Abstract
Cervical cancer control includes primary prevention through vaccination to prevent human papillomavirus (HPV) infection and secondary prevention through screening to detect and treat cervical precancerous lesions. This review summarizes the evidence for the population impact of vaccines against oncogenic HPV types in reducing the prevalence of cervical precancerous lesions. We examine the gradual shift in screening technology from cervical cytology alone to cytology and HPV cotesting, and finally to the recognition that HPV testing can serve alone as the new screening paradigm, particularly in the initial post-vaccination era. We should expect an impact on screening performance and practices, as cohorts of HPV-vaccinated girls and adolescents reach cervical cancer screening age. In preparation for changes in the screening paradigm for the vaccination era, we propose that policymaking on cervical cancer screening should mirror current practices with other cancers as benchmarks. Cervical precancerous lesions will become a very rare condition following the widespread implementation of HPV vaccines with broader coverage in the number of preventable oncogenic types. Irrespective of screening technology, the false positive results will far outnumber the true positive ones, a tipping point that will herald a new period when the harms from cervical cancer screening will outweigh its benefits. We present a conceptual framework to guide decision making when we reach this point within 25–30 years.
Keywords: Human papillomavirus, HPV vaccination, HPV screening, cytology, HPV DNA testing, cervical cancer
1. Introduction
There are two complementary approaches for the prevention of cervical cancer: primary prevention through vaccination to prevent human papillomavirus (HPV) infection, this disease’s causal agent, and secondary prevention through screening to detect and treat cervical precancerous lesions before they become invasive. Broad implementation of HPV vaccination began at the end of 2006, whereas cervical cancer screening with Papanicolaou cytology has been the mainstay of cervical cancer control for at least 50 years. Although implemented independently, these cervical cancer prevention activities are inherently components of a single process [1].
In most countries that implemented it on a large scale and with ongoing quality assurance as part of opportunistic or organized programs, cytology screening has led to significant reductions in cervical cancer incidence and mortality, making screening practices for this disease one of the most successful public health prevention activities worldwide. Yet, cytology has significant limitations, most notably a low sensitivity and poor reproducibility. Knowledge on the biology and natural history of cervical HPV infection and carcinogenesis has shifted the focus of cervical cancer screening to detecting its causal agent, which permits identifying with greater sensitivity the associated high-grade dysplastic changes in the cervix, thus enabling prompt medical intervention.
Several screening methods focusing on the detection of HPV have emerged in recent years, as commercial assays that identify women who harbor cervical infections with at least one of 12–14 high-risk HPV (HR-HPV) types, which are associated with most cases of pre-invasive and invasive cervical neoplasia. Some assays also detect the presence of HPV16 and HPV18 individually and separately from the remaining HR-HPVs [2], given the importance of HPV16 and HPV18 in terms of etiologic fraction in cervical cancer.
Testing for the presence of HPV nucleic acids using clinically validated assays is advantageous largely in its ability to direct early detection further upstream in cervical carcinogenesis relative to conventional cytology. Other documented benefits of HPV testing include its (i) higher sensitivity and reproducibility compared to cytology, which requires subjective appraisal by cytotechnicians [3]; (ii) ability to be easily automated, centralized, and be quality-checked for large specimen throughput, compared to cytology; (iii) adequate safety despite lengthened screening intervals; and (iv) being more cost-effective than cytology, if deployed for high volume testing [4].
Another important dividend of HPV testing is the ability to use self-collected samples, which have the potential to increase coverage of cervical cancer screening to remote areas or to women who are not directly reached by primary healthcare in urban areas. In addition to the above advantages that would come from a change in the screening paradigm from cytology to HPV testing it is also plausible to assume that the latter will serve the needs of cervical cancer screening in the post-vaccination era more efficiently than cytology [5].
In this review, we summarize the evidence for the impact of HPV vaccination in reducing the prevalence of cervical precancerous lesions and the gradual shift in screening technology from cytology alone to cytology and HPV cotesting, and finally to the recognition that HPV testing can serve alone as the anchor technology in screening. We focus on the expected impact on screening performance and practices that will occur as the cohorts of HPV-vaccinated girls and adolescents reach the age to be screened for cervical cancer. We also provide an analogy with other screening activities in prostate, colorectal, lung, and breast cancers prevention. As HPV vaccination becomes a truly universal public health intervention with further coverage in the number of HR-HPV types that can be prevented, cervical precancerous lesions will become a very rare condition. When this happens, guidelines will have to be revisited in the future to realistically consider the balance of benefits to harms from screening, even on the basis of improved molecular HPV tests.
2. Primary prevention through HPV vaccination
As of this writing, there are three commercially available HPV vaccines based on virus-like particles of the L1 capsid protein. They are remarkably efficacious and safe [6–13]. Table 1 shows their characteristics and target population. Vaccine safety and efficacy have been confirmed in pre- and post-licensure studies [11,12,14]. Fewer than the currently recommended three doses of HPV vaccines has been proven to be effective [15,16]. A switch from a three- to a two-dose schedule for primary immunization programmes is hence anticipated, especially in younger adolescents aged between 9 and 13 years [10]. Development and clinical validation of new HPV vaccines by pharmaceutical companies has continued. The next generation of vaccines may increase the range of protection against additional HR-HPV types, as well as to infections and associated diseases caused by mucosal and cutaneous HPV types alike, thus targeting most HPV types that can potentially cause benign or malignant diseases [17].
