Abstract
Background
Direct stimulation of the vagus nerve in the neck via surgically implanted electrodes is protective in animal models of stroke. We sought to determine the safety and efficacy of a non-invasive cervical VNS (nVNS) method using surface electrodes applied to the skin overlying the vagus nerve in the neck in a model of middle cerebral artery occlusion (MCAO).
Methods
nVNS was initiated variable times after MCAO hour in rats (n=33). Control animals received sham stimulation (n=33). Infarct volume and functional outcome were assessed on day 7. Brains were processed by immunohistochemistry for microglial activation and cytokine levels. The ability of nVNS to activate the nucleus tractus solitarius (NTS) was assessed using c-Fos immunohistochemistry.
Results
Infarct volume was 43.15±3.36 percent of the contralateral hemisphere (PCH) in control and 28.75±4.22 PCH in nVNS-treated animals (p<0.05). The effect of nVNS on infarct size was consistent when stimulation was initiated up to 4 hours after MCAO. There was no difference in heart rate and blood pressure between control and nVNS-treated animals. The number of c-Fos positive cells was 32.4±10.6 and 6.2±6.3 in the ipsilateral NTS (p<0.05) and 30.4±11.2 and 5.8±4.3 in the contralateral NTS (p<0.05) in nVNS-treated and control animals, respectively. nVNS reduced the number of Iba-1, CD68, and TNF-α positive cells and increased the number of HMGB1 positive cells.
Conclusions
nVNS inhibits ischemia-induced immune activation and reduces the extent of tissue injury and functional deficit in rats without causing cardiac or hemodynamic adverse effects when initiated up to 4 hours after MCAO.
Keywords: vagus nerve, electrical stimulation, cerebral ischemia, neuroprotection, inflammation
Introduction
There is a surge of interest in brain stimulation to reduce tissue injury and to restore the lost function in a wide variety of neurological disorders. Experimental evidence indicates that stimulation of a group of anatomically connected areas such as the fastigial nucleus [1], periaqueductal gray matter [2], subthalamic vasodilator area [3], sphenopalatine ganglion [4], and cervical vagus nerve [5–8] leads to a reduction in infarct volume by up to 50% in animal models of cerebral ischemia. Unlike intracranial structures that require craniotomy for access, the vagus nerve is accessible in the neck and hence can be stimulated using surgically implanted electrodes following a small incision in the overlying skin. Neural impulses following cervical vagus nerve stimulation (cVNS) project to a wide variety of cortical and subcortical structures via the nucleus tractus solitarius (NTS)[9, 10] and can activate circuits that inhibit neuronal excitability [11] and block microglial response to ischemia-induced inflammation [12].
Although cVNS is protective in experimental models of cerebral ischemia, it is not feasible for human application in the setting of acute ischemic stroke because direct nerve stimulation requires a surgical procedure. This urges a search for less invasive or non-invasive techniques for cVNS. In this study, we explored the safety and efficacy of a non-invasive transcutaneous cervical vagus nerve stimulation (nVNS) approach using surface electrodes applied to the skin overlying the vagus nerve in the neck in a model of middle cerebral artery occlusion (MCAO) in rats.
Material and Methods
All experiments were performed in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Adult male spontaneously hypertensive rats (SHR, 326–420g, n=66; Charles River Laboratories, Wilmington MA) were used. We used SHR in compliance with the Stroke Therapy Academic Industry Roundtable (STAIR) criteria which explicitly require the use of animals with co-morbid conditions in order to increase the quality of translational stroke research.[13]
Experimental Protocols
We studied the following four hypotheses:
nVNS reduces infarct volume and improves neurological outcome after MCAO: Animals were randomly allocated into two experimental groups: treatment (351.00 ± 14.41g; n=7) and control (336.17 ± 16.46g; n=6). Electrical stimulation of the right cervical vagus nerve was initiated 30 minutes after occlusion of the right MCA and repeated every 10 minutes for a period of 1 hour. Control animals received sham stimulation delivered to the right quadriceps femoris muscle. Twenty minutes after the last stimulation animals were returned to their home cages. Functional assessments were performed daily after surgery for 7 days. Infarct volume was assessed on day 7. The following safety parameters were recorded in each animal: arterial blood pressure (ABP), heart rate (HR), arterial blood gases (ABGs) and pH, symptomatic brain hemorrhage, and mortality or development of any of the euthanasia criteria described by AVMA Guidelines for the Euthanasia of Animals 2013.
nVNS activates NTS: We explored whether nVNS caused activation in the NTS after MCAO in rats. We performed the same surgical and stimulation procedures in Experiment-1 but animals were euthanized 3 hours after MCAO and brains were processed for c-Fos immunohistochemistry. There were two experimental groups: treatment (390.25 ± 5.32g; n=4) and control (394.00 ± 4.83g; n=4).
nVNS leads to inhibition of MCAO-induced immune response in the brain: cVNS causes marked inhibition of the immune response to various stimuli both in the periphery and the central nervous system [12, 14]. We explored whether nVNS was able to inhibit microglia activation and normalize altered cytokine levels after MCAO. We performed the same experimental protocol as in Experiment 1 but euthanized animals 3 or 24 hours after MCAO and processed the brains for immunohistochemistry for TNF-α, high mobility group box protein 1 (HMGB1), IL-1β, IL-6, and microglial markers Iba1 and CD68. There were four experimental groups: treatment at 3 hour euthanasia (377.25 ± 31.61g; n=4), control at 3 hour euthanasia (356.25 ± 28.45g; n=4), treatment at 24 hour euthanasia (390.75 ± 2.63g; n=4), control at 24 hour euthanasia (378.00 ± 12.19g; n=4). We have chosen bright-field immunohistochemistry to detect inflammation markers because it allows examination of the morphology of tissue samples and hence provides spatial information on the effect of nVNS on cytokine protein levels.
