Abstract
Functional iron deficiency (FID) incidence is gradually increasing in hemodialysis (HD) patients. Recently, high levels of GDF-15 supressed the iron regulatory protein hepcidin and GDF-15 expression increased in iron-deficient patients. The relationship between FID, GDF-15, and hepcidin is currently unknown. The present study aimed to evaluate the association between GDF-15, hepcidin, and FID in chronic HD patients. Serum GDF-15 and hepcidin concentrations were measured in 105 HD patients and 40 controls. FID is defined as serum ferritin >800 ng/mL, TSAT <25 %, Hb levels <11 g/dL, and reticulocyte haemoglobin content (CHr) <29 pg. Serum GDF-15 and hepcidin levels were increased significantly in HD patients with FID, compared to HD patients without anemia and controls. GDF-15 correlated with ferritin, hepcidin, and CRP in the entire cohort. GDF-15 was related to ferritin and CRP in HD patients with FID. GDF-15 is better diagnostic marker than hepcidin for detection of FID [AUC = 0.982 (0.013) versus AUC = 0.921 (0.027); P = 0.0324]. GDF-15 appears to be a promising tool for detection of FID. High levels of ferritin and CRP correlated with GDF-15. Our results support GDF-15 as a new mediator of FID via hepcidin, chronic inflammation, or unknown pathways.
Keywords: Anemia, GDF-15, Iron, Inflammation
Introductıon
Anemia is a very common complication of chronic kidney disease (CKD) caused by erythropoietin (EPO) [1]. Anemia confers significant risk of cardiovascular disease and contributes to decreased quality of life and overall poor disease outcome [2, 3].
The cause of anemia in patients with CKD is multifactorial: the best-known cause is inadequate EPO production, in addition to iron deficiency, blood loss, hemolysis, chronic inflammation, and other factors, which may include circulating inhibitors of EPO. Erythropoiesis-stimulating agents (ESAs) are the cornerstone of the treatment of anemia secondary to CKD [4]. ESAs increase the absolute demand for iron stores to sustain erythropoiesis [5]. Hyporesponsiveness to ESAs in CKD is very common and generally caused by absolute iron deficiency or functional iron deficiency (FID) [1]. Iron deficiency is almost always present in hemodialysis (HD) patients due to bleeding when needles are removed from the vascular access, blood infiltration of the vascular access, vascular access procedures, frequent blood testing, and clotting or general blood loss in the extracorporeal circuit [4]. Patients receiving ESA and inadequate IV iron therapy are at increased risk for FID [6, 7].
In addition to being an important source of anemia in CKD and HD patients, FID is a gradually increasing source of anemia in general [7–9]. Chronic inflammation and iron replacement contribute to FID development [7]; however, iron sequestration in reticuloendothelial cells is primarily responsible for FID development [7].
Growth differentiation factor-15 (GDF15) is a member of the transforming growth factor β superfamily, which plays important roles in tumorigenesis [10], metastasis [11], atherosclerosis [12], cardiac ischemia/reperfusion injury [13], and cancer-induced anorexia [14]. In addition, recent studies revealed that human erythroblasts secrete GDF15, which suppresses hepcidin expression [15] and contributes to iron overloading in a variety of anemias characterized by profoundly ineffective erythropoiesis [16]. GDF15 is involved in iron homeostasis [17] but is not required to balance iron homeostasis in phlebotomized mice [18].
The relationship between GDF15 and hepcidin is still unknown in HD patients with FID. We aimed to determine the levels of GDF15 and hepcidin in maintenance HD patients with FID.
Material and Methods
This study was approved by the Ethics Committee of Turgut Ozal University and conducted from December 2011 to January 2013. 105 patients with ESRD undergoing HD and 40 healthy controls were recruited. The inclusion criteria were as follows: stable clinical state; adequate HD (urea reduction rate ≥65 %); no thrombosis or acute inflammation (chronically raised but stable C-reactive protein [CRP] 0–50 mg/l using the low-sensitivity method); absence of acute cardiovascular complications (including uncontrolled hypertension, acute coronary syndrome, and acute heart failure), cancer, and connective tissue disease; no absolute iron deficiency, megaloblastic anemia, or hemolytic anemia. We defined absolute iron deficiency (serum ferritin ≤100 ng/mL and TSAT ≤ 20 %), FID (serum ferritin >800 ng/mL, TSAT ≤ 25 %, reticulocyte haemoglobin content (CHr) <29 pg, and Hb levels <11 g/dL) in patients on maintenance HD. Chronic HD patients who had hemoglobin levels of 11–12 g/dL and sufficient iron stores (serum ferritin level greater than 200 ng/mL and TSAT = 30–50 %) after ESA and intravenous (IV) iron therapy were also included in the study. Healthy controls had no anemia (Hb concentration is >13.0 g/dL in males and >12.0 g/dL in females) with sufficient iron stores (ferritin concentration >100 µg/L and transferrin saturation (TSAT) levels >25 %) without chronic disease (hypertension, diabetes mellitus, congestive heart failure, chronic kidney disease, cancer, or chronic infection).
