Abstract
BACKGROUND
Chronic inflammation is commonly observed in benign prostate hyperplasia (BPH), and prostate tissue often contains increased inflammatory infiltrates, including T cells and macrophages. Cytokines are not only key mediators of inflammation but may also play important roles in the initiation and progression of BPH.
METHODS
In order to determine what cytokines might be involved in prostatic enlargement, expressed prostatic secretions (EPS) from ex vivo prostates were analyzed by human cytokine antibody microarray and ELISA. Prostate epithelial cells (PrEC) and prostate stromal cells (PrSC) were used for ELISA, proliferation, and Western blot assays.
RESULTS
Monocyte chemotactic protein-1 (MCP-1/CCL2) was one of the most elevated proteins in secretions from large prostate glands. PrSC were found to secrete MCP-1; Western blotting showed that both PrSC and PrEC express the MCP-1 receptor CCR2 which by RT-PCR was the CCR2b isoform. Proliferation assays showed that MCP-1 stimulates the proliferation of PrEC, but not PrSC, and that a specific MCP-1 antagonist (RS102895) suppressed this effect. Conditioned medium from PrSC stimulated the proliferation of PrEC as well, an effect completely inhibited by both RS102895 and a neutralizing anti-MCP-1 monoclonal antibody. The inflammatory cytokines interleukin (IL)-1β, interferon-γ, and IL-2 enhanced the secretion of MCP-1 from PrEC and PrSC. In addition, MCP-1 levels in EPS correlated with mRNA levels of the macrophage marker CD68 in the same secretions.
CONCLUSIONS
The cytokine MCP-1, of apparent prostatic stromal cell origin, may play an important role in prostatic enlargement and BPH, and is a candidate biomarker for these pathologic processes.
Keywords: BPH, MCP-1, CCL2, PrEC, PrSC
INTRODUCTION
Benign prostate hyperplasia (BPH) is a common disease affecting older men caused by unregulated prostatic stromal and epithelial growth resulting in prostate enlargement, bladder outlet obstruction, and lower urinary tract symptoms [1]. Despite intensive research over the last several decades, the molecular mechanisms underlying prostatic enlargement and symptomatic bladder outlet obstruction remain obscure. However, androgens, estrogens, stromal–epithelial interactions, growth factors, and modifiable risk factors including obesity and diabetes likely play important roles in the etiology of this hyperplastic process [2,3].
BPH is characterized histologically by an increased number of epithelial and stromal cells in the periurethral area of the prostate [4]. In addition, BPH tissues often have infiltrating lymphocytes and macrophages around the glandular elements, and chronic inflammation has been proposed in the pathogenesis of BPH [5–8]. These infiltrating cells produce cytokines, such as interleukin-6 (IL-6) and IL-8, which stimulate epithelial and stromal proliferation. Growth factors from stromal cells, such as epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) also stimulate epithelial cell proliferation.
We used a human cytokine array to search for cytokines in expressed prostatic secretions (EPS) that may be associated with prostate enlargement. In comparison to cytokines found in small volume prostates, a variety of growth factors and cytokines were found to be elevated in EPS from large prostates. One of these was monocyte/macrophage chemoattractant protein-1 (MCP-1/CCL2). MCP-1, or CCL2, is a member of the CC chemokine superfamily that plays a critical role in the recruitment and activation of monocytes during inflammation [9]. MCP-1 has been associated with BPH and chronic pelvic pain syndrome (CPPS) [10,11] and has also been shown to stimulate prostate cancer growth, invasion, and metastasis [12–14].
In this study, we sought to assess the association of MCP-1 levels in EPS with prostate size and further characterize MCP-1 and BPH in vitro using benign prostatic epithelial and stromal cell lines and MCP-1 inhibitors.
MATERIALS AND METHODS
Sample Collection
All samples were collected using IRB-approved protocols. EPS were collected by manually squeezing fresh ex vivo prostate glands immediately following radical prostatectomy for clinically localized prostate cancer, and gathering secretions obtained from the protruding apical urethral stump. The radical prostatectomy specimens were then submitted for routine weighing, formalin fixation, sectioning, and pathologic analysis as per standard protocol [15]. Secretions from patients subsequently determined to have minimal prostate cancer (defined as specimens with minute foci of a maximum tumor area of less than 15 mm2 with Gleason sum ≤6) were chosen for this study. EPS were stored at −8°C until analysis.