Table 1.
Characteristics of currently available HPV vaccines
| Bivalent | Quandrivalent | Nonavalent | |
|---|---|---|---|
| Brand name | CERVARIX | GARDASIL®, SILGARD | GARDASIL®9 |
| Manufacturer | GlaxoSmithKline Biologicals, Rixensart, Belgium | MERCK & CO., INC., Whitehouse station, NJ, USA | MERCK & CO., INC., Whitehouse station, NJ, USA |
| Approval year, FDA HPV types | 2009 16, 18 |
2006 6, 11 16, 18 |
2014 6, 11 16, 18 31, 33, 45, 52, 58 |
| Target population | Females aged 9–25 years | Females aged 9–26 years Males aged 9–26 years |
Females aged 9–26 years Males aged 9–15 years |
| Dose and schedule | Three doses, 0.5ml/dose at 0, 1, and 6 months | Three doses, 0.5ml/dose at 0, 2, and 6 months | Three doses, 0.5ml/dose at 0, 2, and 6 months |
FDA: Food and Drug Administration
Following the introduction of the first two HPV vaccines, the United States, Canada, Australia, and the United Kingdom were the first to implement national vaccination against anogenital HPV infections [18]. High coverage has generally been achieved, especially in countries that implemented school-based vaccination programmes and those that promote routine co-administration of HPV vaccines with other vaccines. Also, HPV vaccines were nationally introduced and demonstration projects were launched in many low-income countries including Rwanda, Bhutan, Ghana, Kenya, Lao, Madagascar, Malawi, Niger, Sierra Leone, and Tanzania [19].
3. Population impact of HPV vaccination
Given the long latency between initial infection and invasive cancer, the full population-level effect of HPV vaccination on the incidence of cervical cancer will not be fully known before several years have passed. Epidemiologic surveillance has addressed early- (prevalence of HPV and/or genital warts), intermediate- (HPV-related precancerous lesions) and long-term (HPV-related cancers) surrogate measures of vaccine efficacy. Overall, HPV vaccination has been effective in protecting against persistent HPV16 and HPV18 infection and in reducing the burden of genital warts (because of the protection against HPV6 and HPV11, which the quadrivalent vaccine provides, Table 1) across diverse populations [19].
3.1. Decline in the prevalence of HPV-related outcomes
A systematic review and meta-analysis of 20 ecologic population-based studies from high-income countries (7 on HPV infection, 11 on anogenital warts, and 2 on high-grade cervical lesions) reported a significant 68% decrease in HPV16 and 18 infections, and a 61% decrease in anogenital warts between pre- and post-vaccination periods with the bivalent and quadrivalent vaccines among girls 13–19 years of age in countries with female vaccination coverage of at least 50% [20]. In countries with less than 50% coverage, significant reductions in HPV16/18 and anogenital warts also occurred in girls younger than 20 years of age [20]. Being the first to adopt HPV vaccination and because of the characteristics of its screening program, Australia was the first country to report a significant decrease in high-grade precancerous cervical lesions in girls aged 15–19 years [21]. Another systematic review of 16 studies documented a significant reduction in the incidence of genital warts as an early outcome of the quadrivalent HPV vaccine, especially in countries with high vaccine uptake [22]. Evidence of cross-type protection, primarily for HPV 45 and, to a lesser extent, for HPVs 31 and 33 was also documented [23].
At the population level, there was a protective effect of the quadrivalent HPV vaccine against cervical precursor lesions among Danish women in the birth cohort 1989 to 1999, based on linkage to individual HPV vaccination status (2006–2012) obtained from nationwide registries [24]. Compared with non-vaccinated women, those vaccinated had a reduced risk of atypical squamous cells of undetermined significance (ASC-US) or worse by up to 60%, and a reduced risk for cervical intraepithelial neoplasia (CIN) grades 2 and 3 by up to 80%. Similarly, evidence of vaccine effectiveness (impact on HPV prevalence and incidence of genital warts) based on individual vaccination status was confirmed by other population-based studies [25–29].
The new and broader spectrum Gardasil 9 vaccine extends protection against five additional HPV types (Table 1). It will have the potential to prevent almost 90% of cervical and other HPV-related cancers worldwide [30]. It is expected that this new nonavalent vaccine will be implemented globally and eventually replace the quadrivalent Gardasil. When this happens, there will be a further impact in reducing the prevalence of cervical precancerous lesions and in decreasing the burden of HPV infections.
4. Secondary prevention through screening
Following the decline in the prevalence of cervical precancerous lesions, which is already evident in the first nine years of the post-vaccination era, is an anticipated decrease in the positive predictive value (PPV) of cytology, otherwise explained as a decline in the probability that a woman who tests positive actually has the disease. This phenomenon would happen with any screening test under conditions of lowered disease prevalence, including HPV testing. However, due to the subjectivity involved in cytotechnicians’ appraisal of cytology smears, there will be an additional strain on the diagnostic accuracy of cytology. For, under conditions of decreased lesion prevalence, sensitivity could drop due to the reduced attention paid to rising proportions of slides that are unremarkable. In turn, this may increase the rate of false-negative cytology reports, thus effectively diminishing the value of cytology from its current primary screening role altogether [31].
