Abstract
The hemorrhagic diseases are characterized by bleeding which can vary considerably according to their severity. The von Willebrand disease (VWD) is the most frequent hereditary hemorrhagic disease and the prevalence of clinically significant disease is probably closer to 1:1000, being an extremely heterogeneous and complex disorder that is related to the deficiency in concentration, structure or function of von Willebrand factor (VWF). The VWD is divided into type 1, with partial deficiency of the VWF, type 2, with qualitative defects in the molecule with four subdivisions, and type 3, with very low or undetectable levels of plasma and platelet VWF and ristocetin cofactor activity. The laboratory diagnosis of VWD is complex. Specific tests that assess the functionality and concentrations of the VWF and FVIII are needed. The routine tests are the bleeding time, the activated partial thromboplastin time and the platelet count, however, singly, they may not suggest the diagnosis of VWD, requiring further specific tests, such as VWF function evaluation through its ristocetin cofactor assay (VWF:RCo), VWF protein concentration immunoassay (VWF:Ag), the factor VIII coagulation assay (FVIII:C), VWF binding to immobilized collagen (VWF:CB), ristocetin-induced platelet aggregation (RIPA), VWF multimers patterns, factor VIII binding of immobilized VWF (VWF:FVIIIB), among others. From the moment the diagnosis is confirmed, the appropriate treatment for each patient is sought, with the purpose of increasing plasma concentrations of the deficient protein, both in bleeding episodes, as for invasive procedures. Although diagnosis facilitates treatment other approach in the present scenario is prenatal diagnosis which, is the need of the hour.
Keywords: Von Willebrand disease, Von Willebrand factor, Platelet count, Platelet aggregation, Ristocetin cofactor
Introduction
Hemostasis maintains fluidity of the blood not allows bleeding or thrombosis [1]. A bleeding disorder usually happens when there is defective hemostasis, meaning that clotting is compromised [2].
Bleeding disorders can be hereditary or acquired. They are characterized by bleedings of varying intensity related to hematologic diseases or systemic situations [1]. Von Willebrand disease (VWD) is the most frequent hereditary hemorrhagic disease and its prevalence varies between 0.8 and 2 % in the population, according to studies of population screening [3]. The prevalence of clinically significant disease is probably closer to 1:1000, being an extremely heterogeneous and complex disorder that is related to deficiency in concentration, structure or function of von Willebrand factor (VWF) [4].
VWF is a multimeric glycoprotein structure, synthesized by endothelial cells in Weibel–Palade bodies and megakaryocytes, it’s found in plasma and in the platelets. [5]. VWF is formed by identical sub-units, which have binding sites for both platelet glycoproteins and collagen [6].
VWF links the platelets to the lesioned subendothelium through its binding to the collagen, this is the function of VWF in the platelet adhesion which triggers the activation of the platelets with consequent aggregation. Another important function is the binding to the plasmatic FVIII, this binding prevents the degradation of the FVIII and extends the plasmatic half-life [3].
In 1926, Erich von Willebrand described the hereditary form of VWD for the first time. At the time, the hypothesis sustained that it was a problem in platelet function or a vascular modification [7]. Years later, it was found that a mutation in the gene responsible for the coding of VWF occurs and it is located in the chromosome 12 short arm [1]. An update classification system has been proposed in 2006, from then the VWD was classified into three major categories: type 1, partial deficiency in VWF concentration; type 2, qualitative defects in VWF’s molecule and it is sub-divided into 2A, 2B, 2M, 2N; and type 3, that shows total deficiency in the concentration of VWF [8].
The qualitative defects sub-divided into four types are described as: type 2A, a variant with altered platelet function and is associated with the loss of VWF high molecular weight multimers; in type 2B, VWF has more affinity to the platelet membrane’s receptor Ib; the type 2M shows alterations in the platelet function without being associated to the loss of high molecular weight multimers; and the type 2N has a smaller affinity to FVIII [9].
The type 2B is responsible for less than 5 % of VWD cases [10]. In these cases, the mutations in a single amino acid in the gene A1 domain result in an increase of the von Willebrand factor binding to the GP-lb platelet receptors, causing the increase in the depuration of platelets and the loss of high molecular weight multimers [10].
In the disease’s type 3, there’s a complete deficiency of VWF, with a reduction in FVIII, which causes severe clinical symptoms [11]. Some patients with type 3 develop alloantibodies against VWF, because of several infusions with the factor concentrates, causing the lack of response to the infusion treatment, including the possibility of allergic reactions, becoming life threatening by anaphylactic reactions [3].