Cytokine Antibody Array
A Raybio™ Human Cytokine Array Kit (Raybiotech, Norcross, GA) including 174 cytokines was used as described elsewhere [16]. Membranes immobilized with capture antibodies were incubated with EPS samples [1 ml, in 10-fold dilution with Tris-buffered saline (TBS) and complete protease inhibitor cocktail tablets (Roche Diagnostics, Indianapolis, IN) for 2 hr at room temperature]. After extensive washing with TBS/0.1% Tween-20 (three times, 5 min each) and TBS (twice, 5 min each), membranes were incubated with biotin-conjugated anticytokine antibodies. Membranes were washed and then incubated with horseradish peroxidase-conjugated streptavidin (2.5 pg/ml) for 1 hr at room temperature. Finally, the signals were detected by the enhanced chemiluminescence system, followed by additional washing. Spots were visualized using enhanced chemiluminescence (ECL plus Western blotting system, Amersham Biosciences, Pittsburgh, PA). Membranes were exposed to Kodak X-Omat radiographic film for 1 min per image. Each film was scanned into TIFF image files, and spots were digitized into densities with Gel-Pro-Analyzer (Media Cybernetics, Bethesda, MD). The densities were exported into Microsoft Excel, and the background intensity was subtracted prior to analysis.
Cell Culture
Human prostate epithelial cells (PrEC) and prostate stromal cells (PrSC) were obtained from Lonza (Walkersville, MD). PrEC and PrSC were maintained in prostate epithelial cell growth media (PrEGM, Lonza) and stromal cell growth media (SCGM, Lonza), respectively, at 37°C containing 5% CO2. Peripheral blood mononuclear cells (PBMC) were isolated from 10 ml blood from a healthy volunteer by density-gradient centrifugation with Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden). PBMC were then seeded in a six-well plate and incubated in RPMI (1% l-glutamate, 1% nonessential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin, 10% heat-inactivated FCS, and 14.4 mol/L beta-mercaptoethanol) for 3 hr. Nonadherent cells were removed by washing several times with HBSS and adherent monocytes on the plate were subjected to further experiment.
Measurement of MCP-1 in Cell Culture Conditioned Media
1×105 of each cell type were seeded in 24-well plates and 24 hr later media were changed to media with or without 10, 100 ng/ml of IFN-γ (R&D Systems), 10, 100 ng/ml IL-1β (R&D Systems), and 10, 100 U/ml IL-2 (Roche, Nutley, NJ). After 48 hr of incubation, supernatants were collected, centrifuged at 1,000g for 10 min to remove cells and subjected to enzyme-linked immunosorbent assay (ELISA).
Enzyme-Linked Immunosorbent Assay (ELISA)
MCP-1 levels in EPS and in cell culture supernatants were measured by a human CCL2 (MCP-1) ELISA Ready-SET-GO kit (eBioscience, San Diego, CA). MCP-1 measurements were performed as per the manufacturer's recommendations. EPS and cell culture supernatants from PrSC were assayed at 16- and 4-fold dilutions, respectively.
Reverse-Transcriptase PCR
Total RNA was extracted from PrEC, PrSC, monocyte/macrophage, or EPS with the RNeasy Mini Kit (Qiagen, Valencia, CA). Total RNA was treated with DNase I (Qiagen). First-strand cDNA was produced with random hexamers as per the manufacturer's recommendations (Omniscript RT kit/Qiagen). PCR amplification of CCR2a and CCR2b was done with HotStar Taq Plus Master Mix (Qiagen) for cDNA of PrEC, PrSC, and monocyte/macrophge. Primers used were as follows: CCR2a, 5′-GAGACTCTTGGGATGACTCAC-3′ (forward) and 5′-ACAGCGATGGAGCGTAT-3′ (reverse); CCR2b 5′-GAGACTCTTGGGATGACTCAC-3′ (forward) and 5′-TTATAAACCAGCCGAGACTTC-3′ (reverse); GAPDH, 5′-ACCAGGGCTGCTTTTAACTCT-3′ (forward) and 5′-GATGACAAGCTTCCCGTTCT-3′ (reverse). Amplification conditions were as follows: 15 min at 95°C (one cycle) and 45 sec at 94°C; 45 sec at the annealing temperature of 56°C; and 60 sec at 72°C (35 cycles) and 72°C for 5 min (one cycle).