nVNS is effective when initiated late after MCAO: First, we tested the effect of nVNS initiated 4 hours after MCAO on functional and tissue outcome. There were two experimental groups: treatment (398.67 ± 16.12g; n=9), control (391.38 ± 6.48g; n=8). Depending on the observed effect size at 4 hours, we planned to repeat experiments by changing the therapeutic window up and down by 1 hour each time until a comparable effect size with 30 minute stimulation was achieved (i.e., at 3 hours after ischemia if 4 hour treatment window failed to show a neuroprotective effect or at 5 hours after ischemia if treatment at 4 hour was as effective as the treatment at 30 minutes after ischemia).
Surgical preparation
Animals were anesthetized using isoflurane (induction: 4–5% in 30% oxygen – 70% nitrous oxide, maintanance: 1–2% in room air). Buprenorphine HCl (0.05 mg/kg; sc) and bupivacaine (8mg/kg; intraincisional) were injected to alleviate pain. Rectal temperature was monitored continously during the anesthesia period and maintained at 37.0 °C by a homeothermic blanket system (Harvard Apparatus, Holliston MA). Temporalis muscle temperature was monitored continously using a T-type thermocouple probe and pod (ADInstruments, Colorado Springs, CO) and kept at 36.0 °C by a heating lamp. The right femoral artery was cannulated for continuous ABP and HR monitoring (ADInstruments) as well as intermittent ABGs and pH measurements (Rapidpoint 300 blood gas system, Siemens Healthcare Diagnostics Inc., Tarrytown NY). Regional cerebral blood flow (rCBF) was recorded over the right parietal cortex (5 mm lateral and 1 mm posterior to bregma) using laser Doppler flowmeter (ADInstruments) to verify induction of MCAO, as described before.[6, 15]
MCA occlusion
A midline neck incision was performed to expose the common, external, and internal carotid arteries. All the visible sections of the external and internal carotid arteries and a 2–3 mm segment of the common carotid artery before the bifurcation were isolated from the surrounding tissues. Caution was applied to avoid injury to the superior cervical ganglion, sympathetic trunk, and vagus nerve during dissection of carotid arteries. Ischemia was induced by intraluminal filament occlusion of the right MCA for 2 hours by a silicone-coated nylon monofilament (diameter: 0.39 ± 0.02 mm or 0.41 ± 0.02 mm; Doccol Corporation, Redlands CA) as reported before.[16] The filament was introduced from the carotid bifurcation. Occlusion was confirmed when ≥60% drop in baseline rCBF was detected. The skin incision was sutured after the occlusion to allow transcutaneous stimulation.
nVNS treatment
We used an experimental non-invasive stimulator developed exclusively for cervical vagus nerve stimulation (gammaCore; ElectroCore, LLC). The stimulator consisted of two surface disc electrodes (6 mm in diameter) separated by 6 mm. Skin was shaved prior to stimulation and a conducting gel (Signa gel, Parker Laboratories, Fairfield NJ) was applied to the electrodes to allow for good electrical contact with the skin. Electrodes were placed on the skin overlying the right cervical vagus nerve (between mid to lower portions of the neck, parallel to the anterior margin of sternocleidomastoid muscle) without applying any extra mechanical pressure. Electrical stimulation (1 msec duration, 5 kHz, 12 V sine waves repeated at 25 Hz; impedance: 350 ohm) was delivered in the form of 2-minute trains, every 10 minutes, for a period of 1 hour.[17] In control animals, electrical stimulation was delivered into the skin overlying right quadriceps femoris muscle using the same stimulation parameters. We selected quadriceps femoris muscle because it provided sufficient contact area for the surface electrodes to achieve selective muscle stimulation in rats. In a pilot study, we demonstrated that electrical stimulation of the skin overlying the ipsilateral quadriceps femoris was not associated with any neuroprotection/increased cerebral blood flow in rats. The infarct volumes were 46.93 ± 5.57 and 44.24 ± 13.27 in active and sham-stimulated animals, respectively (n=4 for each group; unpaired t test: t=−0.374, p=0.7209).
Neurological examination
Functional deficit was assessed by neurological score and grip strength measurements. Neurological score (0=no deficit to 4=no spontaneous walking) was determined immediately after animals awoke from anesthesia and just before euthanasia.[18] Neurological scoring is well accepted and validated method to reliably assess the severity of neurological deficit in the acute phase of ischemia and its sensitivity and specificity for detecting neurological deficit are 88% and 100%, respectively.[19] In order to further enhance the sensitivity of our measurements, we also performed grip strength measurements to quantify the motor deficit. Forelimb muscle strength was measured using a grip strength meter (Columbus Instruments, Columbus OH) that consisted of a base plate with a pedestal that supported the digital force gauge connected with a forelimb grip bar assembly. The rat was hold by the scruff of the neck with one hand and at the base of the tail with the other, leaving the animal’s forelimbs free to grasp the grip bars. Then the animal was pulled back gently along a straight line until its grip was released. This provided a digital reading for the maximum force (in Newtons) of grip strength. Animals were acclimated to the grip strength meter by training them starting a week before the surgery. Measurements were done at baseline (one day before MCAO) and then day 3 and day 7 after MCAO. For each animal, an average of three successive digital readings was used.
