Abstract
Aims
Although the cardiovascular benefits of exercise are well known, exercise induced effects and mechanisms in prevention of cardiomyopathy are less clear during obesity associated type-2 diabetes. The current study assessed the impact of moderate intensity exercise on diabetic cardiomyopathy by examining cardiac function and structure and mitochondrial function.
Methods
Obese-diabetic (db/db), and lean control (db/+) mice, were subjected to a 5 week, 300m run on a tread-mill for 5 days/ week at the speeds of 10-11 m/min. Various physiological parameters were recorded and the heart function was evaluated with M-mode echocardiography. Contraction parameters and calcium transits were examined on isolated cardiomyocytes. At the molecular level: connexin 43 and 37 (Cx43 and 37) levels, mitochondrial biogenesis regulators: Mfn2 and Drp-1 levels, mitochondrial trans-membrane potential and cytochrome c leakage were assessed through western blotting immunohistochemistry and flow cytometry. Ability of exercise to reverse oxygen consumption rate (OCR), tissue ATP levels, and cardiac fibrosis were also determined.
Results
The exercise regimen was able to prevent diabetic cardiac functional deficiencies: ejection fraction (EF) and fractional shortening (FS). Improvements in contraction velocity and contraction maximum were noted with the isolated cardiomyocytes. Restoration of interstitial and micro-vessels associated Cx43 levels and improved gap junction intercellular communication (GJIC) were observed. The decline in the Mfn2/Drp-1 ratio in the db/db mice hearts was prevented after exercise. The exercise regimen further attenuated transmembrane potential decline and cytochrome c leakage. These corrections further led to improvements in OCR and tissue ATP levels and reduction in cardiac fibrosis.
Conclusions
Moderate intensity exercise produced significant cardiovascular benefits by improving mitochondrial function through restoration of Cx43 networks and mitochondrial trans-membrane potential and prevention of excessive mitochondrial fission.
Keywords: Diabetes, obesity, exercise, mitochondrial-biogenesis, Connexin, Drp-1, Mfn-2, cytochrome c, trans-membrane-potential, fibrosis
Introduction
Heart failure is the leading cause of death and accounts for nearly half of the deaths in older adults. Although diabetics have double the risk of heart failure, the mechanisms of diabetes induced heart disease process and the sequence of molecular events leading to heart failure are not entirely known. Diabetes induced cardiovascular changes that lead to heart failure can be divided into three not directly related broad categories: coronary heart disease (CHD), hypertension, and diabetic cardiomyopathy. Diabetic cardiomyopathy involves structural and molecular changes in the myocardial tissue that aggravate heart failure risk, either alone or in combination with the changes in local (CHD) or systemic (hypertension) vascular behavior and hemodynamics [1]. Diabetic cardiomyopathy has been reported to manifest as systolic and diastolic dysfunction and left ventricular hypertrophy thereby enhancing the chances of heart failure [1, 2]. While the functional changes accompanying diabetic cardiomyopathy are relatively well known, the structural and molecular alterations affecting heart contractility remain unknown.
Structural changes during diabetic cardiomyopathy include myocyte uncoupling, mitochondrial uncoupling/failure and endothelial uncoupling, all leading to poor cardiac contractility. Myocyte coupling in the cardiac tissue is maintained through gap junctions. Connexin 43 (Cx43) is the main constituent in the gap junctions of adult hearts [3] and is crucial for nutrient and electrolyte coupling of myocyte networks, mitochondrial networks and microvascular endothelial networks in the cardiac tissue [4, 5]. Significant alterations in the Cx43 levels indicates poor prognosis. Diabetes alters Cx43 levels and/or distribution in different tissues including the heart tissue [3-8]. Diabetes has also been shown to inhibit Cx43 levels in the microvessels [5].However, diabetes can cause either up or down regulation of Cx43 levels in the myocardium and the causes for such variation are unknown [4]. Another important constituent of gap junction proteins, Cx37, has also been implicated as a target for diabetes induced alterations in the cardiac endothelium [9]. Exercise has been shown to reverse the abnormal Cx43 phosphorylation in the rat models of type 1 diabetes [7]. However, in obese type-2 diabetes mouse models, with severe physical limitations, exercise induced changes in Cx43 and Cx37 levels have not been investigated.
Apart from the structural changes that compromise efficient myocyte contraction, defective mitochondrial function also contributes to poor contractility which eventually leads to diabetic cardiomyopathy. Hyperglycemia has been found to alter the balance between mitochondrial fission and fusion, by enhancing Drp-1 (dynamin related protein-1) mediated fission to produce mitochondrial dysfunction and eventual cell death in different tissues [3, 10, 11]. While Drp-1 is necessary for mitochondrial mitophagy, function and ischemic tolerance in the cardiac tissue [12]; diabetes induced changes in Drp-1 levels and consequences of excess Drp-1 presence are unknown. In addition, altered mitochondrial membrane permeability has also been implicated in diabetic cardiomyopathy. Diabetes induced reactive oxygen species (ROS) sensitizes the mitochondrial membrane transition pore (MTP) opening and leads to elevated risk for heart failure [13]. Enhanced MTP opening leads to leakage of cytochrome c into the cytoplasm and may result in enhanced apoptosis especially during diabetes [14, 15] and may lead to poor ATP production as well. However, the modulatory effects of exercise in reversing elevated MTP opening sensitivity during diabetes remain unknown.
Exercise confers numerous cardiovascular benefits during diabetes either directly through modulation of myocardial molecules or indirectly through reducing hyperglycemia [16]. However, there are very few studies that examined direct benefits of exercise on diabetic cardiomyopathy independent of hyperglycemia status during diabetes [17]. Moreover, in severely obese individuals there is often a constraint for rigorous, prolonged and strenuous exercise regimen due to physical limitations, which in turn may lead to lack of motivation to do exercise. Hence, it is highly desirable to know the cardiovascular benefits of a moderate exercise regimen that may not correct glucose levels efficiently but is better suited to obese individuals. Importantly, the relative contribution of connexin and mitochondrial function in causation of diabetes and exercise induced cardiac contractile alterations were not assessed. The current study utilized db/db mice to address the direct benefits of exercise on the cardiovascular system through whole heart and individual myocyte contractility assays and assessed connexin levels, mitochondrial biogenesis, trans-membrane potential, and cytochrome c leakage, which modulate myocyte contractility, as the molecular measures of improvement.