Real-time PCR was done to quantify mRNA levels of CD68 in EPS. PCR amplification mixtures (25 μl) contained 12.5 μl of iQ SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA), 2 μl of a mixture of 2.5 μM reverse and forward primers, 5.5 μl of nuclease-free water, and 5 μl of cDNA template. Quantitative RT-PCR measurements were performed on an iQ5 real-time PCR Detection system with iCycler IQ Software (Bio-Rad Laboratories). PCR cycles proceeded as follows: Taq activation (X min, 95°C), then 40 cycles of denaturation (X sec, 95°C), annealing (X sec, 60°C), and extension (X sec, 72°C). The melting-curve analysis showed the specificity of the amplifications. The relative mRNA levels were estimated by standard method using β-actin as the reference gene. Primers used were as follows: CD68, 5′-CTACATGGCGGTGGAGTACAA-3′ (forward) and 5′-ATGATGAGAGGCAGCAAGATGG-3′ (reverse); β-actin 5′-XXXXX-3′ (forward) and 5′-XXXXX-3′ (reverse).
Western Blot analysis
After washing with ice-cold PBS, cells were harvested in RIPA buffer (Pierce, Rockford, IL) supplemented with Halt protease inhibitor cocktail (Pierce). Total cellular protein concentrations were determined by using a BCA protein assay reagent (Pierce). Twenty-five micrograms protein of lysates were subjected to SDS–PAGE under reducing conditions and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were immunoblotted with rabbit polyclonal antihuman CCR2 antibodies (Abcam, Cambridge, MA) followed by horseradish peroxidase-conjugated secondary antibodies and developed with the Super Signal West Pico Substrate kit (Pierce).
Cell Proliferation Assays
6000 PrEC cells and 1000 PrSC cells were seeded on 96-well plates and 24 hr later media were changed to media containing 0, 1, 10, and 100 ng/ml MCP-1 (R&D Systems) in PrEGM or SCBM. 6000 PrEC cells were also seeded on 96-well plates and 24 hr later media were changed to the media containing 0, 2.5, 5.0, and 10 μM RS102895 in PrEGM with 10 ng/ml MCP-1. The cell proliferation reagent WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) was added to each well 24, 48, and 72 hr later, as specified by the supplier (Roche). After 2 hr of incubation, WST-1 absorbance at 450 nm (OD450) and 610 nm (OD610) was measured and OD450 was subtracted by OD610. PrSC cells were also cultured in PrEGM media for 2 days and the conditioned media (CM) were harvested in 48 hr. The CM with 0, 2.5, 5.0, and 10 μM RS102895 or PrEGM media were also added in 96-well plates 24 hr after seeding. The CM from PrSC were also incubated with or without mouse monoclonal anti-MCP1 antibody (MAB279, R&D Systems) for 2 hr at room temperature and then added in 96-well plates 24 hr after seeding. The WST-1 reagent was added to each well 78 hr later, and absorbance was measured at 450 nm after 2 hr of incubation. Monocytes attached on the plate were incubated in PrEGM and CM were harvested 48 hr later. The CM from monocytes were also added to 96-well plates of PrEC, and 72 hr later the WST-1 reagent was added and absorbance measured in 2 hr.
Data Analysis and Statistics
Results were expressed as mean±SD. Statistical analyses were done using GraphPad Prism 4.0 for Windows. Mann–Whitney tests and Student's t-tests were used to analyze the difference of two categories in clinical samples and in vitro experiments, respectively. Statistical significance was defined as a P-value <0.05.
RESULTS
Cytokine Profile and MCP-1 Levels of Expressed Prostatic Secretions
The array-derived intensity values of cytokines from 21 prostates were calculated. Those from large prostates (>60 g; n=8) were divided by those from small prostates (<40 g; n=6) to calculate the relative n-fold change in intensity value. The ranked cytokine profile of the relative n-fold change is listed in Table I. The 16 most elevated cytokines in large prostates were analyzed, and of these, MCP-1 and IL-1β demonstrated correlations with increasing gland weight in 21 prostates (Spearman's correlation test, r=0.434, P=0.049 and r=0.419, P=0.059, respectively). To validate these findings, we measured MCP-1 and IL-1β levels by ELISA in EPS from a new cohort of 46 patients also with minimal disease postprostatectomy. Spearman's correlation analysis showed that MCP-1 levels were associated with increasing gland weight (r=0.369, P=0.013; Fig. 1) but that IL-1β levels were not (r=−0.015, P=0.9203) (data not shown).
