4, 5a, 9a, and 11) to the cultures of SMCs and determined their effect on cell proliferation using a CellTiter-Glo viability assay. Indeed, the data showed that Wnt2b, Wnt4, Wnt5a, and Wnt9a all stimulated SMC proliferation compared to the control (Figure 5A). The increase of SMC proliferation unlikely resulted from reduced apoptosis, considering that even basal level of apoptosis (active caspase-3) could not be detected unless apoptosis is induced[11]. Wnt11, which did not stabilize β-catenin, did not enhance SMC proliferation at either of the three concentrations used (Figure 5A) thereby reinforcing the functional specificity of the other 4 Wnts. Validating our SMC functional assay, the positive control PDGF-BB produced a three fold stimulation of SMC proliferation, consistent with the data in the literature.
Figure 5. Recombinant Wnts and β-catenin viral expression promote SMC proliferation.
A. SMCs were treated with solvent (control) or recombinant Wnt protein (500 ng/ml) for 96 h and cell proliferation was assessed with CellTiter Glo Assay. PDGF (20 ng/mL) and SKL2001 (40 μM) were used as positive controls. Two additional concentrations (20 ng/ml and 100 ng/ml) were used for Wnt11. * p<0.05 compared to control, n=5 independent experiments.
B. SMCs were treated with solvent (control) or Wnt2b (500 ng/ml) for 96h in the absence or presence of the β-catenin inhibitor JW74 (10 μM), and cell proliferation was determined.
C and D. SMCs were infected with AdNull or Adβ-catenin and then treated with solvent or TGF-β (5 ng/ml). β-catenin expression was determined by Western blotting (C); cellular proliferation was assessed (D). * p<0.05 compared to AdNull, n=3 independent experiments.
In order to verify that stabilization of β-catenin indeed results in increased SMC proliferation, we treated SMCs with SKL2001, a β-catenin stabilizer. We found that SKL2001 treatment stimulated SMC proliferation to an extent similar to those by the 4 Wnts (2b, 4, 5a, and 9a) (Figure 5A). On the other hand, JW74, a widely used β-catenin inhibitor, abolished Wnt2b-stimulated SMC proliferation (Figure 5B). Next, we used an adenovirus to directly increase the expression of β-catenin in SMCs (Figure 5C). We found that while Adβ-catenin numerically increased (albeit not statistically significant) SMC proliferation compared to the AdNull control with no β-catenin overexpression, Adβ-catenin together with recombinant TGF-β1 substantially increased stimulation of SMC proliferation (Figure 5D). In these experiments we observed that viral expression of β-catenin showed a lower band, which presumably is a degraded form of β-catenin (Figure 5C). Addition of recombinant TGF-β1 likely enhanced SMC proliferation by stabilizing the full-length β-catenin.
Taken together, the foregoing data indicate that TGF-β in the background of elevated Smad3 levels up-regulates Wnts 2b, 4, 5a, and 9a, which each stabilizes β-catenin via activation of the canonical signaling resulting in enhanced SMC proliferation. Thus this mechanism may at least partially account for our previous observation that elevated TGF-β/Smad3 signaling promotes SMC proliferation.