Concurrently, a decline in specificity may also result should cytotechnicians, under pressure to abide by strict quality assurance, assign greater importance to reactive atypias and inflammation in smears for fear of missing a relevant abnormality. The main concern with this consequential increase in false-positive results would be a rise in unnecessary referrals to invasive colposcopy procedures and heightened patient stress. The abovementioned qualitative changes affecting clinical utility of conventional cytology screening due to cytotechnicians’ appraisals underscores the importance of effective quality control practices in a post-vaccination era.
4.1. HPV testing
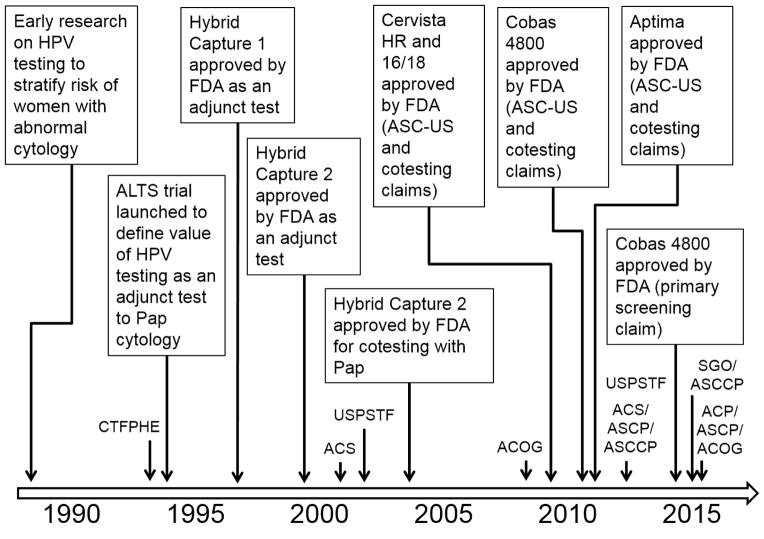
Concomitant to the clinical development and deployment of HPV vaccination in most countries is the gradual penetration of HPV testing in cervical cancer screening. Figure 1 shows a timeline of the adoption of HPV testing, with an emphasis on the U.S. experience. During the 1990’s HPV testing was restricted to an adjunctive role to cytology, as a colposcopy triage test for women with ASC-US smears. Beginning in 2002–03, HPV testing was elevated to a cotesting role, as parallel testing with cytology to permit extended screening intervals for women 30 years and older. The ASC-US triage application prevailed for settings that continued to rely on cytology as the standalone test (or for women up to the age of 29 years). The coexistence of the two clinical applications has persisted until today. The need for triaging cases that were HPV positive and cytology negative gave rise to the need for partial genotyping for HPVs 16/18. The first genotyping test to fulfill this claim was approved in 2009. Others followed subsequently to fill these clinical applications in the US market. Professional guidelines were published in 2012 specifying more liberal screening intervals for cotesting (5 years) for women ages 30–65 [32]. Most recently, end-of-study results from the ATHENA trial, the largest U.S. prospective screening study of HPV primary screening, showed that cotesting provided minimal increased protection against the development of CIN grade 2 or worse compared to HPV primary screening. This finding supported the use of the latter in women aged 25 years and older with triage of HPV-positive using genotyping and reflex cytology [33]. The findings from the ATHENA study led the FDA to approve in 2014 the first assay for a primary screening claim, i.e., as the frontline screening test for women ages 25–65. Women testing positive for HPVs 16 and/or 18 are to be referred for colposcopy, those positive exclusively for one or more of the remainder of the HR-HPVs should be triaged with cytology and, if the latter is positive (ASC-US or worse), colposcopy is indicated, as well. The breakthrough was in the recommendation for the vast majority of women who test HPV negative; they are to be screened again no sooner than 3 years later [34].
Figure 1.
North American timeline for the adoption of clinically-validated HPV assays used in cervical cancer screening. Adoption milestones are shown in the rectangles; publication of professional guidelines is shown close to the time axis. Abbreviations: ALTS, ASCUS-LSIL Triage Study; FDA, Food and Drug Administration; ASC-US, atypical squamous cells of undetermined significance; CTFPHE, Canadian Task Force for Periodic Health Examination; ACS, American Cancer Society; USPSTF, U.S. Preventive Services Task Force; ACOG, American Congress of Obstetricians and Gynecologists; ASCP, American Society for Clinical Pathology; ASCCP, American Society for Colposcopy and Cervical Pathology; SGO, Society of Gynecologic Oncology.
While partial HPV genotyping made inroads in the U.S, cytology remained also as a valid triage option for women who tested positive for any of the 14 HR-HPV types as combined testing. Cervical cancer screening with primary HPV testing followed by cytology triage has gained favor in Europe and in Canada because of the excellent safety relative to cotesting, while reducing the number of required tests by nearly half, with consequent cost reductions for screening programs [35]. Indeed, combining HPV primary screening with cytology triage affords greater reassurance of the absence of cervical lesions. It enables extended intervals between screening rounds for up to twice the maximum duration permitted by conventional cytology [36].