Methods
Animals
Male lean control (db/+) and diabetic obese (db/db) mice with C57BL/6J background were procured from Jackson labs at the age of 2 months and were given ad libitum access to standard chow and water. At the time of final data collection the mice were of age around 4 months. A minimum of four mice were used in each group for physiological function experiments. For molecular experiments, we used a minimum of three different mice per group. Blood glucose levels were estimated using a glucometer and test strips from ‘One touch’ (One touch ultra 2) and the blood drop was taken from the tail vein. At the time of blood glucose measurement, all mice were fed randomly and no active fasting was observed. The western blots from total lysates, Q-PCR, tissue sections (for confocal imaging and Masson's trichrome staining), and ATP levels assessment were done from the same hearts.
Cardiomyocyte isolation
The intact hearts were dissected out quickly from the anaesthetized mice and were perfused with freshly made perfusion buffer with liberase TH as described before [18]. The yield was 80-85% and did not vary between the groups. The isolated myocytes were used immediately for the contractility measurements, calcium transits, flow cytometry analysis, mitochondrial fractionation and oxygen consumption rates (OCR) measurements.
Calcium transit measurement
A sub set of cardiomyocytes were incubated with Fura-2-AM (1.0 μmol/l) for 30 min, and fluorescence measurements are recorded with a dual-excitation fluorescence photomultiplier system (IonOptix) as described before after 1 Hz field stimulation [18].
Cardiomyocyte contractility measurements
Myocytes were field stimulated (at a frequency of 1.0 Hz, pulse duration of 4 ms and amplitude of 10 volts) using IonOptix myopacer and the contractions were recorded through SoftEdge™ Acquisition Software as described before [18]. A batch of 5 randomly-selected myocytes were recorded for the contraction parameters at a time from an unstimulated pool and a total of 20 myocyte recordings per heart were collected for further analysis.
Western blotting
Tissues were homogenized in RIPA lysis buffer supplemented with protein and phosphatase inhibitors and lysates were sonicated before removal of insoluble debris. Total protein concentration was enumerated using Bradford assay. Equal amounts of lysates (30 μg) were resolved using SDS-PAGE gels. Blots were probed with specific antibodies and the reaction was visualized using chemiluminescence reaction as described before [19].
Antibodies
Connexin 37 and 43 are from Invitrogen; HSP-60 and GAPDH are from Millipore; Drp-1 is from Santa Cruz biotech; phospho-Cx43 (Ser 368) is from cell signaling technologies; and Mfn2, phospho-ERK1/2 (Tyr204/187) and cytochrome c are from Abcam.
JC-1 staining
Mitochondrial membrane potential was measured in the freshly isolated cardiomyocytes using JC-1 staining. Briefly, cardiomyocytes were stained with 2 μM JC-1 stain for 20 min in the cell culture incubator and washed with PBS solution twice. Both the red and green fluorescence intensities were detected simultaneously by using Accuri C6 flow cytometer (BD Biosciences, San Jose, CA).
Mitochondrial fractionation
cytosolic and mitochondrial fractions were isolated from the freshly prepared cardiomyocytes by using the Qproteome mitochondria isolation kit (Qiagen) and following the manufacturer's recommended protocol.
OCR estimation
Fresh cardiomyocytes isolated from different groups were washed with PBS and live cell count was determined with trypan blue staining. An equal amount of live cells (~50,000 cells/ well) were plated in black 96 well plates with clear bottom. To measure OCR, MitoXpress® - Xtra HS kit (Cayman chemicals) was used and followed the recommended protocol. Briefly, cells were added with phosphorescent oxygen probe and the wells were sealed with mineral oil to prepare an air tight environment. Appropriate positive (glucose oxidase) and negative (Antimycin A) controls were also prepared simultaneously. The change in the color intensity was measured spectrophotometrically for a period of 16 hrs at five minute intervals.
ATP levels measurement
Tissue levels of ATP were determined spectrophotometrically by using a kit (Bio Vision). After mouse sacrifice, hearts were collected immediately and were flushed and washed in saline solution and were frozen immediately in liquid nitrogen. At the beginning of the assay, heart tissues were thawed, weighed and were homogenized in ATP assay buffer. Deproteinization was carried using perchloric acid precipitation. Equal amounts of neutralized and cleared supernatants were used for ATP level determination along with known ATP standards.
Exercise protocol
Mice were exercised using a small animal treadmill (#1050 RM-E57) from Columbus instruments with zero inclination. Initial adaptation was done at slower speed for 5 days at 7 m / min. Later, the mice were monitored to cover a daily distance of 330 meters with the speed of 10 meters/min for the first two weeks and with the speed of 11 meters /min for the rest of the period (3 weeks) for 5 days/week, for a total of 5 weeks. After every 100 meters the mice were rested for 10 mins. Active monitoring was done to ensure movement. The unmotivated mice were motivated with a gentle push on their backs by using a hanging brush from the top. On any given day, there were few mice that were actually motivated and did well without any assistance throughout the exercise period. Hence, we believe that the stress level is equal among the group. To minimize the stress of excessive motivation, we have given intermittent rest periods. The degree of stress caused by motivational touches during such exercise aversion was not recorded in the current study and could be an important criterion to be included in the future studies.
Confocal imaging
Immunocytochemistry was performed on freshly isolated cardiomyocytes as reported before [18]. After fixation with 4% paraformaldehyde the cells were permeabilized and were washed three times with PBS. After blocking, the cells were incubated with primary and secondary antibodies. After each step, the cells were washed thrice with PBS solution. Cell were analyzed for fluorescence using a laser scanning confocal microscope (Olympus FluoView-1000, 60X). The total fluorescence was calculated using ImageJ software and the measurements are presented as fluorescent intensity units (FIU).
Echocardiogram
Left ventricular function was assessed by transthoracic echocardiography using a Vevo 2100 with an MS550D transducer having a frequency of 22–55 MHz (Visual Sonics). Briefly, the mice were anesthetized using 3.5% isoflurane and depilated with Nair hair removal cream. Maintenance dose of isoflurane was 1.5% and heart rate was at around 450±50 beats/min while recording the cardiac parameters. Percentage ejection fraction (% EF) representing ventricular function, ventricular wall thickness, and chamber diameter was derived from the measurements during short-axis M-mode. Where ever possible, all of the physiological measurements were collected during the afternoon period on the day to minimize the variation.
Masson's trichrome staining
Cryo sections were stained with Masson's trichrome stain (Chromaview, no. KTRA87019, Fisher Scientific, Pittsburgh, PA) by following the manufacturer's recommendations to measure the collagen deposition in the heart tissues as reported before [20]. Images were obtained with the QC-capture software.
Picrosirius red staining
Sections were stained to differentiate type I and III collagen with a Picrosirius red staining kit (Polysciences, Inc.) by following the recommended protocol.
