Abstract
Attention-deficit / hyperactivity disorder (ADHD) is a common childhood psychiatric disorder that often persists into adulthood. While several studies have identified altered functional connectivity in brain networks during rest in children with ADHD, few studies have been performed on adults with ADHD. Existing studies have generally investigated small samples. We therefore investigated aberrant functional connectivity in a large sample of adult patients with childhood-onset ADHD, using a data-driven, whole-brain approach. Adults with a clinical ADHD diagnosis (N = 99) and healthy, adult comparison subjects (N = 113) underwent a 9-minute resting-state fMRI session in a 1.5T MRI scanner. After elaborate preprocessing including a thorough head-motion correction procedure, group independent component analysis (ICA) was applied from which we identified six networks of interest: cerebellum, executive control, left and right frontoparietal and two default-mode networks. Participant-level network maps were obtained using dual-regression and tested for differences between patients with ADHD and controls using permutation testing. Patients showed significantly stronger connectivity in the anterior cingulate gyrus of the executive control network. Trends were also observed for stronger connectivity in the cerebellum network in ADHD patients compared to controls. However, there was considerable overlap in connectivity values between patients and controls, leading to relatively low effect sizes despite the large sample size. These effect sizes were slightly larger when testing for correlations between hyperactivity / impulsivity symptoms and connectivity strength in the executive control and cerebellum networks. This study provides important insights for studies on the neurobiology of adult ADHD; it shows that resting-state functional connectivity differences between adult patients and controls exist, but have smaller effect sizes than existing literature suggested.
Keywords: Adult ADHD, resting-state functional connectivity, independent component analysis, executive control network, cerebellum
1. INTRODUCTION
Attention-deficit / hyperactivity disorder (ADHD2) is one of the most frequent childhood psychiatric disorders. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM) (American Psychiatric Organisation, 2013, 2000) ADHD is characterised by symptoms of inattentiveness or hyperactivity / impulsivity, or by a combination of these two symptom domains. In 65% of the cases ADHD symptoms are chronic and persist into adulthood, with at least 15% of the patients still meeting the full criteria for ADHD in adulthood (Faraone et al., 2006). Despite a prevalence of 2.5% in the adult population (Simon et al., 2009) persistent ADHD has received much less attention in research than ADHD in childhood. While ADHD in adults is characterised by abnormalities in the function of several brain areas (Cortese et al., 2012) the neurobiology of adult ADHD is still poorly understood. Similar to the situation in other psychiatric diseases, etiological modelling of ADHD has now shifted from postulating dysfunctions in isolated brain regions to examining the connectivity of brain networks using both structural and functional measures (Castellanos and Proal, 2012). Structural connectivity depicts anatomical connections, whereas functional connectivity describes the temporal correlations in neural activity between distributed brain regions (Friston, 1994).
In the past five years there has been an increase in studies aimed at discovering functional connectivity differences between patients and controls. Many of these have focused on the default-mode network (DMN), which is characterised by its higher level of activation during rest and deactivation during tasks (Raichle et al., 2001). DMN dysfunction is hypothesized to cause attentional interference and response variability in patients with ADHD (Sonuga-Barke and Castellanos, 2007). In adult patients with persistent ADHD compared to healthy controls, functional connectivity within the DMN was found to be reduced (Castellanos et al., 2008; Uddin et al., 2008), connectivity between the dorsal anterior cingulate and the DMN was found to be less negative (Castellanos et al., 2008) and abnormal (Sato et al., 2012), and coherence between the dorsolateral prefrontal cortex and the DMN was described as stronger (Hoekzema et al., 2014). These findings of aberrant DMN connectivity are generally in line with findings from a slightly larger body of resting-state connectivity studies in children with ADHD (i.e. Cao et al., 2006; Fair et al., 2010; Tian et al., 2006).
Besides the DMN, aberrant connectivity in several other networks has also been associated with ADHD. McCarthy and colleagues observed decreased functional connectivity within the dorsal and ventral attention networks, and increased functional connectivity within the affective, default-mode and right lateralized cognitive control networks, when comparing adult patients with ADHD and healthy controls (McCarthy et al., 2013). Furthermore, Wang and colleagues showed brain-wide increases and decreases in regional resting-state activity (‘regional homogeneity’) in multiple regions, including the DMN, anterior cingulate cortex, cerebellum, insula, and basal ganglia, that could fairly accurately discriminate adult patients with ADHD from controls (Wang et al., 2013). These findings are in line with task-based fMRI studies that have shown aberrant neuronal activation in multiple networks. Problems with working memory, attention and cognitive control in ADHD have been attributed to reduced activity in brain regions in the right and left frontoparietal networks (Valera et al., 2010), while deficits in reward, timing, response inhibition, and impulsivity have been linked to aberrant functioning of frontal-striatal-cerebellar connections (Cubillo et al., 2012).
Taken together, these findings could be interpreted as widespread neuronal dysfunction in adult ADHD. At the same time, however, it seems that findings are difficult to replicate. As most studies described above rely on relatively small sample sizes (typically with n=20 per group), it is difficult to determine whether these findings hold true at the population level (Button et al., 2013). Additionally, the methods to investigate between-group differences in connectivity vary, being either seed-based (Castellanos et al., 2008; McCarthy et al., 2013; Sato et al., 2012), regional homogeneity (Uddin et al., 2008; Wang et al., 2013) or independent component analyses (Hoekzema et al., 2014). Especially findings from seed-based studies, that rely on a specific region of interest (ROI), are difficult to compare with the results from studies using different ROIs or different analysis techniques (Cole et al., 2010). We therefore adopted a data-driven approach that allows the investigation of functional connectivity in all major resting-state networks (RSNs) and that is not biased by the selection of a particular ROI. With independent component analysis (ICA) resting-state fluctuations in neural activity can be separated into spatially independent components that are consistent over time and across subjects (Beckmann et al., 2005; Damoiseaux et al., 2006) and similar to task-based activation networks (Smith et al., 2009). Through subsequent dual-regression analysis one can analyse how the RSNs are manifested in each participant, after which between-group comparisons can be conducted to test if functional connectivity (i.e. temporal coherence) within these networks differs between patients and controls (Filippini et al., 2009). This method has been shown to be successful as an exploratory and data-driven analysis tool in various clinical and non-clinical populations. For example, to identify novel networks involved in major depression (Veer et al., 2010), to distinguish young carriers of the APOE4 allele from non-carriers (Filippini et al., 2009), or to identify networks that can be used as features in a classification model distinguishing autism patients from healthy controls (Uddin et al., 2013).