Venous blood samples were obtained for hematological and biochemical screening tests after fasting (overnight between 8.00 and 9.00 a.m. prior to the midweek dialysis session and before heparin administration) and stored at −80 °C for biochemical analyses. Serum creatinine, urea, aspartate aminotransferase, alanine aminotransferase (ALT), calcium, albumin, uric acid, total cholesterol, high-density lipoprotein cholesterol, triglyceride, iron (Fe), total iron binding capacity, transferrin saturation (TSAT), ferritin, calcium (Ca), and phosphorus (P) were measured by standard laboratory techniques using an autoanalyzer (Roche Diagnostics, COBAS INTEGRA 800, Indianapolis, Indiana, USA). Blood containing EDTA anticoagulant was analyzed by an automatic cell counter (ADVIA 2120i, Siemens, New York, USA) for the determination of complete blood count, including red blood cells, hemoglobin (Hb), white blood cells, and reticulocyte hemoglobin content (CHr). urea reduction rate (URR) values were recorded for all HD patients.
Serum levels of GDF-15 were measured by ELISA (R&D Systems, Minneapolis, MN, USA, Cat no: DGD150) with intra- and inter-assay CVs of <2.8 and <6 %, respectively. Hepcidin levels were measured by ELISA (CUSABIO BIOTECH, Newark, New Jersey, USA. Cat no: CSB-E13062 h) with intra- and inter-assay CVs of <8 and <10 %, respectively. The results of GDF-15 and hepcidin were expressed as pg/mL and ng/mL, respectively. All tests were performed according manufacturer instructions by the same person.
Normality of the variable distribution was tested using a Shapiro–Wilk W-test. The results are presented as the mean ± standard deviation, median (interquartile range), or percentage. Student’s t test or the Mann–Whitney U test were used for comparing the two groups. Analysis of variance (ANOVA) (with post hoc Tukey test for unequal groups) or a Kruskall–Wallis test (the difference between the mean of two variables was calculated by a Mann–Whitney U test) were used to compare differences between groups; P < 0.05 was considered statistically significant. Spearman correlation coefficients were used for correlation analysis between GDF-15, hepcidin, and others (clinical or laboratory variables). The cutoffs of GDF-15 and hepcidin for detection of FID were identified by a receiver-operating characteristic (ROC) curve and the area under the ROC curve (AUC) was calculated. AUCs were compared, following the procedure of Hanley and McNeil [19].
A P value of <0.05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics software version 20.0 (SPSS Inc, Chicago, IL, USA).
Results
One hundred and five (n = 105) HD patients and 40 healthy controls were included in the current study. The demographic characteristics of all patients are shown in Table 1. The patients were classified in three groups: Group 1, HD patients who had FID criteria (n = 53); Group 2, HD patients who had hemoglobin level of 11–12 g/dL and sufficient iron stores (Serum ferritin ≥ 200 ng/mL and TSAT = 30–50 %) (n = 52); and Group 3, healthy controls (n = 40).
Table 1.
