Abstract
To analyze multiple variables, including immunoglobulin subtypes in patients with monoclonal gammopathy of undetermined significance (MGUS) and different types of neuropathy. This was a retrospective, single center study done in a tertiary care hospital in the United States. The data was collected for years 2001–2011. Inclusion criteria were the presence of MGUS and neuropathy. Exclusion criteria were the presence of other factors such as diabetes, vitamin B12 deficiency, alcoholism etc. which can cause neuropathy. Patients with IgM MGUS were compared with patients having Non-IgM MGUS. A total of 281 patients were analyzed in this study. The average age at the time of diagnosis of MGUS and neuropathy was 68 years. The most common type of neuropathy was sensorimotor peripheral neuropathy (46 %). The most common location of neuropathy was the lower extremities (68 %). Among our patients, 52 % had their neuropathy symptoms for 1–5 years before presenting to the clinic. When IgM MGUS was compared with Non-IgM MGUS, a statistically significant difference was found in terms of race (White vs. Others, OR 4.43, 95 % CI 2.13, 9.19, p < 0.001) and survival status (OR 1.98, 95 % CI 1.01, 3.90, p = 0.046). Patients with MGUS are prone to develop different types of neuropathies. Caucasians are more likely to have IgM MGUS as compared to other races. IgM MGUS is generally related to worse outcomes as compared to Non-IgM MGUS. Medical therapies, including gabapentin and pregabalin are effective treatments and the response rate can be as high as 80–90 % with these medications.
Keywords: Monoclonal gammopathy of undetermined significance, Neuropathy, Gabapentin, Pregabalin
Introduction
Over the past few years, the relationship between monoclonal gammopathy of undetermined significance (MGUS) and neuropathy appears to have been well established [1]. However, although most studies have demonstrated a strong association between IgM MGUS and neuropathy, there is lack of data on such an association with non-IgM MGUS. The purpose of this study was to determine the characteristics of patients with IgM MGUS as well as patients with non-IgM MGUS and their relationship with different types of neuropathies. We also wanted to analyze other variables such as types of neuropathy, common location of symptoms, and average duration of symptoms of neuropathy before diagnosis and the patients’ response to different treatment modalities such as pharmacologic therapies and non-pharmacologic approaches.
Materials and Methods
This was a tertiary care hospital based observational study in which a retrospective chart review of 281 subjects was carried out. The study was approved by the institutional review board (IRB approval #8918). Inclusion criteria included patients with MGUS and neuropathy. Exclusion criteria was presence of other known factors which can cause neuropathy such as diabetes mellitus, chronic kidney disease, hypothyroidism, chronic liver disease, amyloidosis, chronic alcoholism, genetic disorders associated with neuropathy (such as Charcot–Marie–Tooth disease), vitamin deficiencies, neurotoxic drug, physical trauma to the affected areas, systemic inflammatory conditions such as systemic lupus erythematosus, Guillain Barre syndrome and multiple sclerosis. The patient population was collected from electronic medical records using The International Classification of Diseases, 9th Revision (ICD-9) coding system. Our initial criterion to extract patients’ charts was the presence of both MGUS and neuropathy in every patient. Initial population size turned out to be approximately 600. Afterwards, exclusion criteria was applied to exclude patients with diseases which can contribute to neuropathy i.e. diabetes mellitus, vitamin B12 deficiency, chronic alcoholism etc. Our final population size after application of exclusion criteria turned out to be 281. The purpose of having such strict exclusion criteria was to isolate a patient population in which the neuropathy was exclusively from MGUS without any confounding factors.
The descriptive part of the study included variables such as gender; race; ages at the time of diagnosis of MGUS and neuropathy; type of immunoglobulin in serum protein electrophoresis; type of neuropathy based on nerve conduction studies testing; location of neuropathy; duration of symptoms; types of treatments used and patients’ response to the treatments. We also analyzed if there was an evolution of MGUS to a more malignant condition such as myeloma or lymphoma. Univariate and multivariate analyses were also carried out. Patients with IgM MGUS were compared with patients having Non-IgM MGUS. Univariate two-group tests were done using Chi square or Fisher’s exact tests for categorical variables and using Wilcoxon rank-sum tests for continuous variables. Statistical significance was set at p < 0.05. All analyses were done using SAS 9.4 (SAS Institute Inc, Cary, NC, USA).
The major types of races in our study were black, white and others (which included all other races including Asians). To determine the type of immunoglobulin, patients’ charts were analyzed for serum protein electrophoresis (SPEP). Most of the subjects had SPEP done multiple times as a part of their regular follow-up.
In terms of the types of neuropathy, we analyzed nerve conduction study results of the patients. We also analyzed office notes from patients’ neurologists or the primary physicians treating their neuropathy. The major types of neuropathies were sensory, motor, sensorimotor, radiculopathy, mononeuropathy and autonomic neuropathy. A small percentage of patients had a mixed pattern i.e. a combination of two or more types of above mentioned neuropathies. The location of symptoms of neuropathy was divided into upper extremities, lower extremities, upper and lower extremities, head and neck or trunk.
