Abstract
Targetable molecular drivers for triple-negative breast cancer (TNBC) have been difficult to identify; therefore, standard treatment remains limited to conventional chemotherapy. Recently, new-generation small-molecule Hsp90 inhibitors (e.g., ganetespib and NVP-AUY922) have demonstrated improved safety and activity profiles over the first-generation ansamycin class. In breast cancer, clinical responses have been observed in a subset of TNBC patients following ganetespib monotherapy; however, the underlying biology of Hsp90 inhibitor treatment and tumor response is not well understood. Glucocorticoid receptor (GR) activity in TNBC is associated with chemotherapy resistance. Here, we find that treatment of TNBC cell lines with ganetespib resulted in GR degradation and decreased GR-mediated gene expression. Ganetespib-associated GR degradation also sensitized TNBC cells to paclitaxel-induced cell death both in vitro and in vivo. The beneficial effect of the Hsp90 inhibitor on paclitaxel-induced cytotoxicity was reduced when GR was depleted in TNBC cells but could be recovered with GR overexpression. These findings suggest that GR-regulated anti-apoptotic and pro-proliferative signaling networks in TNBC are disrupted by Hsp90 inhibitors, thereby sensitizing TNBC to paclitaxel-induced cell death. Thus, GR+ TNBC patients may be a subgroup of breast cancer patients who are most likely to benefit from adding an Hsp90 inhibitor to taxane therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-016-0251-8) contains supplementary material, which is available to authorized users.
Keywords: Paclitaxel, Androgen Receptor, Glucocorticoid Receptor, Mifepristone, Hsp90 Inhibitor
Introduction
The glucocorticoid receptor (GR) is a nuclear receptor and ligand-activated transcription factor that controls signal transduction through the modulation of genes following glucocorticoid (GC) exposure [1]. GCs regulate diverse functions in the human body including immune, metabolic, cardiovascular, and behavioral physiology [2]. This diversity reflects the varying gene expression profiles elicited upon activation in different cell types of various tissues. For example, GC-initiated signaling causes apoptosis in lymphocytes [3] but appears to be anti-apoptotic in estrogen receptor-negative breast cancer cells under cytotoxic conditions such as chemotherapy exposure. Previous studies by our group showed that treatment with the synthetic GC dexamethasone diminished chemotherapy effectiveness in a mouse xenograft model of triple-negative breast cancer (TNBC) [4]. This requires regulation of genes encoding anti-apoptotic proteins such as serum and glucocorticoid-inducible protein kinase 1 (SGK1) [5, 6] and mitogen-activated protein kinase phosphatase 1/dual specificity phosphatase 1 (MKP1/DUSP1) [7]. Overexpression and increased activity of GR [8] and its downstream target SGK1 [9] have been associated with chemotherapy resistance and high recurrence rates in early-stage TNBC. SGK1 is a phosphatidylinositol 3-kinase-dependent serine/threonine kinase, closely related to AKT, and is rapidly induced in response to cellular stressors. Activated SGK1 is an important cell survival signal in mammary epithelial cells [10]. In addition to chemotherapy resistance, increased SGK1 levels are associated with resistance to AKT inhibitors [11]. Blocking GR activity and its downstream targets including SGK1 is therefore an attractive approach for treating chemotherapy-resistant TNBC [12].
It is well established that the activity of the chaperone protein Hsp90 is required for proper GR structure and function [13–15]. Hsp90 activity is crucial for folding of the GR ligand binding domain (LBD) into a conformation that allows GC binding and subsequent GR transcriptional activity [16–18]. A recent study detailing the interaction of GR with Hsp70 and Hsp90 showed that Hsp70 binds and unfolds the GR LBD leading to GR inactivation [18]. Hsp90 binding to this complex promotes an ATP-dependent transfer of GR from Hsp70 to Hsp90, release of Hsp70, and recovery of ligand binding. This process is blocked by the Hsp90 inhibitor 17-AAG [18]. In addition, Hsp90 inhibition results in GR misfolding and subsequent proteasome-dependent degradation with an additional loss of GR activity [19]. We have previously demonstrated that breast cancer cells treated with the Hsp90 inhibitor geldanamycin also exhibited decreased SGK1 activity [20]. Interestingly, unlike GR, steady-state SGK1 protein levels were not immediately decreased by Hsp90 inhibitor treatment [20, 21].
Ganetespib is a second-generation Hsp90 inhibitor currently being evaluated in several large clinical trials including a phase II randomized neoadjuvant trial for women with newly diagnosed, locally advanced breast cancer (I-SPY 2). In vitro treatment of cultured TNBC cells with ganetespib resulted in reduced levels of several oncogenic proteins including hypoxia-inducible factor 1 alpha (HIF-1α), epidermal growth factor receptor (EGFR), Janus kinase 2 (JAK2), AKT, and members of the mammalian target of rapamycin (mTOR) signaling axis. Also depleted were p-EGFR, p-AKT, and p-signal transducer and activator of transcription 3 (STAT3) [22–24]. Ganetespib monotherapy decreased the TNBC xenograft growth and metastases to the lung, and ganetespib enhanced the sensitivity of TNBC cells to taxane chemotherapy in vitro and in vivo [23]. Here, we hypothesized that GR degradation and decreased GR transcriptional activity following treatment with Hsp90 inhibitors would allow enhanced TNBC sensitivity to chemotherapy-induced cytotoxicity. We found that Hsp90 inhibitor-mediated depletion of GR expression was indeed associated with increased tumor sensitivity to paclitaxel; moreover, GR-depleted cells no longer demonstrated a significant Hsp90 inhibitor benefit. These data suggest that GR-regulated gene expression is centrally important to the effectiveness of combining Hsp90 inhibitor activity with paclitaxel in TNBC.
Methods
Cell Culture and Treatments
MDA-MB-231 cells were purchased from the American Type Culture collection, and SUM159PT cells were from Asterand. The identity of all cell lines was authenticated by analysis of short-tandem repeats by DDC Medical. MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Lonza) supplemented with 10 % heat-inactivated fetal calf serum (FCS, Gemini Bio Products) and 1 % penicillin-streptomycin (Lonza). SUM159PT cells were cultured in Hams F12 medium (Cellgro), supplemented with 5 % FCS, hydrocortisone (500 ng/ml, Sigma), and insulin (10 μg/ml, Sigma). For dexamethasone (Dex, Sigma), treatments cells were cultured in 2.5 % stripped FCS instead of full serum for 48 h and treated with Dex ± ganetespib under these conditions. MG132 and Dex were purchased from Sigma. Pharmaceutical-grade paclitaxel was purchased from APP Pharmaceuticals, and NVP-AUY922 was purchased from Selleckchem. Pharmaceutical-grade ganetespib was a kind gift from Synta Pharmaceuticals.
Western Blotting
MDA-MB-231 and SUM159PT Western blotting was performed as previously described [12]. Rabbit polyclonal GR phospho-Ser211 antibody (Cell Signaling Technology, 1:500 dilution, cat. no. 4161) was raised against a synthetic phosphopeptide corresponding to residues surrounding serine 211 of the human GR and recognizes the GRα-A (94 kDa), GRα-B (91 kDa), and GRα-C (82 kDa) translational isoforms. Mouse monoclonal GR antibody (BD Biosciences, 1:500, cat. no. 611226) is raised against a peptide corresponding to amino acids 176–289 of the human GR protein and recognizes the GRα-A (94 kDa), GRα-B (91 kDa), and GRα-C (82 kDa) translational isoforms.