The feasibility and effectiveness of HPV testing with cytology triage for screening cervical cancer in routine practice was assessed within a publicly funded university-affiliated hospital in Montreal, Canada [37]. Relative to the historic cytology-only era, the new screening approach increased detection of precancerous cervical lesions by nearly 3-fold and more than doubled the yield of detecting these lesions per colposcopy performed. Another benefit was to reduce more than 10-fold the cytology workload. In all, these benefits came at the expense of only a moderate increase in the number of colposcopy referrals.
To be sure, not all evidence is overwhelmingly in favor of primary HPV testing. One large study found that cotesting was more sensitive for the detection of CIN grade 3 or worse in women aged 30 to 65 years compared with HPV-only testing, raising concerns about abandoning cytology as a test to be used in parallel with HPV [38].
Other promising, yet not fully validated, novel triage tests of HPV-positive women include slide assessment based on combined detection of the p16INK4a and Ki-67 biomarker protein expression in cervical cytology specimens, [39,40] HPV genotyping [41], and the use of markers including DNA methylation [42,43] and viral load [44].
5. Screening conditional on vaccination status
So far, no country or professional society has yet proposed different screening policies conditional on vaccination status. Standard age-specific guidelines continue to be adopted to screen women irrespective of vaccination history. There is no empirical evidence from randomized controlled trials (RCT) that permit an unequivocal recommendation concerning practice guideline changes towards a later age of onset and less frequent cervical cancer screening for vaccinated women or for the population as a whole in the post-vaccination era. Because of the enormous costs that the design of such trials would incur, it is possible that only evidence from ecologic or observational studies will become available in the future to support changes in practice.
For the US specifically, there are other important considerations that support maintaining the status quo. These include: (i) concerns about low and socioeconomically dependent vaccination uptake; (ii) opportunistic nature of cervical cancer screening, even in more controlled scenarios of managed care, making a call-recall system not viable at present; and (iii) vaccination registries which are yet to become an established norm, precluding the possibility of allowing physicians to have access to reliable vaccination histories when deciding about the level of protection for an individual woman.
Despite the above, physicians may wonder if at an individual risk and benefit management level they would have the option of recommending a more liberal screening strategy to their patients based on self-reported vaccination histories. A woman who was fully vaccinated prior to the onset of sexual debut is substantially protected against cervical lesion development for at least the next decade of her life. Therefore, it stands to reason that the provider would have the option of recommending for such a patient a later age for screening initiation, and, possibly, a longer interval between screenings than are currently accepted as standards of practice. Before such a decision can be reached, however, the clinician must bear in mind several key premises for assuming that the patient’s risk is indeed minimal. First, the woman’s vaccination history must be credible, preferably via medical chart or school records; self-reported histories would be largely unreliable. Second, the provider must ascertain that the full course of vaccination was completed prior to onset of sexual activity, a judicious assurance if the record indicates that the woman received all doses during pre-adolescent years. Third, the clinician must be sufficiently certain, upon deciding on a delayed age at onset for screening, that the patient will not miss the future appointment. Fourth, the provider must have a frank discussion with the patient on lifestyle, sexual behavior, and other characteristics that may expose her to HPV infection during the period preceding the first scheduled screening in order to assign the women an overall risk level which must be judged to be lower than average. Fifth, the first screening done at a later age must be via an acceptable technology that guarantees maximal sensitivity in detecting cervical lesions caused by HPV types other than 16/18. If all the above conditions can be reasonably met, the physician would be able to extend the age at onset of screening for a vaccinated woman to 25 years, from the average-risk recommendation of 21 years, which the guidelines prescribe today [32,34].
The above considerations are obviously intended to err on the side of caution. As knowledge on vaccination impact increases and successive cohorts of vaccinated young women reach screening age, better evidence will have accrued with quantitatively more robust post-vaccination estimates of the reduction in risk. Although some key cohort studies have indicated that the natural history of non-HPV16/18 lesions carries a better prognosis than those elicited by HPVs 16/18 [45], more evidence is needed with respect to the detectability of non-16/18 lesions via different screening methods.
Contrary to concerns that vaccination might negatively impact a woman’s decision to undertake cervical screening, uptake of the HPV vaccine in the United Kingdom was found to be positively correlated with uptake of cervical screening, and cytological abnormalities declined to 13.9% in vaccinated women compared to 16.7% in unvaccinated women [46]. In Sweden, opportunistic HPV-vaccination was associated with an equal or higher attendance after invitation to cervical screening [47].
6. Analogy with other screening activities in cancer prevention
In recent years, policymakers have begun to look at cancer screening through a more conservative prism [48,49]. The recent decision by the US Preventive Services Task Force to recommend against routine prostate cancer screening shows how a full consideration of the balance of benefits to harms is needed for a complete assessment of the evidence concerning a screening activity [50]. A controversial about-face applies also to breast cancer screening [51]. Simple demonstration via RCTs that screening leads to a reduction in cause-specific mortality is no longer the unassailable evidence that is required. Today, policymaking on cancer screening assesses not only the extent of benefit in mortality reduction (and its validity) but also the harms that can come from screening, its costs, utilities, the political risk of inaction, the societal and providers’ tolerance to risk and their preferences, patient choices, and other imponderables. The latter include professional activism for or against changes in the status quo for a particular cancer control strategy because of fear of loss of income.
