Abstract
Objective
The aim of the study was to determine right and left ventricle deformation parameters in patients with transposition of the great arteries who had undergone atrial or arterial switch procedures.
Setting
Patients with transposition are born with a systemic right ventricle. Historically, the atrial switch operation, in which the right ventricle remains the systemic ventricle, was performed. These patients have increased rates of morbidity and mortality. We used cardiac MRI with Velocity Vector Imaging analysis to characterize and compare ventricular myocardial deformation in patients who had an atrial switch or arterial switch operation.
Design
Patients with a history of these procedures, who had a clinically ordered cardiac MRI were included in the study. Consecutive 20 patients (75% males, 28.7±1.8 years) who underwent atrial switch operation and 20 patients (60% males, 17.7±1.9 years) who underwent arterial switch operation were included in the study. Four chamber and short-axis cine images were used to determine longitudinal and circumferential strain and strain rate using Vector Velocity Imaging software.
Results
Compared to the arterial switch group, the atrial switch group had decreased right ventricular ejection fraction and increased end-diastolic and end-systolic volumes; and no difference in left ventricular ejection fraction and volumes. The atrial switch group had decreased longitudinal and circumferential strain and strain rate. When compared to normal controls multiple strain parameters in the atrial switch group were reduced.
Conclusions
Myocardial deformation analysis of transposition patients reveals a reduction of right ventricular function and decreased longitudinal and circumferential strain parameters in patients with an atrial switch operation compared to those with arterial switch operation. A better understanding of the mechanisms of RV failure in TGA may lead to improved therapies and adaptation.
Keywords: Systemic right ventricle, Transposition of great vessels, Vector Velocity Imaging, Cardiac magnetic resonance imaging, Atrial switch operation, arterial switch operation
Introduction
The management of transposition of great arteries (TGA) has evolved since initial attempts at surgical repair began in the late 1940’s with the first published procedure being the Blalock-Hanlon operation in 1950.(1) Varied attempts to physiologically correct TGA by creating an intra-atrial baffle followed. The desired effect of the intra-atrial baffle was to direct pulmonary venous return to the right ventricle (RV) and aorta and systemic venous return to the left ventricle (LV) and pulmonary artery. Over the following years the atrial switch operations pioneered by Ake Senning and William Mustard became the surgical management of TGA. Following advances in neonatal cardiac surgery in the 1980’s, the surgical management of TGA transitioned from the atrial switch operation to the arterial switch operation. The arterial switch operation involves relocating the aorta and coronary arteries to the LV and the pulmonary artery to the RV. As opposed to the atrial switch operation, the arterial switch operation results in a systemic LV. In the United States TGA occurs in 5 of 10,000 live births.(2) Most of the patients that underwent the atrial switch operation as children, are adults now and there is an increasing population of arterial switch operation patients who are reaching adulthood.
Previous studies have indicated a higher incidence of complications after an atrial switch operation.(3, 4) Long-term complications include atrial baffle leaks, baffle obstruction, arrhythmias, right ventricular failure and sudden death. Complications of the arterial switch operation include coronary artery occlusion and subsequent ventricular dysfunction, neo-aortic root dilation, semilunar valve disease, supravalvar pulmonary stenosis and supravalvar aortic stenosis.(5) A prospective study by the Congenital Heart Surgeons' Society (CHSS) evaluating outcomes in TGA patients with the atrial switch and arterial switch operations showed that there is an increased risk of mortality in the atrial switch group.(6) In a single center prospective study most patients had good RV function 14 years after the Mustard operation, but 61% had moderate to severe dysfunction 25 years after the surgery.(7) From these studies there is evidence suggesting that the systemic RV fails over the long-term. The early inciting factors and the stages of progression that finally leads to RV failure are not known.
The current gold standard of evaluation of RV function is cardiac MRI (CMR) which can, measure RV volumes and calculate ejection fraction.(8) Ejection fraction (EF) is of low prognostic significance at lower levels of impairment.(9) Using current clinical imaging modalities, RV failure is likely to be advanced when a significant reduction of ejection fraction is reached. Cardiac deformation (strain) imaging has been applied to other disease processes to better delineate the pre-clinical progression of disease.(10)
The purpose of the study was to evaluate RV global deformation parameters in patients with D-TGA. We hypothesized that the RV global strain parameters will be decreased in the systemic RV in patients with atrial switch operations compared to RV in patients after arterial switch operation or normal controls. Analysis of the RV deformation parameters may provide a way to diagnose and manage patients with repaired TGA prior to the onset of overt failure of the RV.
Methods
Study Population
Patients were identified by retrospective review of hospital records. The most recent 20 consecutive patients who had undergone a CMR and had a history of TGA and an atrial switch operation (n=20) and the most recent 20 patients who had undergone a CMR and had a history of TGA and arterial switch operation (n=20) were identified. A group of control patients with structurally and functionally normal hearts who had undergone CMR earlier were similarly processed to provide a reference normal data set (n=8). Patients were excluded if the CMR images were of poor quality, precluding VVI analysis. Institutional Review Board (IRB) guidelines were met for this study.