Rabbit monoclonal GR XP (DH82) antibody (Cell Signaling Technology, 1:1000, cat. no. 3660) was raised against a peptide corresponding to residues surrounding Leu378 of the human GR protein and recognizes the GRα-A (94 kDa), GRα-B (91 kDa), GRα-C (82 kDa), and GRα-D (54 kDa) translational isoforms. SGK1 DB29 (1:500, Enzo, cat. no. ADI-KAP-PK015-D, a rabbit polyclonal antibody produced by immunization with a COOH-terminal SGK1 peptide, Leu-Gly-Phe-Ser-Tyr-Ala-Pro-Pro-Thr-Asp-Ser-Phe-Leu-Cy), Beta-actin (ACTB) (1:10,000, Santa Cruz, cat. no. sc-47778), Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) (1:5000, Santa Cruz, cat. no. sc-365062), and tubulin (1:5000, Santa Cruz, cat. no. sc-53646) were used as loading controls. Since SGK1 has a short half-life, we treated the cells at each time point with a proteasome inhibitor [MG132 (10 μM)] for 2 h before collection to detect differences in accumulation over time. For densitometry statistics, log (GR/ACTB) was analyzed using a mixed effects analysis of variance (ANOVA) model with treatment as the fixed effect and biological replicate as the random effect. Differences between treatment groups were assessed by constructing appropriate contrasts based on the fitted model. Time course densitometry data were analyzed using a mixed effects model with time, treatment, and time × treatment interaction. Due to the nonlinear pattern of inhibition over time, time was modeled as a categorical variable. A generalized F test was used to test the composite hypothesis of no difference between treatment groups across all time points, effectively comparing the entire curves over time. Contrasts were constructed to estimate differences between treatments at a given time point. Analyses were performed in SAS 9.4.
Quantitative Reverse Transcription PCR
Cells were seeded, RNA was extracted for quantitative reverse transcription PCR (qRT-PCR) as previously published [12], and the reactions were run on a Bio-Rad PCR MyiQ System (Bio-Rad Life Sciences). The following primers were used: SGK1, 5′-AGGCCCATCCTTCTCTGTTT-3′ (forward) and 5′ -TTCACTGCTCCCCTCAGTCT-3′ (reverse); MKP1/DUSP1, 5′CCTGACAGCGCGGAATCT-3′ (forward) and 5′-GATTTCCACCGGGCCAC-3′ (reverse); and RPLP0, 5′-GGAGAAACTGCTGCCTCATATC-3′ (forward), 5′-CAGCAGCTGGCACCTTATT -3′ (reverse). The samples were loaded in triplicate. Relative quantification of gene expression was calculated according to the standard curve method, as described by Applied Biosystems User Bulletin 2, October 2001, based on the ΔΔCt approach [25]. For SGK1 and MKP1/DUSP1 qRT-PCR analysis, a mixed effects ANOVA model was fitted with Ct as the response variable: treatment, time, gene type (target or reference), and all of their interactions as fixed effects and replicate and gene within a replicate as the random effect. A linear contrast was then constructed to estimate ΔΔCt and its confidence interval, and the results were exponentiated to obtain the estimate of 2−ΔΔCt and its confidence intervals.
Quantitative reverse transcription PCR profile multiplex assay
A human glucocorticoid signaling qRT-PCR multiplex assay (RT2 Profiler PCR Array, Qiagen) was used. This is a multiplex assay for 84 known GR target genes from the lung and adipose tissue. MDA-MB-231 cells were pretreated with 100 nM Dex for 1 h, then ganetespib (100 nM) was added for an additional time of 4 h. Samples were prepared according to the manufacturer’s instructions. Data analysis was carried out using the ΔΔCt described above where the fold changes for Dex/vehicle (Veh) and Dex/ganetespib (Gan) samples compared to vehicle were calculated. Genes were considered significantly regulated between the Dex/Veh and Dex/Gan treatment groups if they changed by at least 25 % (Supplementary Table 1).
GR depletion and re-expression in TNBC cells
A stable MDA-MB-231 GR-depleted cell line was created as previously described [26]. Briefly, MDA-MB-231 and SUM159PT cells were transduced with a GR-specific (antisense sequence: TTAATAATCAGATCAGGAG) doxycycline-inducible microRNA-adapted short hairpin RNA (shRNAmir)-expressing lentivirus using the TRIPZ lentiviral shRNAmir system per manufacturer’s instructions (Dharmacon). A negative control (GR-intact) cell line was also produced using an integrated provirus with a controllable shRNAmir that does not target any human gene (non-silencing control antisense sequence CTTACTCTCGCCCAAGCGAGAG). Cells containing the shRNA constructs were selected for with puromycin (400 ng/ml), and stable clones and pools were propagated and tested for GR knockdown by Western blot. For GR depletion experiments, cells were treated once with doxycycline (Dox, 1 μM) 24 h after plating to induce depletion. To re-express GR, a green fluorescent protein (GFP)-GR construct was transiently transfected into GR-depleted TNBC cells 48 h prior to inducing GR depletion by doxycycline. This GFP-GR construct has been previously shown to be capable of translocating to the nucleus and transactivating gene expression [27–29]. A GFP construct was also transiently transfected into GR-depleted TNBC cells as a control. IncuCyte analysis was then performed as described below.
Cell death assay
MDA-MB-231 and SUM159PT cells (1 × 103) were plated in DMEM (10 % FCS) or Hams F12 (5 % FCS) media, respectively, and allowed to adhere for 24 h. Cells were treated with ganetespib (50 nM), paclitaxel (10 nM), or the combination. Each experiment was performed at least twice, and each treatment had three replicate wells per experiment for each treatment condition. To detect dead cells, YOYO-1 iodide (Thermo Fisher Scientific, Y3601) or YOYO-3 iodide (Y3606) was added. YOYO is a cyanine dimer nucleic acid dye that fluoresces if the cellular membrane is compromised. Two images (1.90 × 1.52 mm) in separate regions of each well were captured with a ×10 objective at 4-h intervals using the IncuCyte FLR HD or IncuCyte ZOOM real-time in vitro micro-imaging system (Essen Instruments). Cell death (detected as YOYO-1 positive) and total cell (phase contrast) counts were measured computationally by ImageJ version 1.46r using investigator-coded software for analysis (Supplementary Method S1 [12]). The cytotoxic index represents the number of dead cells/total cells for each image. Images collected between 0 and 72 h were used in the analysis. The cytotoxic index was log transformed to satisfy the normality assumption. Data were analyzed using repeated measures analysis of variance models. A separate model was fitted for each cell line. The fixed effects included were treatment, time, time2, time3, and all corresponding interactions between treatment and time terms. Random effects included random intercept terms for biological and technical replicates and a random slope for the biological replicate. Correlation between serial measurements was modeled using the autoregressive with lag 1 (AR(1)) covariance structure. A generalized F test was used to test the composite hypothesis of no difference between treatment (trt), trt × time, trt × time2, and trt × time3, effectively comparing the entire curves over time.
TNBC Mouse Xenografts
All xenograft experiments were carried out in accordance with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals and approved by either Synta Pharmaceutical Corp. or the University of Chicago Institutional Animals Care and Use Committee. Suspensions of SUM159PT cells (1 × 107) in 50 μL of PBS were injected subcutaneously into the right pectoral mammary fat pad of 6- to 7-week-old female SCID mice (Taconic). MDA-MB-231 (5 × 106) cells were subcutaneously implanted into female SCID mice (Charles River Laboratories, 7 to 12 weeks of age) at Synta as described above. For both xenograft experiments, mice bearing tumors (150–200 mm3) were randomized into groups of eight and treated with ganetespib (100 mg/kg for SUM159PT xenografts, 150 mg/kg for MDA-MB-231 xenografts) or vehicle (DRD = 10 % DMSO, 18 % Cremophor RH 40, 3.6 % dextrose) intravenously via the lateral tail vein. Intraperitoneal (SUM159PT) or intravenous (MDA-MB-231) injection of paclitaxel (10 mg/kg) or vehicle [castor oil (1:10 v/v) for SUM159PT and DRD for MDA-MB-231] was administered an hour later. The longest (L) and shortest (S) diameters of the tumors were measured three times a week with electronic calipers, and tumor volume was calculated using the formula for an ellipsoid sphere: S 2 × L × 0.52 [4]. Animals received four consecutive once weekly treatments of ganetespib, paclitaxel, or the combination of both drugs and were sacrificed 24 h after the last treatment. At time of sacrifice, tumors were minced in lysis buffer and frozen for protein analysis. One ganetespib and paclitaxel-treated SUM159PT xenograft mouse died during the course of the experiment and was removed from the data analysis. Tumor growth data were analyzed using repeated measures analysis of variance models similar to [4], with one exception that the model included treatment day and their interaction as fixed effects.