TABLE I.
Cytokine Profile of Prostatic Fluid
| Cytokine | Ratio (L/S) | Average signal of L group (SD) | Average signal of S group (SD) |
|---|---|---|---|
| IL-1beta | 5.08 | 4.75 (10.33) | 0.94 (0.55) |
| IL-7 | 4.63 | 0.30 (0.37) | 0.06 (0.10) |
| Activin A | 4.47 | 2.75 (3.11) | 0.62 (0.92) |
| MCP-1 | 3.67 | 103.47 (101.64) | 28.2 (12.71) |
| IL-6 | 3.09 | 1.72 (3.56) | 0.56 (0.53) |
| FGF-4 | 2.78 | 0.18 (0.21) | 0.07 (0.10) |
| FGF-7 | 2.76 | 0.33 (0.53) | 0.12 (0.29) |
| IGFBP-4 | 2.75 | 0.77 (0.97) | 0.28 (0.36) |
| IGF-I | 2.67 | 0.87 (1.10) | 0.32 (0.31) |
| Endoglin | 2.37 | 0.56 (0.68) | 0.24 (0.58) |
| AgRP | 1.98 | 3.16 (3.51) | 2.25 (1.71) |
| BLC | 1.96 | 7.10 (7.86) | 3.63 (2.64) |
| I-309 | 1.95 | 1.87 (1.43) | 0.96 (0.94) |
| PARC | 1.85 | 9.11 (9.07) | 4.93 (3.74) |
| FGF-6 | 1.77 | 0.43 (0.56) | 0.24 (0.60) |
| GM-CSF | 1.76 | 3.80 (4.56) | 1.91 (1.69) |
L, large prostate (>60 g; n=8); S, small prostate (<40 g; n=6).
Fig. 1.
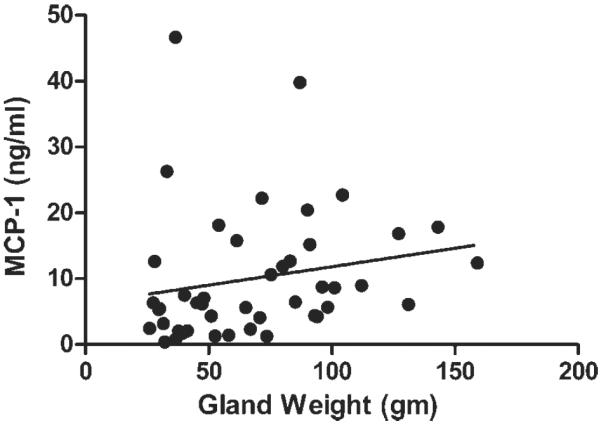
MCP-1 levels in EPS (n = 46) determined by ELISA compared to prostate specimen weight (n = 46); Spearman's r = 0.369, P = 0.013.
Prostate Stromal Cells Secrete MCP-1 and Both Prostate Epithelial and Stromal Cells Express CCR2
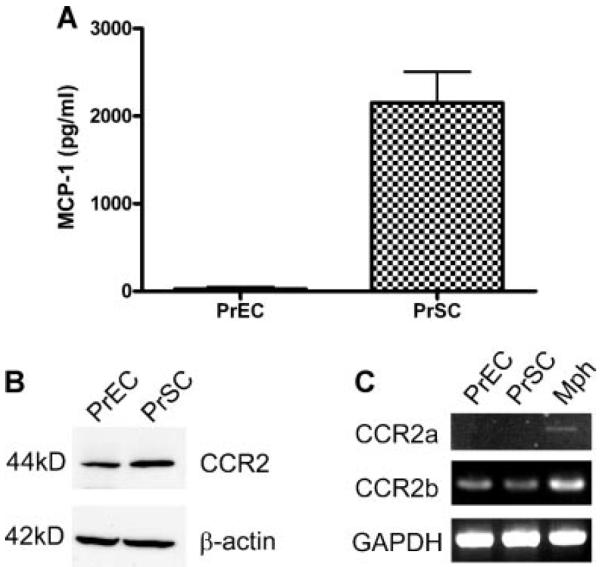
To localize MCP-1 by potential cell of benign prostatic origin, we cultured human PrEC and PrSC. Far higher levels of MCP-1 were found by ELISA in the supernatant of PrSC (2,150±615 pg/ml) compared to that of PrEC (27.0±40.7 pg/ml; Fig. 2A). Western blot analysis demonstrated expression of the MCP-1 receptor protein CCR2, in both PrEC and PrSC (Fig. 2B). CCR2 has two variants, CCR2a and CCR2b, and RT-PCR analysis showed that PrEC and PrSC express primarily the CCR2b variant (Fig. 2C).