Imaging
The images were obtained using a Siemens 1.5T or 3.0T magnet. Retrospectively ECG gated segmented balanced steady-state free precession (bSSFP) cine images were acquired during expiratory breath holds with a slice thickness of 6mm with a 4 mm gap. We scanned our patients using spatial resolution of 1.5mm × 1.5mm/pixel. Temporal resolution was 35 msec. RV and LV volumetric analysis data was previously performed on the manufacturer volumetric analysis software (Siemens Argus). Volumetric measurements were made using a stack of bSSFP images in the short axis plane. For the purpose of this study, ventricular volumes and ejection fraction detailed on the clinical report were used. Ventricular trabeculations were excluded from tracings measuring ventricular volumes.
Ventricular Strain Analysis using Velocity Vector Imaging
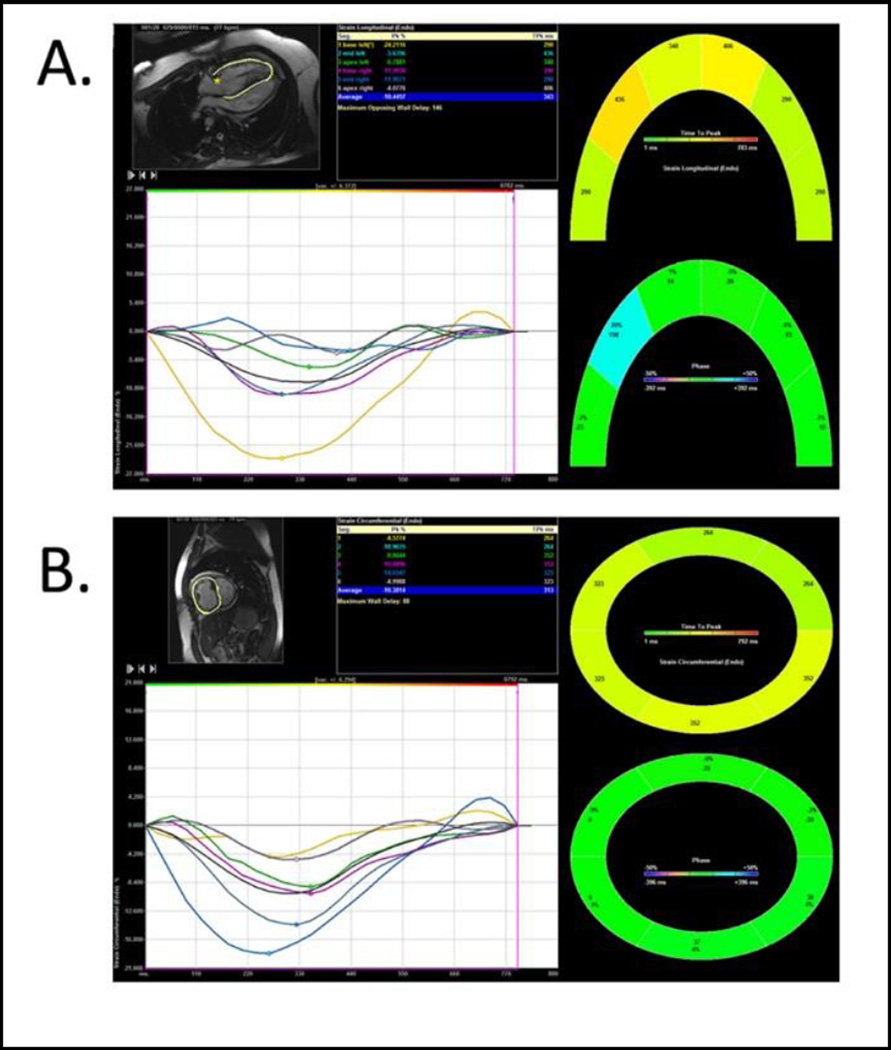
Ventricular deformation parameters were analyzed offline on previously acquired CMR four chamber and short axis images. Longitudinal and circumferential strain and strain rate were obtained by manually tracing the RV and LV sub-endocardial border in the 4-chamber view and mid-ventricular short axis views using Siemens VVI5 software (Siemen’s Medical Solutions, Mountain View, CA). The endocardial borders of the RV were traced from the point of annular attachment of the tricuspid valve to the anterior RV wall to the septal attachment of the annulus. Similarly the LV endocardial border was traced from the annular attachment of the mitral valve to the posterior LV wall to the septal annular attachment. For both the RV and LV tracings in the 4-chamber view the trabeculations was excluded from the tracing. In the mid-chamber short axis views the sub-endocardial borders were traced excluding the papillary muscles. For consistency short axis tracings were begun at 12:00 position and traced clockwise. The built-in 2D tracking algorithm of VVI software was employed to provide dynamic deformation parameters. Automated feature tracking was used for all images in the study (Fig. 1). If poor tracking of the endocardium was noticed then the endocardial tracings were manually readjusted. The software applied automatic segmentation of the left and right ventricular walls into six segments for strain analysis.