To determine the duration of symptoms, we performed a detailed analysis of patients’ charts, mainly office visits to neurologists and internal medicine physicians. We divided the symptom duration into four major categories, i.e. less than a year, 1–5, 6–10 and more than 10 years.
In terms of different treatment modalities, we divided the patients into three major categories. The first category was of patients being managed by conservative approaches such as physical therapy, occupational therapy, acupuncture or careful observation. The second category was non-conservative approaches, which included both pharmacological (gabapentin, pregabalin, tricyclics, narcotic analgesics, non-narcotic analgesics, intravenous immunoglobulin, rituximab) and non-pharmacological approaches (such as surgery). The third category was the patient population that received a combination of one or two of the above mentioned treatments.
To determine response to therapy, documentation from patients’ neurologists as well as other physicians was analyzed. Response to therapy was determined based on patients’ symptoms on follow-up visits as well as physical examination findings (such as numbness, tingling, loss of sensations, motor strength, etc.). A positive response was defined as either resolution in numbness/tingling or return of previously affected sensations (in patients with sensory neuropathy) or improvement in motor strength to baseline (in patients with motor neuropathy). In patients with radiculopathy and/or autonomic neuropathy, resolution of the presenting sign/symptom was considered a positive response to therapy.
Results
A total of 281 subjects were analyzed. Of these 281 patients, 133 (47 %) were males and 148 (53 %) were females. Caucasians comprised the largest portion of our population (58 %), followed by Blacks (30 %) and other races (12 %). The average age at the time of diagnosis of both MGUS and neuropathy was approximately 68 years. The most common immunoglobulin in SPEP analysis was IgG in 194 (69 %) patients, followed by IgM in 58 (21 %) patients and IgA in 29 (10 %) patients (Table 1).
Table 1.
Characteristics of patient population (n = 281)
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 133 (47) |
| Female | 148 (53) |
| Race | |
| Caucasian | 164 (58) |
| Black | 83 (30) |
| Othera | 34 (12) |
| Age at MGUS diagnosis (years) | |
| ≤50 | 32 (11) |
| 51–70 | 109 (39) |
| 71–90 | 138 (49) |
| ≥91 | 2 (1) |
| Age at neuropathy diagnosis (years) | |
| ≤50 | 32 (11) |
| 51–70 | 107 (38) |
| 71–90 | 140 (50) |
| ≥91 | 2 (1) |
| Type of immunoglobulinb | |
| IgG | 194 (69) |
| IgM | 58 (21) |
| IgA | 29 (10) |
| Duration of symptoms | |
| <1 year | 38 (14) |
| 1–5 years | 147 (52) |
| 6–10 years | 62 (22) |
| >10 years | 34 (12) |
| Evolution of MGUS | |
| Yes | 38 (14) |
| No | 243 (86) |
| Survival statusc | |
| Alive | 229 (81) |
| Deceased | 52 (19) |
MGUS monoclonal gammopathy of undetermined significance
aAll races besides Caucasians and Blacks
bFrom the serum protein electrophoresis
cStatus at the time of the study
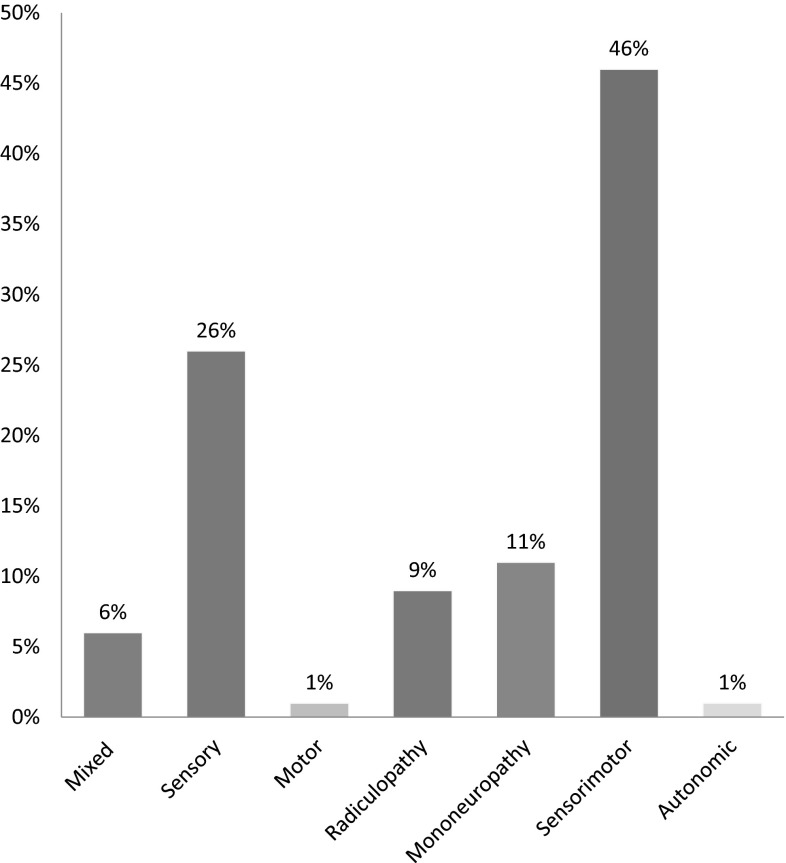
The most common types of neuropathy was found to be sensorimotor peripheral neuropathy in 128 (46 %) patients, followed by sensory neuropathy in 74 (26 %) patients, mononeuropathy in 31 (11 %) patients, and radiculopathy in 25 (9 %) patients. A combination of two or more neuropathy types was seen in 17 (6 %) patients. Motor and autonomic neuropathy comprised the least common types, seen in four (1 %) and two (1 %) patients, respectively (Fig. 1).