This new framework of decision making has up until recently left cervical cancer screening untouched. There was never an RCT of cytology screening that permitted adoption of this technology in the 1960’s. The evidence in support of cytology is based only on observational studies, such as case-control and cohort-type investigations, and ecologic comparisons. The latter include observing cervical cancer rates before and after screening, as well as correlating cervical cancer incidence with screening intensity and coverage [52]. As mentioned above, however, for HPV testing the bar was set higher than for cytology. RCTs were essential in proving the advantages of HPV testing relative to cytology.
Irrespective of the complexity of variables that are considered by policymakers in deciding for or against a screening intervention for cancer, one criterion stands as among the most important: the disease to be prevented must be an important public health problem. There are no benchmarks for deciding what is important enough, however; morbidity and mortality are examined in combination. A common enough cancer that has a poor prognosis satisfies this criterion. Historically, policymaking in cancer screening has been era-dependent. Cervical cancer screening began in Western countries in the 1960’s, an era when incidence rates were well above 20 per 100,000 women per year and less than half of the patients survived 5 years. More importantly, however, the then new screening technology (cytology) required little infrastructure and resources, and clinicians could easily access the target anatomical site. This combination of a simple methodology and observable disease process permitted studying the effects of treatment on cervical carcinogenesis. Cervical cancer screening with the Pap test thus became the paradigm against which all other cancer screening interventions had to be judged.
In Canada and in the US, as well as in most Western industrialized countries, cervical cancer morbidity and mortality is much less today than it was in the 1960’s. Other cancers for which screening modalities exist, i.e., prostate, colorectal, lung, and breast, have become far more common relative to cervical cancer. Moreover, unlike cervical cancer, there are RCTs that show cause-specific mortality reduction from use of the respective screening technologies for these cancers. Yet, there is much less population coverage and clinical conviction that we should screen for prostate, colorectal, lung, and breast cancers than we do for cervical cancer.
A recent cross-cancer comparison of disease burden and screening practices for women in the US showed that guidelines propose the age to start screening for breast and colorectal cancer at a point in life (50 years) when the underlying risk of these diseases is much higher than for cervical cancer at the onset of screening (21 years) [53]. At all ages, breast and colorectal cancers are much more common than cervical cancer. Colorectal cancer in particular is a good example of a disease much like cervical cancer, i.e., screening leads to the discovery of a precancerous state (adenomas) that is amenable to treatment and there is proof of mortality reduction from screening. Yet, risk of high-grade cervical lesions at age 21 is about 50 times lower than the rate of colorectal adenomas in women at age 50, the age of onset of colorectal cancer screening [53]. This indicates that, for the US at least, there is far less tolerance of risk for cervical cancer than there is for colorectal cancer, despite the fact that these two diseases imply comparably unfavourable prognosis for the patients.
7. Will cervical cancer screening be eventually eliminated?
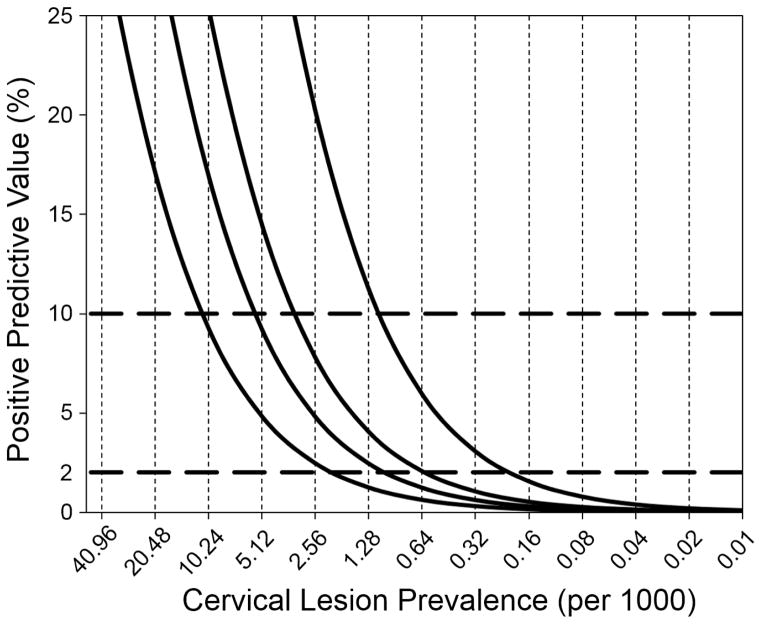
With the progressive impact of HPV vaccination in reducing rates of precancerous cervical lesions, the above anomaly in societal tolerance to cervical cancer risk will become even more inequitable. Even with a new improved technology for cervical cancer screening, i.e., HPV testing, there will be a time when HPV vaccination will have attained sufficiently large coverage and for a sufficiently long time to permit all vaccinated birth cohorts to be in the screening age span (21–65 years in the US). When this happens, the target lesions of screening will have become so rare that the harms from screening may outweigh its benefits. Figure 2 illustrates this situation by considering the PPV of the future screening test. The PPV is a good indicator of the futility (if it is too low) or the utility (if it is high enough) of a screening activity. Today’s HPV tests perform at a primary screening capacity with sensitivity and specificity around 95% and 90%, respectively. The graph shows two risk thresholds to assist clinical decision making in cervical cancer screening: 2% and 10% [54]. The PPV will be remarkably low even for the most optimistic scenario of test performance (99% specificity) when lesion prevalence falls below 0.16 per 1000 women (~0.02%). With more realistic estimates of specificity the futility of screening will be even greater, with PPVs much below 1%. In other words, a positive result under such conditions will most likely be false and trigger diagnostic activities that may cause harm to the woman. Most importantly, test performance at such low disease prevalence will not be coherent with the need for risk stratification based on criteria used in the US today [54].