Baseline characteristics, biochemical parameters, hematologic and iron parameters among three groups
| Group I (n = 53) | Group II (n = 52) | Group III (n = 40) | P value | |
|---|---|---|---|---|
| Age, years | 48.7 ± 18.2 | 49.1 ± 16.5 | 48.5 ± 12.7 | 0.564 |
| Gender, male/female | 26/27 | 24/28 | 19/21 | 0.639 |
| BMI, kg/m2 | 23.7 ± 3.2 | 24.1 ± 2.8 | 23.5 ± 2.7 | 0.456 |
| Hemodialysis duration, months | 39.5 ± 22.1 | 40.2 ± 18.8 | —– | 0.712 |
| Smoking, n (%) | 16 (30.1) | 15 (28.8) | 12 (30) | 0.821 |
| Hemoglobin, g/dL | 9.8 ± 1.2a,b | 11.4 ± 0.7 | 13.2 ± 1.4 | <0.01 |
| MCV, fL | 83.5 ± 5.6 | 84.6 ± 6.2 | 85.1 ± 5.3 | 0.256 |
| MCH, pg/cell | 28.8 ± 3.5 | 29.1 ± 4.1 | 29.5 ± 3.2 | 0.654 |
| MCHC, g/dL | 34.8 ± 2.2 | 35.1 ± 2.5 | 35.5 ± 2.1 | 0.739 |
| WBC, x109/L | 6.4 ± 2.3 | 6.8 ± 2.7 | 6.5 ± 2.8 | 0.852 |
| Platelet, x103/uL | 235.4 ± 100.6 | 248.9 ± 95.8 | 245.7 ± 105.8 | 0.251 |
| CHr, pg | 25.6 ± 3.3a,b | 31.7 ± 2.5 | 32.8 ± 2.9 | <0.01 |
| Iron, ug/dl | 83.8 ± 15.5a,b | 93.8 ± 17.6c | 89.5 ± 14.7 | <0.01 |
| TSAT, % | 18.8 ± 2.7a,b | 38.6 ± 6.5c | 29.1 ± 3.0 | <0.01 |
| Ferritin, ng/ml | 967.2 ± 105.8a,b | 374.3 ± 77.9c | 146.4 ± 34.4 | <0.01 |
| AST, U/L | 12.8 ± 7.9 | 13.5 ± 8.5 | —– | 0.345 |
| ALT, U/L | 14.8 ± 9.2 | 15.1 ± 8.7 | —– | 0.771 |
| Ca (corrected), mg/dl | 8.2 ± 0.8 | 8.1 ± 0.9 | —– | 0.863 |
| P, mg/dl | 5.7 ± 1.2 | 5.9 ± 1.3 | —– | 0.794 |
| iPTH, pg/ml | 295.7 ± 160.5 | 288.6 ± 170.8 | —– | 0.195 |
| Albumin, g/dl | 3.9 ± 0.6 | 3.8 ± 0.7 | —– | 0.809 |
| Total protein, g/dl | 7.6 ± 1.3 | 7.5 ± 1.4 | —– | 0.788 |
| Uric acid, | 6.8 ± 1.8 | 7.1 ± 1.7 | —– | 0.479 |
| Total cholesterol, mg/dl | 180.4 ± 53.2 | 184.1 ± 55.4 | —– | 0.211 |
| Triglycerides, mg/dl | 165.1 ± 45.6 | 162.5 ± 51.2 | —– | 0.672 |
| HDL-C, mg/dl | 38.5 ± 10.5 | 39.2 ± 11.4 | —– | 0.257 |
| URR, % | 69.2 ± 3.4 | 69.3 ± 3.7 | —– | 0.764 |
| GDF-15* | 1239.7 (238.5)d,e | 452.1 (166.5)f | 123.1 (38.6) | <0.001 |
| Hepcidin* | 230.2 (60.3)d,e | 124.7 (45.0)f | 31.6 (14.0) | <0.001 |
| CRP, mg/L | 28.8 ± 15.5b | 24.5 ± 16.2c | 4.5 ± 3.9 | <0.01 |
| Weekly EPO dose, IU | 10 656 (4895) | 5478 (2512) | —– | <0.01 |
| ACEI or ARB, n (%) | 38 (71.6) | 37 (71.1) | —– | 0.704 |
| ß-Blockers, n (%) | 16 (30.1) | 15 (28.8) | —– | 0.318 |
| CCB, n (%) | 21 (39.6) | 20 (38.4) | —– | 0.736 |
| Alpha-blockers, n (%) | 8 (15.0) | 8(15.3) | —– | 0.826 |
* Median (interquartile range), ACEI Angiotensin-converting-enzyme inhibitor, ALT alanine transaminase, ARB angiotensin receptor blockers, AST aspartate transaminase, BMI body mass index, Ca calcium, CHr reticulocyte hemoglobin content, CRP C-reactive protein, Epo erythropoietin, Hb hemoglobin, HDL-C high-density lipoprotein cholesterol, iPTH intact parathyroid hormone, LDL-C low-density lipoprotein cholesterol, MCV mean corpuscular volume, MCH mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, P phosphate, TSAT transferrin saturation, URR urea reduction ratio
ap < 0.01 between Group I and II with post hoc Tukey test
bp < 0.01 between Group I and III with post hoc Tukey test
cp < 0.01 between Group II and III with post hoc Tukey test
dp < 0.001 between Group I and II with Mann–Whitney U test
ep < 0.001 between Group I and III with Mann–Whitney U test
fp < 0.001 between Group II and III with Mann–Whitney U test
There was no difference between Groups 1, 2, or 3 in terms of age, gender, and body mass index (Table 1). The hemoglobin level in Group 1 was significantly lower than the levels in Groups 2 and 3 (9.8 ± 1.2; 11.4 ± 0.7; 13.2 ± 1.4; P < 0.01) (Table 1). The TSAT level in Group 1 was significantly lower than in Groups 2 or 3 (18.8 ± 2.7, 38.6 ± 6.5, 29.1 ± 3.0, P < 0.01), shown in Table 1. On the other hand, the ferritin level in Group 1 was significantly higher than in Groups 2 and 3 (967.2 ± 105.8, 374.3 ± 77.9, 146.4 ± 34.4; P < 0.01), shown in Table 1.
