Abstract
Purpose
To analyze the density and morphology of corneal epithelial cells and keratocytes by in vivo confocal microscopy (IVCM) in patients with herpes zoster ophthalmicus (HZO) as associated with corneal innervation.
Design
Prospective, controlled and masked cross-sectional study.
Methods
SETTING: Single-center study. PATIENTS: Thirty eyes with the diagnosis HZO and their contralateral clinically unaffected eyes, 15 eyes of 15 normal controls. INTERVENTION PROCEDURES: In vivo confocal microscopy and corneal esthesiometry of the central cornea. MAIN OUTCOME MEASURES: Changes in morphology and density of the superficial and basal epithelial cells and stromal keratocytes, and correlation with corneal sensation.
Results
The density of superficial epithelial cells in HZO eyes with severe sensation loss (766.5 ± 25.2 cells/mm2) was significantly lower than both healthy control eyes (1450.23 ± 150.83 cells/mm2) and contralateral unaffected eyes (1974.13 ± 298.24 cells/mm2) (P = .003). Superficial epithelial cell size (1162.5 μm2) was significantly larger in HZO eyes with severe loss of sensation, as compared to contralateral (441.46 ± 298.14) or healthy eyes (407.4 ± 47.2mm2; all P < .05). The density of basal epithelial cells, anterior keratocytes, and posterior keratocytes did not show statistical significance between patients, controls, and contralateral unaffected eyes. Changes in superficial epithelial cell density and morphology correlated strongly with corneal sensation.
Conclusions
In vivo confocal microscopy reveals profound HZO-induced changes in the superficial epithelium, as demonstrated by increase in cell size, decrease in cell density, and squamous metaplasia. We demonstrate that these changes strongly correlate with changes in corneal innervation in eyes affected by HZO.
Herpes zoster ophthalmicus (HZO) has numerous corneal presentations, such as epithelial keratitis, necrotizing stromal and immune keratitis, endotheliitis, and neurotrophic keratopathy. Neurotrophic keratopathy is thought to result from loss of corneal sensation owing to denervation, damaged epithelial basement membrane, stromal inflammation, and toxicity from topical medications.1 Neurotrophic keratopathy may present clinically with decreased visual acuity, persistent corneal epithelial defects, stromal opacification, corneal neovascularization, and stromal melts.
Slit-lamp examination has been the gold standard for detecting epithelial defects, stromal edema and infiltration, keratic precipitates, and iritis attributable to HZO, allowing for diagnosis and monitoring of patients. Recently, in vivo confocal microscopy (IVCM) has become an increasingly popular noninvasive device to image the cornea at the cellular and microstructural level in healthy as well as diseased corneas.2–5 Several studies have demonstrated the feasibility of this technology and have analyzed corneas in ocular rosacea,6 Sjögren's syndrome,6,7 meibomian gland dysfunction,6 dry eye disease,8,9 Stevens-Johnson syndrome,10 and keratitis.3,5,11,12 More recently, our group has demonstrated that patients with unilateral HZO demonstrated bilateral subbasal corneal nerve alterations by IVCM.13 Further, the subbasal corneal nerve changes correlated strongly with loss of corneal sensation in the affected eyes of these patients. However, the consequences of altered sensory nerve function on other corneal layers still remain unknown in patients with HZO. Other studies have demonstrated that epithelial turnover is regulated by corneal nerves in several animal models.14,15 Thus, we hypothesized that corneal nerve loss would lead to loss of epithelial cells and keratocytes in patients with HZO. To test this hypothesis, we analyzed the morphology and density of corneal epithelial cells and keratocytes by IVCM in patients with HZO and correlated these findings with corneal innervation in this group.
Methods
This is a prospective, controlled, and masked cross-sectional study. This study was Health Insurance Portability and Accountability Act (HIPAA) compliant and adhered to the tenets of the Declaration of Helsinki. The Massachusetts Eye and Ear Infirmary institutional review board approved the study. All patients gave informed consent for this research after a detailed explanation of the nature of the study.
Subjects were recruited from the Cornea Service of the Department of Ophthalmology of the Massachusetts Eye & Ear Infirmary, Boston, Massachusetts, between 2006 and 2008. None of the patients was immunocompromised and all eyes were inactive, with no signs of active keratitis. Subjects with a history of ocular trauma, ocular surgery, contact lens use, or diabetes were excluded from the study. Prior to recruitment into the study, all patients and healthy subjects underwent a complete baseline ophthalmologic examination, including visual acuity measurement, anterior segment evaluation with a slit-lamp biomicroscope, fundus examination, and intraocular pressure (IOP) measurement by applanation tonometry.
Central corneal sensation was measured bilaterally with a Cochet-Bonnet esthesiometer (Luneau Ophthalmologie, Chartres, France). The corneal sensation in the unaffected eye was measured first. The esthesiometer stimulates the cornea by a retractable monofilament nylon (6 cm length, 0.12 mm diameter). It was shortened in steps of 1.0 cm if a positive response was not obtained, or advanced by 0.5 cm if a positive response was obtained. The longest final filament length resulting in a positive response was considered the corneal sensitivity threshold. This threshold was verified twice in our study.