Infarct volume
Animals were euthanized by carbon dioxide inhalation on day 7 after MCAO. Infarct volume was measured using 2,3,5-triphenyltetrazolium chloride. This technique was previously shown to reliably identify the region of infarction 7 days after transient MCAO in rats.[20] Briefly, 2 mm-thick brain sections were incubated with the 2% dye solution at room temperature for 30 minutes. [5, 6] From the digital images of each section, infarct area, ipsilateral non-infarct area, and contralateral hemispheric area were outlined manually, in a blinded fashion using Image J (NIH). The infarct volume was calculated by multiplying the infarct area (contralateral hemispheric area minus ipsilateral non-infarct area) by slice thickness and expressed as a percentage of the contralateral hemispheric volume (PCH).
Immunohistochemistry
We used immunohistochemistry to detect neuronal activation (c-Fos), microglial markers (Iba1 to identify activated and resting microglia, CD68 to identify activated microglia), and cytokine markers known to be associated with cVNS-induced anti-inflammatory effects (IL-1β, IL-6, TNF-α, HMGB1). Animals were euthanized by transcardial perfusion (2 IU/ml heparin in 0.9% saline followed by 4% formaldehyde in phosphate buffered saline, pH 7.4) under isoflurane anesthesia 3 or 24 hours after MCAO and the brains were removed for analysis. After incubation in 30% sucrose at 4 °C for 3 days, 20–30 μm-thick coronal brain sections with 1200 μm interslice gap and 30 μm-thick brainstem sections with 500 μm interslice gap were obtained using a cryostat. Immunohistochemistry was performed on either free-floating (for c-Fos staining) or slide-mounted sections (for immunological marker staining). Slide mounted sections were incubated in citrate buffer (pH 6.0, 30 minutes at 90°C) for heat mediated antigen retrieval. All sections were incubated in H2O2 (0.3%; 30 minutes at room temperature) followed by blocking solution (3% normal horse serum in 0.25% Triton X-100; 2 hours at room temperature) and primary antibody [goat polyclonal anti-Iba1 antibody (ab5076, 1:200; Abcam, Cambridge MA), mouse monoclonal anti-CD68 antibody (ab31630, 1:100; Abcam), rabbit polyclonal anti-IL-1β antibody (ab9787, 1:50; Abcam), rabbit polyclonal anti-IL-6 antibody (ab83339, 1:300; Abcam), rabbit polyclonal anti-TNF-α antibody (ab66579, 1:500; Abcam, Cambridge MA), rabbit polyclonal anti-HMGB1 antibody (ab18256, 1:500; Abcam) for overnight at 4°C or rabbit polyclonal anti-cFos antibody (Ab-5, 1:30 000; Calbiochem, San Diego CA) for 72 hours at 4°C]. Then, sections were incubated sequentially in biotinylated horse secondary antibody [anti-rabbit, anti-goat, or anti-mouseIgG (2.25 μg/ml; 2 hours at room temperature; Vector Laboratories, Burlingame, CA)], Vectastain Elite ABC Kit (Vector), DAB substrate kit (Vector), and counterstained with hematoxylin. Slides were inspected using a microscope (Nikon Eclipse 50i, Kodak Scientific Imaging System, New Haven, CT) and digital images were acquired using a camera (SPOT 7.4 Slider RTKE, Diagnostic Instruments, Sterling Heights, MI) connected to a computer. The anatomical structures of tissue sections were identified cytoarchitecturally using The Rat Brain Stereotaxic Coordinates.[21] Primary regions of interest included the NTS, cerebral cortex, and caudate and putamen ipsilateral to the MCAO. Number of positive cells in these regions per microscopic field were counted from three consecutive sections and averaged for each animal blindly to the treatment groups.[22]
Sample size, exclusion criteria, data analysis
Animals were randomly allocated into treatment and control groups. For an alpha of 0.05 and power of 90%, we calculated that 6 animals in each group were needed to detect a 30% reduction in mean infarct volume. The exclusion criteria were MCAO failure as described by <60% decrease in baseline laser Doppler signal upon MCAO, subarachnoid hemorrhage secondary to arterial rupture, and poor systemic physiology (oxygenation, acid-base balance, and hemodynamic failure). Excluded animals were replaced by new animals to attain the calculated sample size. Data analyses were performed by an investigator blinded to the treatment groups. Continuous data were expressed as mean ± SD. Categorical data were expressed as median ± interquartile range (IQR). Infarct volumes were compared using unpaired t test. ABP, HR, arterial blood gases and pH, grip strength measurements were analyzed by repeated measures ANOVA. Neurological scores were compared using Mann-Whitney U test. Positive cell counts were compared using unpaired (for treatment vs. control comparison) or paired t-test (for ipsilateral vs. contralateral comparison). Post hoc analysis was done by Student-Newman-Keuls, when needed. Differences with p <0.05 were considered statistically significant.
Results
We excluded a total of 6 animals: one due to vessel rupture during filament occlusion (Experiment-1, treatment group), three due to premature death secondary to respiratory acidosis that started before electrical stimulation (Experiment-4, two in control, one in treatment group), two due to MCAO failure (Experiment-4, one in each group). Our exclusion rate of 7% was within previously published limits [23].