Median Hepcidin (Table 1) and median GDF-15 levels (Fig. 1) in Group 1 were significantly higher than in Groups 2 and 3 [230.2 (60.3), 124.7 (45.0), 31.6 (14.0), P < 0.001 for hepcidin; 1239.7(238.5), 452.1(166.5), 123.1(38.6), P < 0.001 for GDF-15, respectively]. The serum iron level in Group 1 was significantly lower than in Groups 2 and 3 (83.8 ± 15.5, 93.8 ± 17.6, 89.5 ± 14.7; P < 0.01) (Table 1); however, the serum iron levels were not different between Groups 1 and 3 (83.8 ± 15.5, 89.5 ± 14.7; P = 0.216).
Fig. 1.
Box plot showed that GDF-15 levels in group 1 were higher than its levels in group 2 and 3
There was no difference between Group 1 and 2 in terms of serum Ca, Na, K, ALT, PTH, total protein, albumin, or URR (Table 1). The percentage of antihypertensive drug use was not different between Groups 1 and 2, though the weekly dose of EPO in Group 2 was higher than in Group 1.
We observed a positive correlation between GDF15 levels and hepcidin (r = 0.816, P < 0.01), ferritin (r = 0.897, P < 0.01), and CRP (r = 0.568, P < 0.01), while there was a negative correlation between GDF15 and TSAT (r = -0.565, P < 0.01). No correlation was found between GDF15 and other biochemical parameters, age, VKI, or URR. When only the patients in Group 1 were included, there was a positive correlation between GDF15 level and ferritin (r = 0.745, P < 0.01) and CRP (r = 0.341, P < 0.01). Furthermore, while GDF15 level and serum iron (r = −0.303, P = 0.025) were negatively correlated, no correlation was found with hepcidin (r = −0.008, P = 0.953) or TSAT (r = −0.248, P = 0.068).
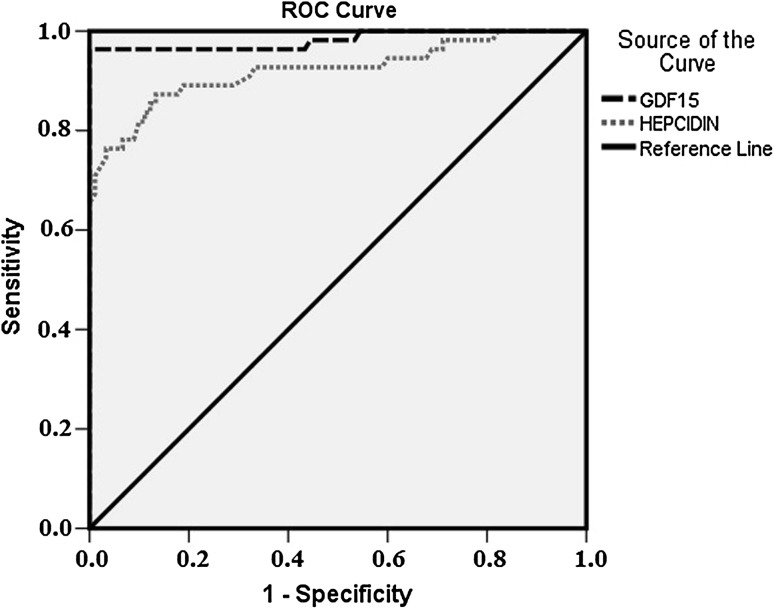
To identify the cutoff values of GDF15 and hepcidin for FID, ROC analysis was performed in whole groups. The optimal cutoff value of GDF15 was 749.5 pg/mL with 96.4 % sensitivity, 100 % specificity, 100 % positive predictive value (PPV), and 98.03 % negative predictive value (NPV); the AUC was 0.982 (Fig. 2). We found the best cutoff value for hepcidin was 167.5 ng/ml with 76.4 % sensitivity, 96.7 % specificity, 93.3 % PPV, and 87 % NPV; the AUC was 0.921 (Fig. 2). We compared the AUC of GDF15 and hepcidin and GDF15 was superior to hepcidin for predicting FID [AUC = 0.982 (0.013) vs AUC = 0.921 (0.027); P = 0.0324].