Fig 1.
Myocardial segmental deformation tracings using VVI software. Longitudinal (4-chamber) Strain (A); and Circumferential (short-axis) Strain (B) The tables in the figure show segmental, peak strain and average peak global strain. The image is color coded for graphical display of segmental and regional velocity. The grayscale image show the endocardial tracing in the 4-chamber and short-axis view.
Statistics
Kruskal-Wallis test was used to analyze the variance between the atrial switch, arterial switch and control groups and followed by post-hoc analysis using Mann-Whitney with Steel–Dwass test for comparisons between two groups. A t-test was used to compare the patient characteristics in Table 1. Inter-observer and intra-observer variability and reproducibility was assessed for 15 randomly selected patients from the atrial switch and arterial switch cohorts for the longitudinal and circumferential strain measurements. Intra-observer variability was assessed by repeating the observations after four weeks by the same observer. The strain measurements were recorded and reproducibility was assessed using Bland-Altman’s plot and Kendall rank correlation coefficient test.
Table 1.
Patient Characteristics of the Atrial Switch and Arterial Switch Group AS
| AS | ASO | p-value | |
|---|---|---|---|
| Number of Patients(males) | 20(15) | 20(12) | |
| Age, yrs. | 28.7±81.8 | 17.7±1.9 | 0.0006 |
| Body Surface Area m2 | 1.9±0.1 | 1.6±0.1 | 0.001 |
| Systolic/Diastolic BP mmHg | 120±3/73±2 | 117±3/67±2 | NS |
| CMR RV | |||
| RV EF (%) | 45±2.5 | 53.3±2.1 | 0.04 |
| RV EDVi(ml/m2) | 100.6 ±6.2 | 79.3±4.7 | 0.01 |
| RV ESVi(ml/m2) | 58.5±4.5 | 37.3±4.0 | 0.004 |
| CMR LV | |||
| LV EF(%) | 57.6±2.0 | 61.4±2.1 | 0.07 |
| LV EDVi(ml/m2) | 63.5±5.0 | 79.4±3.5 | 0.007 |
| LV ESVi(ml/m2) | 28.2±2.9 | 31.1±2.9 | 0.4 |
AS: Atrial Switch Group; ASO Arterial Switch Group; The RV and LV ejection fraction and volumes are listed as shown.
(Values are as shown ±S.E.). End Diastolic and end systolic volumes are normalized to BSA.
Results
Patient Characteristics
The patient characteristics of atrial switch and arterial switch cohorts are shown in Table 1. A total of 48 patients were included in the study and comprised an n=20 in both the atrial and arterial switch groups and n=8 in the control group. The mean age for the control group was 32.6±2.1 years, atrial switch was 28.7± 1.8 years and the arterial switch group was 17.7±1.9 years. The BSA was 1.9 m2 for the control and atrial switch group compared to 1.6 m2 for the arterial switch group. This difference was likely due the difference in the mean age of the population at the time of the study. The systolic and diastolic blood pressures were not significantly different between the groups. Comorbidities among the atrial switch group included one patient with severe and three with moderate tricuspid regurgitation; one patient had severe pulmonary insufficiency; all patients were in normal sinus rhythm except one who was in junctional rhythm; no baffle obstruction or outflow problems were noted. In the arterial switch group two patients had moderate aortic insufficiency; four had moderate aortic root dilatation.
Inter-observer and Intra-observer Variability
Using the Kendall rank correlation coefficient test (τ) for inter- observer variability showed excellent agreement for the RV longitudinal strain measures, (τ =0.84, p<0.0001), and moderate agreement for circumferential strain (τ = 0.52, p<0.005). Intra-observer measures showed similar reproducibility for longitudinal strain (τ =0.84, p<0.0001), and circumferential strain (τ = 0.77, p<0.0001). Using the Bland-Altman’s plot to analyze for inter- and intra-observer variability the bias and the Bland-Altman limits of agreement for longitudinal strain was +0.4±3.1% and +0.3±2.4 % for inter-observer and intra-observer observations respectively. For circumferential strain the bias and Bland-Altman limits of agreement ranged from −0.8±4.0% and −0.2±2.3% for inter-observer and intra-observer observations respectively. Both observers were non-blinded.