Results
Hsp90 Inhibition Results in Rapid Loss of Activated (pSer211) and Total GR
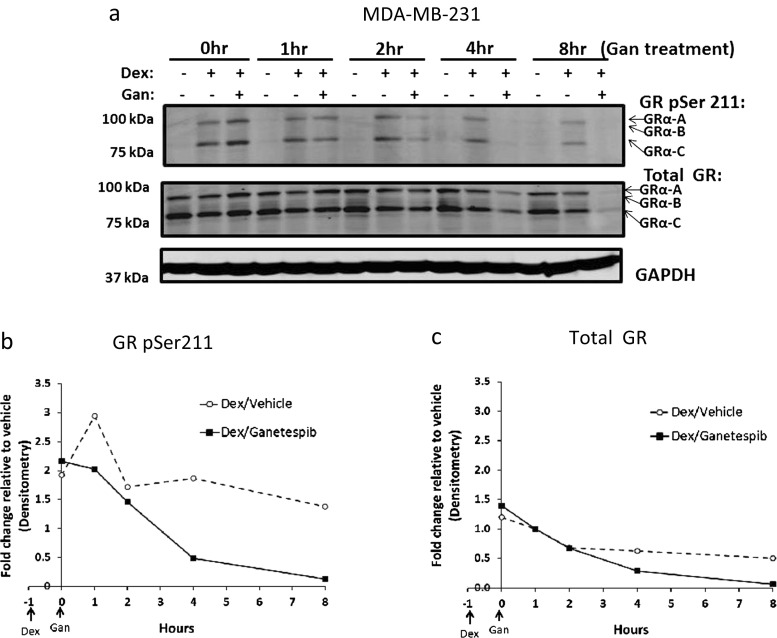
Because GR activation is associated with chemotherapy resistance in TNBC and GR antagonist treatment increases chemotherapy-induced cell death in GR+ TNBC [12], we hypothesized that GR degradation following Hsp90 inhibitor treatment would similarly increase taxane chemotherapy-induced cytotoxicity. To test this hypothesis, we first assessed the stability of total and phosphorylated GR following ganetespib treatment on TNBC cell lines. As predicted, Western analysis showed that total GR levels declined rapidly in MDA-MB-231 (Fig. 1) and SUM159PT (Fig. S1) TNBC cells treated with ganetespib. Because Hsp90 activity is required for the GR LBD to fold correctly and bind glucocorticoid [18], which in turn is required for GR Ser211 phosphorylation (GR pSer211) and transcriptional activity [30, 31], we also determined GR pSer211 levels following Hsp90 inhibition. Decreased Dex-mediated GR Ser211 phosphorylation was detected starting 1 h after ganetespib exposure and continued for the duration of the experiment (Fig. 1). The proteasome-dependent (Fig. S2) decrease in steady-state GR protein also began early on (Fig. 1c). A time course of ganetespib-treated MDA-MB-231 cells showed that Hsp70 levels increased 4 h following ganetespib, while Hsp90 levels increased after 24 h (Fig. S3). Increased Hsp70 is a well-established response to Hsp90 inhibition [23]. Since Hsp70 can bind to and inhibit GR LBD activity [18], the increase in Hsp70 levels may allow sustained GR inactivity.
Fig. 1.
Hsp90 inhibitor treatment results in a rapid decrease of GR activation as measured by reduction in GR Ser211 phosphorylation. In all experiments, cells were pretreated with dexamethasone (Dex, 100 nM) an hour prior to ganetespib (Gan, 100 nM). a Treatment of MDA-MB-231 cells with Gan resulted in decreased Dex-induced GR Ser211 phosphorylation (pSer211) and total GR protein levels. This figure is a representative of two independent experiments. b Densitometry of GR pSer211. c Total GR levels from Fig. 3a show a significant decrease over time following the addition of Gan to Dex compared to Dex alone (p < 0.05, mixed effects ANOVA based on this experiment and one replicate)
Hsp90 Inhibition Significantly Disrupts GR Transcriptional Activity
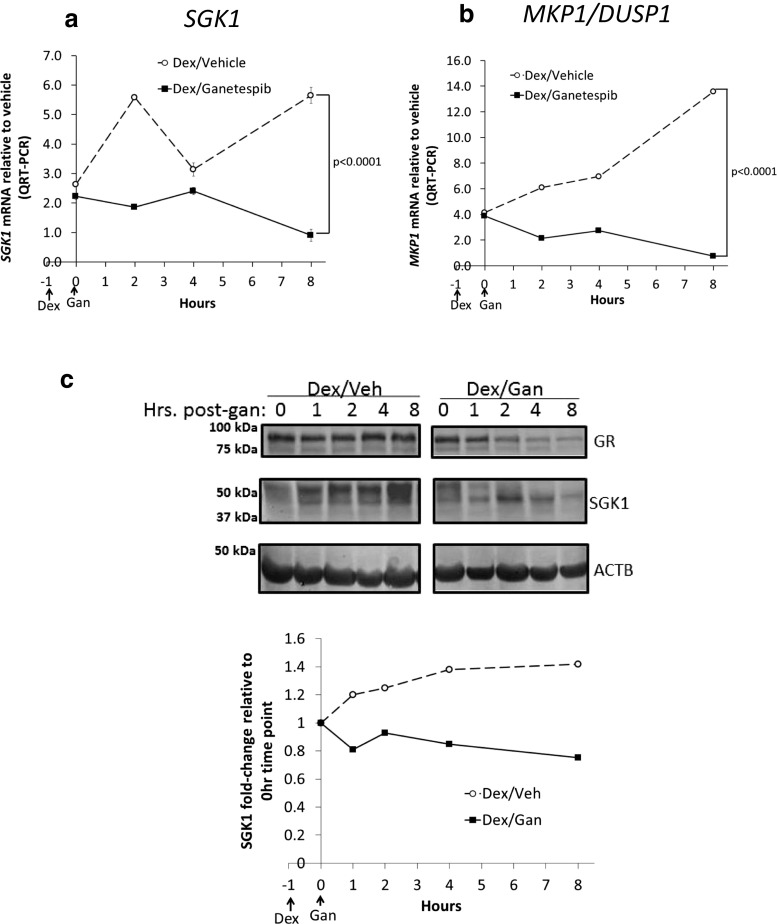
Based on the rapid ganetespib-induced loss of pSer211 GR and the subsequent decrease in total GR steady-state protein, we hypothesized that GR-mediated transcription would be reduced by ganetespib treatment. Indeed, we found that Dex-induced SGK1 and MKP1/DUSP1 steady-state mRNA transcript levels were both significantly decreased following ganetespib treatment of MDA-MB-231 (Fig. 2a, b) and SUM159PT (Fig. S4 A, B) cells. This relative decrease in GR target gene expression was maintained throughout the experiment. We also used a multiplex qPCR assay to determine the effect of ganetespib on a larger transcriptional network of known GR target genes. Table 1 shows the breast cancer-related GR target genes that were decreased (by at least 25 %) following the addition of ganetespib to Dex in MDA-MB-231 cells. These included genes encoding proteins involved in breast cancer growth and proliferation (C/EBPβ, BCL6, ANGPLT4, END1, MT1E, MT2A, and SLC19A2), apoptosis inhibition (BCL6, ANGPLT4, EDN1), and metastasis (ANGPLT4, BCL6, LOX, MT1E, MT2A, USP2).
Fig. 2.