Fig. 2.
Normal prostate epithelial and stromal cell lines secrete MCP-1 and express its receptor. A: MCP-1 in the supernatant of PrEC and PrSC by ELISA. PrEC and PrSC secrete 27.0 ± 40.7 and 2,150 ± 615 pg/ml MCP-1, respectively. B: CCR2 expression in both PrEC and PrSC by Western blot. C: RT-PCR analysis of CCR2 variants from PrEC and PrSC. Both cell lines express CCR2b but not CCR2a (Mph, macrophage).
MCP-1 Stimulates the Proliferation of Epithelial Cells
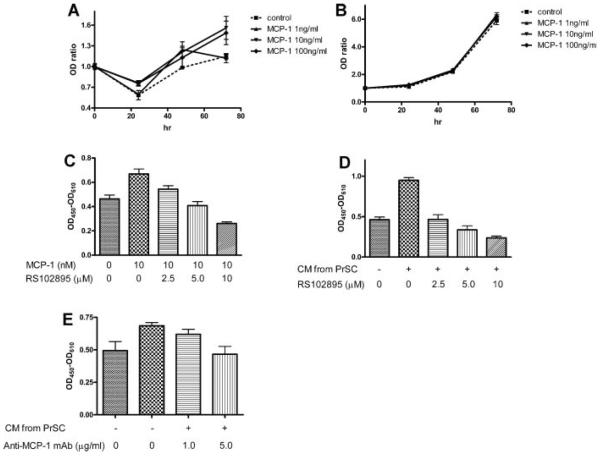
To examine the effects of MCP-1 on benign epithelial and stromal cells, WST-1 proliferation assays were performed. MCP-1 significantly stimulated the proliferation of PrEC in 72 hr (10, 100 ng/ml MCP-1: P<0.05) but not of PrSC (Fig. 3A,B). The growth stimulation by MCP-1 of PrEC was blocked by RS102895, a specific antagonist of CCR2b (P<0.05; Fig. 3C).
Fig. 3.
MCP-1 stimulates the proliferation of epithelial cells. WST-1 proliferation assays showed that MCP-1 stimulated the proliferation of PrEC in 72 hr significantly (10 and 100 ng/ml MCP-1: P < 0.05) (A) but not of PrSC (B). C: WST-1 assay showed that 10 ng/ml MCP-1 stimulated the growth of PrEC, and that RS102895, a CCR2b antagonist, blocked this growth stimulation in a dose-dependent manner (n = 4; 5 μM, P < 0.05; 10 μM, P < 0.01). D: WST-1 proliferation assays showed that conditioned media (CM) from PrSC stimulate the proliferation of PrEC (P < 0.05) and that RS102895 suppresses this effect (n = 4; 5 and 10 μg/ml, P < 0.01). E: Monoclonal anti-MCP-1 neutralizing antibody also suppresses the stimulation of PrEC growth by CM (n = 4; 10 μg/ml, P < 0.05).
To test the impact of MCP-1 secreted from stromal cells on epithelial cells, CM from PrSC were added to PrEC and proliferation was evaluated. CM from PrSC stimulated PrEC growth (P<0.05), and RS102895 completely inhibited this effect (P<0.05; Fig. 3D). Monoclonal anti-MCP-1 neutralizing antibody also inhibited the growth stimulation of PrEC by PrSC-CM (P<0.05; Fig. 3E).
PrSC were also subjected to MCP-1 inhibitors given the presence of MCP-1 receptor on their surface. Proliferation assays showed that only the highest concentration of RS102895 (10 μM) inhibited PrSC proliferation, but that the monoclonal anti-MCP-1 antibody did not (data not shown).