Fig. 1.
Types of neuropathy in MGUS patients along with their prevalence
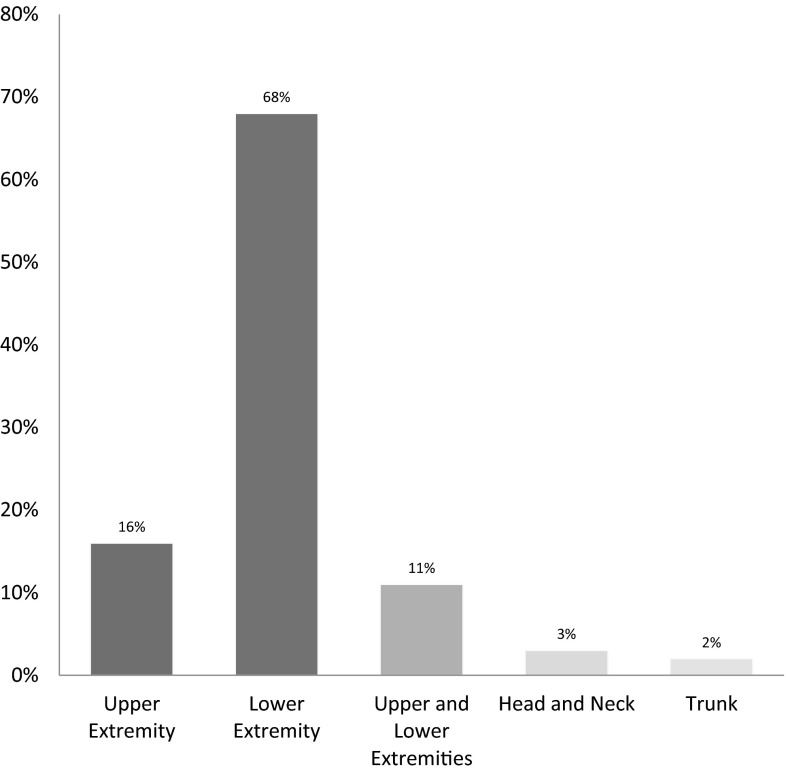
The most common location of neuropathy was found to be in the lower extremities (68 %), followed by the upper extremities (16 %), while in both upper and lower extremities combined it was less common (11 %). Neuropathy involving head and neck was seen in eight (3 %) patients. Involvement of trunk by neuropathy was seen in only six (2 %) patients (Fig. 2).
Fig. 2.
Locations of neuropathy in MGUS patients along with their prevalence