Blood pressure recording
Blood pressure (BP) in the conscious animal was measured by a noninvasive tail-cuff method (CODA 2, Kent Scientific Corp., Torrington, CT) after proper acclimatization and in a rest state as reported before [21].
Semi-Quantitative PCR
Total RNA was isolated by using TRIzol reagent. Equal amounts of total RNA was subjected to cDNA with the usage of RT-PCR reagents from Qiagen. The expression levels of a-MHC were assayed using the following primer pair: Forward: 5’ GGAAGAGTGAGCGGCGCATCAAGG 3’ and Reverse:5’ CTGCTGGAGAGGTTATTCCTCG 3’; and the resulting amplicons were resolved on the agarose gels.
Statistical analysis
All values are presented as mean±SE. Densitometry analysis was performed using ImageJ or Image Lab software. P values of <0.05 were considered significant. Statistical analysis was done using Primer of Biostatistics 7.0 (McGraw-Hill, New York, NY, USA). Comparisons were made by one-way ANOVA (analysis of variance) followed by Bonferroni correction.
Results
Exercise prevented diabetic cardiac dysfunction
We measured various physiological parameters to understand the impact of the moderate exercise regimen (Table 1). There were no significant changes in the blood glucose levels before and after exercise. However, there was significant improvement in the other parameters such as decline in mean arterial blood pressure and reduction in bodyweight. Moreover, there were enhancements in heart weight as well. These results suggested that moderate exercise improves overall body condition and reduces heart stress.
Table 1.
Mean blood pressure, blood glucose levels and gravimetric data in the control and db/db mice were measured following the 6 weeks of exercise protocol.
| Control | db/db | Control+ Ex | db/db+Ex | |
|---|---|---|---|---|
| n=5 | n=6 | n=5 | n=4 | |
| Body wt (gm) | 33.2 ± 1.5 | 58.08 ± 1.4* | 28 ± 2.2 | 45.64 ± 1.2# |
| Mean BP (mmHg) | 92 ± 1.01 | 130 ± 2.11* | 96 ± 1.07 | 112 ± 1.15# |
| heart wt (gm) | 0.156 ± 0.002 | 0.124 ± 0.005* | 0.155 ± 0.004 | 0.142 ±0.003# |
| Blood Glucose (mg/dL) | 188.6 ± 17.5 | 628.8 ± 10.5* | 156.7 ± 4.3 | 621.8 ± 28.1 |
Number of animals (n) in each group are also presented.
p<0.05 vs control non-exercise (Control) and
p<0.05 vs db/db non-exercise (db/db).
Next, to understand the specific improvements in heart function, we performed transthoracic left ventricular echocardiography. As displayed in Fig. 1a and Table 2, the M-mode echocardiography image analysis suggested significant enhancement in the fractional shortening after exercise in the db/db mice. Other parameters such as posterior wall thickness and cardiac output showed a tendency for enhancement. Further, these improvements in heart geometry were also translated into the heart functional output as evidenced by the improvements in the ejection fraction. Together, these results indicate that regular moderate intensity exercise can effectively prevent the diabetes-obesity syndrome induced changes in heart physiology.
Fig. 1.
Evaluation of left ventricular function. (a) Representative M-mode echocardiography images from each are presented. Arrows indicate diastolic (longer) and systolic (shorter) chamber lengths. (b) Line graph represents weekly mean arterial blood pressure before, during and after the exercise. Statistics were done for the last two points. NS represent not significant and * p<0.05.
Table 2.
Various echocardiographic parameters for each group were presented as mean±SE.
| Parameter/Group | Control | db/db | Control+Ex | db/db+Ex | db/db-pre Ex |
|---|---|---|---|---|---|
| IVSd mm | 1.19 ± 0.1 | 1 ± 0.06 | 1.26 ± 0.03 | 1.03 ± 0.04 | 1.16 ± 0.26 |
| IVSs mm | 1.72 ± 0.06 | 1.71 ± 0.09 | 2.14 ± 0.13 | 1.76 ± 0.2 | 1.66 ± 0.19 |
| HR BPM | 399.22 ± 22.04 | 419.29 ± 15.95 | 430.62 ± 13.74 | 416.41 ± 14.95 | 417.94 ± 23.28 |
| SV uL | 39.56 ± 0.6 | 40.12 ± 0.62 | 39.84 ± 1.63 | 49.51 ± 3.42# | 48.52 ± 3.08~ |
| EF % | 88 ± 2.19 | 83.91 ± 1.5 | 90.14 ± 2.1 | 93.03 ± 1.11# | 86.07 ± 3.28 |
| FS % | 57.64 ± 2.97 | 52.68 ± 2.13 | 60.9 ± 2.96 | 65.99 ± 2.34# | 55.8 ± 4.95 |
| CO mL/min | 16.27 ± 0.68 | 16.45 ± 0.93 | 17.16 ± 0.86 | 20.71 ± 1.96 | 20.13 ± 0.16 |
| LVIDd mm | 3.05 ± 0.14 | 3.27 ± 0.31 | 3.21 ± 0.03 | 3.45 ± 0.14 | 3.51 ± 0.3 |
| LVIDs mm | 1.42 ± 0.17 | 1.76 ± 0.17 | 1.5 ± 0.11 | 1.7 ± 0.24 | 1.73 ± 0.27 |
| LVPWd mm | 0.92 ± 0.09 | 0.8 ± 0.11 | 1.39 ± 0.11 | 1.1 ± 0.02 | 0.86 ± 0.03 |
| LVPWs mm | 1.65 ± 0.07 | 1.43 ± 0.19 | 1.92 ± 0.21 | 1.63 ± 0.11 | 1.44 ± 0.15 |
p<0.05 vs db/db non-exercise (db/db) and
p<0.05 vs db/db non-exercise (db/db).