1. The effect of nVNS on infarct volume after MCAO
There were no differences in overall physiological parameters between control and nVNS-treated animals during stimulation (Supplementary Table 1). The rate of symptomatic brain hemorrhage, mortality, or development of any euthanasia criteria were similar between the groups. nVNS resulted in a 33% reduction in infarct volume (Fig. 1A) and improved neurological scores (Fig. 1B) and forelimb grip strength (Fig. 1C). Infarct volume was 43.15 ± 3.36 PCH in control and 28.75 ± 4.22 PCH in nVNS-treated animals (unpaired t test: t= −5.963, p=0.0001). Neurological score on day 7 was 3.000 ± 1.000 in control and 2.000 ± 0.125 in nVNS-treated animals (Mann-Whitney U test: Z=−2.298, p=0.0216 vs. control). Pre MCAO forelimb grip strength was 8.64 ± 1.35 N (n=12; pooled from control and treatment groups). On day 7 after MCAO, forelimb grip strength was 2.34 ± 0.75 N in control and 5.51 ± 1.09 in nVNS-treated animals (repeated measures ANOVA: F(1,10)=28.025, p=0.0004 vs. control).
Figure 1.
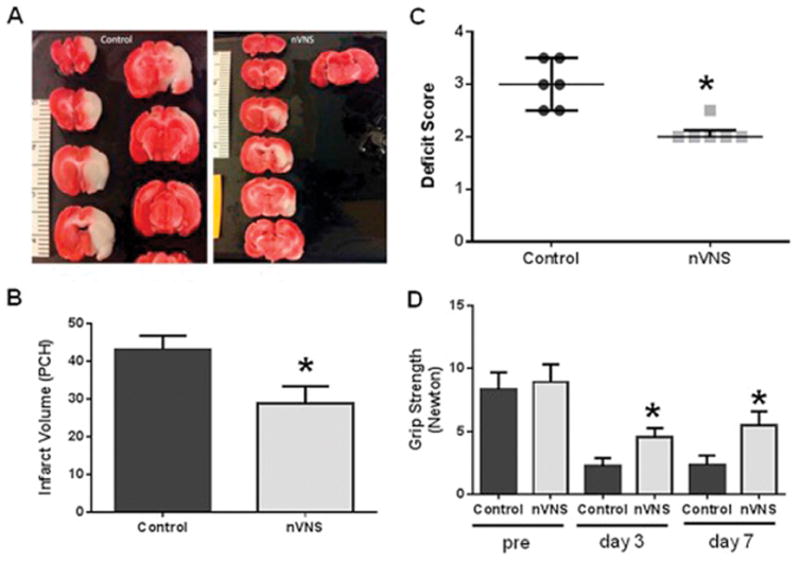
nVNS is neuroprotective in MCAO. (A) TTC-stained sections from representative control and nVNS-treated animals. Effect of nVNS on (B) infarct size, (C) neurological score, and (D) grip strength after MCAO. Treatment was initiated 30 minutes after MCAO. PCH: percentage hemispheric volume. *p<0.05 vs. corresponding control.
2. Activation of NTS by nVNS
nVNS resulted in bilateral activation of the NTS (Fig. 2). In the ipsilateral NTS, the number of c-Fos positive cells was 32.4 ± 10.6 in nVNS-treated and 6.2 ± 6.3 in control animals (unpaired t test: t= 4.246, p=0.0054). In the contralateral NTS, the number of c-Fos positive cells was 30.4 ± 11.2 in nVNS-treated and 5.8 ± 4.3 in control animals (unpaired t test: t= 4.094, p=0.0064). There was no difference in the number of c-Fos positive cells between ipsilateral and contralateral NTS in the treatment arm (paired t test: t=1.775, p=0.1739).
Figure 2.
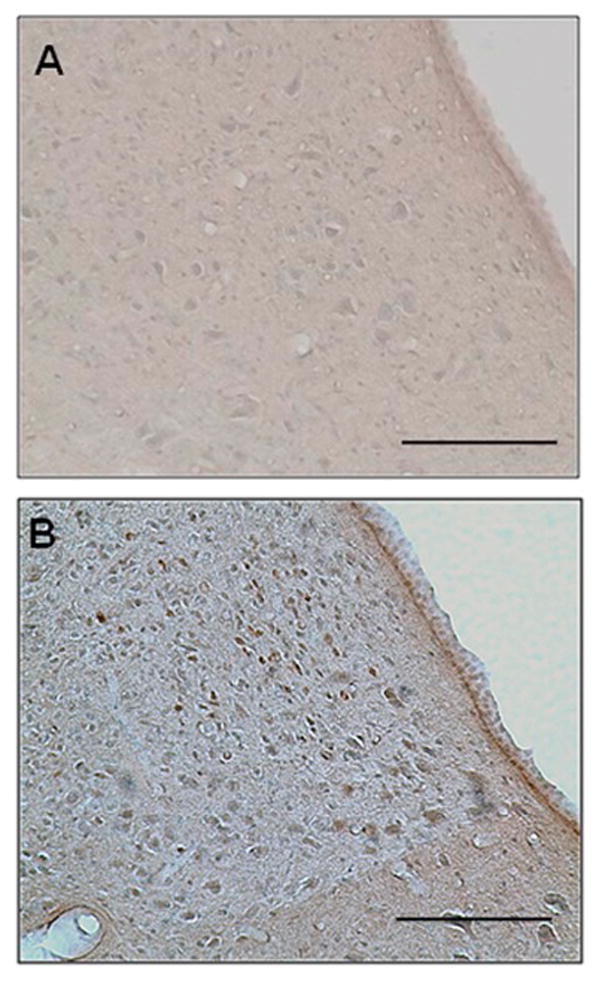
c-Fos immunoreactivity in the ipsilateral NTS. Representative images were taken from control (A) and ct-VNS-treated animals (B). Scale bar: 100μm
3. The effect of nVNS on downstream inflammatory response
nVNS reduced the number of Iba-1- (Fig. 3A and B) and CD68-positive cells (Fig. 3C and D) in the sensory cortex forelimb area (S1FL) at 24 hours but had no effect at 3 hours (Supplementary Fig. 1). There were fewer TNF-α positive cells in nVNS-treated animals at both 3 (Supplementary Fig. 2) and 24 hours (Fig. 4A and B). The number of IL-1β (Supplementary Fig. 3) and IL-6 (Supplementary Fig. 4) positive cells were not different between the control and treated animals. In control animals, there were substantially fewer HMGB1-positive cells at both 3 (Supplementary Fig. 2) and 24 hours after MCAO in the ischemic brain tissue (Fig. 4C). Treatment with nVNS increased HMGB1 levels at both time points (Fig. 4D, Supplementary Fig. 2). Data for the number of positively stained cells are summarized in Table 1.