3.5 β-catenin is up-regulated in the neointima following arterial injury in a rat carotid balloon angioplasty model
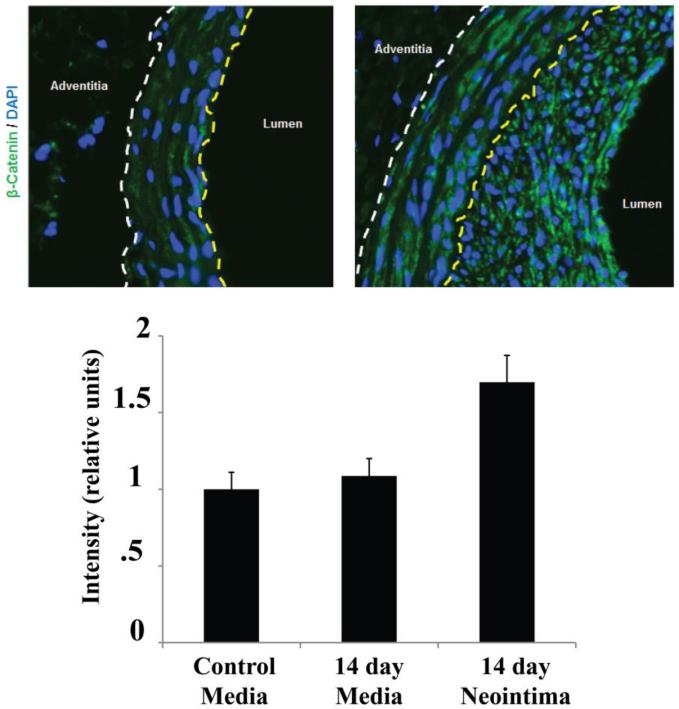
Finally, based on our previous finding that elevated TGF-β/Smad3 signaling in injured arteries contributes to the development of intimal hyperplasia, we explored a possible functional relevance of TGF-β/Smad3 induced β-catenin stabilization and the pathophysiology of intimal hyperplasia. To induce intimal hyperplasia, we performed balloon angioplasty in the rat carotid arteries (Figure 6). Through immunohistochemistry using 14-day injured carotid artery sections, we found that β-catenin increased in the neointima in contrast to that in the media and that in the uninjured arterial wall. Thus, this result shows a consistency between our in vitro observation of TGF-β/Smad3-stimulated increase of β-catenin and the in vivo up-regulation of β-catenin in the neointima, where TGF-β and Smad3 up-regulation occurs as we reported previously[6].
Figure 6. Increase of β-Catenin in the neointima after vascular injury.
Rat carotid arteries were collected 14 days after balloon injury, and β-catenin was immunostained on the sections. Control refers to the uninjured counter-lateral carotid artery. Yellow dashed line indicates the internal elastic lamina (IEL); dashed line indicates external elastic lamina (EEL); neointima is defined between IEL and lumen.
4. Discussion
While the TGF-β/Smad3 and the Wnt/β-catenin pathways each has been extensively studied, the importance of their crosstalk in the context of vascular SMC pathophysiology and IH are little known. Through a systematic analysis we identified that elevated TGF-β/Smad3 in rat aortic SMCs can stabilize β-catenin protein while up-regulating 5 Wnt family members, among which Wnt2b, 4, 5a, and 9a but not Wnt11 can stabilize β-catenin and stimulate SMC proliferation. We have further found that whereas stabilization of β-catenin enhances SMC proliferation, an inhibitor of Wnt receptors abrogates TGF-β/Smad3-induced β-catenin stabilization. Thus a novel mechanism emerges from these results; i.e. elevated TGF-β/Smad3 in SMCs augments canonical Wnt signaling resulting in stabilization of β-catenin, which in turn promotes SMC proliferation. Given that transformation of SMCs into a proliferative phenotype is a key contributor to IH, and that TGF-β/Smad3 and β-catenin are all up-regulated in the hyperplastic neointima, our findings provide important mechanistic implications for the pathophysiology of vascular SMCs with a functional link to IH. In the following discussion, several lines of evidence are put forward in support of the foregoing mechanism.
TGF-β/Smad3 increased the expression of 5 Wnts (2b, 4, 5a, 9a and 11) and also enhanced β-catenin stabilization, suggesting that TGF-β/Smad3 stabilized β-catenin through Wnt signaling. An alternative interpretation would be that up-regulation of those Wnts and the stabilization of β-catenin were unrelated events or that TGF-β/Smad3 stabilized β-catenin by mechanisms without directly involving Wnts. For example, a Smad3/β-catenin complex formed in the nucleus could prevent the shuttling of β-catenin back to the cytoplasm and its subsequent degradation; or Smad3 as a transcription factor could directly activate β-catenin transcription[23, 24]. However, contrary to these scenarios, we did not observe an increase of Smad3/β-catenin co-localization (by immunostaining) following the treatment of SMCs with AdSmad3/TGF-β (data not shown), and our qRT-PCR data showed no increases in β-catenin transcripts (Figure S6). Importantly, a Wnt inhibitor (Niclosamide) that desensitizes Wnt signaling by triggering Frizzled receptor internalization abolished TGF-β/Smad3-initiated β-catenin stabilization. Thus these results consistently support the idea that elevated TGF-β/Smad3 stabilizes β-catenin through upregulated Wnts.