The GR transcriptional network is disrupted by Hsp90 inhibitor treatment. a Dex-induced SGK1 and b MKP1/DUSP1 transcripts were reduced after ganetespib (Gan) treatment of MDA-MB-231 cells. Cells were pretreated with dexamethasone (Dex, 100 nM) for an hour prior to Gan (100 nM) for the indicated times on the x-axis. Real-time quantitative reverse transcription PCR (qRT-PCR) was used to measure mRNA. This figure is a representative of two independent experiments. The error bars are standard error of the mean (SEM) of the triplicate Ct values. For both genes, there is a significant decrease in expression over time with the addition of Gan (p < 0.0001, mixed effects ANOVA based on this experiment and one replicate). c Western blot and densitometry analysis of Dex-induced SGK1 protein levels following Gan treatment. MDA-MB-231 cells were pretreated with 1 μM Dex and 20 % FBS to induce SGK1, 1 h prior to treatment with Veh or Gan (100 nM), for the indicated times. Cells were treated with the proteasome inhibitor MG132 (10 μM) 2 h before lysis to increase the amount of steady-state SGK1. This experiment was repeated twice
Table 1.
GR target gene induction is disrupted by treatment with ganetespib in MDA-MB-231 cells
| Gene symbol | Gene name | Fold change relative to Veh/Veh | p value | Function in ER-negative breast cancer | Reference | |
|---|---|---|---|---|---|---|
| Dex/Veh | Dex/Gan | |||||
| TSC22D3(GILZ) | TSC22 domain family, member 3 | 22.8 | 3.4 | 0.0036 | Unknown | |
| C/EBPβ | CCAAT/enhancer binding protein, beta | 4.1 | 1.2 | 0.0003 | Growth | [52] |
| ANGPTL4 | Angiopoietin-like 4 | 8.5 | 3.5 | 0.0203 | Growth, metastasis, anti-apoptosis | [37] |
| DUSP1 (MKP1) | Dual specificity phosphatase 1 | 3.7 | 1.6 | 0.0041 | Anti-apoptosis | [7] |
| BCL6 | B cell CLL/lymphoma 6 | 2.6 | 1.1 | 0.0063 | Growth, metastasis, anti-apoptosis | [36] |
| SGK1 | Serum/glucocorticoid regulated kinase 1 | 2.9 | 1.4 | 0.0036 | Anti-apoptosis | [6] |
| MT1E | Metallothionein 1E | 2.7 | 1.3 | 0.0159 | Growth, metastasis | [53] |
| LOX | Lysyl oxidase | 2.4 | 1.5 | 0.0055 | Metastasis | [54] |
| SLC19A2 | Solute carrier family 19 (thiamine transporter), member 2 | 2.0 | 1.3 | 0.0206 | Growth | [55] |
| EDN1 | Endothelin 1 | 2.3 | 1.6 | 0.0616 | Growth, anti-apoptosis | [56] |
| MT2A | Metallothionein 2A | 3.0 | 2.1 | 0.0141 | Growth, metastasis | [53] |
| USP2 | Ubiquitin-specific peptidase 2 | 3.1 | 2.3 | 0.0614 | Metastasis | [57] |
Western analysis of SGK1 protein in MDA-MB-231 (Fig. 2c) and SUM159PT (Fig. S4C) cells confirmed decreased SGK1 accumulation as early as 1 h post-Gan treatment. This depletion of SGK1 protein was presumably due to decreased GR activity and transcriptional induction of SGK1 expression (Figs. 2a and S4A) as well as possible direct effects of ganetespib on SGK1 protein stability. Because SGK1 has such a short half-life, its decreased induction was most clearly demonstrated by the addition of the proteasome inhibitor MG132 for 2 h prior to cell lysis. This enhanced accumulation and detection of steady-state SGK1 protein levels over time (Fig. S5 [20]). Interestingly, a treatment of the SUM159PT cell line with ganetespib showed more dramatic depletion of SGK1 than MDA-MB-231 cells, also seen best in a time course with MG132 added prior to each cell lysis (Fig. S4C). Taken together, these results suggest that Hsp90 inhibition both reduces GR target gene expression and SGK1 protein expression, both of which are known effectors of GR-mediated cell survival.
Ganetespib Treatment Potentiates Chemotherapy-Induced TNBC Cell Death
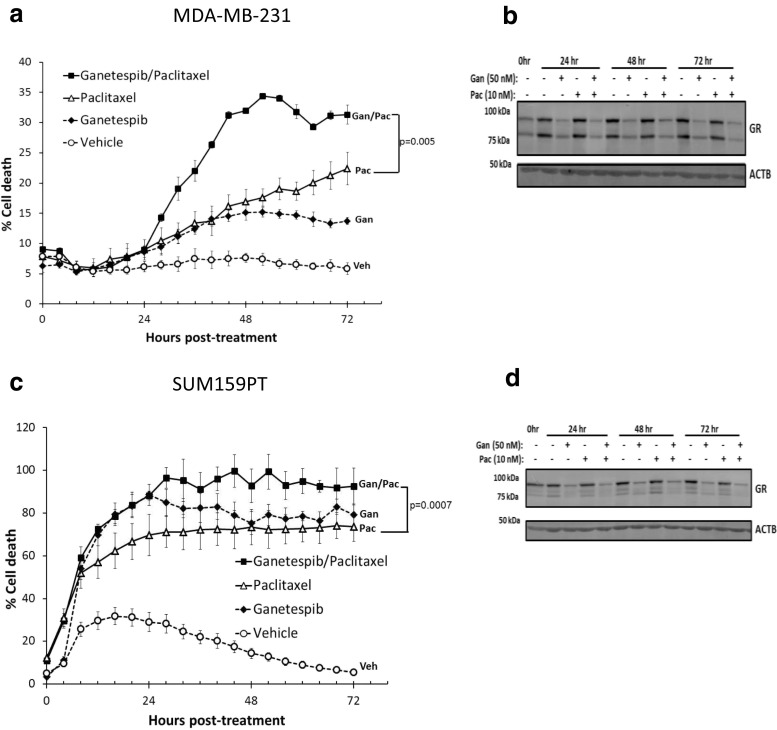
Because ganetespib treatment inhibited GR-mediated gene expression, we next tested whether ganetespib could potentiate paclitaxel cytotoxicity in GR+ TNBC cell lines. We co-treated GR+ MDA-MB-231 and SUM159PT cells with pharmacologically relevant ganetespib (50 nM) and paclitaxel (10 nM) concentrations and measured cellular cytotoxicity using microscopy-based live cell imaging [12, 32]. Figure 3a, c shows that co-treatment caused significantly more cell death compared to paclitaxel treatment alone in both MDA-MB-231 (p = 0.005) and SUM159PT (p = 0.0007) cells. Western analysis (Fig. 3b, d) showed a concomitant loss of total GR following either single agent ganetespib or ganetespib/paclitaxel treatment, but not paclitaxel alone. MDA-MB-231 and SUM159PT live cell counting over time revealed that there was concomitant decreased cell accumulation following treatment with ganetespib and paclitaxel (Fig. S6), with the biggest effect seen in SUM159PT cells, where there were no live cells after 28 h of ganetespib treatment. These data demonstrate that SUM159PT cells were generally more sensitive to either single agent ganetespib or paclitaxel cytotoxicity compared to MDA-MB-231 cells. SUM159PT cells highly express GR and androgen receptor (AR), while MDA-MB-231 cells express GR and low levels of AR [33]. High AR-expressing TNBC (e.g., SUM159PT cells) may be more chemotherapy- and Hsp90 inhibitor- sensitive. Additionally, SUM159PT cells rapidly lost SGK1 protein following ganetespib treatment (Fig. S4C) whereas, for MDA-MB-231, SGK1 protein levels were sustained longer (Fig. 2c).
Fig. 3.