We applied this method to resting-state data from the largest sample of adult patients with ADHD studied to date, comprising 99 patients and 113 healthy controls from the Dutch part of the IMpACT study (Franke et al., 2010). In addition to between-group differences, we investigated dimensional associations between ADHD symptoms of inattention and hyperactivity/impulsivity and within-network functional connectivity strength. Such an approach may provide a closer association between brain and behaviour and has proven to be effective when investigating childhood ADHD (Chabernaud et al., 2012).
Based on previous findings in adult ADHD, we restricted our analyses to RSNs of interest that we identified through high spatial correspondence to the RSNs described by Smith and colleagues (Smith et al., 2009). These networks are the default-mode, cerebellum, executive control, and the left and right frontoparietal networks. The executive control network has also been called the salience (Seeley et al., 2007), ventral attention (Yeo et al., 2011), or affective network (McCarthy et al., 2013) and includes the anterior insula and anterior cingulate cortex. We expected differences in functional connectivity between patients and controls in these networks. Furthermore, we hypothesized that these effects would be more pronounced when taking a dimensional instead of categorical approach.
2. MATERIAL AND METHODS
2.1 Participants
Participants were selected from the Dutch cohort of the International Multicenter persistent ADHD CollaboraTion (IMpACT) (Franke et al., 2010). A total of 212 adult participants were included in the analyses, 113 healthy control participants and 99 patients with ADHD. All participants underwent psychiatric assessments, neuropsychological tests and a MRI session that included functional tasks, functional resting-state, and structural neuroimaging as previously described (Hoogman et al., 2013; Onnink et al., 2014). Patients were included if they had previously been diagnosed with adult ADHD by a psychiatrist according to the DSM (4th edition; DSM-IV-TR; (American Psychiatric Organisation, 2000)) and scored at least five symptoms on either the inattention or hyperactivity / impulsivity domain from the DIVA interview (see below ‘ADHD symptoms’). In case the patient did not participate in the DIVA interview, he/she was included based on scores from the ADHD Self Rating scale (see below ‘ADHD symptoms’), using the same symptom threshold. Controls were included if they scored less than four symptoms on the DIVA interview, or otherwise on the Self Rating scale.
Patients were excluded if they used medication other than psychostimulants or atomoxetine. Other exclusion criteria for both patients and controls were current diagnosis of major depression, substance use disorder or psychosis (assessed with the Structural Clinical Interviews for DSM-IV, SCID-I and SCID-II (First et al., 1997, 1996), estimated IQ below 80 (assessed with two subtests, block design and vocabulary, of the Wechsler Adult Intelligence Scale-III (Wechsler, 1997)), neurological disorders and sensorimotor disabilities, excessive head motion during the resting-state scan (absolute motion > 1.5 mm and/or the root mean square (rms) of relative motion > 0.2 mm) or other MRI contra-indications. Patients using medication at the time of recruitment were asked to withhold medication for 24 hours prior to testing. All participants were asked to refrain from smoking and drinking coffee during testing.
This study was approved by the regional ethics committee (CMO region Arnhem-Nijmegen) and was carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki). After completely describing the study to the subjects, written informed consent was obtained.
2.2 ADHD symptoms
Both patients and controls were assessed using the structured diagnostic interview for adult ADHD (DIVA; http://www.divacenter.eu; (Kooij, 2010)). This interview focuses on the 18 DSM-IV symptoms of ADHD and uses concrete and realistic examples to thoroughly investigate whether a symptom is currently present or was present in childhood. Additionally, all participants were asked to fill out the ADHD-DSM-IV Self Rating scale that assesses current inattention and hyperactivity / impulsivity symptoms (Kooij et al., 2005). For both the DIVA interview and the Self Rating scale two scores can be derived, one for each symptom domain, with a maximum score of 9 per domain.
Based on the DIVA interview, patients were classified as having the inattentive-subtype when they presented with six or more symptoms on the inattention domain, as having the hyperactivity/impulsivity-subtype when they had six or more symptoms on the hyperactivity/impulsivity domain and as having the combined-subtype when they had six or more symptoms on both domains.
2.3 MRI data acquisition
Participants completed a nine-minute resting-state scan consisting of 274 interleaved whole-brain functional volumes using echo planar imaging on a Siemens 1.5-Tesla Avanto scanner (repetition time = 1990 msec; echo time = 45 ms; flip angle = 83, 23 slices, matrix size = 224 × 224 × 115 mm; acquisition voxel size = 3.5 × 3.5 × 5 mm). Participants were verbally instructed to lie still with their eyes closed, but not to fall asleep. A high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) anatomical scan was also obtained (176 sagittal slices, repetition time = 2730 ms, echo time = 2.95 ms, voxel size = 1.0 × 1.0 × 1.0 mm, matrix size = 350 × 263 × 350 mm, inversion time = 1000 ms). The resting-state scan was preceded by the T1 scan and a counting Stroop task (not included in the current analyses) and took place approximately 20 minutes after the participant had entered the scanner.
2.4 Preprocessing of functional MRI images
Image preprocessing was performed using FSL software, version 5.0.5 (http://fmrib.ox.ac.uk/fsl). Preprocessing included deleting the first 5 volumes to allow the magnetization to reach dynamic equilibrium, and retaining the subsequent 269 volumes, motion correction with MCFLIRT (Wilson et al., 2002), removal of non-brain tissue (i.e., skull stripping), grand-mean scaling to normalize the global 4D data and spatial smoothing using a Gaussian kernel of 6 mm full width at half-maximum. Subsequently, we used ICA-AROMA to identify residual motion-related artefacts (Pruim et al. 2015). ICA-AROMA is an automated toolbox that uses single-subject ICA to detect components that are associated with head motion by evaluating each component in light of four parameters: the proportion of high frequencies in the power spectrum of the component, the correlation of the component’s time course with the realignment parameters derived from the motion correction step, the proportion of signal located at the edge of the brain, and the proportion of the signal located in cerebrospinal fluid (CSF). Components identified as head motion were removed from the signal by means of a linear regression (‘non-aggressive denoising’) using the function fsl_regfilt. Details about the identification and removal of motion artefacts, as well as an evaluation of the ICA-AROMA method against alternative motion-correction methods are described elsewhere (Pruim et al. 2015b; Pruim et al. 2015). After removing motion artefacts, signals from the white matter (WM) and CSF were removed using linear regression. WM and CSF signals were derived from conservative anatomical masks that were created using FSL FAST. Lastly, a high-pass temporal filter was used with a cut-off frequency of 0.01Hz. We did not perform global signal regression, as it has been shown to induce anti-correlations in resting-state data (Murphy et al., 2009). The preprocessed functional images were linearly registered with FLIRT to the subject-specific high resolution T1 images using boundary-based registration (Greve and Fischl, 2009). The T1 images were registered to Montreal Neurological Institute (MNI152) standard space using 12-parameter affine transformation and non-linear registration with FSL FNIRT (10 mm warp, 4 mm resampling resolution) (Jenkinson and Smith, 2001; Jenkinson et al., 2002).