Figure 3.
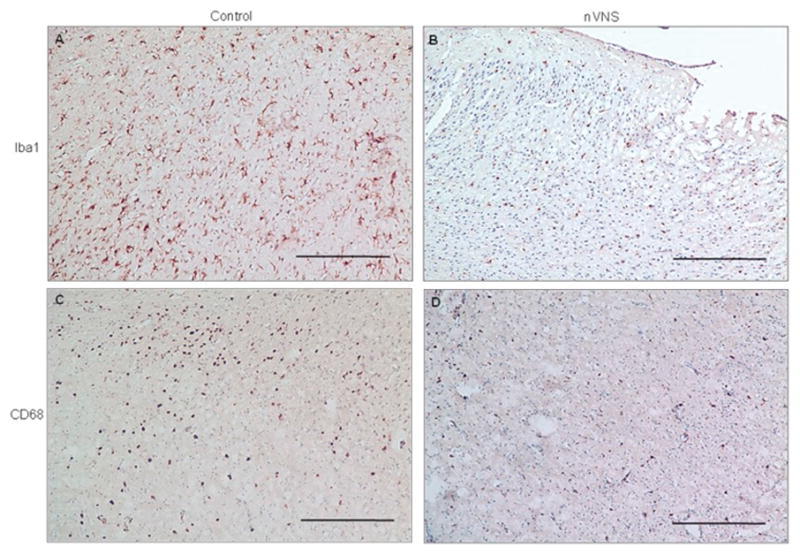
Microglia in the sensory cortex (S1FL) 24 hours after MCAO. Representative Iba-1 (A–B) and CD68 (C- immunopositive cells in the control and nVNS-treated animals. ScaleD) bar: 100μm
Figure 4.
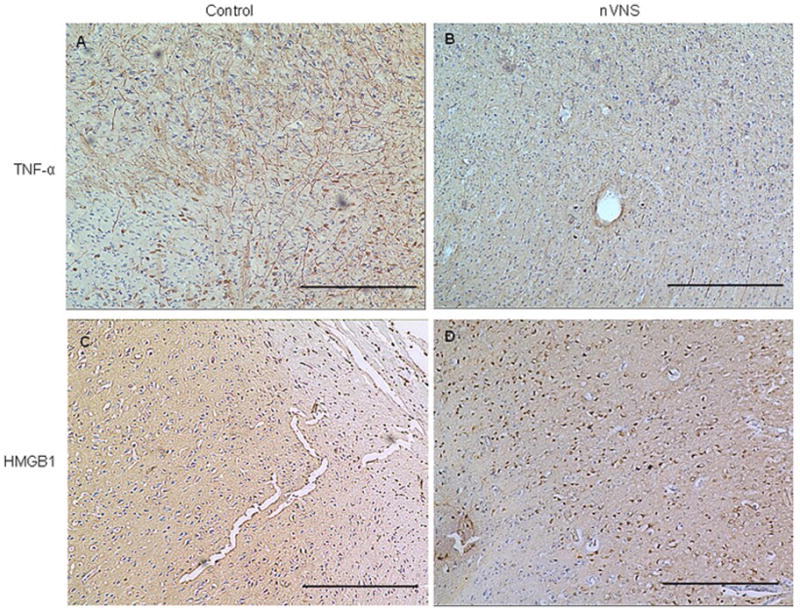
Cytokines in the sensory cortex (S1FL) 24 hours after MCAO. Representative TNF-α (A–B) and HMGB1 (C–D) immunopositive cells in the control and nVNS-treated animals. Scale bar: 100μm
Table 1.