A total of 30 eyes of 30 patients with a diagnosis of HZO, as well as the contralateral, clinically unaffected eyes, were included in this study. All patients have had a history of epithelial herpetic keratitis with no stromal involvement. Fifteen eyes of 15 normal volunteers comprised the control group. HZO keratitis patients were grouped into normal (>5.5 cm), mild (>2.5–5.5 cm), and severe (<2.5 cm) loss of corneal sensation, according to the corneal sensitivity threshold measurements, and results were correlated to confocal microscopic findings. Because contralateral eyes did not show significant differences between HZO subgroups, they were kept together as 1 combined group. No fluorescein was used prior to IVCM during the study. All subjects underwent bilateral examination of the central cornea with a slit-scanning confocal microscope (Confoscan 4; Nidek Technologies, Gamagori, Japan). In order to ensure masking, a randomly generated code was assigned to each patient at the time of scanning. The coding key was kept in a separate file by a technician, who was masked. Therefore, during image analysis, the observers (P.H. and M.H.D.) were masked to patient name, diagnosis, and severity. The microscope was equipped with a 40×/0.75 Zeiss Acroplan numerical aperture immersion objective lens. Topical 0.5% proparacaine eyedrops (Alcaine; Alcon, Fort Worth, Texas, USA) were instilled in both eyes prior to confocal scans, and 0.3% hypromellose (GenTeal gel; Novartis, East Hanover, New Jersey, USA) was used as a coupling medium for the objective lens. The lens was manually advanced until the gel contacted the central surface of the cornea. Three hundred fifty images per scan in every 7 μm were obtained at a speed of 25 frames per second. Moreover, a second scan was obtained for the anterior cornea, obtaining sections every 3 μm. Each image represented a coronal section of 460 × 345 μm, with a minimum axial step of 1 μm, magnification of ×500, and lateral resolution of 1 μm/pixel. A total of 4–8 scans were obtained for each cornea by the same experienced operator (P.H.) in all subjects, depending on full-thickness or anterior scan mode.
The mean of the 3 representative images per layer was used for analysis for each eye. The superficial epithelial layer, basal epithelial layer, subbasal corneal nerves, anterior stromal keratocytes, and posterior stromal keratocytes were included in the analysis. Cell counts were performed manually by Confoscan 4 NAVIS analysis software (Nidek Technologies). This was achieved by marking each clearly defined cell or nucleus in a predefined rectangular frame using the full-frame size on the computer screen. Two independent masked experienced observers counted the cells to determine superficial and basal epithelial cell density, as well as anterior and posterior keratocyte density. Superficial epithelial cells become highly reflective before desquamation.16 To assess epithelial damage, the percentage of highly reflective superficial epithelial cells was calculated by dividing highly reflective cells per frame by total number of superficial epithelial cells per frame and multiplying by 100. For epithelial cell size, 2 masked observers defined the borders of superficial epithelial cells, with Confoscan NAVIS software (Nidek Technologies) calculating epithelial cell area. The areas of superficial epithelial cells were averaged per frame. Cellular changes of the epithelium and stroma were correlated to corneal sensation.
Statistics
Statistical analysis was performed by Student's t test, analysis of variance (ANOVA), and Pearson correlation coefficient. P values less than .05 were considered statistically significant. Analyses were performed with SAS software version 9.2 (SAS Institute Inc, Cary, North Carolina, USA).
Results
Patient Characteristics and Study Groups
Thirty eyes of 30 patients with HZO and 30 contralateral clinically unaffected eyes were included in the study. Fifteen eyes of 15 normal volunteers served as control group. Patient demographics and healthy subjects' characteristics are shown in Table 1. All eyes with HZO were subcategorized into 3 groups based on central corneal sensation as follows: normal sensation, mild sensation loss, and severe sensation loss (described in the methods section). These groups were used in order to correlate corneal sensation to IVCM findings. The correlation of corneal sensation loss to IVCM parameters of superficial epithelium, basal epithelium, and anterior and posterior keratocytes for all groups is presented in Table 2. Demonstrative IVCM images for all layers analyzed for patients with HZO and controls are shown in Figure 1.
Table 1. Demographic Data of Normal Controls and Patients With Herpes Zoster Ophthalmicus.
| Controls | Herpes Zoster Ophthalmicus | |
|---|---|---|
| No. of patients | 15 | 30 |
| Age (mean ± SD; y) | 59 ± 17 | 58 ± 17 |
| Sex (male/female) | 8/7 | 14/16 |
| Corneal sensation (mean ± SD; cm) | 6.0 ± 0 | 3.4 ± 2.3 |
| Disease duration (mean ± SD; y) | - | 5.8 ± 6.7 |
| No. of episodesa (mean ± SD; n) | - | 2.1 ± 1.3 |
Episodes reflect both primary and secondary immune keratitis.