Although several Wnts were up-regulated in SMCs because of increased TGF-β/Smad3, our results clearly demonstrate that it is the canonical (through β-catenin) rather than non-canonical Wnt signaling that produced a functional stimulation of SMC proliferation. The most compelling evidence is that the four Wnts (2b, 4, 5a, and 9a) that stabilized β-catenin also stimulated SMC proliferation. In contrast, Wnt11, which did not change β-catenin levels, was unable to stimulate SMC proliferation. Consistent with our finding in SMCs, multiple papers have identified Wnt11 as a non-canonical ligand in a variety of cell types[25-27]. Although recombinant Wnt11 did not show an effect on SMC proliferation in the current study, we cannot rule out a possibility that Wnt11 may regulate other behaviors of SMCs during neointimal growth.
It is well documented that the 4 canonical Wnts, Wnt2b, Wnt4, Wnt5a, and Wnt9a, promote cell proliferation. Kobayashi et al demonstrate that Wnt2b plays a positive role in the proliferation of mesothelioma[28]. Similarly, Wang et al report that silencing Wnt2b significantly diminishes proliferation in several ovarian cancer cell lines[29]. In addition, Shojima et al report that Wnt5a is capable of promoting proliferation in a variety of oncological cell lines; knockout of Wnt5a reduces proliferation[30]. Wnt9a appears to affect cellular proliferation as well. Embryological research from Matsumoto et al demonstrates that overexpression of Wnt9a in hepatic sinusoids leads to increased hepatocyte proliferation[31]. Most relevant to our current study, the Sarah George group has reported that Wnt4 stimulates vascular SMC proliferation in vitro and in vivo by activating the canonical pathway, which is consistent with our findings here[16]. It is worth noting that although we applied recombinant Wnt4 of the same sequence from the same commercial source at the same concentration (500 ng/mL), in our experiments recombinant Wnt4 stimulated SMC proliferation to a much less extent (32%) compared to that in the previous report (2.5 fold). This discrepancy may be reasonably explained by the difference in the species of cells used in our study (rat aortic SMCs) and that in the George group (mouse aortic SMCs), different proliferation assays, or different protocols used to isolate primary SMCs. Nonetheless, in our study the magnitude of stimulation by Wnt4 in β-catenin stabilization (50%) and that in SMC proliferation (~35%) agreed well. Most significantly, we have made a novel finding that aside from Wnt4, Wnt2b, Wnt5a, and Wnt9a can also produce a functional effect in promoting SMC proliferation via the canonical pathway.
β-catenin as the central effector in the canonical Wnt signaling pathway has been shown to promote cell proliferation in numerous studies. It is known that canonical Wnt signaling regulates embryogenesis, tumor growth, as well as wound healing by promoting β-catenin stabilization and cell proliferation[32, 33] Wnt-induced β-catenin stabilization also stimulates progenitor cell proliferation and self-renewal[34]. Specifically in the cardiovascular system, it has been found that increased levels of β-catenin in vascular endothelial cells, smooth muscle cells, and skeletal myocytes enhanced proliferation[35, 36]. On the other hand, knocking down β-catenin decreased proliferation in airway SMCs and a variety of oncological cell lines[37-40]. In spite of these reports, information concerning the role of β-catenin specifically in vascular SMCs is relatively scarce. Our data clearly shows a proproliferative role of the Wnt/β-catenin pathway in vascular SMCs for the following reasons. Firstly, application of recombinant Wnts (Wnts 2b, 4, 5a, and 9a) that activated canonical β-catenin signaling were able to produce an increase in proliferation. Second, we confirmed that suppression of β-catenin degradation could produce similar increases in SMC proliferation. Third, TGF-β/Smad3-induced upregulation of β-catenin was inhibited through the use of a Frizzled receptor inhibitor (Niclosamide). Fourth, adenoviral overexpression of β-catenin increased SMC proliferation. It is unlikely that the increase of SMC proliferation resulted from reduced apoptosis because we were not able to detect apoptosis (cleaved caspapse-3 assay) in either untreated or Wnt2b (or 5)-treated SMCs (data not shown). In fact, vascular SMCs are hardy cells. Generally they do not undergo apoptosis until induced with an apoptotic factor such as UV[11]. Taken together, these findings demonstrate that increases in β-catenin can increase SMC proliferation.