Although it is the most frequent hereditary coagulopathy, usually it is not recognized because the type 1 has a larger frequency and causes discrete clinical manifestation [12]. The laboratory diagnosis of VWD is complex, and demands patience and perseverance from the doctor and the patient [3]. There’s the possibility that some tests show normal results, therefore specific exams that evaluate the function and VWF and FVIII levels are needed [13].
Mild cases are usually asymptomatic and cause superficial bleedings on the skin or mucous membranes [7]. Yet, in most serious cases, the patient might show bruising, hemarthrosis and positive family history [9].
The occurrence of bleedings in soft tissues and hemarthrosis is rare in VWD, except in type 3 [14]. In patients with skin and mucous membrane bleeding, positive family history and exams that prove quantitative or qualitative defect of VWF, the diagnosis is closed.
A large number of mutations within VWF gene have now been identified. Previous definitions of VWD required the presence of VWF gene mutations. However, because of the genetic complexity of VWD and the practical considerations of VWF gene sequencing is most clinical setting, a VWF gene mutation is no longer included in the criteria for the diagnosis of VWD. These findings form the basis for the simplified classification of VWD that is outlined in Table 1 [8].
Table 1.
Classification of von Willebrand disease
| Types | Features |
|---|---|
| 1 | Partial deficiency of VWF |
| 2 | Qualitative VWF defects |
| 2A | VWF variants with loss of high-molecular-weight multimers and decreased VWF-dependent platelet adhesion |
| 2B | VWF variants with loss of high-molecular-weight multimers caused by increased affinity for platelet glycoprotein Ib |
| 2M | VWF variants with decreased VWF-dependent platelet adhesion not associated with the loss of high-molecular-weight multimers |
| 2N | VWF variants with decreased binding affinity for FVIII |
| 3 | Severe deficiency of VWF |
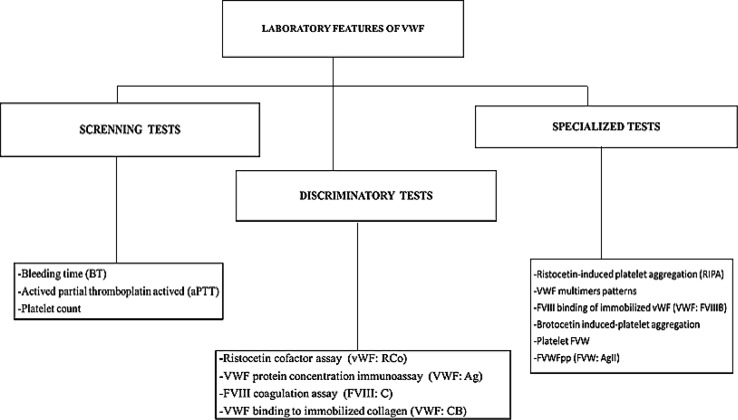
Laboratorial analysis can be divided in screening tests, confirmatory tests and specialized tests [12].
Screening Tests
These tests are characterized by the detection of existing alterations in both primary hemostasis and coagulation cascade [15]. Among them, there are: the bleeding time (BT), activated partial thromboplastin time (aPTT) and platelets count (PC). These exams might be normal and, individually, they might not suggest the diagnosis of VWD [16].
Although the BT is used in the platelet function, there are doubts related to the test, one of them concerning the broad reference caused by different races, ages and different areas all over the world, resulting in the need to determine a normal BT for each geographic region [17]. There is a test of the primary hemostasis that uses the Ivy method. When it’s too long, it suggests a qualitative or quantitative platelet abnormality, a platelet communication defect with vessels or a primary vascular disease [15].
The Ivy method requires the use of a sphygmomanometer, which is placed on the patient’s forearm. A constant pressure of 40 mmHg is applied during the whole test. Later, an incision is performed with a proper lancet on an area with no superficial veins on the inside section of the lower part of the forearm. After the incision, the chronometer is activated. The excess of blood is cleaned every 30 s with filter paper. The time from when the incision is made until the end of the bleeding is the BT value and it varies from 2 to 9 min [17]. The BT presents low reproducibility and diagnostic yield among the published studies [18].
The aPTT is used for the evaluation of most coagulation factors (among them there are the factors XII, XI, IX, VIII, X, V, II, I, HK e PK). When the time is long, it might indicate deficiency of some coagulation factors or the presence of inhibitors, such as some medicines [19]. The aPTT is a reflex of the plasmatic levels of the coagulant FVIII, which might be normal or prolonged [20].