Exercise ameliorated contractile defects with the db/db mouse cardiomyocytes
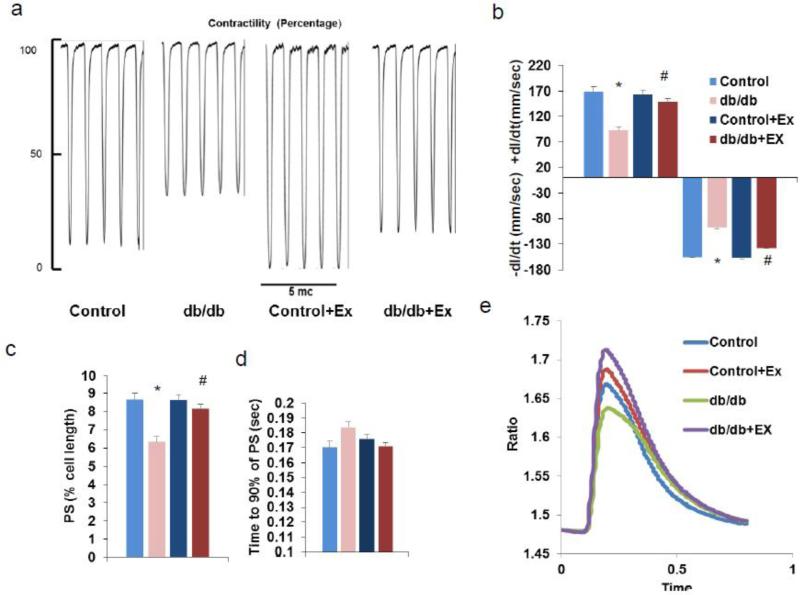
To further know the alterations made by the diabetic condition and the exercise regimen over the contractile properties of cardiomyocytes, we subjected freshly isolated cardiomyocytes to a field electric stimulus and recorded various contractile parameters. Both the velocity of contraction and relaxation were impaired (slower) in the case of db/db cardiomyocytes when compared to that of the control cardiomyocytes (Fig. 2a-b). However, these defects with the velocity were prevented with the exercise regimen. In addition, non-maximal/incomplete contraction occurred as observed by reduced peak shortening (PS) and enhanced time to PS in the db/db mouse cardiomyocytes (Fig. 2c-d). The exercise protocol was able to prevent development of PS defects significantly. Deficiencies associated with calcium transits during contraction were also halted after exercise (Fig. 2e). These findings further indicate that the declined efficiency of cardiac contraction and rate might be the cause of reduced ejection fraction.
Fig. 2.
Cardiomyocyte contractility measurements and calcium transits are presented. (a) Representative contractility tracings. (b) Contraction velocity: relaxation rate (+dl/dt) & contraction rate (−dl/dt). (c) Peak shortening (PS) as percentage of total cell length. (d) Time taken for 90% of the PS. (e) Calcium transit to cytosol in real time. * p<0.05 vs control and # p<0.05 vs db/db. Ex for exercise.
Exercise prevented connexin 43 deficiency in the diabetic myocardium and raised the potential for intercellular communication and myocyte coupling
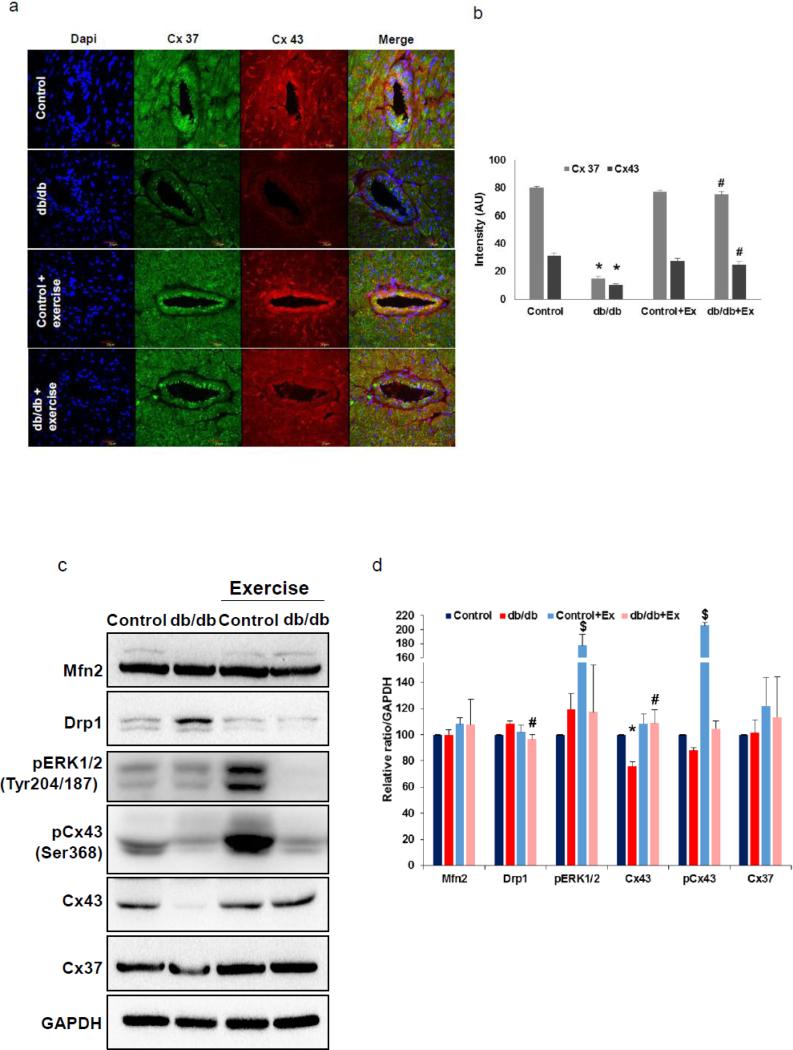
Since reduced heart function could be due to poor networking and less efficient conveyance of action potentials and nutrients among cardiomyocytes, mitochondria and micro-vessels networks; we examined the important constituents of myocardial gap junctional proteins and connexin levels in the db/db mouse heart before and after exercise. Both Cx43 and Cx37 were reduced in myocardial tissue as well as micro-vessels in the cardiac tissue (Fig. 3a-d). However, exercise normalized the altered Cx43 and Cx37 levels in the above mentioned regions of myocardial tissue. We further quantified the levels of Cx43 and Cx37 in the hearts from different groups by western blotting and noted that there was significant reduction in the Cx43 levels in the case of db/db mice and that exercise reversed these levels. Though the exercise tended to slightly enhance the Cx37 levels, the overall levels were not significantly different.
Fig. 3.
Quantification of Connexin and mitochondrial biogenesis regulators. (a-b) connexin (Cx43 and Cx37) expression in IHC tissue sections (60X) and fluorescence intensity quantification. (c-d) connexin and mitochondrial biogenesis regulators: Mfn2 and Drp-1 quantification through western blot imaging and quantification. * p<0.05 vs control and # p<0.05 vs db/db. Ex for exercise.