Figure 2.
Projected impact on the positive predictive value of a future cervical cancer screening test following reductions in precancerous lesion prevalence post HPV vaccination. The X axis indicates in a log scale (base 2) the impact of HPV vaccination on the prevalence of lesions whereas the Y axis shows the positive predictive value of the future screening test. Sensitivity is assumed constant at 99% and specificity as 90%, 95%, 97%, and 99%, (curves from left to right). The horizontal broken lines indicates today’s 2% and 10% risk thresholds of a pre-existing lesion to guide clinical decision regarding immediate colposcopy referral (> 10%), repeat screening in the short-term (2%–10%), or continue low-intensity screening of average-risk women (< 2%).
The above scenario of degradation in screening utility is highly plausible even with the first generation of HPV vaccines targeting HPVs 16/18. With the deployment of the nonavalent HPV vaccine and eventually subsequent generations of HPV vaccines with ever-broader spectra of protection, screening for cervical cancer will be mostly a futile endeavor, both in primary care and via an organized activity. When should we consider that this point has been reached? The analogy with today’s acceptability of risk could be a benchmark for deciding on cessation of screening.
As indicated above, relative to colorectal cancer, cervical cancer screening is much more common. We can also make the analogy to other gynecological malignancies that are related to HPV infection, such as vaginal and vulvar cancers. In the US, except for vulvar cancer in women above the age of 75 years, vaginal and vulvar cancers are much less common than cervical cancer throughout the life span. Cervical and vulvar cancers have comparable survival prognosis, whereas vaginal cancer is a much worse disease. Cytology and molecular HPV testing can also be thought of common screening approaches for these three diseases. Although their natural history is not exactly the same, they can be thought of as within the same niche of clinical interest and with opportunity for early detection with a view to reduce mortality. Yet, there has never been any clinical guidelines recommending regular screening for vaginal and vulvar cancers. Can we accept these two diseases as benchmarks for risk tolerance to gauge the future of cervical cancer screening? These two diseases’ age-specific incidence rates could be thought of as a putative nadir for a future when cervical cancer rates fall below this level and become less common than these two other gynecological malignancies.
8. Concluding reflections
HPV vaccination will provide the ultimate prevention of HPV-associated diseases among young females and males. Likewise, screening will continue to play a key role, constantly evolving so as to remain useful as a clinical and public health activity. HPV testing has evolved in parallel and is arguably the most logical choice for screening women in the post-vaccination era, justifying an eventual transition from cytology screening to HPV primary testing. However, it seems that cytology will remain a central part of cervical cancer prevention via its niche in triaging HPV-positive women.
For countries with a centralized healthcare delivery process, the integration of vaccination and screening via administrative database linkage of vaccination and cancer registries will likely be a cost-effective approach to deploy these two interventions to the population and to provide efficient epidemiologic surveillance.
As cervical cancer control moves forward with subsequent generations of HPV vaccines with increasing breadth of protection, lower costs, and high population coverage, we will have to decide in 25–30 years if cervical cancer screening is to be discontinued. Screening for rare diseases is highly inefficient and can cause harm, despite the improvements in cervical cancer screening technology in the last 20 years. As posited by Gøtzsche, “screening […] always causes harm. Sometimes it also leads to benefits, and sometimes the benefits are sufficiently large to outweigh the harms” [55]. Cervical cancer screening with primary HPV testing and a suitable triage strategy for HPV-positive women fulfills this criterion of a positive benefit-to-harm ratio, but not for long. We have some 25 years to decide what role, if any, screening is to have in cervical cancer prevention.
Highlights.
HPV vaccination was effective in reducing prevalence of HPV and/or genital warts.
Change in the screening paradigm from cytology to HPV primary testing is underway.
Cervical cancer screening policies conditional on HPV vaccination status are lacking.
Role of screening in cervical cancer prevention has to be redefined in 25–30 years.
Acknowledgments
Funding
ELF’s research on cancer screening has been funded by the Canadian Institutes of Health Research (grants MOP-64454, MOP-49396, MCT-54063, CRN-83320), the National Institutes of Health (grant CA70269), and Cancer Research Society.
Footnotes
Competing interests
The authors have no conflict of interest. ELF has served as occasional consultant to pharmaceutical (GSK, Merck) and biotechnology (Roche, Gen-Probe, BD, Qiagen, Ikonisys) companies involved with HPV vaccination, HPV diagnostics, and cervical cytology screening.