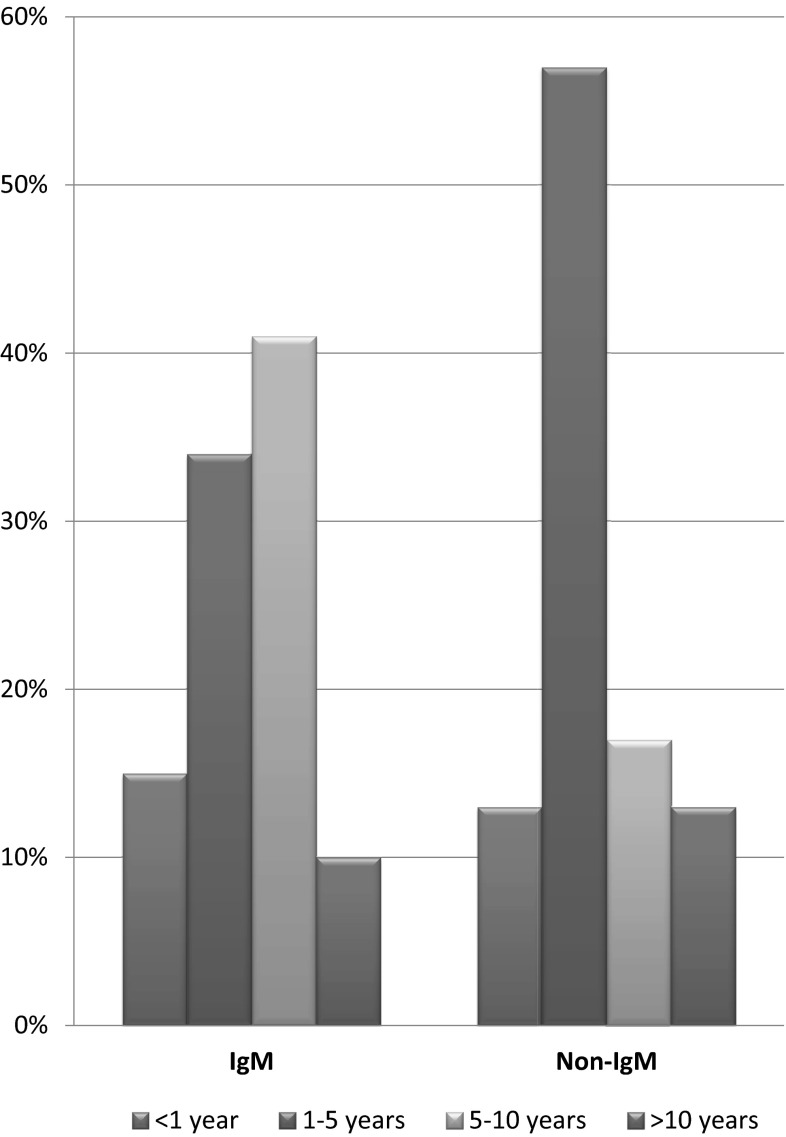
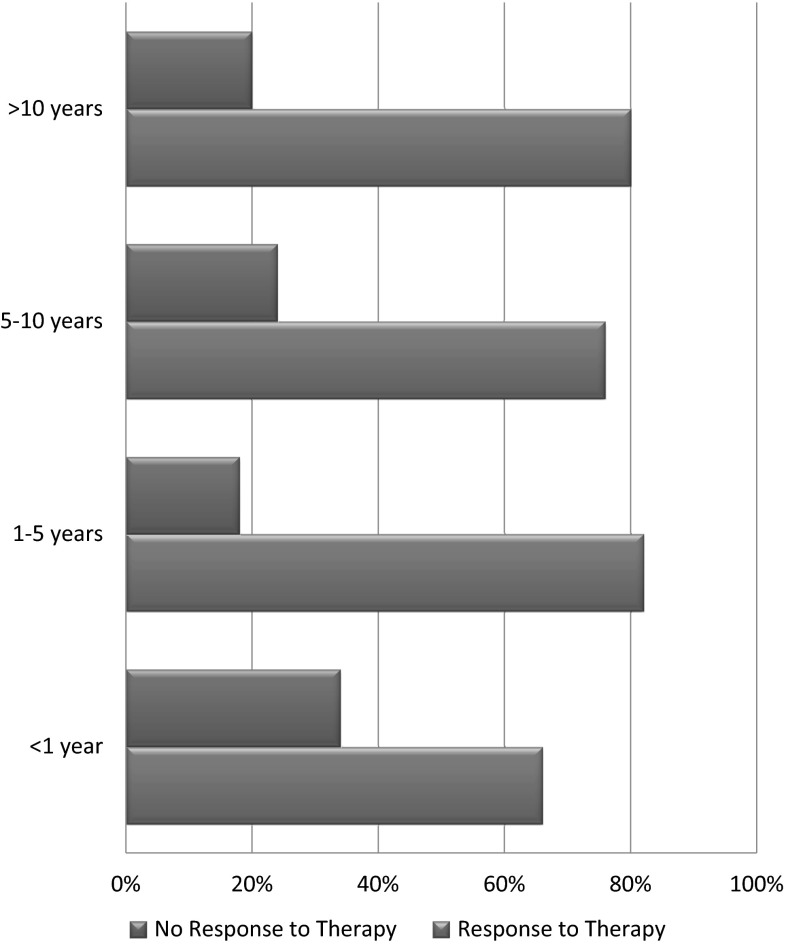
Among our patient population, 147 (52 %) had the symptoms of neuropathy for 1–5 years before presenting to the clinic. Sixty-two (22 %) patients had the symptoms for 6–10 years and 38 (14 %) had the symptoms for less than a year. Only 34 (12 %) patients had the symptoms for more than 10 years before presenting to the physician (Table 1). The relationship between the type of MGUS and duration of symptoms of neuropathy was also analyzed (Fig. 3).
Fig. 3.