Hsp90 inhibition sensitizes TNBC cell lines to paclitaxel-induced cell death and results in GR depletion. a IncuCyte live cell imaging of MDA-MB-231 cell death over 72 h. YOYO-1 fluorescent staining was used to monitor cell death, and ImageJ software was used to analyze percentage cell death [YOYO-1 positive (fluorescent)/total cell number (phase contrast)]. This figure is representative of two independent experiments. The error bars represent the SEM of three cell culture wells. A comparison of cell death following ganetespib (Gan)/paclitaxel (Pac) vs. Pac demonstrates a significant increase in cell death with the addition of Gan (p = 0.005). In both cases, the statistics were done by a mixed effect ANOVA based on two independent experiments. b Time course of Western blot following a treatment of cells with Gan (50 nM) ± Pac (10 nM) that were cultured in parallel to cells in Fig. 3a. c, d The results using the same conditions as in Fig. 3a, b respectively, except SUM159PT cells were used instead. The comparison of percentage cell death following Gan/Pac treatment is again significantly higher compared to Pac alone (p = 0.0007)
In Vivo Ganetespib Treatment Leads to Tumor GR Depletion and a Significant Increase in Paclitaxel Effectiveness
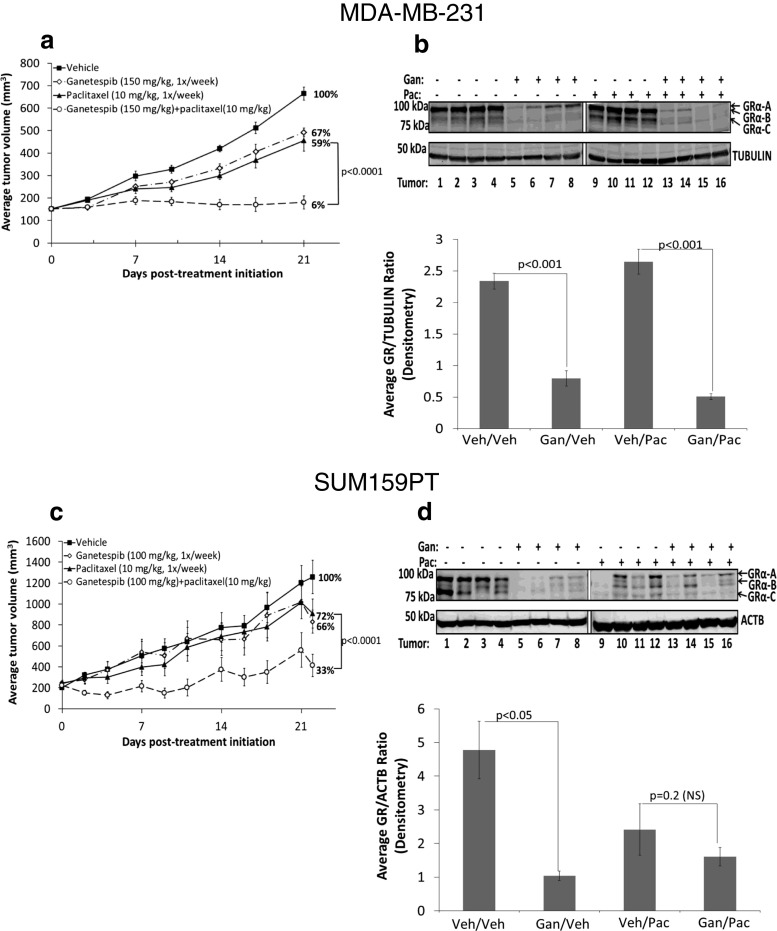
Based on our in vitro results, we hypothesized that the addition of ganetespib to paclitaxel treatment would decrease GR activity, thereby leading to increased paclitaxel-induced cytotoxicity and TNBC tumor shrinkage compared to paclitaxel alone. We found significantly less MDA-MB-231 (Fig. 4a, b) and SUM159PT xenograft (Fig. 4c, d) tumor growth following dual treatment compared to paclitaxel treatment alone (p < 0.0001 for both xenografts). To determine whether ganetespib treatment had in fact reduced GR expression in tumor xenografts, we examined xenografted tumors 24 h following the final paclitaxel ± ganetespib treatment. Western analysis of GR from MDA-MB-231 tumor lysates (Fig. 4b) showed that paclitaxel alone (right panel) did not alter steady-state GR protein levels compared to vehicle-treated tumors (left panel). However, ganetespib treatment, either alone or administered immediately prior to paclitaxel, resulted in significantly reduced tumor GR expression. For SUM159PT tumors, although there was more variation in GR levels in the paclitaxel-treated mice, overall, there was decreased tumor GR expression with ganetespib or with ganetespib/paclitaxel treatment (Fig. 4d).
Fig. 4.
Addition of ganetespib to weekly paclitaxel significantly inhibits TNBC xenograft growth. For a MDA-MB-231 and c SUM159PT SCID mouse xenografts, the addition of ganetespib (Gan) to paclitaxel (Pac) resulted in significantly decreased tumor growth compared to Pac monotherapy (p < 0.0001). There were eight mice per treatment group, and the error bars represent the SEM for each treatment group. b, d Western blot and densitometry analysis of four tumor lysates from four mice in each treatment group show relative depletion of GRα translational isoforms following ganetespib treatment
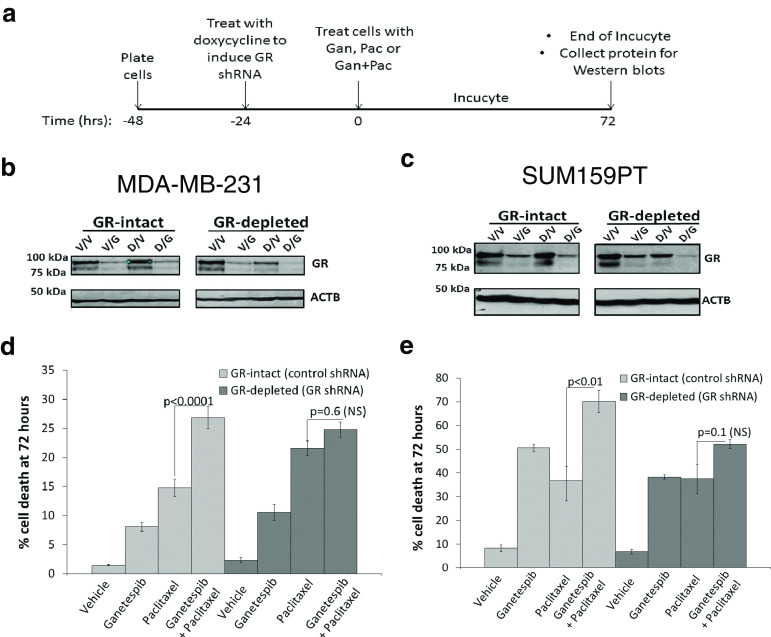
GR Expression Contributes to Ganetespib-Mediated Enhancement of Paclitaxel-Induced Cell Death
Because GR expression and activity are associated with resistance to paclitaxel-induced cytotoxicity [6], we next asked whether GR depletion would increase sensitivity to paclitaxel and reduce the cytotoxic effect of adding ganetespib to paclitaxel. We used a doxycycline-inducible GR shRNA to deplete GR expression (Fig. 5a–c) and then measured the cytotoxicity of tumor cells treated with ganetespib, paclitaxel, or the combination using automated microscopy (Figs. 5d, e, S7, and S8). As expected, MDA-MB-231 and SUM159PT cells expressing a control shRNA exhibited significantly greater cell death with the addition of ganetespib to paclitaxel compared to paclitaxel alone (p < 0.01 for both cell lines). However, the combination of ganetespib and paclitaxel in GR-depleted cells no longer caused a significant increase in paclitaxel-induced cytotoxicity compared to single agent paclitaxel (Fig. 5d, e). GR depletion in MDA-MB-231 cells resulted in more single agent paclitaxel-induced cytotoxicity (Figs. 5d and S7), consistent with the well-established role of GR in mediating chemoresistance. However, a co-treatment of GR-depleted MDA-MB-231 cells no longer demonstrated a significant increase in cytotoxicity compared to paclitaxel alone. As above, AR+/GR+ SUM159PT cells were more sensitive to either single agent ganetespib or paclitaxel compared to MDA-MB-231 cells (Figs. 3, 5e, and S8); however, there was still a significant increase (p < 0.01) in cytotoxicity with drug co-treatment. As with GR-depleted MDA-MB-231, cell death from ganetespib/paclitaxel was blunted in GR-depleted SUM159PT cells compared to treatment with paclitaxel alone. These data suggest that GR expression and activity contribute to the ability of ganetespib to enhance paclitaxel cytotoxicity.