The PC and the platelet evaluation on slides is fundamental to evaluate patients with diseases affecting the platelets, and it is useful to perform an internal control in the quality of automated counts [21]. The result of the PC is usually normal, but in patients with the type 2B, thrombocytopenia might be observed [13]. The most recent method has been proposed by ISLH (International Society for Laboratory Hematology) as the new reference method using specific fluorescent monoclonal antibodies against the superficial glycoproteins from the platelet membranes observed by flow cytometry. The only available haematological equipment that offers this technology is the Cell-Dyn 4000 or Cell-Dyn Sapphire (Abbott Diagnostics) [21].
In clinical practice, the screening shows low reproducibility and performance and this makes it difficult to evaluate platelet function inherited disorders, thus, new tests were developed aiming to identify patients with bleeding diseases more quickly and efficiently [18].
Confirmatory Tests
The confirmatory tests are the most specific laboratory exams to diagnose the disease. They are; the evaluation of VWF function though the ristocetin cofactor assay (VWF:RCo), VWF protein concentration immunoassay (VWF:Ag), the FVIII coagulation assay (FVIII:C) and VWF binding to immobilized collagen (VWF:CB). It is recommended that these tests are taken twice, with the aim of excluding or confirming VWD diagnosis [3].
The purpose of the FVIII:C test is to evaluate VWF’s predisposition to bind to the FVIII and to keep its level in the circulation. If the sample collection and processing are not performed properly, according to the Standard Operational Procedure of the laboratory (SOP) there could be false positives, that in the case of FVIII:C, are justified by the instability of the FVIII [12]. Usually, the levels of the coagulant FVIII are proportional to the levels of VWF [13].
The test VWF:Ag is based on ELISA and uses antibodies against the protein with the aim of measuring it’s total quantity in the plasma. As there is respectively, a partial and a total deficiency in the amount of VWF in types 1 and 3, the levels of VWF:Ag will be low in these cases and normal or limiting in cases of type 2. However, this result cannot measure VWF’s functionality, for this purpose, the test VWF:RCo should be performed [3].
As the factors VIII and Von Willebrand are acute phase proteins, that are raised in inflammatory cases, during pregnancy, stress, physical exercise, among others, its dosage in these conditions could mask the diagnosis [1].
The severity of the bleedings is related to the reduction degree of VWF:RCo and FVIII [14]. The ristocetin is an antibiotic that causes an interaction of VWF to the platelets resulting in a platelet aggregation and shows the functional activity of VWF [20]. The concentration of ristocetin must be elevated in relation to VWF to assure a higher stimulation [3].
The ristocetin cofactor assay (VWF:RCo) is considered the most specific test related to platelet function and might be decreased even in mild cases of the disease [16]. The capacity of the plasmatic VWF to aggregate the platelets in the presence of ristocetin is the most specific way to detect the VWD [7].
The test that determines VWF:RCo uses diluted plasma in different concentrations and in standardized amounts of ristocetin and platelets. After that, a comparison between the reference plasma and that of the patient is performed, in all types of VWD the result must be low [12]. The ristocetin cofactor assay (VWF:RCo) became the most specific test to evaluate the platelet function and can be decreased even in mild cases of the disease [16]. Ristocetin cofactor activity is generally decreased coordinately with VWF:Ag and factor VIII in type 1 VWD patients. VWF:RCo-to-VWF:Ag ratio has been proposed as means to distinguish between type 1 and type 2 VWD, with a ratio of VWF:RCo-to-VWF:Ag <0.7 indicative of a qualitative (type 2) VWF defect. However, in patients with very low VWF:Ag levels, this ratio may not be reliable because of the limits of sensitivity of most VWF:RCo assays [8].
The test of binding capacity of VWF binding to immobilized collagen (VWF:CB) using the immunoenzymatic assay (ELISA), might be considered a test that evaluates the binding capacity of VWF to the collagen as much quantitatively as qualitatively, mainly, the high molecular weight multimers. Usually, VWF:CB is reduced in all types of VWD, being either a qualitative or a quantitative defect [13].
Specialized Tests
The special tests are the ristocetin-induced platelet aggregation (RIPA), VWF multimers patterns, FVIII binding of immobilized VWF (VWF:FVIIIB), botrocetin induced-platelet aggregation, VWF intraplatelet and VWF propeptide (VWFpp) [3].