Difference in time duration for presentation between IgM MGUS and Non-IgM MGUS neuropathies
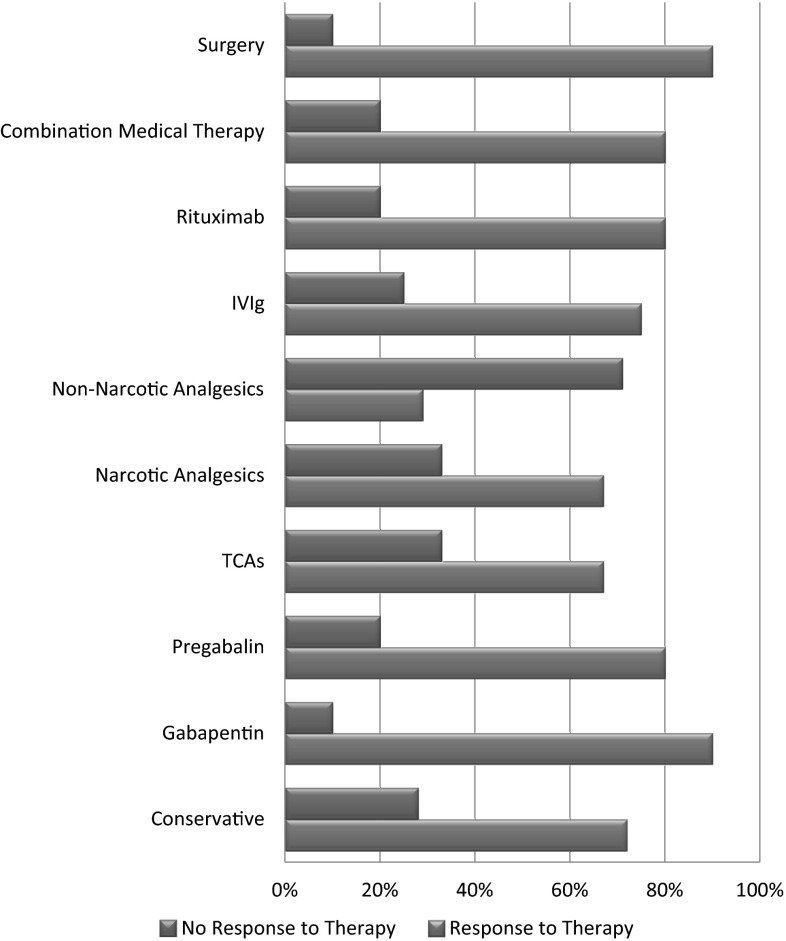
In terms of treatment, the most common approach was conservative in 127 (45 %) patients. Gabapentin was the most commonly used drug in 94 (33 %) patients followed by pregabalin in ten (4 %) patients. Narcotic pain medications (such as hydrocodone and oxycodone) were used in nine (3 %) patients. Intravenous immunoglobulin was used in eight (3 %) patients. The least common management strategies included tricyclics (2 %), non-narcotic analgesics (2 %) and Rituximab (2 %). Surgery was the primary treatment modality in five (2 %) patients (mainly in mononeuropathies such as carpal tunnel syndrome). In ten (4 %) patients, a combination of two or more treatment modalities was used. The response to different modalities of treatment was also analyzed (Fig. 4).
Fig. 4.
Types of treatments used in neuropathies and their response rates
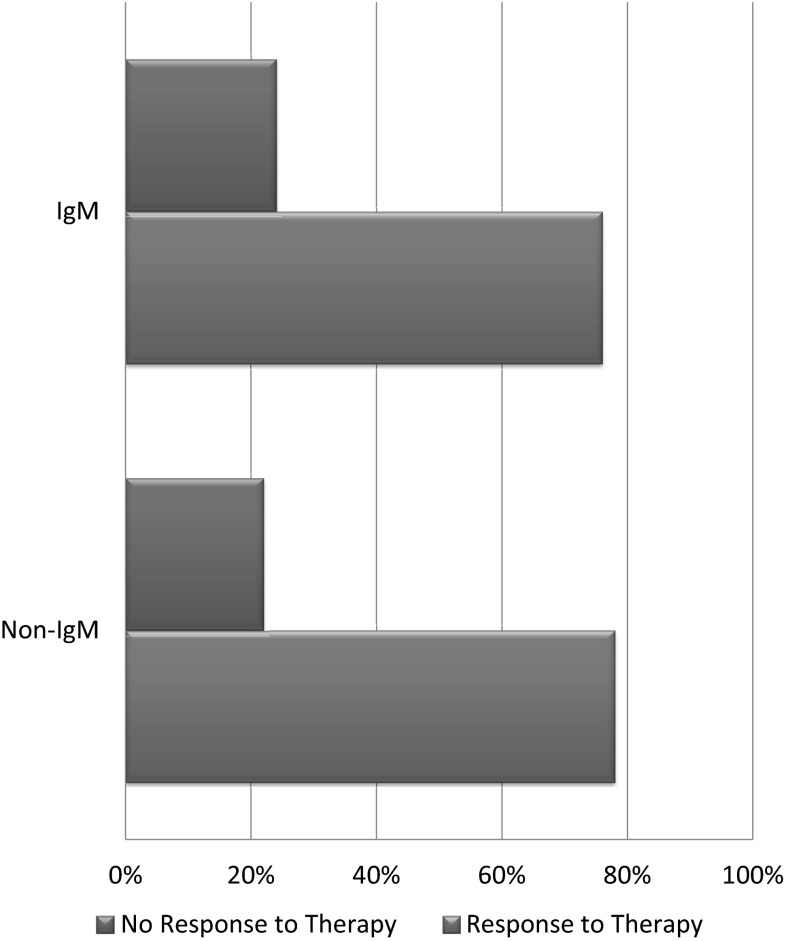
From our patient population, 219 (78 %) reported a response to therapy used and 62 (22 %) reported no significant response to the therapy used. At the time of our study, 229 (81 %) of our patient population were alive and only 52 (19 %) were deceased. We also analyzed the response to different types of therapies after categorizing them based on symptoms duration (Fig. 5) as well as the type of immunoglobulin in SPEP. Surprisingly, the response rates remain high and are not affected much by the total duration of symptoms i.e. longer duration of neuropathy does not change response rates to different therapeutic modalities used. When comparing response rates between IgM MGUS versus Non-IgM MGUS, very similar results were found in both of the groups (Fig. 6).