Ventricular Volumes and Ejection Fraction
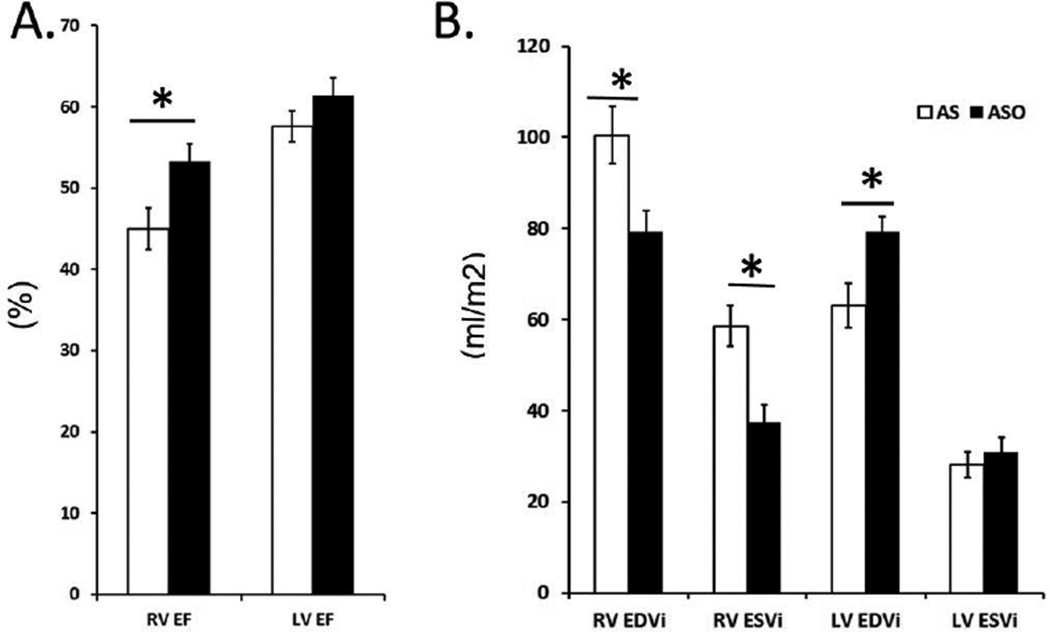
The RV ejection fraction was lower by almost 15% in the atrial switch group compared to the arterial switch group. This reduction in function was accompanied by a significant increase in absolute RV end-diastolic and end-systolic volumes in the atrial switch group (Fig 2). The LV ejection fraction was decreased by 6% in the atrial switch compared to the arterial switch group with significant decrease in end-diastolic LV volumes and no significant difference in the absolute LV end-systolic and end-diastolic volumes.
Fig 2.
Comparison of Right Ventricle (RV) and Left Ventricle (LV) Function and Volume between the Atrial Switch (AS) and Arterial Switch (ASO) group. (*) indicates statistical significance with p values ≤ 0.05. (A) Bar graphs represent significantly decreased RV Ejection Fraction in the AS group. (B) The RV end-diastolic and end-systolic volumes normalized to BSA are significantly increased in the AS group.
RV Deformation parameters
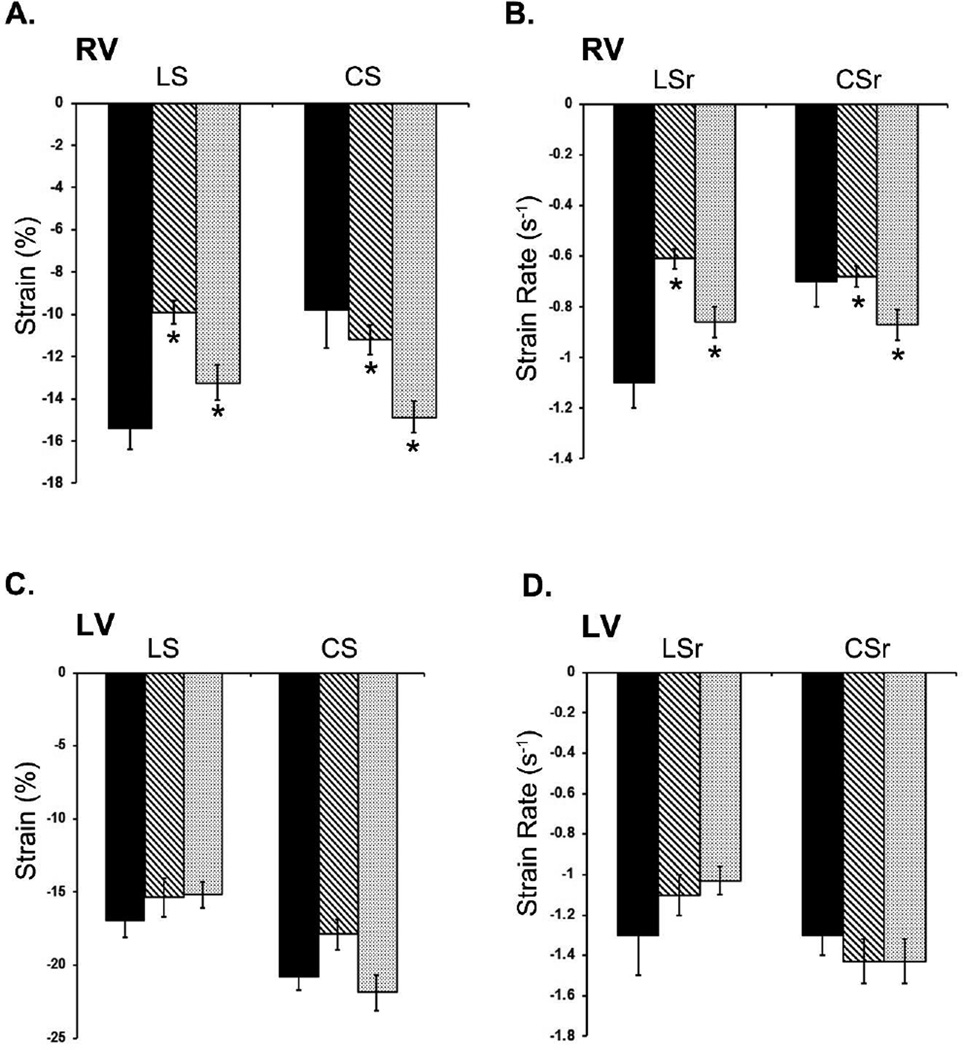
The deformation parameters of the RV and LV of the atrial switch, arterial switch and normal control groups are shown in Tables 2 and Fig 3. When compared across groups, the atrial switch RV strain parameters showed a significant decrease in longitudinal strain and strain rate. A Kruskal-Wallis test showed that there was a statistically significant difference in RV global longitudinal strain between the different groups, χ2(2) = 15.09, p = 0.005. Post-hoc (Mann-Whitney with Steel–Dwass test), showed a significant difference between atrial switch RV global longitudinal strain and arterial switch RV global longitudinal strain (p =0.01) and atrial switch RV global longitudinal strain and control RV global longitudinal strain (p=0.0012). For Strain Rate, there was a statistically significant difference in RV global longitudinal strain rate between the different groups, χ2(2) = 19.34, p = 0.0001. Post-hoc (Mann-Whitney with Steel–Dwass test), showed a significant difference between atrial switch RV global longitudinal strain rate and arterial switch RV global longitudinal strain rate (p =0.003), the atrial switch RV global longitudinal strain rate and control RV global longitudinal strain rate (p=0.0004).
Table 2.
Myocardial Deformation Parameters of the Right and Left Ventricle in the Atrial and Arterial Switch Groups Compared to Controls.
