Abstract
An important means of physiological adaptation to environmental hypoxia is an increased oxygen (O2) affinity of the hemoglobin (Hb) that can help secure high O2 saturation of arterial blood. However, the trade-off associated with a high Hb-O2 affinity is that it can compromise O2 unloading in the systemic capillaries. High-altitude deer mice (Peromyscus maniculatus) have evolved an increased Hb-O2 affinity relative to lowland conspecifics, but it is not known whether they have also evolved compensatory mechanisms to facilitate O2 unloading to respiring tissues. Here we investigate the effects of pH (Bohr effect) and temperature on the O2-affinity of high- and low-altitude deer mouse Hb variants, as these properties can potentially facilitate O2 unloading to metabolizing tissues.
Our experiments revealed that Bohr factors for the high- and low-altitude Hb variants are very similar in spite of the differences in O2-affinity. The Bohr factors of deer mouse Hbs are also comparable to those of other mammalian Hbs. In contrast, the high- and low-altitude variants of deer mouse Hb exhibited similarly low temperature sensitivities that were independent of red blood cell anionic cofactors, suggesting an appreciable endothermic allosteric transition upon oxygenation. In conclusion, high-altitude deer mice have evolved an adaptive increase in Hb-O2 affinity, but this is not associated with compensatory changes in sensitivity to changes in pH or temperature. Instead, it appears that the elevated Hb-O2 affinity in high-altitude deer mice is compensated by an associated increase in the tissue diffusion capacity of O2 (via increased muscle capillarization), which promotes O2 unloading.
Keywords: high-altitude, hypoxia, globin, adaptation, enthalpy of oxygenation, Bohr effect
1. Introduction
Adjustment of blood oxygen (O2)1 affinity in response to ambient O2 availability occurs by two means: (1) by genetically-based modifications of the structure of the hemoglobin (Hb, the O2 carrier of the blood) that affect the intrinsic heme-O2 affinity or the sensitivity to allosteric cofactors, or (2) by changes in the red blood cell concentration of allosteric cofactors, organic phosphates in particular (Weber and Fago 2004; Weber 2007; Mairbaurl and Weber 2012).
Vertebrates living at high altitudes provide excellent examples of adaptive changes in Hb structure and function (Weber 2007; Storz et al. 2010b). To cope with the reduced ambient PO2 at high altitudes, the Hb-O2 affinity of highland natives is often increased (Weber 2007, as this will enhance blood-O2 loading in pulmonary capillaries, thereby securing high saturation of arterial blood. However, there is a trade-off, as a high affinity Hb may compromise O2 unloading to the tissues in the systemic capillaries. To facilitate O2 unloading to metabolizing tissues, mammalian Hbs are typically sensitive to the organic phosphate 2,3-diphosphoglycerate (DPG), chloride, protons and CO2, which all bind to specific sites and stabilize the low-affinity T (tense) state relative to the high-affinity R (relaxed) state of the Hb (Weber and Fago 2004). The well-known effect of a pH change on Hb-O2 affinity, the Bohr effect, facilitates unloading of O2 in systemic capillaries in response to an increase in blood lactic acid and CO2, the end-products of energy metabolism, to locally match O2 supply with O2 consumption. The magnitude of the Bohr effect varies greatly among the Hbs of different species, but it is unclear whether changes in the Bohr effect have evolved as an adaptive response to changes in environmental O2 availability (Jensen 2004; Giardina et al. 2004; Weber 2007).
Another factor that can effectively decrease the O2 affinity and promote O2 delivery is an increase in temperature, due to the exothermic nature of Hb oxygenation. While a substantial temperature effect would be beneficial in favoring O2 unloading to warm exercising muscles, it would also impair O2 delivery to cold limbs and extremities in mammals living in cold environments. The possession of Hbs with reduced temperature sensitivity could compensate for this impairment, and may therefore have adaptive significance in regional heterotherms that live in polar or alpine environments (Brix et al. 1990; Weber and Campbell 2011). Consistent with this idea, a number of arctic or sub-arctic mammals have been shown to possess Hbs with a low temperature sensitivity (Weber and Campbell 2011). Whether the same is true in alpine mammals remains to be investigated.
To better understand how O2 transport is regulated in mammalian species adapted to high altitude hypoxia, we here investigate whether the Hbs of highland and lowland populations of the deer mouse (Peromyscus maniculatus) differ in their sensitivity to pH and temperature. The deer mouse is particularly well suited for such study because this species has the broadest altitudinal distribution of any North American mammal. Moreover, high-altitude deer mice from the Rocky Mountains have evolved an increased Hb-O2 affinity relative to lowland conspecifics from the Great Plains (Storz et al. 2009; Storz et al. 2010a). The increased Hb-O2 affinity in high-altitude mice is attributable to the additive and nonadditive effects of numerous amino acid replacements in duplicated genes that encode the α- and β-type subunits of the Hb tetramer (Storz et al. 2009; Storz et al. 2010a; Natarajan et al. 2013; Natarajan et al. 2015a). The increased Hb-O2 affinity in high-altitude mice is expected to enhance pulmonary O2 loading under hypoxia. Here we investigate whether this modification of Hb function is associated with compensatory mechanisms to promote O2 unloading in the systemic circulation.