Fig. 5.
Relationship between duration of symptoms and response to therapy
Fig. 6.
Differences in response rates to therapy when IgM MGUS patients were compared with Non-IgM MGUS patients
When IgM MGUS was compared with Non-IgM MGUS a statistically significant difference was found between the two groups in terms of race and survival status. IgM-MGUS was found to have a much higher prevalence in Caucasians as compared to all other races (OR 4.43, 95 % CI 2.13–9.19, p < 0.001). Moreover, patients with IgM-MGUS were more likely to be dead at the time of study as compared to patients with Non-IgM MGUS (OR 1.98, 95 % CI 1.01–3.90, p = 0.046) (Table 2). All other differences were found to be statistically insignificant (bold figures are used to describe statisical significance in Table 2 i.e. p < 0.05).
Table 2.
Descriptive statistics with regression analysis
| Factors | Non-IgM MGUS (N = 223) |
IgM MGUS (N = 58) |
Univariate regression analysis | Multivariate regression analysis | |||
|---|---|---|---|---|---|---|---|
| Odds ratio P value (95 % CI) |
Odds ratio P value (95 % CI) |
||||||
| Race | Caucasians All others |
116 (52 %) 107 (48 %) |
48 (83) 10 (17) |
4.43 (2.13, 9.19) |
<0.001 | 3.77 (1.70, 8.38) | <0.001 |
| Gender | Male Female |
103 (46 %) 120 (54 %) |
30 (52 %) 28 (48 %) |
1.25 (0.70, 2.23) |
0.452 | ||
| MGUS Age (in years) | Mean ± SD | 67.9 ± 12.6 | 68.1 ± 14.9 | 1.21 (0.62, 2.36) | 0.495 | ||
| M-spike (quantitative) on presentation (mg/dL) | Mean ± SD | 1541.2 ± 1496.4 | 559.6 ± 569.6 | 0.998 (0.997, 0.998) | <0.001 | 0.977 (0.97, 0.985) | <0.001 |
| Neuropathy age (in years) | Mean ± SD | 68.0 ± 13.0 | 68.2 ± 14.1 | 1.01 (0.53, 1.93) | 0.659 | ||
| Survival status | Alive Deceased |
187 (84 %) 36 (16 %) |
42 (72 %) 16 (28 %) |
1.98 (1.01, 3.90) |
0.046 | ||
| Neuropathy type | Sensory Non-sensory |
58 (26 %) 165 (74 %) |
16 (28 %) 42 (72 %) |
1.08 (0.57, 2.07) |
0.808 | ||
| Location of Neuropathy | Extremities Other |
213 (96 %) 10 (4 %) |
54 (93 %) 4 (7 %) |
1.58 (0.48, 5.23) |
0.455 | ||
| Duration of neuropathy | Less than 1 year 1 year or more |
30 (13 %) 193 (87 %) |
8 (14 %) 50 (86 %) |
0.97 (0.42, 2.25) |
0.946 | ||
| Therapy used | Conservative Non-conservative |
97 (43 %) 126 (57 %) |
30 (52 %) 28 (48 %) |
0.72 (0.40, 1.28) |
0.263 | ||
| Response to therapy | Yes No |
175 (78 %) 48 (22 %) |
44 (76 %) 14 (24 %) |
0.86 (0.44, 1.70) |
0.669 | ||
| Evolution of MGUS | Yes No |
32 (14 %) 191 (86 %) |
6 (10 %) 52 (90 %) |
0.69 (0.27, 1.74) |
0.429 | ||
Discussion
MGUS is a paraproteinemia in which the bone marrow plasma cell burden along with laboratory and radiographic findings don’t qualify to be categorized as multiple myeloma. In contrast to multiple myeloma, MGUS is mainly asymptomatic but can at times be symptomatic such as involving peripheral nerves. There are two distinct clinical types of MGUS, each with a risk of progressing through a unique intermediate (more advanced) premalignant stage and then to a malignant plasma cell dyscrasia or lymphoproliferative disorder. IgM MGUS, which accounts for approximately 15 % of MGUS cases, is considered separately from the Non-IgM MGUS because it has the potential to progress to Waldenstrom’smacroglobulinemia, lymphoma, or AL amyloidosis. Infrequently, IgM MGUS can progress to multiple myeloma.The second type is Non-IgM MGUS (IgG, IgA, or IgD MGUS) which is the most common subtype of MGUS and has the highest potential to progress to multiple myeloma.
The occurrence of MGUS subtypes shows ethnic/racial variations. Black patients have showed increased prevalence of IgG/IgA MGUS and higher rates of un-quantifiable immunoglobulins when compared with Caucasians [2]. Our study results are consistent with these findings. We found Non-IgM MGUS to be more common in Blacks as compared to Caucasians. The difference was statistically significant. Weiss et al. showed that MGUS, in general, was found to be more common in Blacks than Caucasians but the proportion of high risk MGUS was significantly lower in blacks [3]. Our study also showed a statistically significant difference in terms of survival status. A higher proportion of patients with IgM MGUS were deceased at the time of the study as compared to Non-IgM MGUS patients. The likely explanation of this difference is the more aggressive nature of IgM MGUS as compared to Non-IgM MGUS.