Immunohistochemistry cell counts.
| Control 3 hours |
nVNS 3 hours |
Control 24 hours |
nVNS 24 hours |
|
|---|---|---|---|---|
| Iba-1 | 236.6 ±13.4 | 194.1 ±78.7 | 249.3 ± 64.0 | 131.9 ± 28.2* |
| CD68 | 135.5 ± 17.5 | 110.7 ± 42.0 | 129.0 ± 18.8 | 62.2 ± 31.2* |
| TNF-α | 302.7 ± 72.5 | 151.9 ± 9.2* | 348.2 ± 61.0 | 154.2 ± 87.8* |
| IL-1β | 402.8 ± 20.8 | 238.3 ± 132.9 | 394.6 ± 29.3 | 291.1 ± 112.3 |
| IL-6 | 136.8 ± 90.0 | 157.1 ± 42.6 | 193.2 ± 64.5 | 184.8 ± 27.9 |
| HMGB1 | 4.6 ± 3.5 | 139.8 ± 20.1* | 49.8 ± 51.7 | 170.8 ± 101.0* |
p<0.05 vs. corresponding control.
4. The effect of late nVNS
Infarct volume was smaller in animals treated 4 hours after MCAO (Fig. 5A). Mean infarct volume was 43.23 ± 9.34 PCH in control and 26.47 ± 4.84 PCH in nVNS-treated animals (unpaired t test: t= −3.903, p=0.0029). The therapeutic effect of nVNS disappeared at 5 hours: mean infarct volume was 42.82 ± 6.33 PCH in control group (352.50 ± 13.58g; n=6) and 40.31 ± 7.18 PCH in treated animals (365.33 ± 12.60g; n=6, unpaired t test: t= −0.643, p=0.5346). Treatment with nVNS at 4 hours, but not at 5 hours, was associated with improved neurological score (Fig. 5B, Mann-Whitney U test Z=−2.739, p=0.0062 and Z=−1.354, p=0.1757, respectively). There was no change in physiological parameters during treatment with nVNS at 4 hours (Supplementary Table 2).
Figure 5.
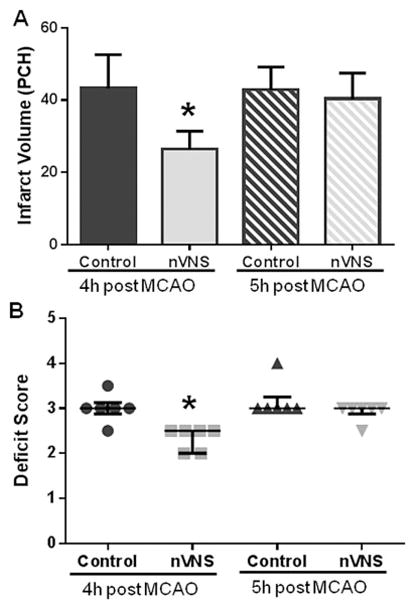
Effect of nVNS on infarct size (A), neurological score (B) when the treatment was initiated 4 and 5 hours after MCAO. PCH: percentage hemispheric volume. *p<0.05 vs. corresponding control.
Discussion
There are four important findings of this study. First, nVNS initiated 30 minutes after MCAO and applied for a period of one hour reduces the extent of tissue injury and functional deficit in rats with comorbid conditions without causing any significant cardiac or hemodynamic adverse effects. Second, nVNS can cause activation of the afferent vagus nerve fibers and stimulate its main afferent relay nuclei (NTS) in the brainstem. Third, nVNS inhibits microglia activation and normalizes altered cytokine levels after MCAO. Lastly, the favorable effect of nVNS on infarct volume is retained when applied up to 4 hours after the induction of ischemia.
Intermittent electrical stimulation of the cervical vagus nerve is an approved treatment by the FDA as an adjunctive therapy for partial epilepsy and drug-resistant depression, and has been used clinically since 1997. Currently, the only FDA-approved VNS device uses an implantable stimulator that delivers electric impulses to the left vagus nerve in the neck via a lead wire placed under the skin. We have previously shown that electrical stimulation of the dermatome corresponding to the auricular branch of the vagus nerve in the external ear using needle electrodes reduces ischemic injury after transient MCAO in healthy adult rats and could be a practical alternative to the invasive cVNS.[22] The present study extends our prior report by showing an infarct reducing effect of another novel transcutaneous stimulation method. We have used a non-invasive stimulator that requires placement of a pair of surface electrodes to the skin overlying the vagus nerve in the neck. nVNS using this particular stimulator provided 33% reduction in infarct size. Although the effect size was statistically significant, it was smaller than previously reported reductions achieved by cVNS (~50%).[5–7] This discrepancy could be due to different animal strains (spontaneously hypertensive rats vs. healthy animals) or differences between the capacity of invasive and noninvasive approaches in selectively stimulating the afferent fibers within the vagus nerve. Activation of vagus nerve fibers via a transcutaneous approach depends on several factors including electrode configuration, stimulus intensity, tissue impedance, and dispersion of the current as a result of highly conductive adjacent muscles and blood vessels. It has been suggested that, at stimulation parameters used in this study, nVNS only stimulates large, myelinated, afferent, type-A fibers that convey sensory information from the viscera to the central nervous system.[24] Increasing the stimulus intensity could provide a greater activation of the central vagal pathways, presumably to a closer extent to that achieved by direct stimulation of the nerve, but high intensity stimulation could potentially cause activation of efferent type-B and type-C fibers within the vagus nerve as well, leading to a number of unwanted side effects such as pain sensation, bronchospasm, laryngeal spasm, cardiac and hemodynamic depression, and contraction of the adjacent skeletal muscles.[25, 26] In addition, activation of carotid sinus afferents and aortic depressor nerve secondary to reduced spatial selectivity could result in an acute hypotensive response via the vasovagal reflex. The lack of cardiovascular side effects in this study supports that electrical field generated by the stimulator exhibits nerve fiber selectivity. Early human experience with the transcutaneous approach used in this study indicates that stimulation is associated with a strong but non-painful sensation and slight contraction of the sternocleidomastoid muscle without hemodynamic changes.[27] nVNS is a rapidly evolving field, and development of stimulation techniques with better spatial, nerve fiber, and directional selectivity could provide stronger activation of central vagal pathways without causing serious adverse events in the future.