2.5 Identification of resting-state networks
To obtain functional connectivity networks we conducted group ICA using MELODIC in FSL (Beckmann et al., 2005) (version 3.14). Functional images of all participants were concatenated in the temporal domain to create a single 4D dataset. This concatenated dataset was then decomposed into 50 spatially independent components (ICs). Due to our large sample we chose this higher-order decomposition (i.e. as compared to the more commonly used 35). Components from the group ICA reflected both functional components (characterised by being located mainly in the grey matter and having a signal within the frequency range of 0.1-0.01 Hz) as well as residual noise components.
Functional connectivity patterns of each participant that corresponded to each group-IC were obtained using a dual-regression approach (Beckmann et al., 2009; Filippini et al., 2009) (dual_regression version 0.5). With this approach, the set of 50 spatial maps from the group ICA was used to generate subject-specific versions of the spatial maps, and associated time series, using two sequential multiple regressions. First, for each subject, the 50 group-level spatial maps were used as spatial regressors against the preprocessed individual subjects’ fMRI data. This resulted in a set of 50 subject-specific time courses corresponding to each group-level IC. Second, these time courses were variance-normalized and used as temporal regressors against the individual subjects’ fMRI data to produce participant-level unique spatial maps for each of the 50 ICs. In this way, the subject-specific spatial maps reflect the relationship (or temporal coherence) between an individual voxel’s time course and the IC time course, thus representing the connectivity strength of each voxel in the network (Janes et al., 2012).
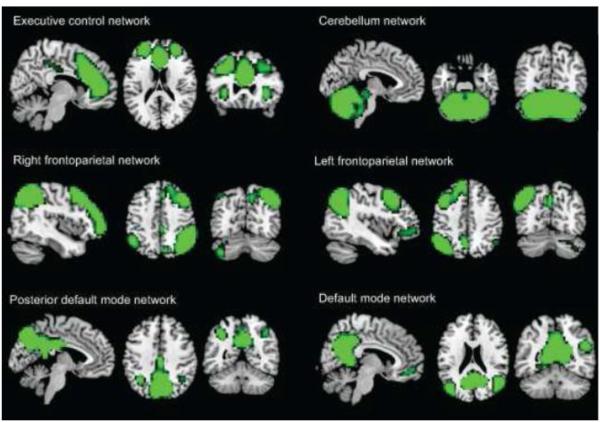
Next, we identified the ICs that showed close correspondence to the networks of interest (the default-mode, cerebellum, executive control, and the left and right frontoparietal networks). We identified these networks in our data by spatial correlation between the components from the group ICA and the five relevant network templates from the study by Smith and colleagues (Smith et al., 2009). Six networks from the group ICA showed high spatial correspondence ( > 0.4) with the five network templates of interest. The cerebellum, executive control, left and right frontoparietal template networks each corresponded to a single component from the group ICA. The DMN template network was represented in two group-level ICs: a full DMN and a posterior part of the DMN. The selected six networks are shown in Figure 1.
Figure 1.
Six components of interest from group ICA representing networks of interest (green), overlayed on a MNI-template brain (grey). Networks were thresholded at Z > 5.
2.6 Categorical comparisons between ADHD patients and controls
Patients with ADHD and healthy controls were compared on age, IQ, education levels, and average head motion during scanning using separate independent samples t-tests. Furthermore, they were compared on gender and handedness using Pearson Chi-square tests. The covariate head motion was computed for each participant as the average root mean square (rms) relative (frame-to-frame) head motion. This parameter was computed with MCFLIRT at the motion correction stage during preprocessing (Jenkinson et al., 2002) and was averaged over all volumes to obtain a single measure of head motion per participant.
To identify group differences within the six networks of interest, for each of these networks the corresponding participant-level spatial maps from the dual regression stage were tested voxel-wise for significant differences between the patients with ADHD and the healthy controls via a general linear model. For this, we employed non-parametric permutation testing (applying 5000 permutations) with Threshold-Free Cluster Enhancement (TFCE, (Smith and Nichols, 2009) using the Randomise tool of FSL (version 2.9). Voxel-wise tests were masked with a whole-brain mask, consisting of only those voxels that were present in all participants. Gender and age were added to the model as covariates of no interest.
Between-group effects were considered significant if they reached two-tailed p-values < 0.004 (family-wise error (FWE) corrected at the voxel level with TFCE; Bonferroni-corrected for two-sided testing in six networks). However, with respect to the exploratory nature of the analyses we also report effects with a p-value < 0.05 and a minimal cluster size of 5 voxels (FWE-corrected at the voxel level with TFCE). MNI coordinates of peak voxels were linked to anatomical locations using the Harvard-Oxford cortical and subcortical atlases and the cerebellum atlas in MNI152 space that are implemented in FSL. Cohen’s d measures of effect size were computed from the t-values of the significant peak voxels of each cluster, using the formula Cohen’s d = 2t /√(df). For visualisation of connectivity strength measures (i.e. Figures 2 and 3), connectivity strength of the peak voxel was extracted as the voxel’s parameter estimate from the second stage of the dual regression (reflecting the coherence of that voxel’s time course with the time course of the entire network).