In terms of neurologic toxicity, MGUS most commonly involves the peripheral nerves, damaging their myelin sheath and presents clinically as polyneuropathies which may have additional features of axonal degeneration. Efforts have been made in the past to differentiate the types of MGUS on the basis of their clinical presentation and initial diagnostic work-up. In a few retrospective studies, it was inferred that four differences set IgM-MGUS neuropathies apart from IgG/IgA-MGUS neuropathies: (1) Statistically significant higher frequency of sensory loss and ataxia in IgM group (2) higher frequency of nerve conduction abnormalities in IgM group-with slowing of conduction velocities and prolonged distal latencies, (3) higher frequency of dispersion of the compound muscle action potential, and (4) frequency of monoclonal IgM was overrepresented in the MGUS neuropathy group [4–6]. It was also stated that polyneuropathy associated with IgM-monoclonal gammopathy better meets the criteria for demyelination as compared to that associated with the IgG type [7–10]. Higher age at onset of IgM type MGUS and polyneuropathy is associated with worst prognosis whereas presence of anti-myelin-associated glycoprotein antibodies indicates good prognosis [11]. A prospective cohort study was conducted to look for the occurrence of tremors in MGUS patients and a positive association was concluded between IgM type MGUS and tremors. It was also found that IgMparaprotenemia increases expression of other tremor types [12].
MGUS is one of the most common premalignant disorders in Western countries. In a retrospective study of multiple myeloma patients, almost every case was preceded by an MGUS stage confirming the premalignant nature of this condition [13].The tendency of MGUS to malignant transformation needs regular follow-up. Patients presenting with peripheral neuropathy should be assessed for type of immunoglobulin in serum and urine. And, in patients found to have MGUS, nerve conduction studies and neurological evaluation should be carried out [14].
The non-symptomatic treatments of peripheral neuropathy in monoclonal gammopathies include corticosteroids, cytotoxic drugs or plasma exchange. Patients with IgG/IgA paraproteins respond more satisfactorily than do those with an IgM paraprotein [15]. Intravenous immunoglobulin is not very effective in management of neuropathies and only has short-term beneficial effect [16, 17]. Rituximab, an anti-CD20 monoclonal antibody, has shown some promise in treating IgM MGUS induced polyneuropathy [18].
In conclusion, patients with MGUS are prone to develop different types of neuropathies. The most common type of neuropathy found in these patients is sensorimotor and the most common location is the lower extremities. Caucasians are more likely to develop IgM MGUS as compared to Blacks that more commonly develop Non-IgM MGUS. IgM MGUS was associated with higher death rates as compared to Non-IgM MGUS. Most of these patients have their symptoms for 1–5 years before being diagnosed. Medical therapies, including gabapentin and pregabalin are effective treatments, and the response rate can be as high as 80–90 % with these medications.
The major limitation of this study is that it was a single center study, done in a tertiary care facility, and the results might not be applicable to the general population. Due to strict exclusion criteria, our patient population was relatively small (which can also be considered strength of this study as confounding was prevented by using such a strict exclusion criteria. Only patients with neuropathy exclusively due to MGUS were included in this study). Another limitation of this study is that we were unable to perform a time to event. For our deceased patients, we are only allowed to see their status in our electronic medical records system as alive or deceased. We were unable to see the date/time of their deaths. There are multiple reasons for this. Firstly, patients can be out of city, state or even out of country at the time of their death, making it difficult to analyze their time/date of death. Also, many of our patients visit different health care systems and their death in another facility is not updated in our system (until a death certificate is issues by the state). For a prospective study, gathering this data should not be a problem, as the investigators are in contact with the patients and their families on a regular basis. However, for a retrospective chart review, it is not possible as we do not have permission to contact the patients or their families. Prospective studies will be helpful to statistically analyze significant differences between the two types of MGUS and their relationship with the associated neuropathy in larger patient populations.
Contributor Information
Shahzaib Nabi, Phone: 1-313-482-8768, Email: snabi1@hfhs.org.
Pushpinderdeep Kahlon, Email: pkahlon1@hfhs.org.
Farshid Bozorgnia, Email: fbozorg1@hfhs.org.
Adeel Arshad, Email: adeelfg@hotmail.com.
Akmam Saleem, Email: akmamsaleem@gmail.com.
Philip Kuriakose, Email: pkuriak1@hfhs.org.