Although a crosstalk between TGF-β/Smad3 and Wnt/β-catenin pathways has been reported in other physiological contexts, we are the first to explore its potential in regulating vascular SMC pathophysiology. Consistent evidence for interactions between TGF-β/Smad3 and β-catenin pathways comes from work involving epithelial to mesenchymal transition. Zhou et al demonstrated with SIS3 that the TGF-β1 induced upregulation of β-catenin is in fact mediated through Smad3[17]. Furthermore, TGF-β1 can promote β-catenin stabilization through rapid phosphorylation of GSK-3β. Most recently, our own study using rat aortic SMCs showed that TGF-β/Smad3 stimulate the expression of osteopontin, a β-catenin target gene[9]. Given the complexities of these two individual pathways, attempts to investigate their crosstalk have also yielded conflicting results. For example, it has also been reported that TGF-β activates Wnt signaling in a Smad3-independent manner (through p38) in human dermal fibroblasts by down-regulating Dkk-1, a Wnt signaling inhibitor protein[41]. On the other hand, opposite effects of TGF-β and Wnt/β-catenin pathways have been reported, e.g. TGF-β treatment is capable of downregulating several β-catenin activated genes such as c-Myc and cyclinD1, underscoring the complexity of the relationship between these two critical pathways that needs to be better understood[42, 43]. These foregoing reports reveal the cell type and signaling context-dependent nature of the crosstalk between the two signaling systems. Nevertheless, here we have shown that in vascular SMCs the Wnt/β-catenin signaling is positively regulated by TGF-β, in a Smad3-dependent manner as demonstrated in experiments either inhibiting or increasing Smad3 activity.
5. Conclusion
This is the first study to show that in vascular SMCs, the canonical Wnt/β-catenin pathway is activated by elevated TGF-β/Smad3 resulting in enhanced cell proliferation. It is Wnt2b, 4, 5a, and 9a but not Wnt11 that mediate the crosstalk between the two canonical signaling pathways. Each of these pathways, but not their crosstalk, has been previously reported to be a contributor to intimal hyperplasia. Thus the mechanistic insights revealed here for the SMC transformation to a proliferative phenotype are new and may guide future studies for better understanding the pathophysiology of vascular SMCs and probably also intimal hyperplasia.
Supplementary Material
Highlights.
A crosstalk between the canonical TGFbeta/Smad3 and Wnt/Beta-Catenin signaling pathways has not been previously investigated in vascular smooth muscle cells
The transition of vascular smooth muscle cells from a normal state to a proliferative phenotype is an important factor in occlusive vascular diseases
We find that elevated TGFbeta/Smad3 increases the expression of several Wnt proteins which in turn promote smooth muscle cell proliferation via stabilization of Beta-Catenin
Acknowledgements
The authors wish to thank Dr. Bo Liu, Dr. Byounghoon Hwang, and Sarah Franco for technical assistance, proofreading of the manuscript, or critical comments. This work was supported by a National Heart Lung and Blood Institute R01 (HL-068673, to KCK) and an institutional T-32 training grant HL-110853 (D DiRenzo, M Chaudhary) and a University of Wisconsin Department of Surgery Start Up Fund (to L-WG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
The authors declare that they have no conflicts of interest.