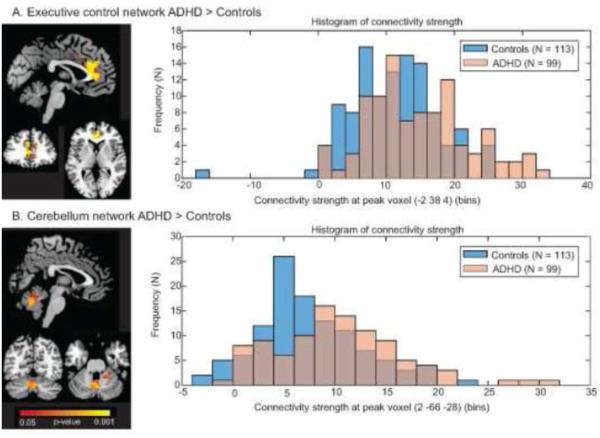
Figure 2.
Stronger connectivity in ADHD patients compared to controls in A) the executive control network and B) the cerebellum network. On the left, significant clusters are depicted in red-yellow at a threshold of p < 0.05 (FWE-corrected). On the right, histograms of connectivity strength of the peak voxel from the clusters on the left are shown for control participants (blue) and ADHD patients (orange). Purple-shaded areas reflect overlap between the two groups.
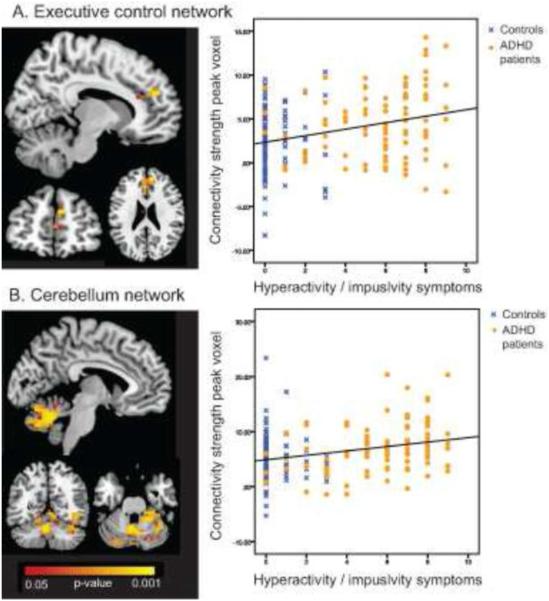
Figure 3.
Significant correlations between hyperactivity/impulsivity symptoms and functional connectivity strength in the executive control network (A) and the cerebellum network (B). Hot colours represent significant regions, thresholded at p < 0.05. Scatterplots represent the correlation between connectivity strength (y-axis; parameter estimates from dual regression, corrected for age and gender) and hyperactivity / impulsivity symptoms (x-axis). In A, the peak voxel is located in the right superior frontal gyrus (MNI 10; 50; 24). In B, the peak voxel is located in the left cerebellum vermis VI (MNI −6; −58; −28). See Table 3 for details.
To assess the robustness of the main group effects, we conducted a series of sensitivity analyses. In these analyses, we added handedness, education, IQ or head motion as additional covariates. Furthermore, within the ADHD group we investigated whether duration of medication treatment correlated with the main effects. Lastly, we investigated whether the main group effects would hold when using a lower-dimensional group ICA (35 components) followed by the same procedure of dual-regression, network selection and between-group testing as described above.
2.7 Dimensional analyses with ADHD symptoms
In those networks that showed categorical between-groups differences we investigated the relationship between functional connectivity strength and ADHD symptom severity across the entire sample (controls and patients combined). Inattention and hyperactivity/impulsivity symptom scores were obtained from the DIVA interview and entered in two separate analyses as variable of interest. As data from the DIVA-interview was missing for 20 participants, we included 104 controls and 88 patients in the dimensional analyses. Voxel-wise effects for a correlation with ADHD symptom scores were tested using permutation testing with Randomise as described above.
3. RESULTS AND STATISTICAL ANALYSES
3.1 Demographics of the sample
Characteristics of the sample and differences in demographics between the groups are reported in Table 1. The patient group did not differ from the control group in terms of age, gender, handedness, estimated IQ, or average frame-to-frame head motion during scanning. Controls were on average higher educated than patients (T = 4.16, p < 0.001). Seventy-five patients with ADHD reported to be taking stimulant medication, with an average treatment duration of 21 months (range 0 – 168 months, SD = 28). Eight patients reported having received medication in the past, and 13 had never been treated with ADHD medication.
Table 1.
Demographics of the participants included in the analyses
| Healthy controls (N = 113) |
Patients with ADHD (N = 99) |
Difference | |
|---|---|---|---|
| Mean age (SD) | 35.75 (11.79) years | 34.71 (10.39) years | T = 0.68 |
| Gender | 46 (40.7%) male | 40 (40.4%) male | X2 = 0.002 |
| Mean IQa (SD) | 111.12 (14.21) | 108.88 | T = 1.15 |
| Mean educationb (SD) | 5.22 (0.78) | 4.76 (0.85) | T = 4.16* |
| Handedness | 102 (90.3%) right | 84 (84.8%) right | X2 = 1.45 |
| Mean head motionc (SD) | 0.08 (0.03) | 0.08 (0.03) | T = −0.31 |
| Mean DIVA inattention symptoms (SD) |
0.37 (0.79) | 7.55 (1.45) | T = −41.46* |
| Mean DIVA hyperactivity/ impulsivity symptoms (SD) |
0.42 (0.83) | 5.74 (2.37) | T = −20.05* |
IQ was estimated based on two subtests, block design and vocabulary, of the Wechsler Adult Intelligence Scale-III (Wechsler, 1997).
Education level was coded from 1 (unfinished primary school) to 7 (post-university).
Head motion was calculated as the mean root mean square (rms) relative motion during scanning.
indicates a p-value < 0.001.
3.2 Group differences in networks of interest
The ADHD patient group showed stronger functional connectivity within the executive control network as compared to controls. This cluster of stronger connectivity was located in the anterior cingulate gyrus (MNI coordinates peak-voxel: −2; 38; 4, p-value = 0.002). There were no significant effects in the other five networks. However, at a more lenient threshold - not correcting for conducting six two-sided tests, while still correcting for family-wise errors at the voxel level - we also observed stronger connectivity in the cerebellum network, with clusters located in the cerebellar vermis VI and crus II regions, and in the lingual gyrus near the tempero-occipital junction (Table 2; Figure 2). As shown in Figure 2, there was a high degree of overlap in the distribution of connectivity values in the executive control and cerebellum networks for the control and patient groups. This was reflected by moderate effect sizes, as shown in Table 2 (Cohen’s d 0.47 – 0.66). Removal of the outlier participant that is apparent in Figure 2 did not alter the results.