References
- 1.Rojas-Garcia R, Gallardo E, Illa I. Paraproteinemic neuropathies. Presse Med (Paris, France: 1983) 2013;42(6 Pt 2):e225–e234. doi: 10.1016/j.lpm.2013.02.329. [DOI] [PubMed] [Google Scholar]
- 2.Leger JM, Chassande B, Bombelli F, Viala K, Musset L, Neil J. Polyneuropathy associated with monoclonal gammopathy: treatment perspectives. Bull Acad Natl Med. 2009;193(5):1099–1110. [PubMed] [Google Scholar]
- 3.Weiss BM, Minter A, Abadie J, Howard R, Ascencao J, Schechter GP, Kuehl M, Landgren O. Patterns of monoclonal immunoglobulins and serum free light chains are significantly different in black compared to white monoclonal gammopathy of undetermined significance (MGUS) patients. Am J Hematol. 2011;86(6):475–478. doi: 10.1002/ajh.22025. [DOI] [PubMed] [Google Scholar]
- 4.Gosselin S, Kyle RA, Dyck PJ. Neuropathy associated with monoclonal gammopathies of undetermined significance. Ann Neurol. 1991;30(1):54–61. doi: 10.1002/ana.410300111. [DOI] [PubMed] [Google Scholar]
- 5.Suarez GA, Kelly JJ., Jr Polyneuropathy associated with monoclonal gammopathy of undetermined significance: further evidence that IgM-MGUS neuropathies are different than IgG-MGUS. Neurology. 1993;43(7):1304–1308. doi: 10.1212/WNL.43.7.1304. [DOI] [PubMed] [Google Scholar]
- 6.Magy L, Chassande B, Maisonobe T, Bouche P, Vallat JM, Leger JM. Polyneuropathy associated with IgG/IgA monoclonal gammopathy: a clinical and electrophysiological study of 15 cases. Eur J Neurol: Off J Eur Fed Neurol Soc. 2003;10(6):677–685. doi: 10.1046/j.1468-1331.2003.00687.x. [DOI] [PubMed] [Google Scholar]
- 7.Vrethem M, Reiser N, Lauermann C, Svanborg E. Polyneuropathy associated with IgM vs IgG monoclonal gammopathy: comparison between clinical and electrophysiological findings. Acta Neurol Scand. 2010;122(1):52–57. doi: 10.1111/j.1600-0404.2009.01259.x. [DOI] [PubMed] [Google Scholar]
- 8.Simovic D, Gorson KC, Ropper AH. Comparison of IgM-MGUS and IgG-MGUS polyneuropathy. Acta Neurol Scand. 1998;97(3):194–200. doi: 10.1111/j.1600-0404.1998.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb AR, Sander HW, Brannagan TH, 3rd, Magda P, Latov N. Characterization of neuropathies associated with elevated IgM serum levels. J Neurol Sci. 2005;228(2):155–160. doi: 10.1016/j.jns.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Nobile-Orazio E. Neuropathy and monoclonal gammopathy. Handb Clin Neurol. 2013;115:443–459. doi: 10.1016/B978-0-444-52902-2.00025-4. [DOI] [PubMed] [Google Scholar]
- 11.Niermeijer JM, Fischer K, Eurelings M, Franssen H, Wokke JH, Notermans NC. Prognosis of polyneuropathy due to IgM monoclonal gammopathy: a prospective cohort study. Neurology. 2010;74(5):406–412. doi: 10.1212/WNL.0b013e3181ccc6b9. [DOI] [PubMed] [Google Scholar]
- 12.Ahlskog MC, Kumar N, Mauermann ML, Klein CJ. IgM-monoclonal gammopathy neuropathy and tremor: a first epidemiologic case control study. Parkinsonism Relat Disord. 2012;18(6):748–752. doi: 10.1016/j.parkreldis.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steingrimsdottir H, Haraldsdottir V, Olafsson I, Gudnason V, Ogmundsdottir HM. Monoclonal gammopathy: natural history studied with a retrospective approach. Haematologica. 2007;92(8):1131–1134. doi: 10.3324/haematol.11284. [DOI] [PubMed] [Google Scholar]
- 14.Filosto M, Cotelli M, Todeschini A, Broglio L, Vielmi V, Rinaldi F, Gregorelli V, Benelle M, Padovani A. Clinical spectrum and evolution of monoclonal gammopathy-associated neuropathy: an observational study. Neurol. 2012;18(6):378–384. doi: 10.1097/NRL.0b013e31826a99e9. [DOI] [PubMed] [Google Scholar]
- 15.Yeung KB, Thomas PK, King RH, Waddy H, Will RG, Hughes RA, Gregson NA, Leibowitz S. The clinical spectrum of peripheral neuropathies associated with benign monoclonal IgM, IgG and IgA paraproteinaemia. Comparative clinical, immunological and nerve biopsy findings. J Neurol. 1991;238(7):383–391. doi: 10.1007/BF00319857. [DOI] [PubMed] [Google Scholar]
- 16.Theaudin M, Lozeron P, Lacroix C, Chretien P, Ducot B, Denier C, Adams D. Short and long-term effect of IVIg in demyelinating neuropathy associated with MGUS, experience of a monocentric study. Rev Neurol. 2011;167(12):897–904. doi: 10.1016/j.neurol.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Lunn MP, Nobile-Orazio E. Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies. Cochrane Database Syst Rev. 2012;5:CD002827. doi: 10.1002/14651858.CD002827.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Koike M, Sugimoto K, Tusui M, Yahata Y. Successful treatment with rituximab in two cases of IgM-monoclonal gammopathy of undetermined significance (MGUS) neuropathy. [Rinsho ketsueki] Jpn J Clin Hematol. 2012;53(4):450–454. [PubMed] [Google Scholar]