Table 2. Corneal Epithelial Cell Density, Morphology, and Corneal Sensation Parameters in Herpes Zoster Keratitis, Contralateral Clinically Unaffected Eyes, and Control Groups.
| Superficial Epithelial Cell Density | Basal Epithelium Cell Density | Anterior Keratocyte Density | Posterior Keratocyte Density | Superficial Epithelial Cell Size | Hyperreflective Superficial Epithelial Cells | |
|---|---|---|---|---|---|---|
| Normal corneal sensation | 1754.23 ± 238.46 cells/mm2 | 4950.2 ± 250.3 cells/mm2 | 629.3 ± 381.4 cells/mm2 | 827.5 ± 227.5 cells/mm2 | 466.68 ± 328.86 μm2 | 30.2% ± 30.55% |
| Mild corneal sensation loss | 946.67 ± 73.18 cells/mm2a | 4521.1 ± 201.02 cells/mm2 | 782.2 ± 58.88 cells/mm2 | 641.22 ± 13.59 cells/mm2 | 902.2 ±216.02 μm2a | 58.3% ± 11.79%a |
| Severe corneal sensation loss | 766.5 ± 25.2 cells/mm2a | 4412.5 ± 120.2 cells/mm2 | 516.5 ± 221.2 cells/mm2 | 586.01 ± 192.84 cells/mm2 | 1162.5 ± 198.04 μm2a | 62.5% ± 12.99%a |
| Contralateral eyes | 1974.13 ± 298.24 cells/mm2 | 5278.32 ± 432.96 cells/mm2 | 822.68 ± 354.84 cells/mm2 | 758.9 ± 276.32 cells/mm2 | 441.46 ± 298.14 μm2a | 10.72% ± 10.65% |
| Controls | 2435.23 ± 224.3 cells/mm2 | 5962.5 ± 83.21 cells/mm2 | 901.2 ± 75.56 cells/mm2 | 603.32 ± 55.34 cells/mm2 | 407.4 ± 47.2 μm2 | 0% |
Values reported as mean ± standard deviation.
Statistically significant (P < .05) compared to controls.
Figure 1.
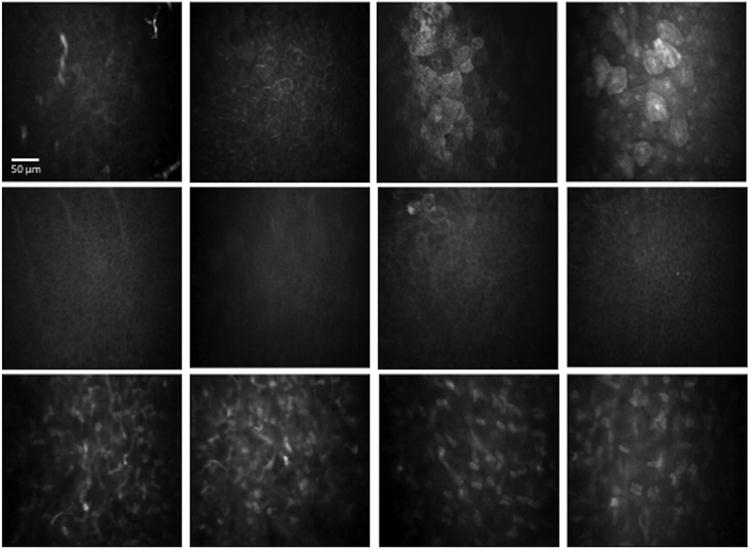
In vivo confocal microscopy images at the level of the corneal epithelium and stroma in patients with herpes zoster ophthalmicus (HZO). (Row 1) Superficial epithelium. Column 1: Normal cornea. Column 2: HZO, normal sensation; no increase in cell size; no decrease in density; prominent nucleoli and bright cell borders are present. Column 3: HZO, mild sensation loss; increase in cell size and decrease in density; further increase in hyperreflectivity. Column 4: HZO, severe sensation loss; very large cells; lower cell density; highly reflective cells. (Row 2) Basal epithelium, no significant changes with loss of sensation. Column 1: Normal cornea. Column 2: HZO, normal sensation. Column 3: HZO, mild sensation loss. Column 4: HZO, severe sensation loss. (Row 3) Corneal stromal keratocytes. Column 1: Normal cornea, anterior stroma. Column 2: HZO, severe sensation loss, anterior stroma. Column 3: normal cornea, posterior stroma. Column 4: HZO, severe loss of sensation, posterior stroma.
Superficial Epithelial Cell Density
We found a significant and gradual decrease in the density of superficial epithelial cells in HZO eyes, with 1754.23 ± 238.46 cells/mm2 in HZO eyes with normal sensation, 946.67 ± 73.17 cells/mm2 in HZO eyes with mild sensation loss, and 766.5 ± 25.2 cells/mm2 in eyes with severe sensation loss as compared to 1974.13 ± 298.24 cells/mm2 in contralateral eyes (P = .01) and 2435.23 ± 224.3 cells/mm2 in control eyes (P = .003) (Table 2 and Figure 2). Clinically unaffected contralateral eyes did not show any significant epithelial or stromal changes as compared to controls.
Figure 2.