Table 2.
Clusters showing stronger within-network connectivity strength in ADHD patients compared to controls a
| Network | Coordinates peak voxel b |
p-value peak |
T-value peak |
Cohen’s d |
Cluster size c |
Region |
|---|---|---|---|---|---|---|
| Executive control |
−2; 38; 4 | 0.002 | 4.12 | 0.57 | 121 | L Anterior cingulate gyrus |
| 6; 18; 40 | 0.019 | 3.37 | 0.47 | 18 | L Paracingulate gyrus | |
| Cerebellum | 2; −66; −28 | 0.01 | 3.79 | 0.53 | 91 | L Cerebellum vermis VI |
| −30; −54; −4 | 0.009 | 4.79 | 0.66 | 23 | L Lingual gyrus | |
| 22; −74; −36 | 0.035 | 3.99 | 0.55 | 9 | R cerebellum crus II |
Effects are shown at a threshold of p < 0.05 (FWE corrected, with TFCE), before correction for multiple testing, and a minimum cluster size of 5 voxels (voxel size = 4 mm). Effects in bold survived correction for testing multiple networks.
Coordinates are in MNI-space.
Number of voxels (voxel size = 4 mm).
Sensitivity analyses showed that handedness, education, IQ, and further correction for head motion did not influence the direction of the effect, nor was medication duration associated with connectivity strength (see Supplementary Table 1 and Supplementary Figure 1 and 2). Defining networks with a group ICA set to find 35 networks yielded very similar networks as the 50-component ICA and highly comparable between-groups effects (see Supplementary Table 2 and Supplementary Figure 3).
3.3 Dimensional analyses in the executive control and cerebellum networks
Based on the findings from the categorical analyses, we tested for positive associations of connectivity strength with inattention and hyperactivity / impulsivity symptoms, respectively, in both the executive control and cerebellum network. We corrected for conducting four tests, considering significant only those results with a p-value < 0.013.
In the executive control network, there was a large cluster of voxels in the right superior frontal gyrus that showed a significant positive correlation between functional connectivity strength and hyperactivity / impulsivity symptoms (Table 3, Figure 3). Another significant cluster was located near the cluster of the categorical group difference, in the anterior cingulate gyrus, although this cluster did not survive multiple comparison correction. In the cerebellum network, hyperactivity / impulsivity symptoms correlated positively with connectivity strength in clusters in the left cerebellar vermis and lingual gyrus. Effect sizes for the dimensional analyses appeared slightly larger compared to the categorical analyses (Cohen’s d 0.57 – 0.76).
Table 3.
Effects for positive correlation between hyperactivity/impulsivity symptoms and connectivity strength a
| Network | Coordinates peak voxel b |
p-value peak |
r-value peak c |
Cohen’s d |
Cluster size d |
Region |
|---|---|---|---|---|---|---|
| Executive control |
10; 50; 24 | 0.005 | 0.305 | 0.64 | 117 | R Superior frontal gyrus |
| −2; 42; 8 | 0.016 | 0.273 | 0.57 | 18 | L Anterior cingulate gyrus | |
| Cerebellum | −6; −58; −28 | 0.004 | 0.294 | 0.62 | 593 | L Cerebellum Vermis VI |
| Cerebellum | −30; −54; −4 | 0.005 | 0.354 | 0.76 | 22 | Left Lingual gyrus |
Effects are shown at a threshold of p < 0.05 (FWE corrected, with TFCE), before correction for multiple testing, and a minimum cluster size of 5 voxels. Effects in bold survived correction for testing multiple networks.
Coordinates are in MNI-space.
R-values reflect the correlation coefficient between the peak voxel connectivity value and hyperactivity/impulsivity symptoms.
Number of voxels (voxel size = 4 mm).
For inattention symptoms, we only found positive correlation with connectivity for a small cluster located in the right frontal pole in the executive control network (MNI-coordinate of the peak voxel: −26; 46; 24, p-value = 0.027, cluster size = 5 voxels). In the cerebellum network, there were no significant clusters of correlation with inattention symptoms.
4. DISCUSSION
In this study, we found that functional connectivity within the anterior cingulate gyrus of the executive control network was stronger in adult patients with ADHD compared to healthy adult control participants. This effect survived stringent correction for both voxel-wise testing (FWE-correction) and testing multiple networks. At a less conservative threshold using only FWE-correction (i.e. ‘nominal significance’), patients with ADHD also showed signs of stronger connectivity within the cerebellum network. Hyperactivity / impulsivity symptoms showed a positive correlation with functional connectivity strength in the executive control and cerebellum networks, with apparent slightly larger effect sizes than the case-control effect and effects surviving correction for multiple tests in both networks. Positive correlations with symptoms of inattention were located in the cerebellum network, but were only seen at nominal significance.
The executive control network encompasses the cingulate cortex, prefrontal cortex, insular cortex, and the striatum and is involved in cognition, the inhibition of actions, emotions, and in pain perception (Smith et al., 2009). It has also been termed “a transitional network linking cognition and emotion/interoception” (Laird et al., 2011). Abnormalities within the executive control network have been widely associated with ADHD (Bush, 2010; Makris et al., 2009; Posner et al., 2014). Furthermore, functional connectivity of the anterior cingulate cortex (ACC) and cerebellum was previously found to be increased in adults with ADHD (McCarthy et al., 2013; Wang et al., 2013). McCarthy et al. however did not observe significant correlations between functional connectivity in the ACC and hyperactivity / impulsivity, which contrasts our findings. Furthermore, a longitudinal study on children and adolescents with ADHD found stronger resting state connectivity in the ACC within the executive control network to be negatively correlated with a decrease in hyperactivity / impulsivity symptoms (Francx et al., 2015). This suggested that stronger integration between the ACC and PFC is important for the remittance of ADHD, at least during childhood and adolescence. Both the ACC and PFC are involved in the ‘cold’ (i.e. response inhibition) aspects (as well as the ‘hot’ (i.e. delay discounting) aspects) of inhibitory control (Bari and Robbins, 2013). In adults with ADHD performing an inhibitory Stop task and a cognitive switching task, activity in the ACC and cerebellum (as well as in other regions of the executive control network) was found to be negatively correlated with symptoms of inattention and hyperactivity (Cubillo et al., 2010). Hence, aberrant functioning of and connectivity between the ACC and PFC within the executive control network may result in inhibitory control problems, which lead to symptoms of hyperactivity and impulsivity (Bush, 2011). Something similar might be true for the cerebellum network. Such a link remains speculative, however, as our analyses are based on correlations. Furthermore, relatively little is known about how altered functional connectivity measured during rest relates to behaviour.