2. Materials and methods
2.1 Sample collection and analysis
We analyzed a set of Hb variants that were representative of high- and low-altitude deer mouse populations (Storz et al. 2009; Storz et al. 2010a; Natarajan et al. 2013; Natarajan et al. 2015a). As described previously (Storz et al. 2010a), deer mice were live-trapped at high-altitude (4347 m, Mount Evans, Clear Creek County, Colorado, 39°15’24”N, 106°10’54”W) and low-altitude locations (1158 m, prairie grassland habitat, Bonny Reservoir, Yuma County, Colorado, 39°37’30”N, 102°10’27”W). After killing each animal by cervical dislocation, blood samples were drawn by cardiac puncture and were snap frozen in liquid nitrogen. Animal handling was in accordance with the guidelines approved by the University of Nebraska Institutional Animal Care and Use Committee (IACUC no. 07-07-030D) and the National Institutes of Health (NIH publication no. 78-23). Purified hemolysates were prepared and examined for Hb multiplicity by isoelectric focusing (IEF) on polyacrylamide gels in the pH range 3–9 (PhastSystem, GE Healthcare) as described previously (Storz et al. 2010a). Individual samples from each elevation that showed identical patterns of Hb multiplicity were pooled. In the present study we examined purified hemolysates representing the largest class of Hb variants from high-altitude and the largest class from the low-altitude population. , corresponding to the “sample B” and “sample I” described in a previous study (Storz et al. 2010a), respectively. Each of these two samples consisted of pooled hemolysates from eight (N=8) individual deer mouse specimen showing identical IEF patterns.
2.2. O2 equilibria and calculations
Pooled hemolysates from high- and low-altitude deer mice were stripped of organic phosphates by using a mixed-bed resin MB-1 AG501-X8 (BioRad), concentrated by ultrafiltration (to [heme]>3 mM), dialyzed in CO-equilibrated 10 mM Hepes buffer, pH 7.6, and stored in aliquots at −80 °C. Hb concentration (heme basis) was measured using previously reported extinction coefficients (Antonini and Brunori 1971). O2 equilibrium curves of 4 µl samples (0.3 mM heme) were measured using a modified diffusion chamber method previously described (Weber 1981; Weber 1992). In this method, samples are stepwise equilibrated to gas mixtures of atmospheric air or O2 and pure (>99.998 %) N2 made by two serially coupled gas mixing pumps (Wösthoff, Bochum, Germany), while absorbance at 436 nm (where oxy and deoxy Hb differ in absorbance) is continuously measured in order to obtain the fractional saturation at each equilibration step (4–6 steps for each curve). Experiments were made in 0.1 M Hepes buffers in the absence (stripped) and presence of anionic cofactors KCl (0.09 ± 0.01 M) and/or DPG (2-fold molar excess over tetrameric Hb) added at physiological levels and approximating conditions existing within red blood cells (Imai 1982; Mairbaurl and Weber 2012). Curves were measured at different pH values to estimate the Bohr effect and at 25° and 37 °C to estimate the enthalpy of oxygenation. Prior to the measurements of O2-equilibrium curves, CO was removed from each sample by repeated equilibrations with pure O2 and nitrogen. In the hemolysate solutions used in O2 equilibrium experiments, Cl− concentration was measured using a model 926S Mark II chloride analyzer (Sherwood Scientific Ldt, Cambridge, UK) and pH was measured using a InLab micro pH-electrode equipped with a SevenCompact pH/Ion Meter S220 (Mettler Toledo, Greifensee, Switzerland) after bringing samples at the same temperature of the experiments using a HLC BioTech block thermostat Model TK23 (Bovenden, Germany).
For each O2 equilibrium experiment, the parameters P50 (the O2 partial pressure at half-saturation) and n50 (Hill cooperativity coefficient) were calculated by fitting the Hill’s sigmoidal equation [SO2 = PO2n50/ (P50n50 + PO2n50)] to the saturation data, where SO2 is the fractional saturation. The nonlinear regression fitting was based on 4–6 saturation steps and yielded means ± sem values for P50 and n50 parameters under each condition used. The Bohr factor (Φ, expressing the number of protons bound per O2) was quantified by the slope of linear plots of logP50 as a function of pH (Φ = ΔlogP50/ΔpH). The overall enthalpy of oxygenation (ΔH, J mol O2−1, expressing the heat liberated upon oxygenation) at pH 7.2 under varying anionic conditions was calculated by using the van’t Hoff equation ΔH = 2.303R (ΔlogP50/Δ1/T), where R is the gas constant (8.314 J·K−1 ·mol O2−1) and T the absolute temperature in Kelvin. Values of logP50 at pH 7.20 were interpolated from the linear logP50 vs pH plots.