Fig. 5.
Sensitization of TNBC cells to paclitaxel by an Hsp90 inhibitor is reduced following GR depletion. a Timeline for GR depletion experiments. Western blots of GR-depleted (GR shRNA) and GR-intact (control shRNA) b MDA-MB-231 or c SUM159PT cells treated with doxycycline (Dox) 24 h after plating, followed by vehicle (Veh), ganetespib (Gan, 50 nM), paclitaxel (Pac, 10 nM), or Gan/Pac 24 h later. d IncuCyte analysis was then performed, and the bar graph represents the percentage cell death at 72 h and is the average of two independent experiments. Gan/Pac vs. Pac cell death is significantly increased for control cells (light gray bars, p < 0.0001) but not significantly (NS) increased for GR-depleted cells (dark gray bars, p = 0.6). e IncuCyte analysis at 72 h for SUM159PT cells. Gan/Pac vs. Pac cell death is significantly increased for control cells (light gray bars, p < 0.01) but NS increased for GR-depleted cells (dark gray bars, p = 0.1)
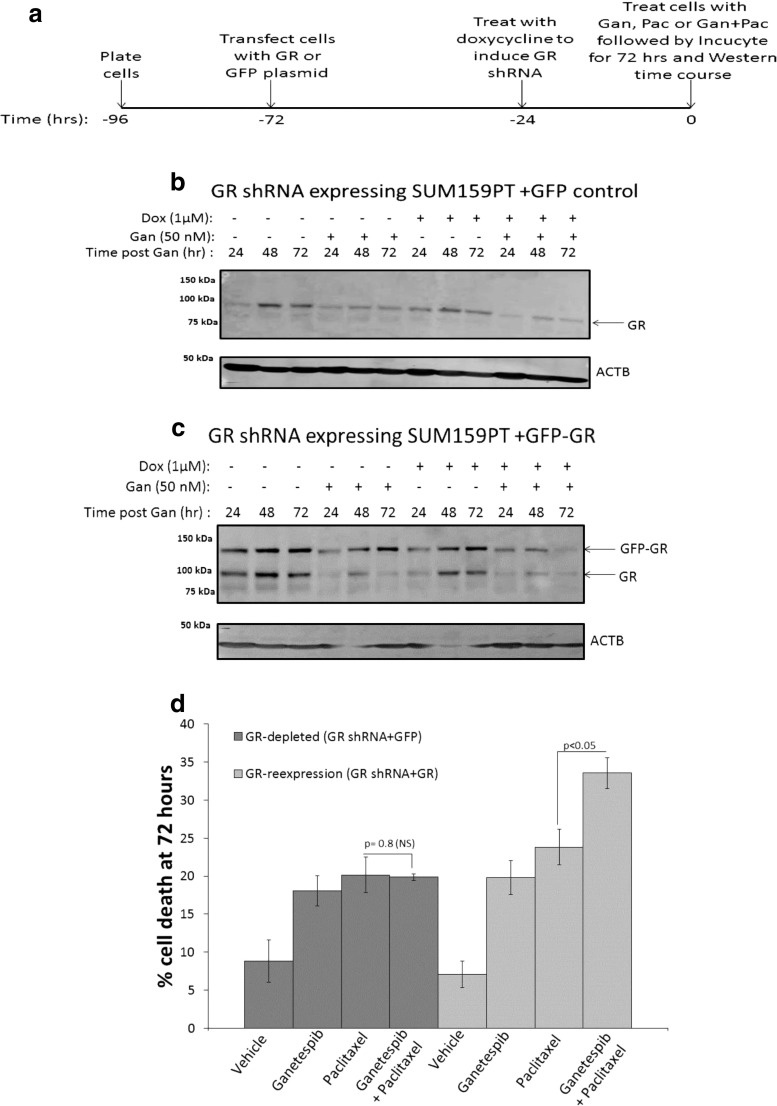
Re-expression of GR Reinstates Ganetespib-Mediated Enhancement of Chemotherapy Sensitivity
To determine whether ganetespib-mediated chemotherapy sensitization was in fact mediated, at least in part, by GR expression, we ectopically expressed a GFP control or a GFP-GR construct in GR-depleted SUM159PT cells (Fig. 6a–6c). GR-depleted MDA-MB-231 cells proved to be extremely resistant to efficient transient transfection, and therefore, GR re-expression for MDA-MB-231 could not be achieved. Western analysis of SUM159PT GR-depleted cells showed expression of GFP-GR (Fig. 6c, band at approximately 130 kDa) despite co-treatment of doxycycline (to induce GR knockdown) and ganetespib which lead to further GR degradation (Fig. 6c). The end result was increased expression of GR compared to SUM159PT GR-depleted cells transfected with a GFP control vector (Fig. 6b). Cytotoxicity of cells treated with ganetespib/paclitaxel compared to paclitaxel was measured (Figs. 6d and S9) and demonstrated that with GR re-expression, there was again a significant increase in cytotoxicity following ganetespib/paclitaxel treatment compared to paclitaxel-treated cells (p < 0.05).
Fig. 6.
Re-expression of GR recovers the benefit of adding an Hsp90 inhibitor to paclitaxel. a Timeline for GR depletion and re-expression experiments. Western blot time course analysis of GR expression following transfection of b GFP control or c GFP-GR into GR shRNA-expressing SUM159PT cells. Cells were treated 48 h later with vehicle or doxycycline (Dox, 1 μM) and then treated ± ganetespib (Gan, 50 nM) 24 h after Dox treatment. d IncuCyte live cell imaging analysis of GR-depleted (GR shRNA-expressing cells transfected with GFP) or GR-re-expressed (GR shRNA-expressing cells transfected with GFP-GR) cells. The bar graph represents percentage cell death at 72 h. Gan/Pac vs. Pac cell death is not significantly (NS) increased for GR-depleted cells (dark gray bars, p = 0.8) but significantly increased for GR-re-expressing cells (light gray bars, p < 0.05). This graph represents the average of two independent experiments
Discussion
GR expression is associated with chemotherapy resistance in TNBC [4, 6]. Here, we show that destabilization and transcriptional inactivation of GR following Hsp90 inhibitor treatment (Figs. 1 and 2) increased the sensitivity of GR+ TNBC cells to paclitaxel (Figs. 3 and 4). As shown previously with the GR antagonist mifepristone, we demonstrate that inhibition of GR activity in GR+ TNBC (here with the Hsp90 inhibitor ganetespib) decreased the expression of GR target genes associated with paclitaxel resistance (Table 1, Fig. 2). Mifepristone inhibited GR-mediated SGK1 and MKP1/DUSP1 expression and increased paclitaxel-induced caspase 3 and PARP-associated cell death [12]. Loss of GR activity following Hsp90 inhibition primed GR+ TNBC cells for more efficient cell death by paclitaxel (Figs. 3 and 6). The kinetics of GR loss after ganetespib treatment (4 h) occurred prior to the onset of paclitaxel-induced cell death (Fig. 3); therefore, we hypothesize that by the time paclitaxel initiated cell death, GR, and its downstream oncogenic signaling were diminished by Hsp90 inhibition, thereby increasing paclitaxel sensitivity. Thus, GR appears to be required, at least in part, for ganetespib-mediated sensitization to paclitaxel (Fig. 5) and re-expression of GR restored the sensitivity of TNBC to the combination of ganetespib and paclitaxel compared to paclitaxel alone (Fig. 6). These studies highlight the importance of disrupting the GR-mediated transcriptional network in GR+ TNBC to increase sensitivity to chemotherapy-induced cell death.