Demonstration that the vagus nerve is being stimulated by the transcutaneous approach requires simultaneous recording of action potentials from the proximal nerve. Because the electrical field generated by the transcutaneous stimulator covered the entire cervical portion of the vagus nerve, nerve recording in the neck was not possible in rats. We, however, demonstrate that nVNS causes the same alterations known to occur after direct cVNS in key neural structures. In the brainstem, unilateral nVNS leads to bilateral activation in NTS, the main sensory relay nucleus of the vagus nerve, as demonstrated by c-Fos staining. More distally, in the cerebral cortex, nVNS reduces the number of activated microglia and TNF-α level, and increases HMGB1 level after ischemia. A well-known effect of cVNS in the periphery is inhibition of innate immune responses to tissue injury; cVNS exerts a powerful anti-inflammatory effect via activation of α7 nicotinic acetylcholine receptors (α7nAChRs) on peripheral macrophages in experimental models of sepsis, hemorrhagic shock, myocardial ischemia, and arthritis.[14] Activation of α7nAChRs via the cholinergic anti-inflammatory pathway inhibits cytokines such as TNF-α and HMGB1 and reduces inflammation.[28, 29] The exact mechanism of vagus nerve stimulation-mediated ischemic protection is not known. Under non-ischemic conditions, cVNS exerts anti-inflammatory effect via inhibition of cytokine release, leads to suppression of excitotoxicity, and may cause vasodilation secondary to activation of parasympathetic cerebrovascular postganglionic fibers from the sphenopalatine ganglion.[30, 31] We have previously shown that cVNS does not lead to increased rCBF within the ischemic tissue and parasympathetic system-mediated vasodilation is not the primary mechanism of ischemic protection by cVNS.[6, 15] Here, we report that nVNS treatment is associated with reduced microglia activation and cytokine expression/release. This finding underscores the importance of anti-inflammatory mechanism in ischemic neuroprotection by vagus nerve stimulation. Indeed, recent studies show that invasive cVNS decreases TNF-α, IL-1β, IL-6, and increases expression of α7nAChRs on microglia following MCAO and peroxisome proliferator-activated receptor gamma and microRNA210 may play a mechanistic role in cVNS-induced neuroprotection.[8, 32, 33] We did not observe a significant reduction in IL-1β and IL-6 levels with transcutaneous stimulation. This could be due to differences in animal strains (Sprague-Dawley vs. SHR) as well as the method used to detect interleukins (ELISA vs. immunohistochemistry).
While intraluminal filament occlusion of the MCA is a widely used and reproducible method, it requires manipulation, clipping, and puncture of the carotid artery and hence poses a risk of injury to the carotid baroreceptors and its nerve. This may have caused underestimation of carotid baroreceptor-mediated cardiac and hemodynamic depression by nVNS. Additionally, isolation and dissection of the carotid artery during filament occlusion could cause damage to the vagus nerve and blunt the effect of nVNS on infarct size. Since unilateral nVNS causes bilateral NTS activation, future studies where nVNS is applied contralateral to the MCAO could address such concerns. Additional limitations include utilization of a single MCAO model, challenges related to quantification and interpretation of non-automated immunohistochemistry experiments, and lack of monitorization of neurological function over 1 week using detailed sensori-motor function tests.[34] Also, our results on immunohistochemistry should be interpreted within the context of cerebral ischemia.
Conclusions
We demonstrate that transcutaneous stimulation applied to the neck reaches to the brain, causes blockage of ischemia-induced inflammation, and provides protection against ischemic tissue injury. Our results provide proof of concept evidence with implications for a variety of other neurological conditions where nVNS can potentially be effective such as partial epilepsy, acute migraine headache, drug-resistant depression, Alzheimer’s disease, and traumatic brain injury.[35, 36] If proven safe and effective in humans, nVNS could help avoid missed opportunities to reduce ischemic brain injury as transcutaneous stimulation can be easily applied by trained medical personnel even in a non-hospital setting early after stroke. Its relatively large therapeutic window (up to 4 hours) suggests utility as a potential adjunctive therapy in acute ischemic stroke.
Supplementary Material
Highlights.
Noninvasive VNS reduces tissue injury and functional deficit after cerebral ischemia.
Therapeutic effect of noninvasive VNS on infarct volume in retained when applied up to 4 hours after ischemia.
Noninvasive VNS does not cause any significant cardiac or hemodynamic adverse effects.
Noninvasive VNS inhibits microglia activation and normalizes altered cytokine levels after cerebral ischemia.
Acknowledgments
This study was supported by NIH-NINDS (R21NS081395 to I.A.) and an unrestricted research gift from Electrocore, LLC. This research was carried out in whole at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41RR14075, a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), National Institutes of Health.