Since Cx43 is the major constituent of the cardiac gap junctions (GJ) and phospho-Cx43 was shown to modulate gap junction performance [22, 23], we have evaluated phosphorylation status at the Ser368 residue of the CX43, which is mediated by the PKC. We found that CX43 levels were significantly elevated in the control db/+ mice hearts, but there seems to be overall decline in the phopsho-Cx43/total Cx43 ratio in the db/db mice hearts after exercise. As it has been suggested that MAPK activation is also involved in the inhibition of gap junction intercellular communication (GJIC) through Cx43 phosphorylation (at Ser255, Ser262, and Ser279/Ser282 ) [22, 23], we assayed for the ERK1/2 activity levels by detecting their phosphorylation status. As demonstrated in Fig. 3c & 3d, ERK1/2 activation was elevated in the db/+ mice hearts but not in the db/db mice hearts after the exercise. These results suggest that gap junction mediated intercellular communication was interrupted only in the db/+ mice. Conversely, the GJIC is enhanced in the db/db mice through Cx43 after exercise.
Exercise attenuated adverse mitochondrial biogenesis regulation by enhancing Mfn2/Drp-1 ratio in the db/db mice hearts
As mitochondria are the energy powerhouses and could affect the myocyte contractile properties, we assayed for the mitochondrial biogenesis regulators. As noted in Fig. 3c-d, we did not observe significant change in one of the mitochondrial fusion regulators, Mfn2, in the db/db mouse hearts before and after exercise. In contrast to the Mfn2 levels, levels of the one of the key fission regulators, Drp-1, were significantly elevated in the db/db mouse hearts and were normalized after exercise. These findings indicate that obesity associated diabetic condition imbalances the mitochondrial biogenesis through excessive fission and might contribute to the contractile defects observed with the myocytes, and that exercise through reducing the excess fission, leads to balanced biogenesis.
Exercise ameliorated mitochondrial transmembrane potential decline and leakage of cytochrome c
As diabetic condition was reported to cause abnormal membrane permeability transition pore opening, we examined whether the exercise could reverse decline in the transmembrane potential and prevent excessive leakage of inner mitochondrial protein cytochrome C. For this purpose, we isolated cardiomyocytes from the hearts of different groups and assessed the JC-1 aggregates in the mitochondria through flow cytometry. Around 16% of the myocytes from db/db hearts exhibited considerably higher JC-1 monomers indicating reduced transmembrane potential (Fig. 4a). After exercise, the myocytes with abnormal transmembrane depolarization were reduced to 10%; indicating betterment of mitochondrial function. Next, to know if correction in the transmembrane potential leads to prevention of cytochrome C leakage into the cytosol, we collected the cytosolic and mitochondrial fractions from the freshly isolated cardiomyocytes and examined the cytochrome c levels (Fig. 4b-c). Though exercise considerably reduced the Cytochrome c leakage into the cytosol, the difference was not significantly different from the db/db mice group. However, exercise was able to enhance the mitochondrial cytochrome c levels as expected. In contrast to the db/db mice, exercise further reduced the residual cytochrome c leakage in the control group.
Fig. 4.
Mitochondrial membrane potential (ΔΨm) and cytochrome c leakage. (a) % cells with: right upper (UR) quadrant represents red fluorescence by the JC-1 aggregates (normal) and lower right (LR) quadrant represents green fluorescence by the JC-1 monomers (defective). (b) Bar graph depicting the quantification of cardiomyocytes with defective membrane potential (with JC-1 monomers)in each group. (c-) Representative western blot images of mitochondrial and cytosolic proteins of freshly isolated cardiomyocytes are presented. (d-e) quantification of cytosolic (d) and mitochondrial (e) cytochrome c (CytoC)protein levels in bar graphs. * p<0.05 vs control, $ p<0.05 vs control and # p<0.05 vs db/db non-exercise (db/db)..
Exercise halted decline in ATP generation and OCR
To know if exercise also ameliorated the mitochondrial functional deficiencies, we evaluated two parameters: OCR from isolated cardiomyocytes (Fig. 5a) and total ATP content from the ventricular tissue (Fig. 5b). The mean OCR was lower when compared to that of the control cardiomyocytes throughout the measuring period after the 5 hrs of incubation and there were more significant differences in the OCR after 15 hrs of measurement (Fig. 5a). In contrast to the non-exercise controls, the exercised cardiomyocytes from both the groups fared well throughout the measuring period. These results suggested that exercise reinvigorated the mitochondria to enhance the myocyte ability to utilize oxygen, presumably through prevention of excessive mitochondrial fission and decline in mitochondrial transmembrane potential.
Fig. 5.
Oxygen consumption rate (OCR) and ATP levels: (a) Line graph represent OCR (mean±SE). (b) Bar graph depicting the ATP levels (mean +SE). * p<0.05 vs control and # p<0.05 vs db/db. Ex for exercise.
Next, to assess if there was significant enhancement in the ATP production in the cardiac tissue, we examined the ATP content from snap frozen tissues. The ATP content was significantly lower in the db/db hearts and the exercise regimen prevented such decline in the ATP levels (Fig. 5b). These findings further corroborate the findings with the mitochondrial OCR and indicate that exercise significantly precluded the mitochondrial deficiencies associated with the diabetic condition.
Exercise prevented adverse myocardial tissue remodeling
Next, we examined whether the above changes also lead to prevention of adverse matrix remodeling and cardiac structure. To this end, we looked at interstitial collagen content and perivascular fibrosis after exercise. Overall collagen accumulation at both the perivascular regions and in interstitial regions of heart tissue was significantly higher in db/db mice and was normalized with the adoption of exercise indicating that exercise enhanced heart function and contractility by reducing fibrosis in the db/db mice (Fig.6a-b). We further confirmed the collagen accumulation and its reversal after exercise with Picrosirius red staining as well (Fig. 6c).
Fig. 6.
Collagen staining. (a-b) Masson's trichrome staining for collagen (blue) and quantification of area of collagen as percentage in bar graph. (c) Picrosirius staining for collagen (bright red). (d) Representative agarose gel image showing semi-Q-PCR amplicons for α-MHC mRNA. (e) Bar graph depicting the quantification of α-MHC mRNA levels from 3 different experiments. A.U arbitrary units. * p<0.05 vs control, $ p<0.05 vs control and # p<0.05 vs db/db.
In addition to the tissue fibrosis, we also assayed for the levels of fast twitch cardiac myosin heavy chain (MHC) isoform, α-MHC, which at higher levels endow the heart with higher contractility and better systolic function [24]. The results (Fig. 6d & 6e) suggest that exercise prevented the tendency for decline in the α-MHC isoform in the db/db mice. In the lean controls, there was significant increase in the levels of α-MHC. Altogether, the reduced fibrosis and prevention of α-MHC isoform decline after exercise in the db/db mice could also explain the improved heart function after exercise apart from improvements in the myocyte coupling and mitochondrial function.