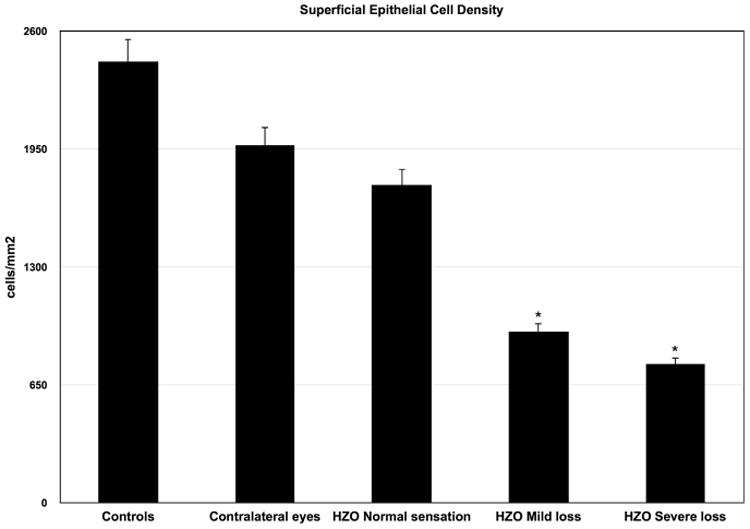
Superficial epithelial cell density in herpes zoster ophthalmicus (HZO) according to corneal sensation loss classification. Error bars represent standard error from the mean. *P < .05 compared to control group. Statistical analysis by ANOVA (analysis of variance between groups). As the corneal sensation decreases in patients with HZO, the superficial epithelial cell density decreases.
Superficial Epithelial Cell Size
There was a gradual increase in superficial epithelial cell size with loss of corneal sensation in HZO eyes. Superficial epithelial cell size was almost 3.0-fold larger in HZO eyes with severe loss of sensation (1162.5 ± 198.04 μm2) as compared to contralateral (441.46 ± 298.14; P = .012) or normal eyes (407.4 ± 47.2 μm2; P = .008) (Table 2 and Figure 3).The IVCM scans in the mild sensation loss group also revealed a significant increase in mean superficial epithelial cell size (902.2 ± 216.02 μm2), as compared with the contralateral eyes (441.46 ± 298.14; P = .03) and normal control group (407.4 ± 47.2 μm2; P = .001), while HZO eyes with normal sensation did not demonstrate statistically significant changes from normal controls and contralateral eyes (both P > .05) (Table 2).
Figure 3.
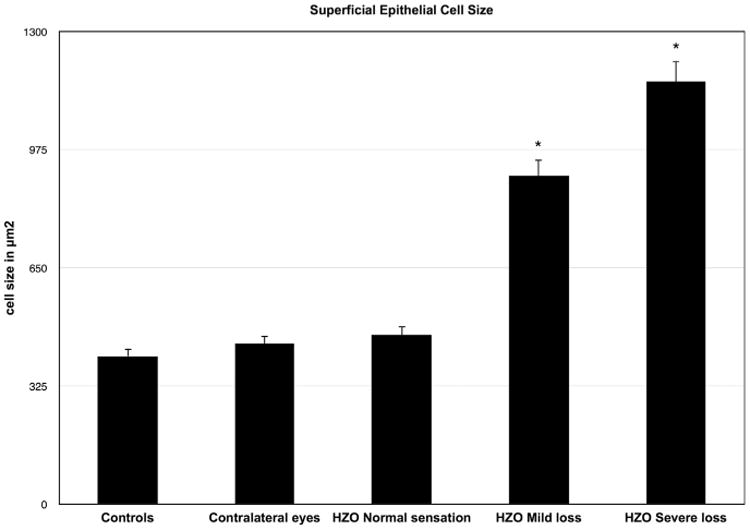
Superficial epithelial cell size in herpes zoster ophthalmicus (HZO) according to corneal sensation loss classification. Error bars represent standard error from the mean. *P < .05 compared to control group. Statistical analysis by ANOVA (analysis of variance between groups). As the corneal sensation decreases in patients with HZO, the superficial epithelial cell size increases significantly.
Hyperreflective Superficial Epithelial Cells
Apart from cell density and size, we looked for other morphologic epithelial characteristics such as reflectivity of cell nucleoli. There was minimal reflectivity of cell nucleoli in controls or contralateral eyes. However, in eyes with HZO, we consistently found increased reflectivity of superficial epithelial cell nucleoli. Another striking finding was the presence of hyporeflective rings around the hyperreflective cell nucleoli in patients with severe sensation loss. Moreover, we observed bright cell borders and increased cell hyperreflectivity in HZO eyes with normal sensation. Interestingly, these findings were more pronounced in patients with mild and severe loss of sensation. A significant number of hyperreflective desquamating superficial epithelial cells were present in HZO eyes with normal sensation (30.2% ± 30.55%, P = .09) and mild (58.3% ± 11.79%, P = .002) and severe (62.5% ± 12.99%, P = .0003) loss of sensation but were absent in controls or minimal (10.72% ± 10.65%) in contralateral eyes (Table 2 and Figure 4).
Figure 4.