| RV | Control(n=8) | AS(n=20) | ASO(n=20) | p-value |
|---|---|---|---|---|
| Longitudinal Strain (%) | −15.3±0.9 | −9.9±0.5 | −13.2±0.8 | (0.0005) |
| Circumferential Strain (%) | −9.8±1.8 | −11.2±0.7 | −14.8±0.06 | (0,002) |
| Longitudinal Strain Rate (s−1) | −1.1±0.1 | −0.6±0.03 | −0.8±0.05 | (0.0001) |
| Circumferential Strain Rate (s−1) | −0.7±0.1 | −0.6±0.04 | −0.8±0.06 | (0.02) |
| LV | ||||
| Longitudinal Strain (%) | −16.9±1.3 | −15.37±1.3 | −15.19±0.9 | (0.76) |
| Circumferential Strain (%) | −20.8±0.9 | −17.89±1.0 | −21.89±1.2 | (0.06) |
| Longitudinal Strain Rate (s−1) | −1.3±0.2 | −1.1±0.09 | −1.03±0.07 | (0.25) |
| Circumferential Strain Rate (s−1) | −1.3±0.1 | −1.17±0.08 | −1.43±0.1 | (0.34) |
AS -Atrial Switch Group; ASO-Arterial Switch Group; RV – Right Ventricle; LV – Left Ventricle.
p-value ≤ 0.05 was considered significant. (Values are as shown ±S.E.)
Fig 3.
Changes in Right Ventricle (RV) and Left Ventricle (LV) Strain and strain rate between the atrial switch (AS) and the arterial switch (ASO) groups compared to controls. (*) indicates statistical significance with p values ≤ 0.05. Bar graphs show significant decrease in RV longitudinal strain (LS), longitudinal strain rate (LSr) in the AS group (A, B). The circumferential strain (CS) and circumferential strain rate (CSr) is increased in both the AS and ASO group compared to controls (A, B) LV strain and strain rates were not significantly changed in either the AS and ASO groups (C, D).
The circumferential strain was higher in the atrial and arterial switch group compared to controls. RV global circumferential strain between the different groups, χ2(2) = 12.33, p = 0.002. A significant difference between atrial switch RV global circumferential strain and arterial switch RV global circumferential strain (p =0.005; Steel-Dwass) whereas, atrial switch RV global circumferential strain and control RV global circumferential strain showed no significant difference (p=0.61; Steel-Dwass). RV global circumferential strain rate between the different groups was significant, χ2(2) = 7.04, p = 0.02. A significant difference between atrial switch RV global circumferential strain rate and arterial switch RV global circumferential strain rate (p =0.05; Steel-Dwass) whereas, atrial switch RV global circumferential strain rate and control RV global circumferential strain rate showed no significant difference (p=0.96; Steel-Dwass).