Fig. 2.
ROC curve showed that GDF-15 is better indicator of FID than Hepcidin when the AUCs were compared [AUC = 0.982 (0.013) vs AUC = 0.921 (0.027); P = 0.0324]
Discussion
The results of the present study are: both GDF15 and hepcidin levels are significantly high in HD patients who have functional iron deficiency; when all patients were evaluated, GDF15 level correlated with hepcidin, TSAT, ferritin, and CRP, though GDF15 correlated only with ferritin and CRP when HD patients with FID were evaluated alone; in ROC analysis, GDF15 was a better indicator of FID than hepcidin.
While anemia is a frequently-seen complication in HD patients, a significant reduction occurred with ESA [4]. In some guidelines, if the ferritin level is >800 ng/ml, intravenous iron is not recommended for 3 months [20, 21] ). In clinical practice, FID develops when intravenous iron is not given and ESA therapy is continued in patients with low transferrin saturation and high ferritin levels, [22]. FID is the most common cause of incomplete ESA response [23]. In the current study, we detected that hepcidin, GDF15, and ferritin levels are significantly high in HD patients who have FID, compared to non-anemic HD patients who have sufficient iron stores,. In both chronic inflammation and cancer, IL-6 level increases and hepcidin synthesis in liver is stimulated [22, 23]. Increased hepcidin causes ferroportin degradation which, in turn, decreases iron absorption from the gastrointestinal system and the availability and mobilization of the iron stored in macrophages [23]. As a result, in spite of sufficient iron stores, a clinical picture of iron deficiency develops [23]. Thus, when compared to the patients in which the target hemoglobin level is reached and who have sufficient iron stores, the high levels of hepcidin in patients with FID is expected. In some studies, hepcidin levels in HD patients who have FID were high, consistent with the results of the present study [24, 25]. We also observed high hepcidin in patients with FID.
The mechanisms that cause high GDF15 levels in HD patients who have FID might be as follows: 1. GDF15 may increase to suppress the high hepcidin level in HD patients who have FID; 2. Expression of GDF 15 is upregulated in response to chronic inflammation [8, 26], present in patients with FID [27]; 3. Iron depletion, which independently causes GDF15 induction, develops in erythroid precursor cells as a result of iron sequestration in macrophages [8, 28].
There was no correlation between GDF 15 level and hepcidin, both in patients with FID and overall. The negative correlation between GDF15 and hepcidin was demonstrated in renal allograft recipients [29]. In cancer patients, when the GDF15 level is less than 2000 pg/ml, GDF15 and hepcidin levels have a positive correlation [30]. On the other hand, no correlation was found between GDF15 and hepcidin in patients with iron deficiency and chronic disease anemia [31]. As already known, GDF15 is a hepcidin suppression factor [15]; however, although the GDF15 level increased, no hepcidin inhibition occurred. Elevated GDF15 (mean 66.000 pg/ml) [15] and high levels of GDF15 (>5000 pg/ml) [32] inhibit hepcidin expression; in the current study, because the mean GDF15 level was 1250 pg/ml, is was not expected to inhibit hepcidin expression. On the other hand, the non-suppression of the hepcidin level, despite the increase in GDF15 might be related to resistance development against the hepcidin suppression effect of GDF15. According to the present study, the GDF15 level is the only predictor that might determine FID. GDF15 might cause FID development by hepcidin-independent or –dependent mechanisms. In a study by Malyszko et al., they found no difference in GDF15 levels in HD patients with or without FID [25]. The most important difference of the current study from that of Malyszko et al. is that they defined FID in a different manner in HD patients and the number of patients with FID (n = 23) is quite low in their study. They defined FID as ferritin above 200 ng/ml and TSAT below 20 %, whereas we defined FID as serum ferritin >800 ng/mL, TSAT <25 %, Hb levels <11 g/dL, and CHr <29 pg. CHr is the one of most established variables for the identification of FID, according to current guidelines [23]. Our definition, by using CHr, strengthened the diagnosis of FID. 68 patients in which FID was detected were included in the study.