Ethical approval
Not applicable for a review article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franco EL, Cuzick J. Cervical cancer screening following prophylactic human papillomavirus vaccination. Vaccine. 2008;26:A16–A23. doi: 10.1016/j.vaccine.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 2.Isidean SD, Coutlee F, Franco EL. cobas 4800 HPV Test, a real-time polymerase chain reaction assay for the detection of human papillomavirus in cervical specimens. Expert Rev Mol Diagn. 2014;14:5–16. doi: 10.1586/14737159.2014.865521. [DOI] [PubMed] [Google Scholar]
- 3.Arbyn M, Ronco G, Anttila A, Meijer CJLM, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 4.Dillner J, Rebolj M, Birembaut P, Petry K, Szarewski A, Munk C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco EL, Mahmud SM, Tota J, Ferenczy A, Coutlée F. The expected impact of HPV vaccination on the accuracy of cervical cancer screening: the need for a paradigm change. Arch Med Res. 2009;40:478–85. doi: 10.1016/j.arcmed.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.McKee SJ, Bergot A-S, Leggatt GR. Recent progress in vaccination against human papillomavirus-mediated cervical cancer. Rev Med Virol. 2015;25:54–71. doi: 10.1002/rmv.1824. [DOI] [PubMed] [Google Scholar]
- 7.Gardasil 9 – A Broader HPV Vaccine. Med Lett Drugs Ther. 2015;57 [PubMed] [Google Scholar]
- 8.Kirby T. FDA approves new upgraded Gardasil 9. Lancet Oncol. 2014;2045:426485. doi: 10.1016/S1470-2045(14)71191-X. [DOI] [PubMed] [Google Scholar]
- 9.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 10.Schiller JT, Müller M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol. 2015;16:e217–e225. doi: 10.1016/S1470-2045(14)71179-9. [DOI] [PubMed] [Google Scholar]
- 11.Kash N, Lee M, Kollipara R, Downing C, Guidry J, Tyring S. Safety and Efficacy Data on Vaccines and Immunization to Human Papillomavirus. J Clin Med. 2015;4:614–633. doi: 10.3390/jcm4040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stillo M, Santisteve PC, Lopalco PL. Safety of human papillomavirus vaccines: a review. Expert Opin Drug Saf. 2015;14:697–712. doi: 10.1517/14740338.2015.1013532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrosky E, Bocchini M, Hairi S, Chesson H, Curtis R, Saraiya M, et al. Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Reccomendations of the Advisory Committee on Immunization Practices. Morb Mortal Wkly Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 14.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. N Engl J Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 15.Jit M, Brisson M, Laprise JF, Choi YH. Comparison of two dose and three dose human papillomavirus vaccine schedules: cost effectiveness analysis based on transmission model. BMJ. 2015;350:g7584–g7584. doi: 10.1136/bmj.g7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laprise JF, Drolet M, Boily MC, Jit M, Sauvageau C, Franco EL, et al. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: A transmission-dynamic modelling study. Vaccine. 2014;32:5845–5853. doi: 10.1016/j.vaccine.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 17.Isidean SD, Tota JE, Gagnon JA, Franco EL. Human papillomavirus vaccines: key factors in planning cost-effective vaccination programs. Expert Rev Vaccines. 2015;14:119–133. doi: 10.1586/14760584.2015.964213. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, et al. Human papillomavirus vaccine introduction--the first five years. Vaccine. 2012;30(Suppl 5):F139–48. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Herrero R, González P, Markowitz LE. Present status of human papillomavirus vaccine development and implementation. Lancet Oncol. 2015;16:e206–e216. doi: 10.1016/S1470-2045(14)70481-4. [DOI] [PubMed] [Google Scholar]
- 20.Drolet M, Bénard E, Boily M, Brisson J, DSc, Lemieux-Mellouki P, Mboup A, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brotherton JML, Fridman M, May CL, Chappell G, Saville MA, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 22.Mariani L, Vici P, Suligoi B, Checcucci-Lisi G, Drury R. Early Direct and Indirect Impact of Quadrivalent HPV (4HPV) Vaccine on Genital Warts: a Systematic Review. Adv Ther. 2015;32:10–30. doi: 10.1007/s12325-015-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 24.Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia - Nationwide follow-up of young danish women. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt460. [DOI] [PubMed] [Google Scholar]
- 25.Blomberg M, Dehlendorff C, Munk C, Kjaer SK. Strongly decreased risk of genital warts after vaccination against human papillomavirus: Nationwide follow-up of vaccinated and unvaccinated girls in Denmark. Clin Infect Dis. 2013;57:929–934. doi: 10.1093/cid/cit436. [DOI] [PubMed] [Google Scholar]
- 26.Leval A, Herweijer E, Ploner A, Eloranta S, Fridman Simard J, Dillner J, et al. Quadrivalent human papillomavirus vaccine effectiveness: A swedish national cohort study. J Natl Cancer Inst. 2013;105:469–474. doi: 10.1093/jnci/djt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabrizi SN, Brotherton JML, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645–1651. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 28.Powell SE, Hariri S, Steinau M, Bauer HM, Bennett NM, Bloch KC, et al. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine. 2012;31:109–113. doi: 10.1016/j.vaccine.2012.10.092. [DOI] [PubMed] [Google Scholar]
- 29.Mahmud SM, Kliewer EV, Lambert P, Bozat-Emre S, Demers AA. Effectiveness of the Quadrivalent Human Papillomavirus Vaccine Against Cervical Dysplasia in Manitoba, Canada. J Clin Oncol. 2014;32:438–443. doi: 10.1200/JCO.2013.52.4645. [DOI] [PubMed] [Google Scholar]