3. Results
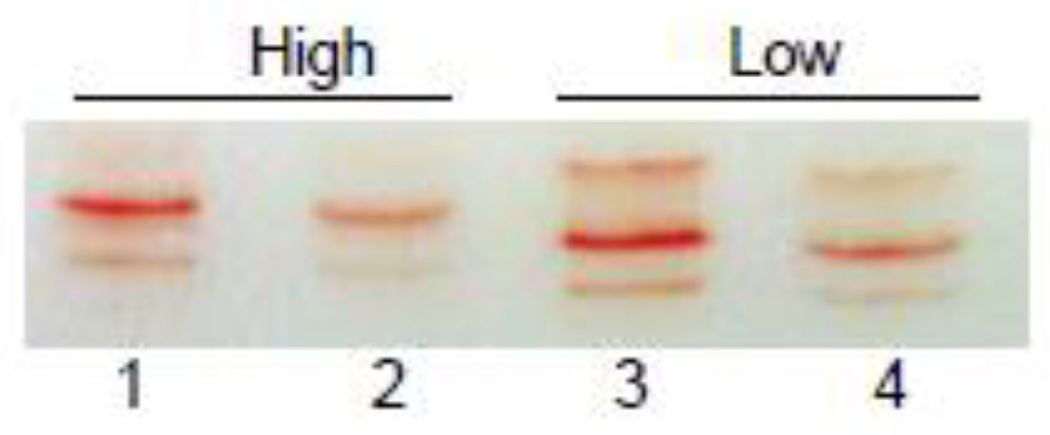
The common patterns of Hb multiplicity in deer mouse populations living at high and low altitudes are shown in the IEF gel images (Fig. 1). The high-altitude deer mice that we examined primarily expressed one major and one minor Hb component, whereas low altitude mice expressed one major and two minor Hb components (Fig. 1). This difference in Hb multiplicity in the hemolysates reflects altitudinal variation in the frequencies of alternative α- and β-chain amino acid variants (Storz and Kelly 2008; Storz et al. 2009; Storz et al. 2010a; Storz et al. 2012; Natarajan et al. 2015a). In particular, the set of Hb variants comprising high- and low-altitude samples exhibit fixed differences at four sites in the β-chain subunit, reflecting the fact that the four-site β-globin haplotype ‘62Gly/72Gly/128Ala/135Ala’ is nearly fixed in the high-altitude, Rocky Mountain population, and the alternative four-site haplotype ‘62Ala/72Ser/128Ser/135Ser’ is nearly fixed in the low-altitude, Great Plains population (Storz et al. 2009; Storz et al. 2010a; Storz et al. 2012).
Figure 1. Expression of different Hb isoforms in high- and low-altitude deer mice.
Isoelectrofocusing (pH 3–9) of the pooled hemolysates from high-altitude (left) and low-altitude deer mice (right). Samples in lanes 2 and 4 are dilutions of samples in lanes 1 and 3, respectively. Cathode is at the bottom.
O2 equilibrium curves of the purified hemolysates from highland and lowland deer mice were measured at different pH values and two different temperatures to estimate possible altitude-related differences in the Bohr effect and in the enthalpy of oxygenation. These experiments were performed in the absence and presence of anionic cofactors to determine whether their allosteric effects were influenced by changes in pH or temperature. An overview of all O2 equilibrium data is presented in Table 1.
Table 1.
O2 affinities (P50, mmHg) and Hill’s cooperativity coefficients (n50) for high- and low-altitude deer mouse Hbs measured at 37° and 25°C in 0.1 M Hepes buffer at varying pH values (heme 0.3 mM) and in the absence (stripped) and presence of KCl and DPG, as indicated. P50 and n50 values are derived from nonlinear regression of the sigmoidal Hill equation to 4–6 saturation points measured for each condition and are expressed as means±SEM. The corresponding overall enthalpy of oxygenation (kJ mol O2−1) calculated at pH 7.20 is indicated.