As mentioned above when the atrial and arterial switch groups are compared to each other, there was a global reduction in the RV deformation parameters of the atrial switch group. The global longitudinal strain was decreased in the atrial switch group compared to the arterial switch group (atrial switch −9.9±0.5; arterial switch= −13.2±0.8) with a significant decrease by −3.33 % (p=0.01). The RV global circumferential strain for the atrial switch group was decreased by −3.67% (atrial switch −11.2±0.7; arterial switch −14.8±0.7; p=0.005). The global strain rate was significantly decreased for global longitudinal and circumferential parameters. The global longitudinal strain rate was decreased by −0.2 (s−1) (atrial switch−0.6±0.03; arterial switch −0.8±0.05; p=0.005). The global circumferential strain rate was decreased in the atrial switch group (atrial switch−0.6±0.04; arterial switch −0.8±0.05; p=0.05).
LV Deformation parameters
The LV deformation parameters of the atrial switch group and the arterial switch group were compared to the normal control group. None of the LV deformation parameters of either group were statistically different from the normal group (Fig 3). No significant difference was also detected when comparing the LV deformation parameters between the atrial switch and arterial switch groups.
Discussion
Advances in medical management and surgical techniques for TGA have improved the survival rates into adulthood. (11) Previous long-term studies have shown various degrees of diastolic and systolic RV dysfunction in both cohorts of TGA patients.(12, 13). Over the long-term many of these patients develop RV failure and the incidence is higher in patients who underwent an atrial switch operation. (14) The pathogenesis of systemic RV failure in the atrial switch cohort is unclear. A failing pressure overloaded systemic RV, if uncorrected, can secondarily cause diastolic LV dysfunction.(15) Early recognition of RV failure can help modify management and possibly prevent long-term morbidity and mortality in this patient population.
Strain Imaging
Cardiac volumetric analysis and ejection fraction are typically used in clinical practice to assess ventricular function. Echocardiographically these measures have been shown to be limited by inaccuracies, lack of reproducibility and delay in detecting early changes in myocardial function.(16) Initially, myocardial deformation imaging or strain imaging was applied to echocardiography for assessment of systolic and diastolic function.(17). When assessed by tissue Doppler imaging, strain and strain rate measurements are limited by the angle of insonation. While this limitation is decreased with the use of 2D echo imaging, CMR permits even less anisotropy to confound a 2D feature tracking analysis tool such as Velocity Vector Imaging.(18, 19). This modality allows for feasible and reproducible strain analysis retrospectively on CMR images.(20) Segmental changes in myocardial length have been defined as strain (S); rate of this change is strain rate (Sr). Changes in the longitudinal and circumferential planes are defined as longitudinal (LS) and circumferential strain (CS).
This is the first study to use feature tracking software on CMR images to evaluate the RV and LV deformation parameters in patients with TGA who have undergone the atrial switch or arterial switch operations. The application of feature tracking to CMR is important in this patient population as CMR is a prominent imaging modality in adults with repaired TGA. This is also the first study that determines function and strain parameters close to the third decade post-surgical intervention in the atrial switch group.
The present study demonstrates a global reduction in multiple deformation parameters of the systemic right ventricle in the atrial switch group when compared to the normal control group and the arterial switch group. This coincides with an increase in CMR measured ventricular volumes and a decrease in ejection fraction in the atrial switch group when compared to the normal and the arterial switch groups. Similar to this study, Diller et al noted a decrease in RV global longitudinal strain by speckle-tracking echocardiography in TGA patients who had undergone an atrial switch operation. They correlated these changes with adverse clinical outcomes.(21) In the present study, circumferential strain and strain rates were increased when compared to controls suggesting a progressive adaptive response by the RV. Previously, Pettersen et al reported similar increase in circumferential strain and strain rate of the right ventricle.(22) The major difference between the current study and the previous study is the time point from the surgical intervention. The present study looks at RV function and deformation parameters at a mean age of 28.7 years in the atrial switch group whereas the earlier study reported a mean age of 18.4 years. This likely indicates a continuum of the disease process with decreasing ventricular function.(23) The RV deformation parameters of the arterial switch group were similar to normal controls, consistent with its position as the subpulmonary ventricle after arterial switch operation. The LV deformation parameters of the atrial switch group were mostly unchanged when compared to the arterial switch or the control groups.. It is somewhat surprising that more of the deformation parameters were not different given that the LV of the atrial switch group is subpulmonary whereas the LV of the arterial switch group is subaortic. It is likely that the LV is more adaptive to a wider variation of pressures unlike the RV. A recent study looked into RV and LV function at medium-term follow up after arterial switch operation. The findings of this study suggest that LV systolic and diastolic function returns to normal while RV systolic and diastolic performance continued to be impaired a year after surgery.(24)
Future studies should be directed at evaluating changes in the RV and LV of atrial switch and arterial switch TGA patients over time, especially as the atrial switch cohort moves into middle and later age. This will provide insight into the changes in strain parameters of the RV and LV. Linking these findings with clinical outcome data will help formulate clinical management options before the onset of irreversible RV myocardial changes and progressive heart failure. Analysis of segmental strain parameters of the RV and LV can also provide temporal deformation data which may permit for the assessment of ventricular dyssynchrony.(25) This study determines the changes in the RV primarily related to pressure overload; however, it would be interesting to see how RV myocardial mechanics change with primary volume overload (e.g.: repaired tetralogy of Fallot with pulmonary insufficiency) or combined loading conditions (e.g.: congenitally corrected transposition of the great arteries with hemodynamically significant tricuspid regurgitation).The methods used in this study can be applied to these and other diseases including pulmonary stenosis and single morphologic RV’s that can lead to pressure loaded RV failure.(8, 15)
Limitations
The limitations of our current study include the absence of a larger cohort of age matched normal controls. This would allow us to evaluate the extent of change that is related to the disease process and would not be confounded by changes with age.(26) The mean age of the two cohorts are separated by a greater than 10 years. This again alters the characteristics of the cohort. Also of note is this being the first study to use VVI CMR to evaluate RV myocardial deformation in TGA patients and is limited by no previous VVI RV validation.