- 30.FDA. Approves Gardasil 9 for More Types of HPV. Cancer. 2015:1156–1157. doi: 10.1002/cncr.29374. [DOI] [PubMed] [Google Scholar]
- 31.Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine. 2006;24(Suppl 3):S3/171–7. doi: 10.1016/j.vaccine.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 32.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain JM, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16:175–204. doi: 10.1097/LGT.0b013e31824ca9d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189–197. doi: 10.1016/j.ygyno.2014.11.076. [DOI] [PubMed] [Google Scholar]
- 34.Huh WK, Ault K, Chelmow D, Davey D, Goulart R, Garcia F, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Gynecol Oncol. 2015;136:178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Katki Ha, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663–72. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuzick J, Szarewski A, Mesher D, Cadman L, Austin J, Perryman K, et al. Long-term follow-up of cervical abnormalities among women screened by HPV testing and cytology-Results from the Hammersmith study. Int J Cancer. 2008;122:2294–300. doi: 10.1002/ijc.23339. [DOI] [PubMed] [Google Scholar]
- 37.Louvanto K, Chevarie-Davis M, Ramanakumar AV, Franco EL, Ferenczy A. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210 doi: 10.1016/j.ajog.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 38.Blatt AJ, Kennedy R, Luff RD, Austin RM, Rabin DS. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015:282–288. doi: 10.1002/cncy.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petry KU, Schmidt D, Scherbring S, Luyten A, Reinecke-Lüthge A, Bergeron C, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual-stained cytology. Gynecol Oncol. 2011;121:505–509. doi: 10.1016/j.ygyno.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 40.Uijterwaal MH, Polman NJ, Witte BI, van Kemenade FJ, Rijkaart D, Berkhof J, et al. Triaging HPV-positive women with normal cytology by p16/Ki-67 dual-stained cytology testing: Baseline and longitudinal data. Int J Cancer. 2015;136:2361–2368. doi: 10.1002/ijc.29290. [DOI] [PubMed] [Google Scholar]
- 41.Schiffman M, Vaughan LM, Raine-Bennett TR, Castle PE, Katki H, Gage JC, et al. A study of HPV typing for the management of HPV-positive ASC-US cervical cytologic results. Gynecol Oncol. 2015;138:573–578. doi: 10.1016/j.ygyno.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, Rozendaal L, Heideman DaM, Hesselink AT, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315–322. doi: 10.1016/S1470-2045(14)70019-1. [DOI] [PubMed] [Google Scholar]
- 43.Louvanto K, Franco EL, Ramanakumar AV, Vasiljević N, Scibior-Bentkowska D, Koushik A, et al. Methylation of viral and host genes and severity of cervical lesions associated with human papillomavirus type 16. Int J Cancer. 2015;136:E638–45. doi: 10.1002/ijc.29196. [DOI] [PubMed] [Google Scholar]
- 44.Depuydt CE, Jonckheere J, Berth M, Salembier GM, Vereecken AJ, Bogers JJ. Serial type-specific human papillomavirus (HPV) load measurement allows differentiation between regressing cervical lesions and serial virion productive transient infections. Cancer Med. 2015;4:1294–1302. doi: 10.1002/cam4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 46.Beer H, Hibbitts S, Brophy S, Rahman M, Waller J, Paranjothy S. Does the HPV vaccination programme have implications for cervical screening programmes in the UK? Vaccine. 2014;32:1828–1833. doi: 10.1016/j.vaccine.2014.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herweijer E, Feldman AL, Ploner A, Arnheim-Dahlström L, Uhnoo I, Netterlid E, et al. The Participation of HPV-Vaccinated Women in a National Cervical Screening Program: Population-Based Cohort Study. PLoS One. 2015;10:e0134185. doi: 10.1371/journal.pone.0134185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franco EL, Shinder Ga, Tota JE, Isidean SD. Sobering realizations in cancer prevention and screening and their lessons. Prev Med (Baltim) 2015;76:129–131. doi: 10.1016/j.ypmed.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Saquib N, Saquib J, Ioannidis JP. Does screening for disease save lives in asymptomatic adults? Systematic review of meta-analyses and randomized trials. Int J Epidemiol. 2015;44:264–277. doi: 10.1093/ije/dyu140. [DOI] [PubMed] [Google Scholar]
- 50.Moyer V. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 51.Biller-Andorno N, Jüni P. Abolishing Mammography Screening Programs? A View from the Swiss Medical Board. N Engl J Med. 2014;370:1965–7. doi: 10.1056/NEJMp1401875. [DOI] [PubMed] [Google Scholar]
- 52.Franco EL, Duarte-Franco E, Rohan TE. Evidence-based policy recommendations on cancer screening and prevention. Cancer Detect Prev. 2002;26:350–361. doi: 10.1016/s0361-090x(02)00118-6. [DOI] [PubMed] [Google Scholar]
- 53.Whitham HK, Kulasingam SL. The significantly lower risk of cervical cancer at and after the recommended age to begin and end screening compared to breast and colorectal cancer. Prev Med (Baltim) 2015;76:135–140. doi: 10.1016/j.ypmed.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 54.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. Am J Obstet Gynecol. 2007;197:356.e1–6. doi: 10.1016/j.ajog.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gøtzsche PC. Commentary: Screening: a seductive paradigm that has generally failed us. Int J Epidemiol. 2015;44:278–280. doi: 10.1093/ije/dyu267. [DOI] [PubMed] [Google Scholar]