Abbreviations
- MCAO
Middle cerebral artery occlusion
- cVNS
cervical vagus nerve stimulation
- nVNS
non-invasive cervical vagus nerve stimulation
- SHR
spontaneously hypertensive rat
- PCH
percent of the contralateral hemisphere
- NTS
nucleus tractus solitarius
- ABP
arterial blood pressure
- HR
heart rate
- rCBF
regional cerebral blood flow
- α7nAChR
α7 nicotinic acetylcholine receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- 1.Reis DJ, Berger SB, Underwood MD, Khayata M. Electrical stimulation of cerebellar fastigial nucleus reduces ischemic infarction elicited by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 1991;11:810–8. doi: 10.1038/jcbfm.1991.139. [DOI] [PubMed] [Google Scholar]
- 2.Glickstein SB, Ilch CP, Golanov EV. Electrical stimulation of the dorsal periaqueductal gray decreases volume of the brain infarction independently of accompanying hypertension and cerebrovasodilation. Brain Res. 2003;994:135–45. doi: 10.1016/j.brainres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Glickstein SB, Ilch CP, Reis DJ, Golanov EV. Stimulation of the subthalamic vasodilator area and fastigial nucleus independently protects the brain against focal ischemia. Brain Res. 2001;912:47–59. doi: 10.1016/s0006-8993(01)02602-6. [DOI] [PubMed] [Google Scholar]
- 4.Henninger N, Fisher M. Stimulating circle of Willis nerve fibers preserves the diffusion-perfusion mismatch in experimental stroke. Stroke. 2007;38:2779–86. doi: 10.1161/STROKEAHA.107.485581. [DOI] [PubMed] [Google Scholar]
- 5.Ay I, Lu J, Ay H, Gregory Sorensen A. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459:147–51. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–5. doi: 10.1016/j.brainres.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Z, Baker W, Hiraki T, Greenberg JH. The effect of right vagus nerve stimulation on focal cerebral ischemia: an experimental study in the rat. Brain Stimul. 2012;5:1–10. doi: 10.1016/j.brs.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y, Li L, Liu B, Zhang Y, Chen Q, Li C. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One. 2014;9:e102342. doi: 10.1371/journal.pone.0102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chae JH, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE, et al. A review of functional neuroimaging studies of vagus nerve stimulation (VNS) J Psychiatr Res. 2003;37:443–55. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 10.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980;193:435–65. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- 11.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–14. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheyuo C, Jacob A, Wu R, Zhou M, Coppa GF, Wang P. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31:1187–95. doi: 10.1038/jcbfm.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–50. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ay I, Ay H. Ablation of the sphenopalatine ganglion does not attenuate the infarct reducing effect of vagus nerve stimulation. Auton Neurosci. 2013;174:31–5. doi: 10.1016/j.autneu.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 17.Oshinsky ML, Murphy AL, Hekierski H, Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155:1037–42. doi: 10.1016/j.pain.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 20.Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PloS one. 2009;4:e4764. doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Elsevier Academic Press; 2005. [Google Scholar]
- 22.Ay I, Napadow V, Ay H. Electrical Stimulation of the Vagus Nerve Dermatome in the External Ear is Protective in Rat Cerebral Ischemia. Brain Stimul. 2015;8:7–12. doi: 10.1016/j.brs.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strom JO, Ingberg E, Theodorsson A, Theodorsson E. Method parameters’ impact on mortality and variability in rat stroke experiments: a meta-analysis. BMC Neurosci. 2013;14:41. doi: 10.1186/1471-2202-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundar S, Gonzalez-Cueto JA. On the activation threshold of nerve fibers using sinusoidal electrical stimulation. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2908–11. doi: 10.1109/IEMBS.2006.259667. [DOI] [PubMed] [Google Scholar]
- 25.Evans MS, Verma-Ahuja S, Naritoku DK, Espinosa JA. Intraoperative human vagus nerve compound action potentials. Acta Neurol Scand. 2004;110:232–8. doi: 10.1111/j.1600-0404.2004.00309.x. [DOI] [PubMed] [Google Scholar]
- 26.McAllen RM, Spyer KM. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. The Journal of physiology. 1978;282:353–64. doi: 10.1113/jphysiol.1978.sp012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goadsby PJ, Grosberg BM, Mauskop A, Cady R, Simmons KA. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986–93. doi: 10.1177/0333102414524494. [DOI] [PubMed] [Google Scholar]
- 28.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–74. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 30.Mollet L, Grimonprez A, Raedt R, Delbeke J, El Tahry R, De Herdt V, et al. Intensity-dependent modulatory effects of vagus nerve stimulation on cortical excitability. Acta Neurol Scand. 2013;128:391–6. doi: 10.1111/ane.12135. [DOI] [PubMed] [Google Scholar]
- 31.Bansal V, Ryu SY, Lopez N, Allexan S, Krzyzaniak M, Eliceiri B, et al. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation. 2012;35:214–20. doi: 10.1007/s10753-011-9307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y, Li L, Liu B, Zhang Y, Chen Q, Li C. PPARgamma upregulation induced by vagus nerve stimulation exerts anti-inflammatory effect in cerebral ischemia/reperfusion rats. Med Sci Monit. 2015;21:268–75. doi: 10.12659/MSM.891407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Y, Li L, Tan X, Liu B, Zhang Y, Li C. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J Neurochem. 2015;134:173–81. doi: 10.1111/jnc.13097. [DOI] [PubMed] [Google Scholar]
- 34.Taylor CR, Levenson RM. Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49:411–24. doi: 10.1111/j.1365-2559.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- 35.Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjogren MJ, Hellstrom PT, Jonsson MA, Runnerstam M, Silander HC, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J Clin Psychiatry. 2002;63:972–80. doi: 10.4088/jcp.v63n1103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