Generally, increased ferritin levels are seen in HD patients. When the serum ferritin levels are evaluated alone, it is insufficient for the diagnosis of iron deficiency or iron overload; however, markedly elevated ferritin levels are generally associated with chronic inflammation [33–35]. CRP, an acute phase reactant that is frequently high in HD patients due to microinflammation [36], is more than 50 mg/L in approximately 10 % of HD patients and shows acute infection [36]. CRP is between 0 and 50 mg/L in approximately 90 % of HD patients [36, 37]. Both high levels of ferritin and increased CRP demonstrate that there is a chronic inflammation background in HD patients with FID and, as a result of this inflammatory response, erythrocyte stem cell proliferation decreases, erythropoesis is suppressed, destruction of erythrocytes accelerates, the response to erythropoetin becomes blunt, iron sequestration in reticuloendothelial cells is increased, and hepatic hepcidin secretion is suppressed [37–39]. Thus, high levels of ferritin and CRP, and their correlation with GDF15 in HD patients with FID, suggest that GDF15, in addition to being a strong indicator of chronic inflammation, might be a mediator that leads to FID development.
The KDIGO practice guidelines suggest directed IV iron use be limited to patients with TSAT <30 % and ferritin <800 ng/mL (500 µg/L) [20]. Intravenous iron therapy for FID anemia in HD patients improves anemia parameters despite the long-term safety and efficacy of this treatment strategy is still unknown [21]. Furthermore, GDF15 may be a novel potential candidate for therapy of FID anemia in HD patients.
The serum hepcidin level has been considered to be the best predictor of iron-restricted erythropoiesis. Some studies have shown a relationship between the serum ferritin and hepcidin levels of dialysis patients [40, 41]. They reported an inverse correlation between serum hepcidin levels and epoetin doses and a decline in the hepcidin level after the start of epoetin therapy. On the other hands, Kato et al. showed no difference between the hepcidin levels of epoetin-responsive and epoetinresistant dialysis patients [42], and Ford et al. revealed no relationship between blood hepcidin levels and epoetin doses [43]. Serum hepcidin measurements can be benefical a biomarker to detect FID and monitorize the iron status and iron demand in dialysis patients according to our results in consistent with these studies.
This cross-sectional study had some limitations. First, it was not possible to establish causal relationships between GDF15 and FID. Second, serial measurements of GDF15 and hepcidin and prospective follow-up of these parameters could be more appropriate for determination of the relationship. Third, this study had a relatively small number of patients.
In conclusion, FID is frequently seen in HD patients and its most important known reason is insufficient iron mobilization from macrophages. Hepcidin has an important role in the physiopathology of iron deficiency anemia. GDF-15 could be considered to be a risk marker for FID. When the current study is supported with prospective randomized trials and experimental studies, the exact role of GDF15 in functional anemia will become clear and treatment modalities targeting GDF15 will become a current issue.
Acknowledgments
Conflict of interest
We have no conflict of interest.