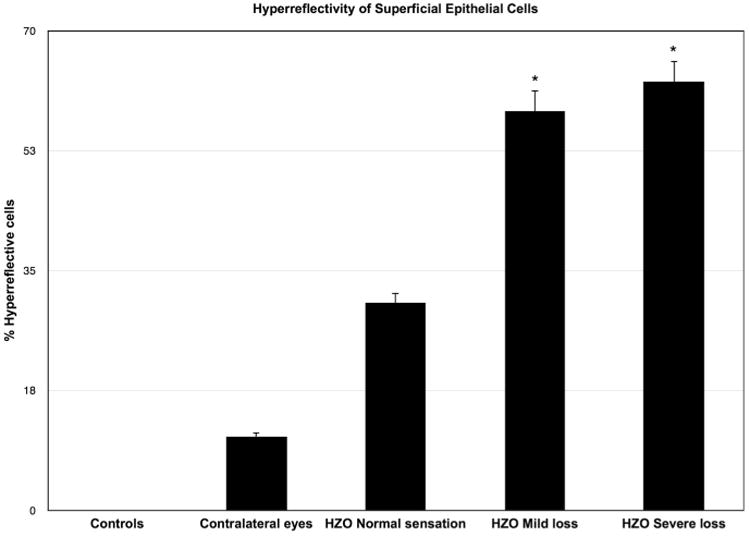
Hyperreflective desquamating superficial epithelial cells in herpes zoster ophthalmicus (HZO) according to corneal sensation loss classification. Error bars represent standard error from the mean. *P < .05 compared to control group. Statistical analysis by ANOVA (analysis of variance between groups). As the corneal sensation decreases, the number of hyperreflective superficial epithelial cells increases significantly.
Basal Epithelial Cell And Keratocyte Density
Although a decreased density in basal epithelial cells and anterior keratocytes was noted, the density of basal epithelial cells, anterior keratocytes, and posterior keratocytes did not show statistical significance between patients, contralateral eyes, and controls, despite the demonstration of a bilateral loss of subbasal nerve plexus, which we have previously described.13 When we compared the clinical location of the herpetic lesion analyzing epithelial cell and keratocyte alterations between the central and peripheral herpetic lesions, no statistical difference was observed in the patients. Finally, in order to confirm the appropriate sample size or our study, we performed power calculations, which demonstrated a power of >91% for all statistically significant variables of the superficial epithelium.
Correlation Between Number of Disease Recurrences, Duration, and in Vivo Confocal Microscopic Findings
There was no significant correlation of the number of superficial epithelial cells, superficial epithelial cell size, hyperreflective superficial epithelial cells, and basal epithelial cell and keratocyte density with the number of recurrences or duration of the disease (P > .05).
Discussion
In vivo confocal microscopy provided us with new insights about our understanding of corneal physiology and homeostasis.2–4,9,17 IVCM allowed us to study corneal epithelial cell morphologic characteristics and nerve density in health and in various corneal diseases. This relatively new imaging tool is noninvasive, precise, and much more reproducible as compared to slit-lamp examination.
In the present study, we found that changes in superficial epithelial cell density and morphology correlated strongly with corneal sensation. This finding is in line with our previous findings that showed compromised neural integrity in HZO.13 The corneal epithelium has important homeostatic functions in maintaining corneal transparency. Any injury to the corneal epithelium and/or corneal nerves caused by physical or microbial/viral insults causes the activation of stromal fibroblasts and inflammatory cell infiltration, leading to stromal edema and, eventually, loss of corneal transparency.18 Although there is no study evaluating corneal epithelial changes by means of IVCM in HZO, there are several studies that were performed in various corneal diseases such as in dry eye disease and herpes simplex virus (HSV) keratitis.12,19–21 Our group recently showed that HSV keratitis induces a significant increase in corneal epithelial cell size, a decrease in cell density, and squamous metaplasia. We also demonstrated that these changes strongly correlate with alterations in the subbasal nerve plexus.21 Interestingly, the epithelial changes observed in this study are more severe than in our group's previous study, which was performed in patients with HSV keratitis. The superficial epithelial cell size is significantly larger in HZO eyes as compared to HSV eyes. This difference may be explained by the differential pattern of corneal nerve loss in HZO and HSV. Nagasato and associates22 recently showed that the subbasal nerve destruction did not seem remarkable in the endothelial type of HSV keratitis, while nerve alterations were shown in HSV keratitis with epithelial and stromal involvement. Additional studies are required to study the underlying mechanisms in both HSV keratitis and HZO. Although their study was performed in HSV keratitis patients, Rosenberg and associates12 reported enlarged corneal epithelial cells by IVCM, similar to our study. Prominent large nuclei and size changes in epithelial cells have been seen in conditions such as rapid cellular turnover and regenerative atypia as during wound healing.19 In dry eye conditions, where subbasal nerve loss is apparent, IVCM analysis showed hyperreflectivity of the nucleus and a decrease in the nucleocytoplasmic ratio in the superficial corneal epithelial cells.10,20 Moreover, the mean superficial and intermediate epithelial cell densities were found to be significantly lower than in healthy subjects.9 These findings had been speculated to be attributable to decreased subbasal nerve density, a common finding in dry eye patients.7