The platelet aggregation (PA) is used to evaluate the platelet function, once it allows the exploration, in vitro, of the different platelet activation pathways [22]. The aggregation method by turbidimetry or platelet rich plasma (PRP) is performed by the action of many agonists like adenosine diphosphate (ADP), collagen, arachidonic acid and adrenalin, and the result is measured by transmittance. According to the formation of platelet aggregates, the transmittance increases, the speed and intensity of the transmittance increase reflect the platelet function [23].
Although this method is extensively studied and allows the use of different agonists in varied concentrations, it still has limitations related to the speed of test performance; proper analysis of the results is also required. Thereby, new tests are developed to enable the platelet aggregation interpretation to be simpler and adequate [24].
The concept of the RIPA is to evaluate the propensity of different concentrations of ristocetin to aggregate platelets in the presence of VWF [13]. The ristocetin interacts with sequences flanking the A1 domain of VWF’s molecules, inducing VWF binding to the glycoprotein GPIb in vitro [7]. Through the addition of the ristocetin to the platelet rich plasma, an evaluation of the affinity of VWF to the platelets in the patient’s plasma is performed [12].
Three concentrations between 0.5 and 1.5 mg/mL are used. In patients with type 3 VWD, RIPA is absent as the presence of VWF is necessary for platelet aggregation to occur. In patients with the type 1, depending on the level of VWF present in the plasma, aggregation might happen. In patients with the type 2, the absence of RIPA might happen at the concentration of 1.0 mg/mL and mild platelet aggregation at ristocetin levels of 1.0 mg/mL Patients with type 2B will have a greater response at the ristocetin concentration of 0.5 mg/mL [13].
A platelet coagglutinin, platelet aggregation induced by botrocetin, is also found in Bothrops jararaca snake venom [25]. In the presence of VWF, this substance has the same features as ristocetin, however, the binding occurs among platelets and different residues of VWF’s A1 domain. Generally, VWD variants that present a reduction in high molecular weight multimers, have a greater activity with the botrocetin than with the ristocetin [7].
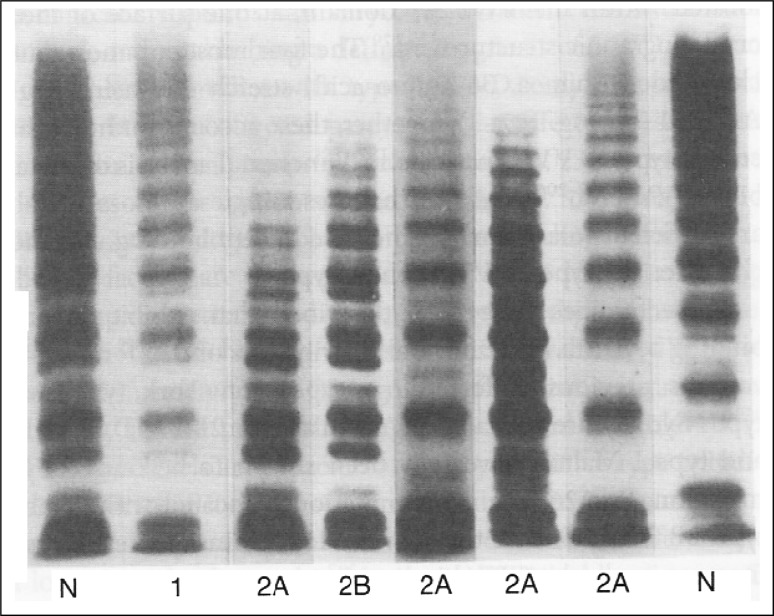
VWF multimers patterns performed by agarose gel electrophoresis, enables the study of VWF structure, distinguishing the different types of the disease [12]. VWF multimers patterns have different sizes and therefore, have different migration patterns, enabling the identification of the types and subtypes of VWD [3]. It is possible to separate the multimers based on their molecular weight using a platelet lysate or isolated VWF. They are visualized by autoradiography after being incubated with anti-VWF antibodies marked with immunoperoxidase or alkaline phosphatase [7].
In type 1, all multimers are present, however, in a reduced amount. In type 3, there is a reduction or even absence of multimers. On the other hand, in type 2, the large multimers are absent, except in the type 2M, which presents a similar pattern to type 1 [12]. The Fig. 1 shows VWF multimers patterns performed by agarose gel electrophoresis of plasma VWF from plasma of patients with various subtypes of VWD.
Fig. 1.