We observed only a small, positive association between inattention symptoms and connectivity in the executive control network, at nominal significance. This suggests that inattentive symptoms in adult ADHD are associated with different neurobiological mechanisms than hyperactivity/impulsivity symptoms, at least in terms of resting-state functional connectivity. Possibly, the aetiology of inattention symptoms is different from that of hyperactivity / impulsivity symptoms (Larsson et al., 2011).
In contrast to expectation, and despite our large sample size, we did not observe the differences in the default-mode and lateralized frontoparietal networks earlier reported. Furthermore, the effects in the executive control and cerebellum networks were small in both categorical and dimensional analyses. As can be seen in Figure 2, connectivity strength of the peak-voxels from case-control difference showed strong overlap between the patient and control groups. Although the means of the two distributions differed significantly, the difference between the means was small and the variability large. This was also reflected by the moderate effect sizes (Cohen’s d between 0.46 and 0.66) of the observed effects. Based on the literature, including several review articles (e.g. (Konrad and Eickhoff, 2010; Liston et al., 2011; Posner et al., 2014), we had expected more wide-spread and stronger effects associated with ADHD status. We propose several explanations for the differences.
First, small and underpowered studies are susceptible to the so-called ‘winner’s curse’, which means that the estimate of the effect can be inflated by chance (Button et al., 2013). When early studies report findings with inflated effects, subsequent studies that do not find any differences are often not published, which results in a biased effect estimate. Functional connectivity differences between adult patients with ADHD and controls may therefore actually be smaller than previously thought. Related to this, effects are likely to be small due to the heterogeneity of (adult) ADHD (e.g. (Hervey et al., 2004; Nigg et al., 2005). Patients with ADHD differ in the number of symptoms in the clinical domains of inattention and hyperactivity / impulsivity (i.e. different clinical subtypes), in the cognitive domains in which they show impairment (Coghill et al., 2013), in the comorbidity with various other psychiatric disorders (Biederman et al., 1991; Wåhlstedt et al., 2009), and in medication use. Although sensitivity analyses showed no direct effects of age, IQ, or medication use, it is likely that different neural mechanisms underlie behavioural symptoms in different patients. This is likely to reduce effect sizes and makes it difficult to compare samples across studies, or to extend research findings to the general patient population (Nigg et al., 2005). To further investigate the aetiology of adult ADHD we conducted additional analyses that were better able to account for heterogeneity. In those dimensional analyses, we investigated the association between functional connectivity and ADHD symptoms subdivided by domain, disregarding the categorical patient-control distinction. Indeed, this enhanced the findings, indicating that the functional connectivity alterations may be better explained by symptom severity than disease status (Chabernaud et al., 2012).
In addition, we were very careful to remove effects from head movements during scanning from the functional data. The issue of spurious effects induced by head motion has received widespread attention in the past few years (e.g. (Fair et al., 2013; Van Dijk et al., 2012). To control for this, we adopted a rigorous new approach to remove motion-related signals that were identified with single-subject ICA from each individual’s functional data (Pruim et al. 2015). This method has been shown to outperform alternative methods, such as linear regression with 24 motion parameters or the removal of volumes associated with head motion (‘scrubbing’), in terms of the number of motion-related artefacts removed, reproducibility of resting-state networks across samples, and preservation of temporal degrees of freedom (Pruim et al. 2015). Furthermore, we confirmed that the addition of another covariate for average frame-to-frame head motion in the group level analyses did not yield different results.
Another potential difference between the current and previous studies is that our sample included a relatively high proportion of women. Although in childhood ADHD is more prevalent in boys than in girls (Biederman et al., 2004), this gender difference is absent in adulthood. This difference between childhood and adulthood may be due to a referral bias in children, as girls tend to be less disruptive than boys and therefore less easily diagnosed, while adult women are more likely to seek treatment compared to men (Biederman et al., 2004). Nonetheless, previous resting-state functional connectivity studies in adults have included either only male participants (Hoekzema et al., 2014) or a majority of male participants (Castellanos et al., 2008; McCarthy et al., 2013; Uddin et al., 2008). Interestingly, Valera and colleagues showed that neural activity differences between patients with ADHD and healthy controls were only observed when comparing male participants, and not between females (Valera et al., 2010). Although our study set-up is ecologically valid, the high proportion of women may explain why our findings differed from those of previous studies.
The current findings should be viewed in light of several strengths and limitations. Obvious strengths were the large sample size and extensive motion correction. These make our findings more robust against inflated estimates of effect sizes and spurious effects of head motion as compared to previous studies. Of course, we also faced some limitations. First, we did not preselect patients according to subtype of ADHD (inattentive, hyperactive/impulsive and combined subtypes) or for the absence of comorbidity. While this may have led to suboptimal control of heterogeneity, it made the current sample most representative of the adult ADHD population. The resulting wide spread in symptoms also increased the power of our dimensional analyses.
A second limitation of our study was medication use by the patients with ADHD. The use of stimulant medication may affect functional connectivity between brain regions (Rubia et al., 2009; Sripada et al., 2013). Our study included patients that were using medication, that had used medication in the past, and those that were medication-naïve. Those actively using medication withheld it for ≥24 hours before testing. Although we cannot entirely rule out that medication differences between patients and controls may have influenced our findings, we found no correlations between the duration of medication treatment and connectivity strength in the identified clusters in the main group contrast (Supplementary Figure 2).
In light of the heterogeneity of (adult) ADHD and the low reproducibility of disease-specific findings across resting-state fMRI studies, we propose that future studies should focus more on dimensional aspects of the disorder rather than the categorical patient-control distinction. Such an approach is in line with the Research Domain Criteria (RDoC) proposed by the U.S. National Institute of Mental Health (NIMH) (Insel et al., 2010) and may enhance our understanding of neurobiological causes for aberrant behaviour and find new targets for treatment. In order to accurately model inter-individual differences in both behaviour and neurobiology, large samples are essential. To achieve this goal, collaborations between institutes and ‘consortium science approaches’ are becoming increasingly important.