Another interesting finding in our study was the significantly increased number of hyperreflective corneal epithelial cells in HZO patients. In our recent study conducted on patients with HSV keratitis, we observed similar alterations of the superficial epithelial cells. However, the frequency was much lower as compared to patients with HZO. Interestingly, Hovakimyan and associates23 reported hyperreflectivity of superficial epithelial cells 2 weeks after collagen cross-linking, which is known to induce inflammation in the corneal stroma as well as in the epithelium. This type of hyperreflective corneal epithelial cell was also found in patients with pterygium.24 Fuchs' patches were observed as islets of hyperreflective polygonal cells in front of the pterygium head with blurred limits. Given the strong correlation of superficial epithelial cell parameters with corneal sensation and innervation in our study, our findings are suggestive that the changes in the superficial epithelium may be direct and/or indirect results of the diminished subbasal nerve plexus in HZO patients. Diminishment in corneal nerve and function in HZO patients commonly results in neurotrophic keratopathy, a condition that may be very difficult to treat.1,25 Thus, IVCM may provide a valuable tool for monitoring patients with HZO and potentially predicting the risk for neurotrophic keratopathy or clinically apparent epithelial disease. Because neurotrophic keratopathy initially lacks signs and symptoms and may rapidly progress to corneal perforation, quantification of epithelial cell density and morphology by IVCM may serve as a tool to detect disease early on. However, prospective longitudinal studies are required to assess this role.
In the present study, we also compared affected HZO eyes with unaffected contralateral eyes and healthy controls. We demonstrated that the contralateral epithelium is not affected, although the subbasal corneal nerve plexus is affected bilaterally in apparently unilateral HZO13 and HSV.3 Moreover, our group has recently developed a new animal model to further study bilateral nerve alterations in unilateral neurotrophic keratopathy.26 In this animal model, we found that the subbasal nerve density in the contralateral nonsurgical eyes also decreased significantly in the center as early as day 1 after unilateral ciliary nerve axotomy. Although the study was performed in an animal model, this finding further supports our hypothesis that subbasal corneal nerve plexus is affected bilaterally in clinically unilateral HZO keratitis.
We did not find any changes in superficial epithelial cell morphology, density, and corneal sensation in contralateral eyes of our patients, despite reduced nerve density.3,13 We had demonstrated that corneal nerve density higher than 1032 μm/frame is sufficient for a patient to have near-normal sensation.13 Thus, it seems that this nerve density is sufficient to maintain epithelial integrity as well. These findings also explain why the previous study by Rosenberg and associates12 did not find a difference in subbasal nerve and epithelial changes between the affected and unaffected contralateral eyes.
The corneal epithelial turnover is controlled, in part, by sensory nerves.27 Neuropeptides such as substance P, nerve growth factor (NGF), vasoactive intestinal peptide, and glucagon-like peptides15,27–30 secreted by these nerve endings play a key role in cell proliferation and differentiation in vitro.14 Partial or complete loss of corneal sensation or denervation leads to impaired corneal epithelial homeostasis owing to the lack of these neuropeptides. It has recently been shown that denervation induces apoptosis of corneal epithelial cells31 and reduction of epithelial cell mitosis.32 Corneal wound healing and permeability are also effected.27 After sensory denervation, an inflammatory response is triggered.27,33 Superficial epithelial cells start losing their tight junctions and attach weakly to each other.34 Further, inflammation can activate keratocytes, which synthesize NGF and other nerve growth factors.35 Activated keratocytes can then stimulate epithelial cells to migrate and proliferate, leading to changes in their shape and size owing to modifications in actin cytoskeleton.27,33,36–38 Thus, it is conceivable that enlarged superficial epithelial cells as seen by IVCM could be representative of cells in a particular state of metabolic activation induced by proinflammatory cytokines or as a result of apoptosis of neighboring cells. Moreover, the reduced number of superficial epithelial cells and the increased hyperreflectivity may be attributable to extensive apoptotic response widely seen in denervated corneas.31 On the other hand, a proliferative response is triggered to balance apoptosis in denervated corneas. This response may explain why we did not see a decrease in the basal epithelial layer. It is thus likely that decreased neural innervation in the cornea may lead to changes in neuropeptide levels, resulting in epithelial cell changes detected by IVCM in our HZO patients.
There are limitations to our study that we would like to point out. First, we were only able to evaluate the central corneal epithelium and these findings cannot necessarily be extrapolated to the peripheral cornea. Second, the Cochet-Bonnet esthesiometer only measures mechanical nociceptors;39 however, there are also thermal receptors in the cornea, which cannot be measured with the Cochet-Bonnet esthesiometer. Gallar and associates40 sophisticatedly demonstrated that HSV keratitis disrupts mechanical nociceptors, as well as heat and cold thermoreceptors.