Agarose gel electrophoresis of plasma VWF
The laboratorial test that evaluates VWF intraplatelet enables, when combined with VWF multimers patterns, type 1 VWD to be classified. The binding capacity to FVIII (VWF:FVIIIB) provides the diagnosis of the subtype 2N of VWD, distinguishing it from a light or mild hemophilia [13]. Through the multimeric pattern characterization and composition of VWF subunits, the qualitative and quantitative analysis using Western immunoblotting is studied [26]. VWFpp assay is currently only available in a few reference laboratories or for research purposes. Figure 2 shows a flowchart proposed for VWD diagnosis. Following the given coordinates, it is possible to distinguish the type of the researched disease through laboratorial exams [3].
Fig. 2.
Flowchart proposed for VWD diagnosis
Conclusion
The laboratorial diagnosis of VWD has been considered difficult because usually it goes unnoticed, with mild symptoms and also because of its complexity of laboratorial proof, once there is the chance that some exams yield normal results. In a small laboratory, the routine tests used for screening of VWD are the BT, aPTT and PC. However, these tests standing alone, cannot confirm the disease diagnosis, once in some cases they show normal results. Thus, small and big laboratories, need more specific exams, for instance, ristocetin cofactor assay (VWF:RCo), VWF protein concentration immunoassay (VWF:Ag), VWF multimers patterns, FVIII binding of immobilized VWF (VWF:FVIIIB), RIPA and platelet aggregation induced by botrocentin. From the moment the diagnosis is confirmed, a proper treatment is sought aiming the elevation of plasmatic concentrations of the deficient protein in crisis or invasive procedures.
Acknowledgments
We are thankful to the Pontifical Catholic University of Parana and Federal University of Parana for the support.
References
- 1.Rezende SM. Distúrbios da hemostasia: doenças hemorrágicas. Rev Med Minas Gerais. 2010;20(4):534–553. [Google Scholar]
- 2.Ratnoff OD. Distúrbios Hemorrágicos: defeitos da coagulação: tratado de medicina interna, vol 2, 14th edn. Rio de Janeiro: Interamericana; 1977. [Google Scholar]
- 3.BRASIL-Ministério-da-Saúde-Secretaria-de-Atenção-à-Saúde-Departamento-de-Atenção-Especializada (2008) Manual de diagnóstico e tratamento da doença de von Willebrand. Editora do Ministério da Saúde. http://www.saude.pr.gov.br/arquivos/File/MANUAISHEMEPAR/Manual_de_diagnostico_tratamentodadoencadevonWillebrand.pdf. Accessed 14 Dec 2014
- 4.Sap F, Kavakli T, Kavakli K, Dizdarer C. The prevalence of von Willebrand disease and significance of in vitro bleeding time (PFA-100) in von Willebrand disease screening in the Izmir region. Turk J Haematol. 2013;30(1):40–47. doi: 10.4274/tjh.2011.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopes AAB, Maeda NY, Bydlowski SP. Fator von Willebrand e disfunção endotelial pulmonar. Implicações prognósticas. Arq Bras Cardiol. 1998;70(3):141–145. doi: 10.1590/S0066-782X1998000300001. [DOI] [PubMed] [Google Scholar]
- 6.Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2014;25(3):206–216. doi: 10.1097/MBC.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.João C. Doença de von Willebrand. Med Interna. 2001;8(1):28–36. [Google Scholar]
- 8.Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM, Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F, Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 9.Rocha HHAG, Maia EG (2005) Doença de Von Willebrand. Revista Laes & Haes Rio de Janeiro, ed 155
- 10.Mikhail S, Aldin ES, Streiff M, Zeidan A. An update on type 2B von Willebrand disease. Expert Rev Hematol. 2014;7(2):217–231. doi: 10.1586/17474086.2014.868771. [DOI] [PubMed] [Google Scholar]
- 11.Paro MO (2012) Peptídeos antigênicos indutores de anticorpos específicos para identificação de alterações de maior prevalência do subtipo 2B da doença de von Willebrand e do fator de von Willebrand normal. Universidade Federal de Ouro Preto. http://www.repositorio.ufop.br/handle/123456789/3494. Accessed 03 Aug 2014