| High | Low | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| temperature | 37 °C | 25 °C | ΔH | 37 °C | 25 °C | ΔH | ||||||
| pH | 7.41 | 6.92 | 6.53 | 7.26 | 6.56 | 7.20 | 7.41 | 6.91 | 6.55 | 7.27 | 6.57 | 7.20 |
| P50 (mmHg) | ||||||||||||
| stripped | 9.17±0.41 | 17.3±0.5 | 32.6±2.2 | 6.32±0.27 | 14.4±0.7 | −11.0 | 9.2±0.22 | 17.8±0.5 | 28.5±1.1 | 6.20±0.20 | 14.9±0.4 | −11.4 |
| +KCl | 13.5±0.5 | 23.5±0.9 | 37.3±3.2 | 8.40±0.33 | 16.6±1.0 | −12.3 | 16.7±0.5 | 25.1±0.6 | 40.1±0.9 | 9.84±0.32 | 20.1±0.6 | −12.3 |
| +DPG | 12.0±0.4 | 26.8±0.6 | 43.8±2.6 | 10.4±0.6 | 19.0±0.8 | −7.9 | 13.6±0.4 | 29.3±1.3 | 48.0±1.5 | 10.5±0.3 | 23.2±0.6 | −9.6 |
| +KCl+DPG | 15.1±0.5 | 27.5±0.7 | 44.7±1.8 | 10.7±0.4 | 24.7±1.3 | −10.1 | 16.3±0.3 | 30.5±0.5 | 52.3±1.8 | 11.7±0.4 | 28.1±1.0 | −9.6 |
| Hill coeff. (n50) | ||||||||||||
| stripped | 1.94±0.14 | 1.75±0.07 | 1.79±0.18 | 1.63±0.11 | 1.46±0.10 | 2.03±0.09 | 1.86±0.09 | 1.76±0.09 | 1.61±0.08 | 1.02±0.01 | ||
| +KCl | 2.02±0.13 | 2.04±0.13 | 1.92±0.26 | 1.87±0.13 | 1.96±0.21 | 2.03±0.11 | 2.04±0.09 | 2.43±0.12 | 2.06±0.12 | 1.87±0.10 | ||
| +DPG | 2.00±0.13 | 2.48±0.12 | 2.11±0.22 | 1.81±0.20 | 1.95±0.15 | 2.20±0.14 | 2.30±0.22 | 2.51±0.17 | 2.11±0.15 | 2.26±0.13 | ||
| +KCl+DPG | 2.11±0.11 | 2.32±0.13 | 2.33±0.19 | 2.05±0.15 | 1.72±0.13 | 2.27±0.08 | 2.53±0.10 | 2.40±1.76 | 2.08±0.14 | 2.00±0.13 | ||
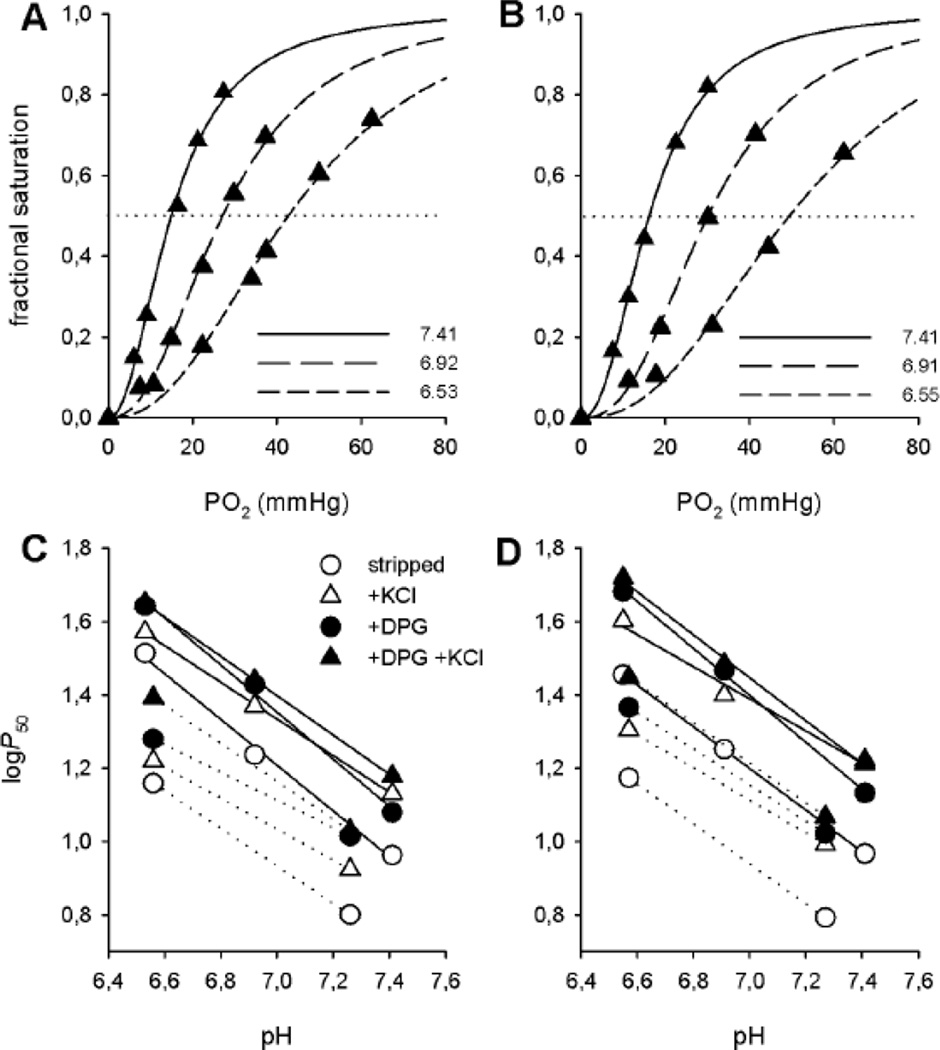
An increase in P50 with decreasing pH indicated the presence of Bohr effects of roughly similar magnitudes in high- and low-altitude samples, as shown by similar slopes of the logP50 vs pH linear plots (Fig. 2). However, the measured P50 values in the presence of anionic cofactors were consistently lower in high-altitude samples, indicating an increased O2 affinity under physiological conditions (Table 1, Fig. 2C,D). Overall, we observed similar shifts in logP50 with pH under the buffer and temperature conditions here investigated in Hbs from highland and lowland deer mice, indicating that the allosteric binding of anionic cofactors (DPG or Cl−) was largely independent of both temperature and pH (Fig. 2C,D). Consistently, the Bohr factors (Φ, indicating the magnitude of the Bohr effect and the slope of the plots in Fig. 2) were roughly similar in Hbs from highland and lowland deer mice (Table 2). Slight differences in Bohr factors were observed in the presence of Cl− possibly related to small variations in Cl− concentration in the samples.