As expected, following GR depletion by ganetespib, GR transcription was effectively disrupted (Fig. 2, Tables 1 and S1). Previously, the GR transcriptional network has been implicated in TNBC oncogenesis by Shah and colleagues who demonstrated that somatic mutations in NR3C1 (GR gene) result in significant changes in GR network gene expression [34]. Here, we show that a number of GR target genes involved in breast cancer growth and metastasis were downregulated by treatment with ganetespib.
Ga Ganetespib-induced decreased live cell count of the TNBC cell lines (Fig. S6) could be the result of increased apoptosis or decreased cell proliferation, in turn related to the decreased expression of anti-apoptotic and pro-proliferative genes (Table 1). For example, in addition to known GR target genes, SGK1, MKP1/DUSP1, and GILZ; BCL6, and ANGPTL4 were also downregulated following Hsp90 inhibitor treatment. BCL6 promotes proliferation and inhibits apoptosis in cytokeratin 5-positive/ER-negative [35] and TNBC [36] cells. ANGPTL4 is involved in proliferation and metastasis [37, 38] and is upregulated in TNBC cell lines [39]. A number of other proteins closely associated with GR function are key Hsp90 inhibitor drug targets. For example, HIF-1α and GR interact to modulate gene transcription during the stress response [40]. Interestingly, ANGPTL4 expression has been shown to also be regulated by HIF-1α in breast cancer [39]. Ganetespib blocks HIF-1α activation and inhibits TNBC tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in mice [24]. The PI3K and mTOR pathways are both upstream of SGK1 phosphorylation and activation [20, 41], and mTOR signaling pathways are both disrupted by Hsp90 inhibition in TNBC [22, 23]. We also found that exposure to Hsp90 inhibitors resulted in degradation of both the androgen receptor (AR) and JAK in TNBC cells (Agyeman and Conzen, unpublished). AR activity has been implicated in TNBC proliferation and apoptosis [33], and GR and AR have been shown to have overlapping roles in activating oncogenic gene expression in prostate cancer [42]. SUM159PT cells express significantly more AR than MDA-MB-231 cells [33], and these cells were more sensitive to ganetespib alone. This suggests that ganetespib’s ability to decrease both AR and GR expression may be associated with increased SUM159PT cytotoxic sensitivity compared to MDA-MB-231 cells. The role of JAK/STAT signaling in TNBC tumorigenesis and chemoresistance is well established [43, 44]; interestingly, GR and JAK/STAT pathways crosstalk to inhibit cytokines and promote apoptosis of immune cells, leading to immunosuppression and suppressing inflammation [45]. The interplay between GR signaling and Hsp90 client proteins suggests that proteins encoded by the GR transcriptional network can also be inactivated by Hsp90 inhibition. Thus, inhibiting GR and its transcriptional network proteins simultaneously via Hsp90 inhibition may effectively block oncogenic bypass mechanisms arising following GR depletion.
Small-molecule Hsp90 inhibitors appear potent and yet relatively well tolerated [46]. NVP-AUY922 was in phase II clinical trials for ER+ and Her2+ breast cancer ([47], NCT00526045 and NCT01271920), while PU-H71 [48] and PF-4942847 [49] showed some preclinical anti-tumor activity in TNBC; PU-H71 is in a phase I clinical trial (NCT01393509). Ganetespib monotherapy is currently in a phase II clinical trial for metastatic breast cancer that includes TNBC (ENCHANT Trial; NCT01677455) [23, 50]. The combination of ganetespib and paclitaxel is being analyzed as neoadjuvant treatment for breast cancer, including TNBC, in the ongoing I-SPY 2 trial (NCT01042379). In summary, our results suggest that high GR-expressing TNBCs will demonstrate increased sensitivity to paclitaxel-induced cytotoxicity following the addition of an Hsp90 inhibitor to paclitaxel therapy.
Immunohistochemical studies have shown that anywhere between 30 and 50 % of early-stage TNBC patients express significant levels of GR ([51] and our unpublished observations). However, we suspect that the percentage with recurrent, chemoresistant TNBC will be much higher due to selection of GR+ cells following an adjuvant chemotherapy treatment. A neoadjuvant clinical trial examining 2 weeks of ganetespib monotherapy followed by 12 weeks of ganetespib and paclitaxel in TNBC is planned to address the hypothesis that GR depletion will occur in GR-overexpressing TNBC and that adding ganetespib to paclitaxel will therefore improve TNBC complete response rates compared to using paclitaxel alone.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(PDF 1632 kb)
Acknowledgments
Synta Pharmaceuticals Corporation provided pharmaceutical-grade ganetespib but no financial support for these studies. We thank Dr. Nick Lu from Northwestern University for his helpful discussions concerning GR isoforms and Mr. Ani Solanki from the University of Chicago Animal Resource Center for his assistance with tail vein injections. We also thank Dr. Larissa Belova and Nicole Lee for initial experiments with geldanamycin and paclitaxel. This work was supported by the National Institutes of Health (NIH) [R01CA089208 (S. D. Conzen) and R21CA149472 (S. D. Conzen)], the University of Chicago’s Breast Cancer SPORE NIH P50CA125183 (O. I. Olopade, S.D. Conzen), the University of Chicago Comprehensive Cancer Center Support Grant NIH P30CA014599 (M. M. Le Beau, S. D. Conzen), and the Susan G. Komen Postdoctoral Fellowship Grant (PDF15333330, A. Agyeman).