Discussion
Regular physical activity has been shown to provide a multitude of cardiovascular benefits. Often, obese-diabetics encounter physical and psychological barriers to exercise and which in turn leads to lack of motivation or inability to do t prolonged/ high intensity exercise. Hence, our goal in this study was to assess the benefits of moderate intensity exercise on the diabetic cardiomyopathy. In the current study, we focused on the myocardial tissue networking through connexins and mitochondrial integrity and biogenesis. The results suggested that exercise reversed changes in cardiac function, myocyte contractility and calcium transits; Cx43 levels; mitochondrial biogenesis and permeability; restored OCR and ATP production; and prevented cardiac fibrosis. Further studies are needed to understand the longevity of the exercise mediated benefits on the cardiac improvements observed here during diabetic condition.
Previous studies on diabetes induced modulation of Cx43 levels in the heart tissue have been found to vary, but the causes for such variation are currently unknown. Most of the studies have been done with rats and almost all of them utilized streptozotocin treatment for induction of diabetes [4-7]. While it is very convenient and cost effective, diabetes induced by streptozotocin may not entirely represent the broad spectrum of human diabetic condition associated changes and also may have unknown organ-specific adverse/undesirable side effects [25]. The current study used obese spontaneous diabetic mouse model (db/db) to unravel the diabetes and exercise induced changes. This mouse model represents only a subset of human population with type 2 diabetes and with extreme obesity. Though this model is ideal to study the obesity induced diabetes and insulin resistance, unlike other models of diabetes with insulin insufficiency (type 1 diabetes), it may also not entirely represent human heterogeneity associated with the type 2 diabetes because of genetic nature of the disease (mutation in the leptin gene). The mono-genetic nature of disease in the db/db mouse model causes less inter-animal variation and requires fewer animals per group for experimentation [26]. However, the model may be less suitable for diabetes caused by multi-genic involvement. In contrast to most of the reports [4], the current study found that Cx43 levels were reduced in the db/db hearts. Though the causes for such reduction in Cx43 levels are currently unknown, it is plausible that excessive activation of JNK might have played a role. Previous reports have demonstrated that db/db mice hearts exhibit excessive JNK activation [27] and excessive activation of JNK pathway was linked to the downregulation of Cx43 levels in the cardiomyocytes [28].
Interestingly, the same exercise regimen which enhanced the ratio between phospho-Cx43 and total Cx43 in the lean control mice resulted in decline in this ratio in the db/db mice hearts. These results suggest that exercise significantly raised the threshold for Cx43 internalization and inhibition of GJIC in the db/db mice hearts. Conversely, exercise enhanced both Cx43 internalization and inhibition of GJIC in the hearts of lean control mice. The apparent differences might have been due to the differential induction of growth factors and cytokines and differences in adoption process after exercise. It has been reported that growth factors and cytokines such as EGF and VEGF, through activation of PKC and MAPK signaling, were able to cause post-translational modifications on Cx43 and resulted in inhibition of GJIC through Cx43 internalization [22, 23]. Additional support for the differential regulation of Cx43 internalization and inhibition of GJIC between the groups comes from our observation that indicated ERK1/2 activation after exercise in the lean mice but not in the db/db mice. It has been demonstrated that ERK1/2 activation enhances Cx43 phosphorylation at Ser262, Ser279/282 amino acids and results in Cx43 internalization and inhibition of GJIC [22, 23]. Though we did not test the status of these ERK1/2 mediated phosphorylations on Cx43 in our study, the potent activation of ERK1/2 indicates such possibility. Consistent with the observations: 1) ERK1/2 activation and 2) potential for PKC activation as indicated by the raise in CX43 Ser368 phosphorylation in the lean controls after exercise; previous studies have also reported that exercise enhances ERK1/2 activation and PKC activation [29, 30]. These reports [29, 30] also illustrated that exercise differentially induces ERK1/2 and PKC activities depending on whether the rodents are active or not prior to the final bout of exercise and how long they have been active. Furthermore, exercise induced ERK1/2 activation can be seen as early as 4 weeks, however the outcome of activated ERK1/2, the cardiac hypertrophy, can only be seen after 12 weeks and at this point of time it is not accompanied with ERK1/2 activation [29]. Analogous to these reports, the current study also suggests that differential activation of ERK1/2 and regulation of Cx43 internalization in the lean and obese-diabetics after exercise might further explain the presence or absence of cardiac hypertrophy and the differences in functional improvements after the exercise regimen. Future studies are necessary to test whether hypertrophic responses observed after exercise are maladaptive or adaptive in nature. Another limitation of the current study is sample size. Inclusion of more mice per group would not only consolidate the results reported here, but also enhance the impact of the message. Nonetheless, based on enhancement in the collagen content and reduction in GJIC which reduces ventricular compliance, our data predict that db/db mice are prone to diastolic dysfunction. The exercise regimen prevented such a diastolic dysfunction. Improvements in the stroke volume following the exercise further support such a notion. Given the significance of diastolic dysfunction in prediction of heart failure and its prevalence in type 2 diabetics [31, 32], inclusion of this criterion would enable critical evaluation of the nature of hypertrophic responses observed with the db/db mice following the exercise.
We also found that exercise prevented Cx43 and Cx37 level downregulation in the db/db heart vasculature. Though the vascular function is not assessed in the current study, others have reported improvement in the coronary vascular function with similar intensity exercise without hyperglycemic correction in db/db mice [17]. Taken together, these results indicate that exercise might directly regulate vascular signaling independent of glucose levels and improve the function of heart vasculature through restoration of connexin coupling.
Rigorous exercise training (~70% of the animal's V̇O2 max), in rats enhanced Mfn2 levels in the heart tissue [33]. Though the Mfn-2 levels were not significantly altered in the current study, they showed enhanced tendency to increase after exercise, as was also observed by the others, suggesting that Mfn2 enhancement needs increased duration and/or intensity of exercise. Nonetheless, the Mfn2/Drp-1 ratio was reversed after exercise through suppression of Drp-1 levels indicating that excessive mitochondrial fission was halted in the db/db mouse. Though not assessed here, mitochondrial biogenesis improvement in the db/db hearts through enhanced PGC-1α and Akt signaling after exercise was reported [34] and might potentially contribute to the enhanced mitochondrial density and ATP levels after exercise.