Figure 2. Similar Bohr effects in Hbs of high- and low-altitude deer mice.
Representative O2 equilibrium curves of high- (A) and low-altitude deer mice Hb (B) measured in 0.1 M Hepes buffer at different pH values at 37°C in the presence of KCl (0.09 ± 0.01 M) and DPG (2-fold molar excess over tetrameric Hb), as indicated. Fitting of the data according to the Hill’s sigmoidal equation is shown. Bohr plots (logP50 vs pH) of high- (C) and low-altitude deer mouse Hb (D) in the absence (stripped) and presence of KCl and DPG, as indicated, at 37°C (continuous lines) and 25°C (dotted lines). The slope of each of these linear plots corresponds to the magnitude of the Bohr effect (Bohr factor, Φ) and is reported in Table 2.
Table 2.
Bohr factors for high- and low-altitude deer mouse Hbs measured at 37° and 25°C in 0.1 M Hepes buffer (heme 0.3 mM) and in the absence (stripped) and presence of KCl and DPG, as indicated. For each condition, values correspond to the linear regression of logP50 vs pH data shown in Fig. 2C,D.
| High | Low | |||
|---|---|---|---|---|
| Bohr factor (Φ) | 37 °C | 25 °C | 37 °C | 25 °C |
| stripped | −0.62±0.04 | −0.51 | −0.57±0.00 | −0.55 |
| +KCl | −0.50±0.01 | −0.42 | −0.44±0.06 | −0.44 |
| +DPG | −0.64±0.05 | −0.38 | −0.64±0.02 | −0.49 |
| +KCl+DPG | −0.54±0.00 | −0.52 | −0.59±0.03 | −0.54 |
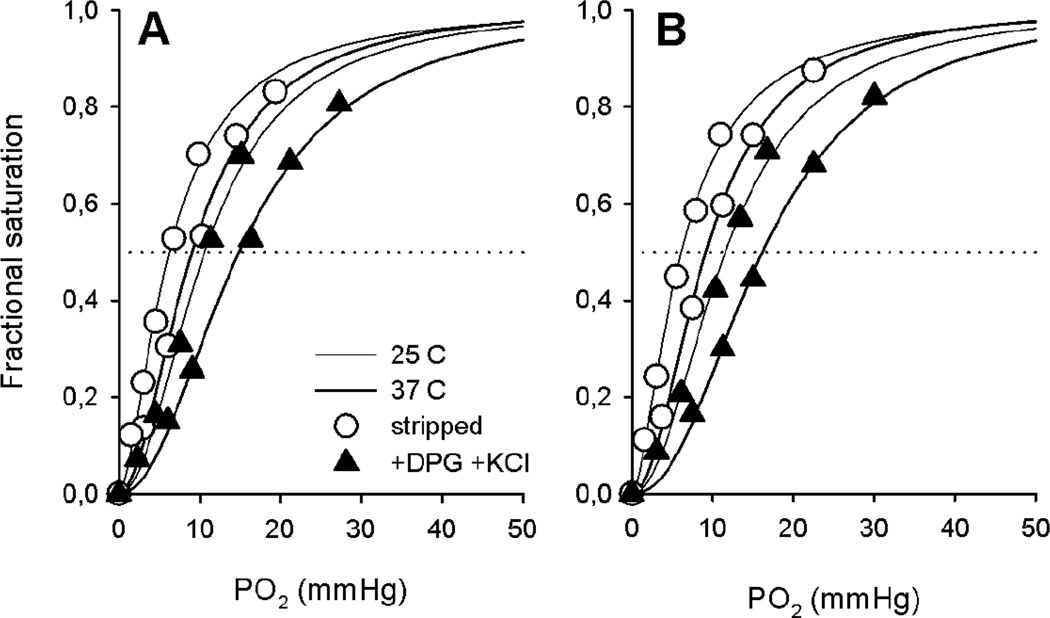
An increase in temperature from 25° to 37°C produced a small right-shift of the O2 binding curve (Fig. 3, Table 1), with an average ΔlogP50 shift of 0.02 per °C. The correlated changes in P50 and ΔH were similar for high- and low-altitude samples under all anionic conditions investigated (Table 1), although addition of DPG alone resulted in a less exothermic oxygenation (i.e., a less negative overall ΔH), particularly in high-altitude samples (Table 1). Interestingly, addition of Cl− had no noticeable effect on ΔH, even though it exerted a substantial effect on P50 (Table 1).