Compliance with Ethical Standards
Conflict of Interest
D. A. Proia is the Director of Cancer Biology at Synta Pharmaceuticals Corp.
References
- 1.Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- 2.Quax RA, Manenschijn L, Koper JW, Hazes JM, Lamberts SW, van Rossum EF, et al. Glucocorticoid sensitivity in health and disease. Nat Rev Endocrinol. 2013;9:670–686. doi: 10.1038/nrendo.2013.183. [DOI] [PubMed] [Google Scholar]
- 3.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 4.Pang D, Kocherginsky M, Krausz T, Kim SY, Conzen SD. Dexamethasone decreases xenograft response to paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol Ther. 2006;5:933–940. doi: 10.4161/cbt.5.8.2875. [DOI] [PubMed] [Google Scholar]
- 5.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. doi: 10.1158/0008-5472.CAN-03-2546. [DOI] [PubMed] [Google Scholar]
- 6.Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem. 2001;276:16649–16654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 2005;280:4117–4124. doi: 10.1074/jbc.M411200200. [DOI] [PubMed] [Google Scholar]
- 8.Pan D, Kocherginsky M, Conzen SD. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011;71:6360–6370. doi: 10.1158/0008-5472.CAN-11-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speers C, Tsimelzon A, Sexton K, Herrick AM, Gutierrez C, Culhane A, et al. Identification of novel kinase targets for the treatment of estrogen receptor-negative breast cancer. Clin Cancer Res. 2009;15:6327–6340. doi: 10.1158/1078-0432.CCR-09-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leong ML, Maiyar AC, Kim B, O’Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem. 2003;278:5871–5882. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 11.Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J. 2013;452:499–508. doi: 10.1042/BJ20130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skor MN, Wonder EL, Kocherginsky M, Goyal A, Hall BA, Cai Y, et al. Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin Cancer Res. 2013;19:6163–6172. doi: 10.1158/1078-0432.CCR-12-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertorelli G, Bocchino V, Olivieri D. Heat shock protein interactions with the glucocorticoid receptor. Pulm Pharmacol Ther. 1998;11:7–12. doi: 10.1006/pupt.1998.0119. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz OR, Freiburger L, Rutz DA, Krause M, Zierer BK, Alvira S, et al. Modulation of the Hsp90 chaperone cycle by a stringent client protein. Mol Cell. 2014;53:941–953. doi: 10.1016/j.molcel.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Pratt WB, Morishima Y, Murphy M, Harrell M (2006) Chaperoning of glucocorticoid receptors. Handb Exp Pharmacol 111–138 [DOI] [PubMed]
- 16.Bresnick EH, Dalman FC, Sanchez ER, Pratt WB. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989;264:4992–4997. [PubMed] [Google Scholar]
- 17.Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 18.Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157:1685–1697. doi: 10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitesell L, Cook P. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol Endocrinol. 1996;10:705–712. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]
- 20.Belova L, Brickley DR, Ky B, Sharma SK, Conzen SD. Hsp90 regulates the phosphorylation and activity of serum- and glucocorticoid-regulated kinase-1. J Biol Chem. 2008;283:18821–18831. doi: 10.1074/jbc.M803289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, et al. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedland JC, Smith DL, Sang J, Acquaviva J, He S, Zhang C, et al. Targeted inhibition of Hsp90 by ganetespib is effective across a broad spectrum of breast cancer subtypes. Invest New Drugs. 2014;32:14. doi: 10.1007/s10637-013-9971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proia DA, Zhang C, Sequeira M, Jimenez JP, He S, Spector N, et al. Preclinical activity profile and therapeutic efficacy of the HSP90 inhibitor ganetespib in triple-negative breast cancer. Clin Cancer Res. 2014;20:413–424. doi: 10.1158/1078-0432.CCR-13-2166. [DOI] [PubMed] [Google Scholar]
- 24.Xiang L, Gilkes DM, Chaturvedi P, Luo W, Hu H, Takano N, et al. Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J Mol Med (Berl) 2014;92:151–164. doi: 10.1007/s00109-013-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melhem A, Yamada SD, Fleming GF, Delgado B, Brickley DR, Wu W, et al. Administration of glucocorticoids to ovarian cancer patients is associated with expression of the anti-apoptotic genes SGK1 and MKP1/DUSP1 in ovarian tissues. Clin Cancer Res. 2009;15:3196–3204. doi: 10.1158/1078-0432.CCR-08-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer. 2014;5:72–89. doi: 10.1007/s12672-014-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijsing SH, Elbi C, Luecke HF, Hager GL, Yamamoto KR. The ligand binding domain controls glucocorticoid receptor dynamics independent of ligand release. Mol Cell Biol. 2007;27:2442–2451. doi: 10.1128/MCB.01570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji JY, Jing H, Diamond SL. Shear stress causes nuclear localization of endothelial glucocorticoid receptor and expression from the GRE promoter. Circ Res. 2003;92:279–285. doi: 10.1161/01.RES.0000057753.57106.0B. [DOI] [PubMed] [Google Scholar]
- 29.Htun H, Barsony J, Renyi I, Gould DL, Hager GL. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc Natl Acad Sci U S A. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, et al. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol. 2008;22:1754–1766. doi: 10.1210/me.2007-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stringer-Reasor EM, Baker GM, Skor MN, Kocherginsky M, Lengyel E, Fleming GF, et al. (2015) Glucocorticoid receptor activation inhibits chemotherapy-induced cell death in high-grade serous ovarian carcinoma. Gynecol Oncol [DOI] [PMC free article] [PubMed]
- 33.Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14:769–778. doi: 10.1158/1535-7163.MCT-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman CR, Sato T, Peck AR, Girondo MA, Yang N, Liu C, et al. (2015) Steroid induction of therapy-resistant cytokeratin-5-positive cells in estrogen receptor-positive breast cancer through a BCL6-dependent mechanism. Oncogene [DOI] [PMC free article] [PubMed]
- 36.Walker SR, Liu S, Xiang M, Nicolais M, Hatzi K, Giannopoulou E, et al. The transcriptional modulator BCL6 as a molecular target for breast cancer therapy. Oncogene. 2015;34:1073–1082. doi: 10.1038/onc.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan MJ, Teo Z, Sng MK, Zhu P, Tan NS. Emerging roles of angiopoietin-like 4 in human cancer. Mol Cancer Res. 2012;10:677–688. doi: 10.1158/1541-7786.MCR-11-0519. [DOI] [PubMed] [Google Scholar]
- 38.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, Chaturvedi P, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Kodama T, Shimizu N, Yoshikawa N, Makino Y, Ouchida R, Okamoto K, et al. Role of the glucocorticoid receptor for regulation of hypoxia-dependent gene expression. J Biol Chem. 2003;278:33384–33391. doi: 10.1074/jbc.M302581200. [DOI] [PubMed] [Google Scholar]
- 41.Hall BA, Kim TY, Skor MN, Conzen SD. Serum and glucocorticoid-regulated kinase 1 (SGK1) activation in breast cancer: requirement for mTORC1 activity associates with ER-alpha expression. Breast Cancer Res Treat. 2012;135:469–479. doi: 10.1007/s10549-012-2161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73:1570–1580. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- 43.Marotta LLC, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J Clin Invest 121 [DOI] [PMC free article] [PubMed]
- 44.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, et al. (2009) The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 16 [DOI] [PMC free article] [PubMed]
- 45.Gupta N, Mayer D. Interaction of JAK with steroid receptor function. JAKSTAT. 2013;2:e24911. doi: 10.4161/jkst.24911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 1823;2012:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen MR, Schoepfer J, Radimerski T, Massey A, Guy CT, Brueggen J, et al. NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res. 2008;10:R33. doi: 10.1186/bcr1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A. 2009;106:8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta PP, Whalen P, Baxi SM, Kung PP, Yamazaki S, Yin MJ. Effective targeting of triple-negative breast cancer cells by PF-4942847, a novel oral inhibitor of Hsp 90. Clin Cancer Res. 2011;17:5432–5442. doi: 10.1158/1078-0432.CCR-11-0592. [DOI] [PubMed] [Google Scholar]
- 50.Jhaveri K, Chandarlapaty S, Lake D, Gilewski T, Robson M, Goldfarb S, et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer. 2014;14:154–160. doi: 10.1016/j.clbc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Abduljabbar R, Negm OH, Lai CF, Jerjees DA, Al-Kaabi M, Hamed MR, et al. Clinical and biological significance of glucocorticoid receptor (GR) expression in breast cancer. Breast Cancer Res Treat. 2015;150:335–346. doi: 10.1007/s10549-015-3335-1. [DOI] [PubMed] [Google Scholar]
- 52.Zahnow CA. CCAAT/enhancer-binding protein beta: its role in breast cancer and associations with receptor tyrosine kinases. Expert Rev Mol Med. 2009;11:e12. doi: 10.1017/S1462399409001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bay BH, Jin R, Huang J, Tan PH. Metallothionein as a prognostic biomarker in breast cancer. Exp Biol Med (Maywood) 2006;231:1516–1521. doi: 10.1177/153537020623100910. [DOI] [PubMed] [Google Scholar]
- 54.El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci U S A. 2012;109:17460–17465. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zastre JA, Hanberry BS, Sweet RL, McGinnis AC, Venuti KR, Bartlett MG, et al. Up-regulation of vitamin B1 homeostasis genes in breast cancer. J Nutr Biochem. 2013;24:1616–1624. doi: 10.1016/j.jnutbio.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Tamkus D, Sikorskii A, Gallo KA, Wiese DA, Leece C, Madhukar BV, et al. Endothelin-1 enriched tumor phenotype predicts breast cancer recurrence. ISRN Oncol. 2013;2013:385398. doi: 10.1155/2013/385398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qu Q, Mao Y, Xiao G, Fei X, Wang J, Zhang Y, et al. USP2 promotes cell migration and invasion in triple negative breast cancer cell lines. Tumour Biol. 2015;36:5415–5423. doi: 10.1007/s13277-015-3207-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1632 kb)