Although our exercise regimen did not produce significant changes in the cytosolic cytochrome c levels in the db/db mouse, when we consider exercise induced enhancement in the cytochrome c levels (Fig. 4c & 4e), the results strongly indicate significant suppression of cytochrome c leakage after exercise in the db/db mouse hearts. Further evidence supporting this notion comes from the data that suggested enhanced ATP generation and OCR after exercise. Moreover, in addition to the functional improvements, we were able to observe enhancements in the contractile velocity and calcium transits after exercise. These data together supported the improvements in the mitochondrial function after exercise in the db/db hearts. In contrast to db/db mouse scenario, exercise induced gains in the control mouse heart are rather modest. When considered that db/db mouse nearly weighed double the weight of the control mouse, the adopted exercise regimen might have produced relatively stronger effect in the db/db mice than in the control mice. A similar trend was also reported earlier with regard to the vascular function in the db/db [17] and control mice. Given that, we could not observe significant reductions in blood glucose levels, the results suggest that the exercise benefits observed are through the direct modulation of cardiac signaling but not through the control of hyperglycemic status as has also been observed by others [17]. Interestingly, shorter duration of exercise (3 weeks) was reported to enhance oxidative stress whereas longer duration (8 weeks), similar to the one used here, was reported to suppress oxidative stress [17, 35]. Though the exact causes are not clear, duration of diabetic status and age of the animals in addition to the changes in the exercise protocol might have played a significant role in the opposite antioxidant effects observed with the exercise. Nonetheless, exercise dependent induction of MnSOD has been reported in the cardiac tissue [36] and might potentially contributes to the enhanced mitochondrial function observed in this report.
Exercise mediated reduction in aging-induced cardiac fibrosis [37] has been reported. Moreover, moderate-intensity exercise was able to reduce cardiac fibrosis and adverse remodeling associated with diet-induced obesity [38]. Similar to these reports, our findings also suggested reduction in both the perivascular and interstitial fibrosis after exercise. Though we could not assay for the specific matrix regulators in the current study, others have suggested exercise potentially suppresses adverse remodeling [38]. In addition to the matrix regulators, connexin downregulation has also been implicated in fibrosis [39]. Apart from prevention of connexin level downregulation, exercise was also able to reduce blood pressure (Fig. 1b) which might also aid in lowering the cardiac stress and result in cardiac fibrosis suppression. It would be interesting to know which fibrosis regulators are altered by diabetes and exercise in the myocardium. Diabetes induced cardiac remodeling also involves changes in expression of alpha and beta MHC isoforms. Progressive decline in the α-MHC/β-MHC ratio during diabetes coincides with heart function deterioration [40-43]. Consistent with other studies, our data also indicate a trend of decline in α-MHC levels in the db/db mice. Exercise prevented such a decline, which might also have contributed to enhanced cardiac function.
In summary, the current study demonstrated that moderate intensity exercise improved gap junctional communication and restored mitochondrial function, thereby ameliorating myocardial contractile dysfunction of obese-diabetic mice with severe physical limitations for intense prolonged exercise. Our findings suggest that obese-diabetic patients in early stages of cardiac dysfunction can be benefited from adoption of moderate intensity exercise with brief rest periods during exercise. The findings also implied that the cardiovascular benefits observed with exercise do not require alterations in diet or correction of hyperglycemic state.
Highlights.
-
1)
Exercise prevented loss of gap junctional inter cellular communication in obese-diabetic mice.
-
2)
Exercise prevented enhancement in the Drp1/Mfn2 ratio and improved contractile function.
-
3)
Exercise ameliorated decline in mitochondrial transmembrane potential and resulted in enhanced ATP production.
Acknowledgement
We thank Lee J Winchester for his help with design of exercise protocol.
Funding: Part of this study was supported by National Institutes of Health Grants HL-74185, HL-108621 and NS-84823.
Abbreviations
- Akt
Protein kinase B
- CHD
Coronary heart disease
- Cx
Connexin
- Drp-1
Dynamin related protein 1
- EF
Ejection fraction
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HSP60
Heat shock protein 60
- JC-1
Tetraethylbenzimidazolylcarbocyanine iodide
- LVIDd
Left ventricular internal dimension during diastole
- LVPWd
Left ventricular posterior wall thickness during diastole
- Mfn-2
Mitofusin 2
- MTP
Mitochondrial membrane transition pore
- OCR
Oxygen consumption rate
- PS
Peak shortening
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator-1-alpha
- ROS
Reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution statement: SV, SG, SK, NM, SP and SCT conceived and designed the experiments. SV, SG, SK, SP and NM performed the experiments and analyzed and interpreted the data. SV wrote the manuscript. All the authors approved the final version.
Duality of interest: The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Reviews in endocrine & metabolic disorders. 2010;11:31–9. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102–7. doi: 10.1161/01.cir.103.1.102. [DOI] [PubMed] [Google Scholar]
- 3.Givvimani S, Pushpakumar S, Veeranki S, Tyagi SC. Dysregulation of Mfn2 and Drp-1 proteins in heart failure. Canadian journal of physiology and pharmacology. 2014;92:583–91. doi: 10.1139/cjpp-2014-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz R, Gorge PM, Gorbe A, Ferdinandy P, Lampe PD, Leybaert L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacology & therapeutics. 2015;153:90–106. doi: 10.1016/j.pharmthera.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato T, Haimovici R, Kao R, Li AF, Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002;51:1565–71. doi: 10.2337/diabetes.51.5.1565. [DOI] [PubMed] [Google Scholar]
- 6.Lin H, Ogawa K, Imanaga I, Tribulova N. Alterations of connexin 43 in the diabetic rat heart. Advances in cardiology. 2006;42:243–54. doi: 10.1159/000092573. [DOI] [PubMed] [Google Scholar]
- 7.Hesari FS, Khajehnasiri N, Khojasteh SM, Soufi FG, Dastranj A. Attenuation of phosphorylated connexin-43 protein levels in diabetic rat heart by regular moderate exercise. Archives of Iranian medicine. 2014;17:569–73. [PubMed] [Google Scholar]