5. CONCLUSION
To conclude, in a large sample of adults with persistent ADHD and healthy adult controls, we found stronger functional connectivity in the executive control network in the ADHD group, and - at a lower significance threshold - also in the cerebellum network. Hyperactivity / impulsivity symptoms correlated positively with connectivity in these networks, showing slightly stronger effects as compared to the case-control findings. Unexpectedly, we did not observe significant differences in the lateralized fronto-parietal and in the default-mode networks. Furthermore, effects were relatively small despite the large sample size. Future studies should include even larger sample sizes and focus more on brain-behaviour relationships rather than categorical disease status in order to get a better understanding of the aetiology of heterogeneous disorders such as adult ADHD.
Supplementary Material
Highlights.
We investigated functional connectivity in adults with ADHD and healthy controls
Patients showed stronger connectivity in the executive control network
ADHD symptoms were positively correlated with functional connectivity strength
Effect sizes were moderate, but larger for the correlational analyses
Dimensional approaches are therefore promising to understand ADHD aetiology
ACKNOWLEDGMENTS
This study was supported by grants from the Netherlands Organization for Scientific Research (NWO), i.e. the NWO Brain & Cognition Excellence Program (grant 433-09-229) and a Vici grant to BF (grant 016-130-669), and by grants from the Netherlands Brain Foundation (grant 15F07[2]27) and BBMRI-NL (grant CP2010-33). The research leading to these results also received funding from the European Community’s Seventh Framework Programme (FP7/2007 – 2013) under grant agreements n° 602805 (Aggressotype) and n° 602450 (IMAGEMEND), and from the European Community’s Horizon 2020 Programme (H2020/2014 – 2020) under grant agreement n° 643051 (MiND). In addition, the work was supported by a grant for the ENIGMA Consortium (grant number U54 EB020403) from the BD2K Initiative of a cross-NIH partnership.
The authors with to thank Paul Gaalman for technical assistance with MRI scanning. We also thank Thomas Wolfers and Janneke Dammers for assistance with recruitment and testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- ADHD
- attention-deficit/hyperactivity disorder
- RSN
- resting-state network
- DMN
- default-mode network
- ECN
- executive control network
- CER
- cerebellum network
- ICA
- independent component analysis
- DIVA
- diagnostic interview for adult ADHD
CONFLICT OF INTEREST
Authors report not to have conflicts of interest. Jan Buitelaar has been in the past 3 years a consultant to / member of advisory board of / and/or speaker for Janssen Cilag BV, Eli Lilly, Shire, Novartis, Roche and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties. Barbara Franke received a speaker fee from Merz.
REFERENCES
- American Psychiatric Organisation . Diagnostic and Statistical Manual of Mental Disorders. 5th. American Psychiatric Association; Arlington, VA: 2013. doi:10.1176/appi.books.9780890425596. [Google Scholar]
- American Psychiatric Organisation . Diagnostic and Statistical Manual of Mental Disorders. Fourth. American Psychiatric Association; Arlington, VA: 2000. Text Revision (DSM-IV-TR), 4th ed. doi:10.1176/appi.books.9780890423349. [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. doi:10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. doi:10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Hum. Brain Mapp. Conf. 2009;181 [Google Scholar]
- Biederman J, Faraone SV, Monuteaux MC, Bober M, Cadogen E. Gender effects on attention-deficit/hyperactivity disorder in adults, revisited. Biol. Psychiatry. 2004;55:692–700. doi: 10.1016/j.biopsych.2003.12.003. doi:10.1016/j.biopsych.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am. J. Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69:1160–7. doi: 10.1016/j.biopsych.2011.01.022. doi:10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. doi:10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis J.P. a, Mokrysz C, Nosek B. a, Flint J, Robinson ESJ, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–76. doi: 10.1038/nrn3475. doi:10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–6. doi: 10.1097/01.wnr.0000224769.92454.5d. doi:10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJS, Rotrosen J, Adler LA, Milham MP, Di Martino A, Biswal B, Sonuga-Barke EJS, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. doi:10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: Beyond the prefrontalstriatal model. Trends Cogn. Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. doi:10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabernaud C, Mennes M, Kelly C, Nooner K, Di Martino A, Castellanos FX, Milham MP, Di Martino A, Castellanos FX, Milham MP. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2012;71:434–442. doi: 10.1016/j.biopsych.2011.08.013. doi:10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol. Med. 2013;44:1–13. doi: 10.1017/S0033291713002547. doi:10.1017/S0033291713002547. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. doi:10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: A meta-analysis of 55 fMRI sudies. Am. J. Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. doi:10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder ( ADHD ) and persisting symptoms during tasks of motor inhibition and cognitive switching. J. Psychiatr. Res. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. doi:10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. doi:10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts S. a R.B., Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. doi:10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NUF, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang Y-F, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front. Syst. Neurosci. 2013;6:80. doi: 10.3389/fnsys.2012.00080. doi:10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TGC, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68:1084–91. doi: 10.1016/j.biopsych.2010.07.003. doi:10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol. Med. 2006;36:159–65. doi: 10.1017/S003329170500471X. doi:10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. doi:10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Wiliams GBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) American Psychiatric Press; Washington D.C.: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis i disorders: SCID-I/P (version 2) Biometrics Resarch Department; New York: 1996. [Google Scholar]