MCP-1 Levels Correlate With Inflammatory Cytokines and Inflammation in BPH
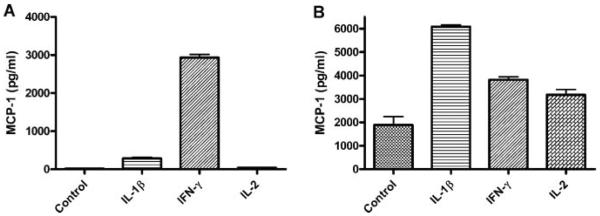
IL-1β, which appeared on the cytokine array data, is a pro-inflammatory molecule produced from monocyte/macrophages. MCP-1 also attracts T cells, and IFN-γ and IL-2, which are produced by activated T cells, are known to be upregulated 10-fold in BPH compared with normal prostate [17]. To test the effects of IL-1β, IFN-γ, and IL-2 on MCP-1 secretion from PrEC and PrSC, these lines were incubated with 10 ng/ml of IL-1-β, 10 ng/ml of IFN-γ, and 10 U/ml of IL-2. PrEC secreted 286±50, 2,933±148, and 37.1±6.7 pg/ml of MCP-1 on stimulation by IL-1β, IFN-γ, and IL-2, respectively, but only 16.0±7.5 pg/ml of MCP-1 without stimulation (Fig. 4A). PrSC secreted 1,888±631 pg/ml of MCP-1 without stimulation, which increased to 6,084±131, 3,818±212, and 3,179±392 pg/ml of MCP-1 on stimulation by IL-1β, IFN-γ, and IL-2, respectively (Fig. 4B).
Fig. 4.
Inflammatory cytokines enhance MCP-1 secretion from prostatic epithelial and stromal cells. A: ELISA of culture media showed that PrEC secrete 286 ± 50, 2,933 ± 148, and 37.1 ± 6.7 pg/ml of MCP-1 upon stimulation by 10 ng/ml of IL-1β, 10 ng/ml of IFN-γ, and 10 U/ml of IL-2, respectively, in contrast to 16.0 ± 7.5 pg/ml of MCP-1 without any stimulation (P < 0.05). B: PrSC secreted 6,084 ± 131, 3,818 ± 212, and 3,179 ± 392 pg/ml of MCP-1 upon stimulation by IL-1β, IFN-γ, and IL-2, respectively, while PrSC secreted 1,888 ± 631 pg/ml of MCP-1 without any stimulation (P < 0.05).
MCP-1 is known primarily as a chemotactic factor for monocyte/macrophages. We therefore examined the correlation of MCP-1 levels in EPS with the presence of the macrophage marker CD68 in those same specimens. Real-time PCR levels of CD68 mRNA in 25 available EPS samples correlated with MCP-1 levels from the same fluid (r=0.448, P<0.05; Fig. 5). There was statistically no correlation between gland weight and CD68 mRNA (r=0.169, P=0.478) or between gland weight and MCP-1 (r=0.364, P=0.115) in this limited set of 25 samples.
Fig. 5.
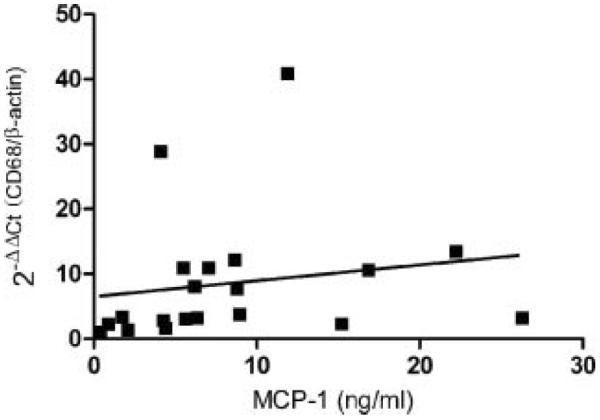
Correlation between MCP-1 levels and macrophages in 25 EPS samples. CD68 mRNA levels normalized by β-actin were measured by real-time PCR and correlated with MCP-1 levels measured by ELISA (r = 0.448, P < 0.05)
DISCUSSION
To date, much research on MCP-1 has been focused on its role in the progression of prostate cancer and in prostatic conditions other than benign prostatic hyperplasia. MCP-1 levels in EPS were found to be elevated in young men with CPPS IIIA and IIIB compared to a cohort of men with a variety of other benign prostatic pathologies including BPH [10]. In prostate cancer studies, MCP-1 has been shown to be a possible paracrine and autocrine factor in prostate cancer growth and migration [12,18], and anti-MCP-1 antibody has induced prostate cancer regression in vivo [14]. MCP-1 transcript is, however, expressed primarily by stromal smooth muscle cells and basal cells of the benign prostate [11]. Immunohistochemistry demonstrates a strong MCP-1 reaction in the fibromuscular stroma but not in the basal layer of these glands [11].