Figure 3. Similar temperature-dependent shifts of the O2 equilibrium curves of high- and low-altitude deer mouse Hb.
Representative O2 equilibrium curves of high- (A) and low-altitude deer mice Hb (B) measured in 0.1 M Hepes buffer at 37°C (pH 7.41; thick lines) and 25°C (pH 7.26 in A and pH 7.27 in B; thin lines) in the absence (stripped) and presence of KCl (0.09 ± 0.01 M) and DPG (2-fold molar excess over tetrameric Hb) as indicated. Fitting of the data according to the Hill’s sigmoidal equation is shown.
4. Discussion
A major finding of this study is that deer mice living at high- and low-altitudes have Hbs with similar Bohr effects. In this study we examined purified hemolysates with IEF profiles that were representative of the high- and low-altitude deer mouse populations (Storz et al. 2010a). The fact that Hbs of high-altitude deer mice have an unaltered Bohr effect implies that the increase in O2 delivery in response to an increase in energy metabolism in tissues does not involve particular molecular adaptations of the Hb molecule. Instead, other physiological adjustments of the cardio-circulatory system may facilitate O2 unloading in the systemic circulation. For example, high-altitude deer mice have evolved significantly higher muscle capillary densities relative to lowland mice (Lui et al. 2015; Scott et al. 2015), a change which should increase the O2 diffusion capacity, thereby enhancing aerobic capacity under hypoxia (Wagner 1996; Scott and Milsom 2006). Moreover, deer mice exhibit plasticity in a number of hematological traits such as hematocrit, Hb concentration, and the intraerythrocytic concentration of DPG that can potentially enhance blood-O2 carrying capacity in response to hypoxia (Tufts et al. 2013; Lui et al. 2015). Thus, it appears that genetic adaptation to high-altitude in deer mice involves an evolved increase in Hb-O2 affinity, which maximizes pulmonary O2 loading, in conjunction with an evolved increase in tissue diffusion capacity of O2 (via increased muscle capillarization) that should promote O2 unloading. The results of this study are in overall good agreement with those of a previous investigation on the same samples (Storz et al. 2010a) but performed under slightly different experimental conditions (e.g. in the presence of an enzymatic met-reducing system and at a lower heme concentration) and confirm the consistently higher O2-affinity of the high-altitude deer mouse Hbs (Table 1).
Overall, the magnitudes of the Bohr effect found in this study (Table 2) are close to those typical of mammalian Hbs measured under in vitro standard conditions (e.g. ~−0.5 in human HbA) (Shih et al. 1993; Giardina et al. 2004; Revsbech et al. 2013), although other studies have found that the Bohr effect scales with body size and is larger in Hbs from small animals with higher mass-specific metabolic rates (Riggs 1960). The Hbs from high- and low-altitude deer mice have similar Bohr effects at each of the two temperatures and in the presence and absence of anionic allosteric cofactors (Table 2). This suggests that there is no difference in the number of Bohr groups between the Hb samples, corresponding to the O2-linked dissociation of ~1.5–2.5 protons per tetrameric Hb. That DPG does not increase appreciably the magnitude of the Bohr effect implies that negatively-charged DPG does not alter the affinity for protons of positively-charged groups in the DPG binding central cavity of the T-state Hb molecule. Similarly to the deer mouse, Hbs from closely related pairs of high- and low-altitude waterfowl from the Andes exhibited differences in O2-affinity (higher affinities in the high-altitude taxa) but no associated differences in the Bohr factor (Natarajan et al. 2015b).