Conclusions
VVI CMR analysis of TGA patients reveals a reduction of RVEF and multiple RV strain parameters in patients with an atrial switch operation when compared to normal controls and those with an arterial switch operation. All of the LV strain and strain rate parameters in the atrial switch group are unchanged compared to the atrial switch and control groups.. This is the first study to use VVI CMR to evaluate RV myocardial deformation in TGA patients. A better understanding of the mechanisms of RV failure in TGA may lead to improved therapies. The findings can also be used to better understand myocardial mechanics in RV failure due to other congenital heart diseases.
Acknowledgments
NHLBI-funded T-32 Pediatric Cardiology Training Grant.
Footnotes
Conflict of Interest: None
- Bijoy Thattaliyath, M.D.: Collection of patient data, analysis, statistics, interpretation and preparation of manuscript.
- Daniel E. Forsha, M.D.: Data analysis, analysis for inter-observer variability, manuscript review.
- Chad Stewart M.D.: Collection and analysis of control group and manuscript review.
- Piers Barker, M.D: Concept and design of project, training on the use of VVI software, Manuscript review.
- Michael Jay Campbell, M.D.: Concept and design, supervision of the project, help with selection of patients for the study, access to CMR data and manuscript review.
References
- 1.Blalock A, Hanlon CR. The surgical treatment of complete transposition of the aorta and the pulmonary artery. Surgery, gynecology & obstetrics. 1950 Jan;90(1):1–15. illust. [PubMed] [Google Scholar]
- 2.Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth defects research Part A, Clinical and molecular teratology. 2006 Nov;76(11):747–756. doi: 10.1002/bdra.20294. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 3.Meijboom F, Szatmari A, Deckers JW, Utens EM, Roelandt JR, Bos E, et al. Long-term follow-up (10 to 17 years) after Mustard repair for transposition of the great arteries. The Journal of thoracic and cardiovascular surgery. 1996 Jun;111(6):1158–1168. doi: 10.1016/s0022-5223(96)70217-9. [Comparative Study Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar D, Bull C, Yates R, Wright D, Cullen S, Gewillig M, et al. Comparison of long-term outcomes of atrial repair of simple transposition with implications for a late arterial switch strategy. Circulation. 1999 Nov 9;100(19 Suppl):II176–II181. doi: 10.1161/01.cir.100.suppl_2.ii-176. [Comparative Study Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 5.Dobson R, Danton M, Nicola W, Hamish W. The natural and unnatural history of the systemic right ventricle in adult survivors. The Journal of thoracic and cardiovascular surgery. 2013 Jun;145(6):1493–1501. doi: 10.1016/j.jtcvs.2013.02.030. discussion 501–3. [DOI] [PubMed] [Google Scholar]
- 6.Williams WG, McCrindle BW, Ashburn DA, Jonas RA, Mavroudis C, Blackstone EH, et al. Outcomes of 829 neonates with complete transposition of the great arteries 12–17 years after repair. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. [Multicenter Study] 2003 Jul;24(1):1–9. doi: 10.1016/s1010-7940(03)00264-1. discussion -10. [DOI] [PubMed] [Google Scholar]
- 7.Roos-Hesselink JW, Meijboom FJ, Spitaels SE, van Domburg R, van Rijen EH, Utens EM, et al. Decline in ventricular function and clinical condition after Mustard repair for transposition of the great arteries (a prospective study of 22–29 years) European heart journal. 2004 Jul;25(14):1264–1270. doi: 10.1016/j.ehj.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Davlouros PA, Niwa K, Webb G, Gatzoulis MA. The right ventricle in congenital heart disease. Heart. 2006 Apr;(92 Suppl 1):i27–i38. doi: 10.1136/hrt.2005.077438. [Research Support, Non-U.S. Gov't Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005 Dec 13;112(24):3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [Randomized Controlled Trial Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Nagueh SF. Current perspectives on cardiac function in patients with diastolic heart failure. Circulation. 2009 Mar 3;119(8):1146–1157. doi: 10.1161/CIRCULATIONAHA.108.822676. [Review]. [DOI] [PubMed] [Google Scholar]
- 11.Gorler H, Ono M, Thies A, Lunkewitz E, Westhoff-Bleck M, Haverich A, et al. Long-term morbidity and quality of life after surgical repair of transposition of the great arteries: atrial versus arterial switch operation. Interactive cardiovascular and thoracic surgery. [Comparative Study] 2011 Apr;12(4):569–574. doi: 10.1510/icvts.2010.253898. [DOI] [PubMed] [Google Scholar]