- 12.Matos R, Magalhães SR. Doença de Von Willebrand. Rev Iniciaç Cient Univ Vale do Rio Verde. 2011;1(2):17–20. [Google Scholar]
- 13.Santos KF (2009) Enzimas que hidrolisam nucleotídeos de adenina em plaquetas, agregação plaquetária e polimorfismo do gene α2 da integrina α2β1 em pacientes com a doença de von Willebrand. Centro de Ciências Naturais e Exatas. Universidade Federal de Santa Maria. http://cascavel.ufsm.br/tede/tde_busca/arquivo.php?codArquivo=2589. Accessed 15 Sept 2013
- 14.Pauleta J, Osório F, Alho C, Tavares A, Calhaz-Jorge C. Histerectomia totalmente laparoscópica numa mulher com Doença de von Willebrand tipo 3 e aloanticorpos contra o factor de von Willebrand. Acta Obstet Ginecol Port. 2011;5(4):189–192. [Google Scholar]
- 15.Rizzati EG, Franco RF. Investigação diagnóstica dos distúrbios hemorrágicos. Med Ribeirão Preto. 2001;34:238–247. [Google Scholar]
- 16.Barbosa FT, Cunha RMd, Barbosa LT. Doença de von Willebrand e Anestesia. Rev Bras Anestesiol. 2007;57(3):315–323. [PubMed] [Google Scholar]
- 17.Maleki A, Roohafza H, Rashidi N, Aliyari F, Ghanavati R, Foroughi S, Nabatchi B, Torkashvand M. Determination of normal range of bleeding time in rural and urban residents of Borujerd, Iran: a pilot study. ARYA Atheroscler. 2012;8(3):136–142. [PMC free article] [PubMed] [Google Scholar]
- 18.Lombana MA, Ramos-Ramos G, Torres AM. Utilidad del PFA-100 en una población colombiana como método de tamizaje en enfermedad de von Willebrand y trastornos de la función plaquetaria. Rev Hematol Mex. 2013;14(2):71–77. [Google Scholar]
- 19.Pereira JPdM, Faustino SMM, Rodrigues ÁSdN. Análise dos problemas encontrados na execução do coagulograma em laboratórios da cidade de Macapá-Amapá. Ciência Equat. 2011;1(1):50–57. [Google Scholar]
- 20.Abreu MP, Porto AM, MinariIII AL, Caseli HG, Porto AM, Minari AL, Caseli HG. Anestesia para Septoplastia e Turbinectomia em Paciente Portador de Doença de von Willebrand: Relato de Caso. Rev Bras Anestesiol. 2003;53(3):382–387. doi: 10.1590/S0034-70942003000300009. [DOI] [PubMed] [Google Scholar]
- 21.Comar SR, Danchura HSM, Silva PH. Contagem de plaquetas: avaliação de metodologias manuais e aplicação na rotina laboratorial. Rev Bras Hematol Hemoter. 2009;31(6):431–436. doi: 10.1590/S1516-84842009005000087. [DOI] [Google Scholar]
- 22.Piedade PR, Gagliardi RJ, Damiani IT, Junior APN, Fuzaro MM, Sanvito WL. Papel da curva de agregação plaquetária no controle da antiagregação na prevenção secundária do acidente vascular cerebral isquêmico. Arq Neuropsiquiatr. 2003;61(3B):764–767. doi: 10.1590/S0004-282X2003000500011. [DOI] [PubMed] [Google Scholar]
- 23.Silva LL, D’Amico EA. Estudo comparativo entre agregação plaquetária por turbidimetria e impedância elétrica em pacientes sob terapia antiplaquetária à base de ácido acetilsalicílico. Rev Bras Hematol Hemoter. 2010;32(6):463–468. doi: 10.1590/S1516-84842010000600010. [DOI] [Google Scholar]
- 24.Soares JS, Brito FCF, Pena FM, Mesquita ET, Medina-Acosta E. Aspectos Farmacogenéticos Associados à Resistência Terapêutica Antiplaquetária em Pacientes com Síndrome Coronariana Aguda. Rev Bras Cardiol. 2010;23(2):131–142. [Google Scholar]
- 25.Novello JC (1995) Purificação e Caracterização de uma proteína (SIII-3rp) do veneno de Bothrops alternatus que se liga ao fator de von willebrand (vWF). Universidade Estadual de Campinas, Instituto de Biologia. http://www.bibliotecadigital.unicamp.br/document/?code=vtls000093709. Accessed 07 Dec 2013
- 26.Nascimento NM (2010) Atividade enzimática da ADAMTS-13 e padrão de fragmentação do fator de von Willebrand em crianças hipoxêmicas portadoras de cardiopatias congênitas. Faculdade de Medicina da Universidade de São Paulo. Programa de Ciências Médicas. São Paulo. http://www.teses.usp.br/teses/disponiveis/5/5167/tde-22092010-104951/pt-br.php. Accessed 17 Apr 2014