References
- 1.Drüeke T. Hyporesponsiveness to recombinant human erythropoietin. Nephrol Dial Transplant. 2001;16:25–28. doi: 10.1093/ndt/16.suppl_7.25. [DOI] [PubMed] [Google Scholar]
- 2.Gouva C, Nikolopoulos P, Ioannidis JP, et al. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66:753–760. doi: 10.1111/j.1523-1755.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 3.Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol. 2005;16:3403–3410. doi: 10.1681/ASN.2005030226. [DOI] [PubMed] [Google Scholar]
- 4.Hörl WH. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol. 2013;9(5):291–301. doi: 10.1038/nrneph.2013.21. [DOI] [PubMed] [Google Scholar]
- 5.Cavill I. Iron status as measured by serum ferritin: the marker and its limitations. Am J Kidney Dis. 1999;34(Suppl 2):S12–S17. doi: 10.1053/ajkd.1999.v34.aajkd0344b0012. [DOI] [PubMed] [Google Scholar]
- 6.Lankhorst CE, Wish JB. Anemia in renal disease: diagnosis and management. Blood Rev. 2010;24(1):39–47. doi: 10.1016/j.blre.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi T, Kuragano T, Kaibe S, Nagasawa Y, Hasuike Y. Should we reconsider iron administration based on prevailing ferritin and hepcidin concentrations? Clin Exp Nephrol. 2012;16(6):819–826. doi: 10.1007/s10157-012-0694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanWyck DB, Bailie G, Aronoff G. Just the FAQs: frequently asked questions about iron and anemia in patients with chronic kidney disease. Am J Kidney Dis. 2002;39:426–432. doi: 10.1053/ajkd.2002.30566. [DOI] [PubMed] [Google Scholar]
- 9.Besarab A. Evaluating iron sufficiency: a clearer view. Kidney Int. 2001;60:2412–2414. doi: 10.1046/j.1523-1755.2001.00078.x. [DOI] [PubMed] [Google Scholar]
- 10.Unsicker K, Spittau B, Krieglstein K. The multiple facets of the TGF-β family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev. 2013;24(4):373–384. doi: 10.1016/j.cytogfr.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Aw Yong KM, Zeng Y, Vindivich D, Phillip JM, Wu PH, Wirtz D, Getzenberg RH. Morphological effects on expression of growth differentiation factor 15 (GDF15), a marker of metastasis. J Cell Physiol. 2014;229(3):362–373. doi: 10.1002/jcp.24458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggers KM, Kempf T, Lind L, Sundström J, Wallentin L, Wollert KC, Siegbahn A. Relations of growth-differentiation factor-15 to biomarkers reflecting vascular pathologies in a population-based sample of elderly subjects. Scand J Clin Lab Invest. 2012;72(1):45–51. doi: 10.3109/00365513.2011.626072. [DOI] [PubMed] [Google Scholar]
- 13.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98(3):351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 14.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13(11):1333–1340. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 15.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 16.Tanno T, Miller JL. GDF15 expression and iron overload in ineffective erythropoiesis. Rinsho Ketsueki. 2011;52(6):387–398. [PubMed] [Google Scholar]
- 17.Lakhal S, Talbot NP, Crosby A, Stoepker C, Townsend AR, Robbins PA, Pugh CW, Ratcliffe PJ, Mole DR. Regulation of growth differentiation factor 15 expression by intracellular iron. Blood. 2009;113:1555–1563. doi: 10.1182/blood-2008-07-170431. [DOI] [PubMed] [Google Scholar]
- 18.Casanovas G, Spasic MV, Casu C, Rivella S, Strelau J, Unsicker K, Muckenthaler MU. The murine growth differentiation factor 15 is not essential for systemic iron homeostasis in phlebotomized mice. Haematologica. 2013;98(3):444–447. doi: 10.3324/haematol.2012.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO Clinical Practice Guideline for anemia in chronic kidney disease. Kidney Int. 2012;2 (Suppl):279–335. [Google Scholar]
- 21.Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, Kuriyama S, Komatsu Y, Suzuki M, Nakai S, Hattori M, Babazono T, Hiramatsu M, Yamamoto H, Bessho M, Akizawa T. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial. 2010;14(3):240–275. doi: 10.1111/j.1744-9987.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi T, Kuragano T, Kaibe S, Nagasawa Y, Hasuike Y. Should we reconsider iron administration based on prevailing ferritin and hepcidin concentrations? Clin Exp Nephrol. 2012;16(6):819–826. doi: 10.1007/s10157-012-0694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas DW, Hinchliffe RF, Briggs C, Macdougall IC, Littlewood T, Cavill I. British Committee for Standards in Haematology. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161(5):639–648. doi: 10.1111/bjh.12311. [DOI] [PubMed] [Google Scholar]