- 12.Pettersen E, Fredriksen PM, Urheim S, Thaulow E, Smith HJ, Smevik B, et al. Ventricular function in patients with transposition of the great arteries operated with arterial switch. The American journal of cardiology. 2009 Aug 15;104(4):583–589. doi: 10.1016/j.amjcard.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Martin RP, Qureshi SA, Ettedgui JA, Baker EJ, O'Brien BJ, Deverall PB, et al. An evaluation of right and left ventricular function after anatomical correction and intra-atrial repair operations for complete transposition of the great arteries. Circulation. 1990 Sep;82(3):808–816. doi: 10.1161/01.cir.82.3.808. [DOI] [PubMed] [Google Scholar]
- 14.Buch J, Wennevold A, Jacobsen JR, Hvid-Jacobsen K, Lauridsen P. Long-term follow-up of right ventricular function after Mustard operation for transposition of the great arteries. Scandinavian journal of thoracic and cardiovascular surgery. 1988;22(3):197–202. doi: 10.3109/14017438809106062. [DOI] [PubMed] [Google Scholar]
- 15.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006 Oct 24;114(17):1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [Congresses Research Support, N.I.H., Extramural]. [DOI] [PubMed] [Google Scholar]
- 16.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. American heart journal. 2009 Aug;158(2):294–301. doi: 10.1016/j.ahj.2009.05.031. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 17.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? Journal of the American College of Cardiology. 2006 Apr 4;47(7):1313–1327. doi: 10.1016/j.jacc.2005.11.063. [Research Support, Non-U.S. Gov't Review]. [DOI] [PubMed] [Google Scholar]
- 18.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010 Apr;23(4):351–369. doi: 10.1016/j.echo.2010.02.015. quiz 453–5. [DOI] [PubMed] [Google Scholar]
- 19.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007 Nov 27;116(22):2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [Research Support, N.I.H., Extramural Review]. [DOI] [PubMed] [Google Scholar]
- 20.Williams LK, Urbano-Moral JA, Rowin EJ, Jamorski M, Bruchal-Garbicz B, Carasso S, et al. Velocity vector imaging in the measurement of left ventricular myocardial mechanics on cardiac magnetic resonance imaging: correlations with echocardiographically derived strain values. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013 Oct;26(10):1153–1162. doi: 10.1016/j.echo.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Diller GP, Radojevic J, Kempny A, Alonso-Gonzalez R, Emmanouil L, Orwat S, et al. Systemic right ventricular longitudinal strain is reduced in adults with transposition of the great arteries, relates to subpulmonary ventricular function, and predicts adverse clinical outcome. American heart journal. [Comparative Study] 2012 May;163(5):859–866. doi: 10.1016/j.ahj.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Pettersen E, Helle-Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B, et al. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. Journal of the American College of Cardiology. 2007 Jun 26;49(25):2450–2456. doi: 10.1016/j.jacc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 23.Phan TT, Shivu GN, Abozguia K, Gnanadevan M, Ahmed I, Frenneaux M. Left ventricular torsion and strain patterns in heart failure with normal ejection fraction are similar to age-related changes. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009 Aug;10(6):793–800. doi: 10.1093/ejechocard/jep072. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 24.Klitsie LM, Roest AA, Kuipers IM, Hazekamp MG, Blom NA, Ten Harkel AD. Left and right ventricular performance after arterial switch operation. The Journal of thoracic and cardiovascular surgery. 2014 May;147(5):1561–1567. doi: 10.1016/j.jtcvs.2013.07.048. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 25.Delgado V, Tops LF, Trines SA, Zeppenfeld K, Marsan NA, Bertini M, et al. Acute effects of right ventricular apical pacing on left ventricular synchrony and mechanics. Circulation Arrhythmia and electrophysiology. 2009 Apr;2(2):135–145. doi: 10.1161/CIRCEP.108.814608. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 26.Codreanu I, Pegg TJ, Selvanayagam JB, Robson MD, Rider OJ, Dasanu CA, et al. Normal values of regional and global myocardial wall motion in young and elderly individuals using navigator gated tissue phase mapping. Age. 2013 Apr 21; doi: 10.1007/s11357-013-9535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]