- Francx W, Oldehinkel M, Oosterlaan J, Heslenfeld D, Hartman C. a., Hoekstra PJ, Franke B, Beckmann CF, Buitelaar JK, Mennes M. The executive control network and symptomatic improvement in attention-deficit/hyperactivity disorder. Cortex. 2015;73:62–72. doi: 10.1016/j.cortex.2015.08.012. doi:10.1016/j.cortex.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hümmer A, Heine M, Jacob CP, Lesch K-P, Casas M, Ribasés M, Bosch R, Sánchez-Mora C, Gómez-Barros N, Fernàndez-Castillo N, Bayés M, Halmøy A, Halleland H, Landaas ET, Fasmer OB, Knappskog PM, Heister AJGAM, Kiemeney LA, Kooij JJS, Boonstra AM, Kan CC, Asherson P, Faraone SV, Buitelaar JK, Haavik J, Cormand B, Ramos-Quiroga JA, Reif A. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology. 2010;35:656–64. doi: 10.1038/npp.2009.170. doi:10.1038/npp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Hum. Brain Mapp. 1994:56–78. [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. doi:10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. doi:10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernández V, Bosch R, Soliva JC, Rovira M, Bulbena A, Tobeña A, Casas M, Vilarroya O. An independent components and functional connectivity analysis of resting state FMRI data points to neural network dysregulation in adult ADHD. Hum. Brain Mapp. 2014;35:1261–1272. doi: 10.1002/hbm.22250. doi:10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Onnink M, Cools R, Aarts E, Kan C, Arias Vasquez A, Buitelaar J, Franke B. The dopamine transporter haplotype and reward-related striatal responses in adult ADHD. Eur. Neuropsychopharmacol. 2013;23:469–478. doi: 10.1016/j.euroneuro.2012.05.011. doi:10.1016/j.euroneuro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. doi:10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick B. de B., Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. doi:10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum. Brain Mapp. 2010;31:904–16. doi: 10.1002/hbm.21058. doi:10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij JJS. Adult ADHD: Diagnostic Assessment and Treatment. 1st Pearson Assessment and Information BV; Amsterdam: 2010. [Google Scholar]
- Kooij JJS, Buitelaar JK, van den Oord EJ, Furer JW, Rijnders C. a T., Hodiamont PPG. Internal and external validity of attention-deficit hyperactivity disorder in a population-based sample of adults. Psychol. Med. 2005;35:817–827. doi: 10.1017/s003329170400337x. doi:10.1017/S003329170400337X. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PTM, Eickhoff SB, Turner J. a, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011;23:4022–37. doi: 10.1162/jocn_a_00077. doi:10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Dilshad R, Lichtenstein P, Barker ED. Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: genetic effects, family risk and associated psychopathology. J. Child Psychol. Psychiatry. 2011;52:954–63. doi: 10.1111/j.1469-7610.2011.02379.x. doi:10.1111/j.1469-7610.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- Liston C, Cohen MM, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: Pathway to disease or pathological end point? Biol. Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. doi:10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev. Neurosci. 2009;31:36–49. doi: 10.1159/000207492. doi:10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J, Johnson K, Fagan A, Gill M, Meaney J, Frodl T. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA psychiatry. 2013;70:1329–37. doi: 10.1001/jamapsychiatry.2013.2174. doi:10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. doi:10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol. Psychiatry. 2005;57:1224–30. doi: 10.1016/j.biopsych.2004.08.025. doi:10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Onnink AMH, Zwiers MP, Hoogman M, Mostert JC, Kan CC, Buitelaar J, Franke B. Brain alterations in adult ADHD: effects of gender, treatment and comorbid depression. Eur. Neuropsychopharmacol. 2014;24:397–409. doi: 10.1016/j.euroneuro.2013.11.011. doi:10.1016/j.euroneuro.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol. Rev. 2014;24:3–15. doi: 10.1007/s11065-014-9251-z. doi:10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting-state fMRI. NeuroIm. 2015a;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. doi:10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015b;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. doi:10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. doi:10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad A-M, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–52. doi: 10.1016/j.neuropharm.2009.08.013. doi:10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Sato JR, Hoexter MQ, Castellanos XF, Rohde LA. Abnormal Brain Connectivity Patterns in Adults with ADHD: A Coherence Study. PLoS One. 2012;7:e45671. doi: 10.1371/journal.pone.0045671. doi:10.1371/journal.pone.0045671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. doi:10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br. J. Psychiatry. 2009;194:204–11. doi: 10.1192/bjp.bp.107.048827. doi:10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PTM, Miller KL, Glahn DC, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. doi:10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TETE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. doi:10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31:977–86. doi: 10.1016/j.neubiorev.2007.02.005. doi:10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Kessler D, Welsh R, Angstadt M, Liberzon I, Phan KL, Scott C. Distributed effects of methylphenidate on the network structure of the resting brain: a connectomic pattern classification analysis. Neuroimage. 2013;81:213–21. doi: 10.1016/j.neuroimage.2013.05.016. doi:10.1016/j.neuroimage.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci. Lett. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. doi:10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly a M.C., Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler L. a, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J. Neurosci. Methods. 2008;169:249–54. doi: 10.1016/j.jneumeth.2007.11.031. doi:10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, Ryali S, Menon V. Salience network-based classification and prediction of symptom severity in children with autism. JAMA psychiatry. 2013;70:869–79. doi: 10.1001/jamapsychiatry.2013.104. doi:10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Ph D, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, Sc D, Whitfield-Gabrieli S, Vitulano M, Schiller M, Seidman LJ. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am. J. Psychiatry. 2010;167:86–94. doi: 10.1176/appi.ajp.2009.09020249. doi:10.1176/appi.ajp.2009.09020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R. a., Sabuncu MR, Buckner RL. The Influence of Head Motion on Intrinsic Functional Connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. doi:10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, van Tol M-J, Ferrarini L, Milles J, Veltman DJ, Aleman A, van Buchem M. a, van der Wee NJ, Rombouts S. a R.B. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front. Syst. Neurosci. 2010;4:1–10. doi: 10.3389/fnsys.2010.00041. doi:10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wåhlstedt C, Thorell LB, Bohlin G. Heterogeneity in ADHD: neuropsychological pathways, comorbidity and symptom domains. J. Abnorm. Child Psychol. 2009;37:551–64. doi: 10.1007/s10802-008-9286-9. doi:10.1007/s10802-008-9286-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiao Y, Tang T, Wang H, Lu Z. Altered regional homogeneity patterns in adults with attention-deficit hyperactivity disorder. Eur. J. Radiol. 2013:4–9. doi: 10.1016/j.ejrad.2013.04.009. doi:10.1016/j.ejrad.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale (3rd edition) 3rd The Psychological Corporation; London: 1997. [Google Scholar]
- Wilson JL, Jenkinson M, de Araujo I, Kringelbach ML, Rolls ET, Jezzard P. Fast, Fully Automated Global and Local Magnetic Field Optimization for fMRI of the Human Brain. Neuroimage. 2002;17:967–976. doi:10.1006/nimg.2002.1172. [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. doi:10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.