References
- 1.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 3.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 4.Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular Inflammation and Repair: Implications for Reendothelialization, Restenosis, and Stent Thrombosis. JACC Cardiovasc Interv. 2011;4:1057–1066. doi: 10.1016/j.jcin.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edlin RS, Tsai S, Yamanouchi D, Wang C, Liu B, Kent KC. Characterization of primary and restenotic atherosclerotic plaque from the superficial femoral artery: Potential role of Smad3 in regulation of SMC proliferation. J Vasc Surg. 2009;49:1289–1295. doi: 10.1016/j.jvs.2008.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai S, Hollenbeck ST, Ryer EJ, Edlin R, Yamanouchi D, Kundi R, Wang C, Liu B, Kent KC. TGF-beta through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am J Physiol Heart Circ Physiol. 2009;297:H540–549. doi: 10.1152/ajpheart.91478.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther. 2005;315:1005–1012. doi: 10.1124/jpet.105.091249. [DOI] [PubMed] [Google Scholar]
- 8.Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ, Wang C, Tsai S, Liu B, Kent KC. Arterial gene transfer of the TGF-beta signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF. Cardiovasc Res. 2009;84:326–335. doi: 10.1093/cvr/cvp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi X, DiRenzo D, Guo LW, Franco SR, Wang B, Seedial S, Kent KC. TGF-beta/Smad3 stimulates stem cell/developmental gene expression and vascular smooth muscle cell de-differentiation. PLoS One. 2014;9:e93995. doi: 10.1371/journal.pone.0093995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Si Y, Ren J, Wang P, Rateri DL, Daugherty A, Shi XD, Kent KC, Liu B. Protein kinase C-delta mediates adventitial cell migration through regulation of monocyte chemoattractant protein-1 expression in a rat angioplasty model. Arterioscler Thromb Vasc Biol. 2012;32:943–954. doi: 10.1161/ATVBAHA.111.244921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi X, Guo LW, Seedial SM, Si Y, Wang B, Takayama T, Suwanabol PA, Ghosh S, DiRenzo D, Liu B, Kent KC. TGF-beta/Smad3 inhibit vascular smooth muscle cell apoptosis through an autocrine signaling mechanism involving VEGF-A. Cell Death Dis. 2014;5:e1317. doi: 10.1038/cddis.2014.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Mill C, Monk BA, Williams H, Simmonds SJ, Jeremy JY, Johnson JL, George SJ. Wnt5a-induced Wnt1-inducible secreted protein-1 suppresses vascular smooth muscle cell apoptosis induced by oxidative stress. Arterioscler Thromb Vasc Biol. 2014;34:2449–2456. doi: 10.1161/ATVBAHA.114.303922. [DOI] [PubMed] [Google Scholar]
- 15.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ. Wnt4/beta-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–436. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, Nguyen C, Flodby P, Zhong Q, Krishnaveni MS, Liebler JM, Minoo P, Crandall ED, Borok Z. Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP) J Biol Chem. 2012;287:7026–7038. doi: 10.1074/jbc.M111.276311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clowes MM, Lynch CM, Miller AD, Miller DG, Osborne WR, Clowes AW. Long-term biological response of injured rat carotid artery seeded with smooth muscle cells expressing retrovirally introduced human genes. J Clin Invest. 1994;93:644–651. doi: 10.1172/JCI117016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryer EJ, Hom RP, Sakakibara K, Nakayama KI, Nakayama K, Faries PL, Liu B, Kent KC. PKCdelta is necessary for Smad3 expression and transforming growth factor beta-induced fibronectin synthesis in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006;26:780–786. doi: 10.1161/01.ATV.0000209517.00220.cd. [DOI] [PubMed] [Google Scholar]
- 20.Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/beta-catenin pathway. PLoS One. 2011;6:e29290. doi: 10.1371/journal.pone.0029290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arend RC, Londono-Joshi AI, Samant RS, Li Y, Conner M, Hidalgo B, Alvarez RD, Landen CN, Straughn JM, Buchsbaum DJ. Inhibition of Wnt/beta-catenin pathway by niclosamide: a therapeutic target for ovarian cancer. Gynecol Oncol. 2014;134:112–120. doi: 10.1016/j.ygyno.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Gwak J, Hwang SG, Park HS, Choi SR, Park SH, Kim H, Ha NC, Bae SJ, Han JK, Kim DE, Cho JW, Oh S. Small molecule-based disruption of the Axin/β-catenin protein complex regulates mesenchymal stem cell differentiation. Cell Res. 2012:237–247. doi: 10.1038/cr.