- 8.Bobbie MW, Roy S, Trudeau K, Munger SJ, Simon AM, Roy S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Investigative ophthalmology & visual science. 2010;51:3758–63. doi: 10.1167/iovs.09-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. American journal of physiology Cell physiology. 2008;295:C221–30. doi: 10.1152/ajpcell.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Men X, Wang H, Li M, Cai H, Xu S, Zhang W, et al. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. The international journal of biochemistry & cell biology. 2009;41:879–90. doi: 10.1016/j.biocel.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell metabolism. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circulation research. 2015;116:264–78. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 13.Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, Lust RM, et al. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. Journal of molecular and cellular cardiology. 2012;52:1009–18. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxidants & redox signaling. 2010;12:537–77. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb E, Armour SM, Harris MH, Thompson CB. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–17. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- 16.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes care. 2010;33:e147–67. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moien-Afshari F, Ghosh S, Elmi S, Khazaei M, Rahman MM, Sallam N, et al. Exercise restores coronary vascular function independent of myogenic tone or hyperglycemic status in db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1470–80. doi: 10.1152/ajpheart.00016.2008. [DOI] [PubMed] [Google Scholar]
- 18.Givvimani S, Pushpakumar SB, Metreveli N, Veeranki S, Kundu S, Tyagi SC. Role of mitochondrial fission and fusion in cardiomyocyte contractility. International journal of cardiology. 2015;187:325–33. doi: 10.1016/j.ijcard.2015.03.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veeranki S, Winchester LJ, Tyagi SC. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochimica et biophysica acta. 2015;1852:732–41. doi: 10.1016/j.bbadis.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veeranki S, Lominadze D, Tyagi SC. Hyperhomocysteinemia inhibits satellite cell regenerative capacity through p38 alpha/beta MAPK signaling. Am J Physiol Heart Circ Physiol. 2015;309:H325–34. doi: 10.1152/ajpheart.00099.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Givvimani S, Kundu S, Pushpakumar S, Doyle V, Narayanan N, Winchester LJ, et al. Hyperhomocysteinemia: a missing link to dysfunctional HDL via paraoxanase-1. Canadian journal of physiology and pharmacology. 2015:1–9. doi: 10.1139/cjpp-2014-0491. [DOI] [PubMed] [Google Scholar]
- 22.Fong JT, Nimlamool W, Falk MM. EGF induces efficient Cx43 gap junction endocytosis in mouse embryonic stem cell colonies via phosphorylation of Ser262, Ser279/282, and Ser368. FEBS letters. 2014;588:836–44. doi: 10.1016/j.febslet.2014.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimlamool W, Andrews RM, Falk MM. Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Molecular biology of the cell. 2015;26:2755–68. doi: 10.1091/mbc.E14-06-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circ Physiol. 2000;278:H412–9. doi: 10.1152/ajpheart.2000.278.2.H412. [DOI] [PubMed] [Google Scholar]
- 25.King AJ. The use of animal models in diabetes research. British journal of pharmacology. 2012;166:877–94. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. The Indian journal of medical research. 2007;125:451–72. [PubMed] [Google Scholar]
- 27.He Q, Pu J, Yuan A, Yao T, Ying X, Zhao Y, et al. Liver X receptor agonist treatment attenuates cardiac dysfunction in type 2 diabetic db/db mice. Cardiovascular diabetology. 2014;13:149. doi: 10.1186/s12933-014-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrich BG, Gong X, Lerner DL, Wang X, Brown JH, Saffitz JE, et al. c-Jun N-terminal kinase activation mediates downregulation of connexin43 in cardiomyocytes. Circulation research. 2002;91:640–7. doi: 10.1161/01.res.0000035854.11082.01. [DOI] [PubMed] [Google Scholar]
- 29.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Kasuya Y, Miyauchi T. Activation pattern of MAPK signaling in the hearts of trained and untrained rats following a single bout of exercise. Journal of applied physiology. 2006;101:151–63. doi: 10.1152/japplphysiol.00392.2005. [DOI] [PubMed] [Google Scholar]
- 30.Carson LD, Korzick DH. Dose-dependent effects of acute exercise on PKC levels in rat heart: is PKC the heart's prophylactic? Acta physiologica Scandinavica. 2003;178:97–106. doi: 10.1046/j.1365-201X.2003.01131.x. [DOI] [PubMed] [Google Scholar]
- 31.Boyer JK, Thanigaraj S, Schechtman KB, Perez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–5. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Poulsen MK, Henriksen JE, Dahl J, Johansen A, Gerke O, Vach W, et al. Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ Cardiovasc Imaging. 2010;3:24–31. doi: 10.1161/CIRCIMAGING.109.855510. [DOI] [PubMed] [Google Scholar]
- 33.Kavazis AN, Smuder AJ, Powers SK. Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. Journal of applied physiology. 2014;117:223–30. doi: 10.1152/japplphysiol.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Bei Y, Lu Y, Sun W, Liu Q, Wang Y, et al. Exercise Prevents Cardiac Injury and Improves Mitochondrial Biogenesis in Advanced Diabetic Cardiomyopathy with PGC-1alpha and Akt Activation. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;35:2159–68. doi: 10.1159/000374021. [DOI] [PubMed] [Google Scholar]
- 35.Laher I, Beam J, Botta A, Barendregt R, Sulistyoningrum D, Devlin A, et al. Short-term exercise worsens cardiac oxidative stress and fibrosis in 8-month-old db/db mice by depleting cardiac glutathione. Free Radic Res. 2013;47:44–54. doi: 10.3109/10715762.2012.737463. [DOI] [PubMed] [Google Scholar]
- 36.French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J. 2008;22:2862–71. doi: 10.1096/fj.07-102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwak HB, Kim JH, Joshi K, Yeh A, Martinez DA, Lawler JM. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25:1106–17. doi: 10.1096/fj.10-172924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hafstad AD, Lund J, Hadler-Olsen E, Hoper AC, Larsen TS, Aasum E. High- and moderate-intensity training normalizes ventricular function and mechanoenergetics in mice with diet-induced obesity. Diabetes. 2013;62:2287–94. doi: 10.2337/db12-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jong S, van Veen TA, van Rijen HV, de Bakker JM. Fibrosis and cardiac arrhythmias. Journal of cardiovascular pharmacology. 2011;57:630–8. doi: 10.1097/FJC.0b013e318207a35f. [DOI] [PubMed] [Google Scholar]
- 40.Yue P, Arai T, Terashima M, Sheikh AY, Cao F, Charo D, et al. Magnetic resonance imaging of progressive cardiomyopathic changes in the db/db mouse. Am J Physiol Heart Circ Physiol. 2007;292:H2106–18. doi: 10.1152/ajpheart.00856.2006. [DOI] [PubMed] [Google Scholar]
- 41.Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105:1861–70. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 42.Pelzer T, Jazbutyte V, Arias-Loza PA, Segerer S, Lichtenwald M, Law MP, et al. Pioglitazone reverses down-regulation of cardiac PPARgamma expression in Zucker diabetic fatty rats. Biochem Biophys Res Commun. 2005;329:726–32. doi: 10.1016/j.bbrc.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circulation research. 2000;86:386–90. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]