In conclusion, IVCM is a promising tool to study epithelial cell morphology. An objective quantitative evaluation of epithelial cells can provide valuable clues to detect sub-clinical neurotrophic keratopathy before intractable clinical findings occur. Moreover, there is a need for objective IVCM evaluation of treatment response through quantification of cellular changes in the cornea, since new treatment options are being investigated for neurotrophic keratopathy.
Acknowledgments
Funding support: NIH K08-EY020575 (Bethesda, Maryland, USA) (P.H.), NIH K24-EY019098 (Bethesda, Maryland, USA) (R.D.), Falk Medical Research Foundation (Chicago, Ilinois, USA) (P.H.), Research to Prevent Blindness Career Development Award (New York, New York, USA) (P.H.), Johnstone Research Foundation (Chicago, Illinois, USA) (D.P.L.), Stevens Fund (Boston, Massachusetts, USA) (D.P.L.), TUBITAK 111S437 (Ankara, Turkey) (A.S.). The funding organizations had no role in the design or conduct of this research. All authors attest that they meet the current ICMJE requirements to qualify as authors.
Biography

Dr Pedram Hamrah, a clinician-scientist, is an Assistant Professor of Ophthalmology with Harvard Medical School. He is the Director of Ocular Surface Imaging Center, is on the full-time staff of the Cornea and Refractive Surgery Service at the Massachusetts Eye and Ear Infirmary. He was the former Henry Allen Cornea Scholar and is an assistant scientist at the Schepens Eye Research Institute, where he runs an NIH-funded laboratory.
Footnotes
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest.
Financial disclosures: Pedram Hamrah: consultancy (Allergan, Alcon, Valeant, Revision Optics, Jade Therapeutics, GlaxoSmith-Kline); grants/grants pending (Allergan, GlaxoSmithKline, Alcon, Dompe, NIH); royalties (UpToDate); travel/accommodations/meeting expenses unrelated to activities listed (Allergan). Afsun Sahin: grants/grants pending (TUBITAK 111S437). Reza Dana: consultancy (Alcon, Cambium, Eleven Biotherapeutics, Gurnet Point Capital, NovaBay, Rigel); grants/grants pending (NEI EY012963, EY020889); stock/stock options (Eleven Biotherapeutics). Deborah-Pavan Langston: consultancy (Valeant Inc).
References
- 1.Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 Suppl):S3–12. doi: 10.1016/j.ophtha.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Efron N, Perez-Gomez I, Mutalib HA, Hollingsworth J. Confocal microscopy of the normal human cornea. Cont Lens Anterior Eye. 2001;24(1):16–24. doi: 10.1016/s1367-0484(01)80005-9. [DOI] [PubMed] [Google Scholar]
- 3.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillenaar T, Weenen C, Wubbels RJ, Remeijer L. Endothelial involvement in herpes simplex virus keratitis: an in vivo confocal microscopy study. Ophthalmology. 2009;116(11):2077–2086.e1–2. doi: 10.1016/j.ophtha.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Martone G, Alegente M, Balestrazzi A, et al. In vivo confocal microscopy in bilateral herpetic keratitis: a case report. Eur J Ophthalmol. 2008;18(6):994–997. doi: 10.1177/112067210801800622. [DOI] [PubMed] [Google Scholar]
- 6.De Nicola R, Labbe A, Amar N, Dupas B, Baudouin C. In vivo confocal microscopy and ocular surface diseases: anatomical-clinical correlations. J Fr Ophtalmol. 2005;28(7):691–698. doi: 10.1016/s0181-5512(05)80980-5. [DOI] [PubMed] [Google Scholar]
- 7.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren's syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48(5):2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Chen Q, Chen W, et al. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011;118(5):902–907. doi: 10.1016/j.ophtha.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 9.Erdelyi B, Kraak R, Zhivov A, Guthoff R, Nemeth J. In vivo confocal laser scanning microscopy of the cornea in dry eye. Graefes Arch Clin Exp Ophthalmol. 2007;245(1):39–44. doi: 10.1007/s00417-006-0375-6. [DOI] [PubMed] [Google Scholar]
- 10.Vera LS, Gueudry J, Delcampe A, et al. In vivo confocal microscopic evaluation of corneal changes in chronic Stevens-Johnson syndrome and toxic epidermal necrolysis. Cornea. 2009;28(4):401–407. doi: 10.1097/ICO.0b013e31818cd299. [DOI] [PubMed] [Google Scholar]
- 11.Gabison EE, Alfonsi N, Doan S, et al. Archipelago keratitis: a clinical variant of recurrent herpetic keratitis? Ophthalmology. 2007;114(11):2000–2005. doi: 10.1016/j.ophtha.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg ME, Tervo TM, Muller LJ, Moilanen JA, Vesaluoma MH. In vivo confocal microscopy after herpes keratitis. Cornea. 2002;21(3):265–269. doi: 10.1097/00003226-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120(1):40–47. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neuro-trophic influences on corneal epithelial cells. Exp Eye Res. 1994;59(5):597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- 15.Klyce SD, Beuerman RW, Crosson CE. Alteration of corneal epithelial ion transport by sympathectomy. Invest Ophthalmol Vis Sci. 1985;26(4):434–442. [PubMed] [Google Scholar]