The temperature effect of deer mouse Hbs, quantified as the enthalpy of oxygenation ΔH (on average −10.5 kJ mol O2−1, Table 1), was significantly lower than that of human HbA (−50.7 kJ mol O2−1) (Weber et al. 2014) and similar to that of bovine Hb, (Coletta et al. 1992; Weber et al. 2014), a known example of a temperature-insensitive Hb. ΔH values of deer mouse Hbs were also similar to those of ground squirrels (Revsbech et al., 2013) and moles (Signore et al. 2012). Moreover, the enthalpy of oxygenation was similar in high-altitude and low-altitude deer mouse Hb and was largely unaffected by the presence of anionic cofactors. This observation suggests that – as in bovine Hb (Weber et al. 2014) – the T-R shift of deer mouse Hb upon oxygenation is considerably endothermic in nature, whereas the endothermic dissociation of ionic cofactors upon oxygenation plays a minor role. This endothermic transition in quaternary structure correlates with a tighter α1β2 sliding subunit interface identified in crystallographic studies of deer mouse recombinant Hb (Inoguchi et al. 2013), with additional interactions between αArg92 and βGln39 and between αArg92 and βAsp43, which are both lacking in human HbA. However, the molecular mechanisms underlying why DPG markedly affects O2 affinity but not the enthalpy of oxygenation remains to be clarified.
In conclusion, adaptive modifications of the oxygenation properties of deer mouse Hbs do not affect the magnitude of the Bohr effect, indicating that O2 unloading at high-altitude is instead facilitated by an increased muscle capillary density, and possibly also by plastic changes in hematocrit, Hb concentration, and intraerythrocytic DPG concentration that increase blood-O2 carrying capacity (Tufts et al. 2013; Lui et al. 2015). Furthermore, the low temperature effect on Hb-O2 affinity, which is comparable to that of temperature-insensitive ruminant Hbs, suggests that O2 delivery to extremities that are below the body core temperature may be facilitated by a lower heat of oxygenation of the Hb, as reported in polar mammals (Weber and Campbell 2011).
Acknowledgments
We thank Roy E. Weber for helpful discussions and Elin E. Petersen for assistance in the lab. This work was supported by the National Institutes of Health/National Heart, Lung and Blood Institute grant HL087216 to JFS), the National Science Foundation (grants IOS-1354390 and MCB-1517636 to JFS), and the Danish Council for Independent Research, Natural Sciences (grant 10-084-565 to AF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: O2, oxygen; Hb, hemoglobin; P50, oxygen partial pressure at half-saturation; PO2, oxygen partial pressure; n50, Hill cooperativity coefficient; DPG, 2,3-diphosphoglycerate; ΔH, enthalpy of oxygenation; IEF, isoelectrofocusing; Φ, Bohr factor.
Contributor Information
Birgitte Jensen, Email: x.birgitte.jensen@gmail.com.
Jay F. Storz, Email: jstorz2@unl.edu.
Angela Fago, Email: angela.fago@bios.au.dk.
References
- Antonini E, Brunori M. Hemoglobin and Myoglobin in their Reactions with Ligands. Amsterdam: North-Holland Publishing Company; 1971. [Google Scholar]
- Brix O, Bardgard A, Mathisen S, Tyler N, Nuutinen M, Condò SG, Giardina B. Oxygen transport in the blood of arctic mammals: adaptation to local heterothermia. J. Comp. Physiol. B. 1990;159:655–660. doi: 10.1007/BF00691710. [DOI] [PubMed] [Google Scholar]
- Coletta M, Clementi ME, Ascenzi P, Petruzzelli R, Condò SG, Giardina B. A comparative study of the temperature dependence of the oxygen-binding properties of mammalian hemoglobins. Eur. J. Biochem. 1992;204:1155–1157. doi: 10.1111/j.1432-1033.1992.tb16741.x. [DOI] [PubMed] [Google Scholar]
- Giardina B, Mosca D, De Rosa MC. The Bohr effect of haemoglobin in vertebrates: an example of molecular adaptation to different physiological requirements. Acta Physiol. Scand. 2004;182:229–244. doi: 10.1111/j.1365-201X.2004.01360.x. [DOI] [PubMed] [Google Scholar]
- Imai K. Allosteric Effects in Haemoglobin. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- Inoguchi N, Oshlo JR, Natarajan C, Weber RE, Fago A, Storz JF, Moriyama H. Deer mouse hemoglobin exhibits a lowered oxygen affinity owing to mobility of the E helix. Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 2013;69:393–398. doi: 10.1107/S1744309113005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FB. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol. Scand. 2004;182:215–227. doi: 10.1111/j.1365-201X.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- Lui MA, Mahalingam S, Patel P, Connaty AD, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R779–R791. doi: 10.1152/ajpregu.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairbaurl H, Weber RE. Oxygen transport by hemoglobin. Compr. Physiol. 