- 24.Małyszko J, Małyszko JS, Hryszko T, Pawlak K, Mysliwiec M. Is hepcidin a link between anemia, inflammation and liver function in hemodialyzed patients? Am J Nephrol. 2005;25(6):586–590. doi: 10.1159/000089266. [DOI] [PubMed] [Google Scholar]
- 25.Małyszko J, Koc-Żórawska E, Levin-Iaina N, Małyszko J, Koźmiński P, Kobus G, Myśliwiec M. New parameters in iron metabolism and functional iron deficiency in patients on maintenance hemodialysis. Pol Arch Med Wewn. 2012;122(11):537–542. [PubMed] [Google Scholar]
- 26.Kempf T, Wollert KC. Growth-differentiation factor-15 in heart failure. Heart Fail Clin. 2009;5:537–547. doi: 10.1016/j.hfc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Goodnough LT. Iron deficiency syndromes and iron-restricted erythropoiesis (CME) Transfusion. 2012;52(7):1584–1592. doi: 10.1111/j.1537-2995.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez JM, Schaad O, Durual S, Cossali D, Docquier M, Beris P, Descombes P, Matthes T. Growth differentiation factor 15 production is necessary for normal erythroid differentiation and is increased in refractory anaemia with ring-sideroblasts. Br J Haematol. 2009;144:251–262. doi: 10.1111/j.1365-2141.2008.07441.x. [DOI] [PubMed] [Google Scholar]
- 29.Malyszko J, Koc-Zorawska E, Malyszko JS, Glowinska I, Mysliwiec M, Macdougall IC. GDF15 is related to anemia and hepcidin in kidney allograft recipients. Nephron Clin Pract. 2013;123(1–2):112–117. doi: 10.1159/000351810. [DOI] [PubMed] [Google Scholar]
- 30.Jiang F, Yu WJ, Wang XH, Tang YT, Guo L, Jiao XY. Regulation of hepcidin through GDF-15 in cancer-related anemia. Clin Chim Acta. 2014;428:14–19. doi: 10.1016/j.cca.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Theurl I, Finkenstedt A, Schroll A, Nairz M, Sonnweber T, Bellmann-Weiler R, Theurl M, Seifert M, Wroblewski VJ, Murphy AT, Witcher D, Zoller H, Weiss G. Growth differentiation factor 15 in anaemia of chronic disease, iron deficiency anaemia and mixed type anaemia. Br J Haematol. 2010;148(3):449–455. doi: 10.1111/j.1365-2141.2009.07961.x. [DOI] [PubMed] [Google Scholar]
- 32.Tanno T, Noel P, Miller JL. Growth differentiation factor 15 in erythroid health and disease. Curr Opin Hematol. 2010;17(3):184–190. doi: 10.1097/MOH.0b013e328337b52f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fishbane S, Kalantar-Zadeh K, Nissenson AR. Serum ferritin in chronic kidney disease: reconsidering the upper limit for iron treatment. Semin Dial. 2004;17:336–341. doi: 10.1111/j.0894-0959.2004.17359.x. [DOI] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in hemodialysis patients. Nephrol Dial Transplant. 2004;19:141–149. doi: 10.1093/ndt/gfg493. [DOI] [PubMed] [Google Scholar]
- 35.Rambod M, Kovesdy CP, Kalantar-Zadeh K. Combined high serum ferritin and low iron saturation in hemodialysis patients: the role of inflammation. Clin J Am Soc Nephrol. 2008;3(6):1691–1701. doi: 10.2215/CJN.01070308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenvinkel P, Wanner C, Metzger T, et al. Inflammation and outcome in end-stage renal failure: does female gender constitute a survival advantage? Kidney Int. 2002;62:1791–1798. doi: 10.1046/j.1523-1755.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- 37.Wanner C, Richardson D, Fouque D, Stenvinkel P. OPTA—Influence of inflammation/infection on anaemia therapy in haemodialysis patients. Nephrol Dial Transplant. 2007;22(Suppl 3):iii7–iii12. [Google Scholar]
- 38.Nitta K, Akiba T, Takei T, et al. Inflammation and resistance to erythropoietin in hemodialysis patients. Acta Haematol. 2002;108:168–170. doi: 10.1159/000064704. [DOI] [PubMed] [Google Scholar]
- 39.Weiss G, Goodnough LT. Anemia of chronic disease. New Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 40.Ashby DR, Gale DP, Busbridge M, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–981. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 41.Weiss G, Theurl I, Eder S, et al. Serum hepcidin concentration in chronic haemodialysis patients: associations and effects of dialysis, iron and erythropoietin therapy. Eur J Clin Invest. 2009;39:883–890. doi: 10.1111/j.1365-2362.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- 42.Kato A, Tsuji T, Luo J, Sakao Y, Yasuda H, Hishida A. Association of prohepcidin and hepcidin-25 with erythropoietin response and ferritin in hemodialysis patients. Am J Nephrol. 2008;28:115–121. doi: 10.1159/000109968. [DOI] [PubMed] [Google Scholar]
- 43.Ford BA, Eby CS, Scott MG, Coyne DW. Intra-individual variability in serum hepcidin precludes its use as a marker of iron status in hemodialysis patients. Kidney Int. 2010;78:769–773. doi: 10.1038/ki.2010.254. [DOI] [PubMed] [Google Scholar]