- 16.Wiegand W, Thaer AA, Kroll P, Geyer OC, Garcia AJ. Optical sectioning of the cornea with a new confocal in vivo slit-scanning videomicroscope. Ophthalmology. 1995;102(4):568–575. doi: 10.1016/s0161-6420(95)30981-5. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20(4):374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Saito J, Yanai R, et al. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003;22(2):113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 19.Alomar TS, Nubile M, Lowe J, Dua HS. Corneal intraepithelial neoplasia: in vivo confocal microscopic study with histopathologic correlation. Am J Ophthalmol. 2011;151(2):238–247. doi: 10.1016/j.ajo.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45(9):3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 21.Hamrah P, Sahin A, Dastjerdi MH, et al. Cellular changes of the corneal epithelium and stroma in herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2012;119(9):1791–1797. doi: 10.1016/j.ophtha.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagasato D, Araki-Sasaki K, Kojima T, Ideta R, Dogru M. Morphological changes of corneal subepithelial nerve plexus in different types of herpetic keratitis. Jpn J Ophthalmol. 2011;55(5):444–450. doi: 10.1007/s10384-011-0068-5. [DOI] [PubMed] [Google Scholar]
- 23.Hovakimyan M, Guthoff R, Reichard M, et al. In vivo confocal laser-scanning microscopy to characterize wound repair in rabbit corneas after collagen cross-linking. Clin Experiment Ophthalmol. 2011;39(9):899–909. doi: 10.1111/j.1442-9071.2011.02634.x. [DOI] [PubMed] [Google Scholar]
- 24.Labbe A, Gheck L, Iordanidou V, et al. An in vivo confocal microscopy and impression cytology evaluation of pterygium activity. Cornea. 2010;29(4):392–399. doi: 10.1097/ICO.0b013e3181bd44ce. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman SC. Anterior segment complications of herpes zoster ophthalmicus. Ophthalmology. 2008;115(2 Suppl):S24–32. doi: 10.1016/j.ophtha.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Turhan A, Harris DL, et al. Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy. PLoS One. 2013;8(8):e70908. doi: 10.1371/journal.pone.0070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69(1):196–201. doi: 10.1016/0014-4886(80)90154-5. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura M, Chikama T, Nishida T. Synergistic effect with Phe-Gly-Leu-Met-NH2 of the C-terminal of substance P and insulin-like growth factor-1 on epithelial wound healing of rabbit cornea. Br J Pharmacol. 1999;127(2):489–497. doi: 10.1038/sj.bjp.0702550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347–1351. doi: 10.1016/s0161-6420(00)00163-9. discussion 1351–1352. [DOI] [PubMed] [Google Scholar]
- 30.Lambiase A, Manni L, Bonini S, et al. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41(5):1063–1069. [PubMed] [Google Scholar]
- 31.Ferrari G, Chauhan SK, Ueno H, et al. A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain. Invest Ophthalmol Vis Sci. 2011;52(5):2532–2539. doi: 10.1167/iovs.10-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigelman S, Friedenwald JS. Mitotic and wound-healing activities of the corneal epithelium; effect of sensory denervation. AMA Arch Ophthalmol. 1954;52(1):46–57. doi: 10.1001/archopht.1954.00920050048005. [DOI] [PubMed] [Google Scholar]
- 33.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood) 2001;226(7):653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 34.Araki K, Ohashi Y, Kinoshita S, et al. Epithelial wound healing in the denervated cornea. Curr Eye Res. 1994;13(3):203–211. doi: 10.3109/02713689408995778. [DOI] [PubMed] [Google Scholar]
- 35.Tuominen IS, Konttinen YT, Vesaluoma MH, et al. Corneal innervation and morphology in primary Sjogren's syndrome. Invest Ophthalmol Vis Sci. 2003;44(6):2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 36.Beuerman RW, Thompson HW. Molecular and cellular responses of the corneal epithelium to wound healing. Acta Ophthalmol Suppl. 1992;(202):7–12. doi: 10.1111/j.1755-3768.1992.tb02161.x. [DOI] [PubMed] [Google Scholar]
- 37.Crosson CE, Klyce SD, Beuerman RW. Epithelial wound closure in the rabbit cornea. A biphasic process. Invest Ophthalmol Vis Sci. 1986;27(4):464–473. [PubMed] [Google Scholar]
- 38.Beuerman RW, Schimmelpfennig B, Burstein N. Anatomy of the denervated corneal epithelium. Invest Ophthalmol Vis Sci. 1979;18:126. [Google Scholar]
- 39.Patel DV, Tavakoli M, Craig JP, Efron N, McGhee CN. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea. 2009;28(7):735–740. doi: 10.1097/ICO.0b013e318193e0e3. [DOI] [PubMed] [Google Scholar]
- 40.Gallar J, Tervo TM, Neira W, et al. Selective changes in human corneal sensation associated with herpes simplex virus keratitis. Invest Ophthalmol Vis Sci. 2010;51(9):4516–4522. doi: 10.1167/iovs.10-5225. [DOI] [PubMed] [Google Scholar]