2012;2:1463–1489. doi: 10.1002/cphy.c080113. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Hoffmann FG, Lanier HC, Wolf CJ, Cheviron ZA, Spangler ML, Weber RE, Fago A, Storz JF. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol Biol Evol. 2015a;32:978–997. doi: 10.1093/molbev/msu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF. Epistasis among adaptive mutations in deer mouse hemoglobin. Science. 2013;340:1324–1327. doi: 10.1126/science.1236862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Munoz-Fuentes V, Green AJ, Kopuchian C, Tubaro PL, Alza L, Bulgarella M, Smith MM, Wilson RE, Fago A, McCracken KG, Storz JF. Convergent evolution of hemoglobin function in high-altitude andean waterfowl involves limited parallelism at the molecular sequence level. PLoS Genet. 2015b;11:e1005681. doi: 10.1371/journal.pgen.1005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech IG, Tufts DM, Projecto-Garcia J, Moriyama H, Weber RE, Storz JF, Fago A. Hemoglobin function and allosteric regulation in semi-fossorial rodents (family Sciuridae) with different altitudinal ranges. J. Exp. Biol. 2013;216:4264–4271. doi: 10.1242/jeb.091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. The nature and significance of the Bohr effect in mammalian hemoglobins. J. Gen. Physiol. 1960;43:737–752. doi: 10.1085/jgp.43.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol. Biol. Evol. 2015;32:1962–1976. doi: 10.1093/molbev/msv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Milsom WK. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir. Physiol. Neurobiol. 2006;154:284–301. doi: 10.1016/j.resp.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Shih D, Luisi BF, Miyazaki G, Perutz MF, Nagai K. A mutagenic study of the allosteric linkage of His(HC3)146β in haemoglobin. J. Mol. Biol. 1993;230:1291–1296. doi: 10.1006/jmbi.1993.1242. [DOI] [PubMed] [Google Scholar]
- Signore AV, Stetefeld J, Weber RE, Campbell KL. Origin and mechanism of thermal insensitivity in mole hemoglobins: a test of the 'additional' chloride binding site hypothesis. J. Exp. Biol. 2012;215:518–525. doi: 10.1242/jeb.063669. [DOI] [PubMed] [Google Scholar]
- Storz JF, Kelly JK. Effects of spatially varying selection on nucleotide diversity and linkage disequilibrium: insights from deer mouse globin genes. Genetics. 2008;180:367–379. doi: 10.1534/genetics.108.088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Natarajan C, Cheviron ZA, Hoffmann FG, Kelly JK. Altitudinal variation at duplicated β-globin genes in deer mice: effects of selection, recombination, and gene conversion. Genetics. 2012;190:203–216. doi: 10.1534/genetics.111.134494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, Fago A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA. 2009;106:14450–14455. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 2010a;213:2565–2574. doi: 10.1242/jeb.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR, Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 2010b;213:4125–4136. doi: 10.1242/jeb.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts DM, Revsbech IG, Cheviron ZA, Weber RE, Fago A, Storz JF. Phenotypic plasticity in blood-oxygen transport in highland and lowland deer mice. J. Exp. Biol. 2013;216:1167–1173. doi: 10.1242/jeb.079848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD. A theoretical analysis of factors determining VO2 max at sea level and altitude. Respir. Physiol. 1996;106:329–343. doi: 10.1016/s0034-5687(96)00086-2. [DOI] [PubMed] [Google Scholar]
- Weber RE. Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J. Appl. Physiol. 1992;72:1611–1615. doi: 10.1152/jappl.1992.72.4.1611. [DOI] [PubMed] [Google Scholar]
- Weber RE, Campbell KL. Temperature dependence of haemoglobin-oxygen affinity in heterothermic vertebrates: mechanisms and biological significance. Acta Physiol. 2011;202:549–562. doi: 10.1111/j.1748-1716.2010.02204.x. [DOI] [PubMed] [Google Scholar]
- Weber RE, Fago A. Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir. Physiol. Neurobiol. 2004;144:141–159. doi: 10.1016/j.resp.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Weber RE. Cationic control of O2 affinity in lugworm erythrocruorin. Nature. 1981;292:386–387. [Google Scholar]
- Weber RE. High-altitude adaptations in vertebrate hemoglobins. Respir. Physiol. Neurobiol. 2007;158:132–142. doi: 10.1016/j.resp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Weber RE, Fago A, Campbell KL. Enthalpic partitioning of the reduced temperature sensitivity of O2 binding in bovine hemoglobin. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014;176:20–25. doi: 10.1016/j.cbpa.2014.06.012. [DOI] [PubMed] [Google Scholar]