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Wang M, Tan X, Li TF, Zhang YE, Chen D. Smad3 prevents beta-catenin degradation and facilitates beta-catenin nuclear translocation in chondrocytes. J Biol Chem. 2010;285:8703–8710. doi: 10.1074/jbc.M109.093526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20:666–674. doi: 10.1101/gad.1388806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P, Cai Y, Soofi A, Dressler GR. Activation of Wnt11 by transforming growth factor-beta drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells. J Biol Chem. 2012;287:21290–21302. doi: 10.1074/jbc.M112.357202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uysal-Onganer P. I.C.L. Department of Surgery and Cancer, London, UK, R.M. Kypta, I.C.L. Department of Surgery and Cancer, London, UK, C.f.C.R.i.B.C.b. Department of Cell biology and Stem Cells Unit, Bilbao, Spain, Wnt11 in 2011 – the regulation and function of a non-canonical Wnt. Acta Physiologica. 2015;204:52–64. doi: 10.1111/j.1748-1716.2011.02297.x. [DOI] [PubMed] [Google Scholar]
- 27.Toyama T, Lee HC, Koga H, Wands JR, Kim M. Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res. 2010;8:254–265. doi: 10.1158/1541-7786.MCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi M, Huang CL, Sonobe M, Kikuchi R, Ishikawa M, Kitamura J, Miyahara R, Menju T, Iwakiri S, Itoi K, Yasumizu R, Date H. Intratumoral Wnt2B expression affects tumor proliferation and survival in malignant pleural mesothelioma patients. Exp Ther Med. 2012;3:952–958. doi: 10.3892/etm.2012.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Fan L, Xia X, Rao Y, Ma Q, Yang J, Lu Y, Wang C, Ma D, Huang X. Silencing Wnt2B by siRNA interference inhibits metastasis and enhances chemotherapy sensitivity in ovarian cancer. Int J Gynecol Cancer. 2012;22:755–761. doi: 10.1097/IGC.0b013e3182540284. [DOI] [PubMed] [Google Scholar]
- 30.Shojima K, Sato A, Hanaki H, Tsujimoto I, Nakamura M, Hattori K, Sato Y, Dohi K, Hirata M, Yamamoto H, Kikuchi A. Wnt5a promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively. Sci Rep. 2015 doi: 10.1038/srep08042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto K, Miki R, Nakayama M, Tatsumi N, Yokouchi Y. Wnt9a secreted from the walls of hepatic sinusoids is essential for morphogenesis, proliferation, and glycogen accumulation of chick hepatic epithelium. Dev Biol. 2008;319:234–247. doi: 10.1016/j.ydbio.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. 2006 doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 33.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 34.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 35.Zhong W, Oguljahan B, Xiao Y, Nelson J, Hernandez L, Garcia-Barrio M, Francis SC. Serum and glucocorticoid-regulated kinase 1 promotes vascular smooth muscle cell proliferation via regulation of beta-catenin dynamics. Cell Signal. 2014;26:2765–2772. doi: 10.1016/j.cellsig.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim KI, Cho HJ, Hahn JY, Kim TY, Park KW, Koo BK, Shin CS, Kim CH, Oh BH, Lee MM, Park YB, Kim HS. Beta-catenin overexpression augments angiogenesis and skeletal muscle regeneration through dual mechanism of vascular endothelial growth factor-mediated endothelial cell proliferation and progenitor cell mobilization. Arterioscler Thromb Vasc Biol. 2006;26:91–98. doi: 10.1161/01.ATV.0000193569.12490.4b. [DOI] [PubMed] [Google Scholar]
- 37.Nunes RO, Schmidt M, Dueck G, Baarsma H, Halayko AJ, Kerstjens HA, Meurs H, Gosens R. GSK-3/beta-catenin signaling axis in airway smooth muscle: role in mitogenic signaling. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1110–1118. doi: 10.1152/ajplung.00500.2007. [DOI] [PubMed] [Google Scholar]
- 38.Sinnberg T, Menzel M, Ewerth D, Sauer B, Schwarz M, Schaller M, Garbe C, Schittek B. beta-Catenin signaling increases during melanoma progression and promotes tumor cell survival and chemoresistance. PLoS One. 2011;6:e23429. doi: 10.1371/journal.pone.0023429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng G, Apte U, Cieply B, Singh S, Monga SP. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. 2007;9:951–959. doi: 10.1593/neo.07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C, Zhao M, Tian A, Zhang X, Yao Z, Ma X. Aberrant activation of Wnt/beta-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget. 2015;6:17570–17583. doi: 10.18632/oncotarget.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terada Y, Nakashima O, Inoshita S, Kuwahara M, Sasaki S, Marumo F. TGF-|[bgr]|-activating kinase-1 inhibits cell cycle and expression of cyclin D1 and A in LLC-PK1 cells. Kidney International. 1999;56:1378–1390. doi: 10.1046/j.1523-1755.1999.00665.x. [DOI] [PubMed] [Google Scholar]
- 43.Pietenpol JA, Holt JT, Stein RW, Moses HL. Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci U S A. 1990;87:3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.