We found that the benign prostate stromal line PrSC secretes much higher levels of MCP-1 than the benign prostate epithelial line PrEC. However, PrEC can be induced to secrete high levels of MCP-1 by IL-1β and particularly IFN-γ. Thus, primary sources of MCP-1 in the prostate may indeed be stromal cells and inflamed epithelial cells. We also observed that MCP-1 levels in EPS correlated with increasing prostate volume, and therefore hypothesized that MCP-1 secreted from stromal cells may stimulate the proliferation of prostatic epithelial cells, Both epithelial and stromal cells were found to express the MCP-1 receptor (CCR2). CCR2 has two alternatively spliced forms, CCR2a and CCR2b, which differ only in their carboxyl-terminal tails [19]. CCR2a is the major isoform expressed by mononuclear cells, while CCR2b is expressed by satellite cells, regenerative muscle fibers, and endometrium [20,21]. Prostatic epithelial and stromal cells were demonstrated to express primarily the CCR2b variant.
Exogeneous MCP-1 stimulated the proliferation of PrEC but not of PrSC, and conditioned medium from PrSC stimulated the growth of PrEC. While such medium contains MCP-1 it also likely contains numerous other growth factors such as KGF, IGF, and FGF, which play important roles in BPH development. However, when we added a specific MCP-1 antagonist or anti-MCP-1 antibody to the conditioned PrSC medium, we inhibited its growth effects on PrEC completely. These data strongly suggest that MCP-1 is an important regulator of prostatic epithelial proliferation, perhaps involved in conditions such as BPH. On the other hand, proliferation assays showed that only the highest concentration of RS102895 (10 μM) inhibited the proliferation of PrSC, and that addition of monoclonal anti-MCP-1 antibody did not. This suggests a possible toxic effect of the antagonist molecule on PrSC rather than implying the existence of an autocrine MCP-1 pathway affecting PrSC. MCP-1 is known to promote the fibrosis in lung and kidney [22] and may stimulate collagen synthesis from prostatic stromal cells, but the effect of MCP-1 on PrSC remains to be determined.
MCP-1 is a chemotactic factor for lymphocytes and macrophages [23], immune cells that are often observed in BPH specimens. Typical infiltrates in BPH may consist of ~70% T lymphocytes, ~15% B cells, and ~15% macrophages as well as mast cells [24]. Our analyses showed a strong correlation between MCP-1 levels and CD68 mRNA levels in EPS. CD68 is a transmembrane glycoprotein, which is highly expressed by monocytes and macrophages and acts as a marker for them. Its association with MCP-1 levels suggests that the bioactive MCP-1 may be exerting a chemotactic role for monocyte/macrophages and those attracted monocyte/macrophages may secrete other factors to stimulate the proliferation of epithelial and stromal cells, though further studies would have to be performed to elucidate any causal relationship. CCR2 is expressed on activated and memory effector T cells [25,26], and MCP-1 is also a chemoattractant for T cells [27]. The effect of MCP-1 on T-cell differentiation remains controversial: MCP-1 stimulates Th2 polarization in the lymph node, whereas CCR2 activation stimulates Th1 polarization. In organ inflammation, MCP-1 acts to attract effector cells, which are abundant sources of IFN-γ [26].
IL-1β has been found to be elevated in prostatic inflammation [28], and IFN-γ and IL-2 are elevated in BPH tissues [29]. IL-2 and IFN-γ may support fibromuscular growth in BPH [30]. In vitro, we noted that IL-1β, IFN-γ, and IL-2 enhanced the secretion of MCP-1 from both epithelial and particularly from stromal cells. Surprisingly, the MCP-1 secretion from epithelial cells on stimulation by IFN-γ was almost as much as from stromal cells, although there was only trace MCP-1 secretion from epithelial cells without added cytokines.
An inflammatory stimulus and MCP-1 from stromal cells in the prostate may induce infiltration of T lymphocytes and macrophages, which in turn produce inflammatory cytokines. These cytokines might hypothetically stimulate the secretion of MCP-1 from stromal and epithelial cells, and secreted MCP-1 could in turn stimulate the growth of epithelial cells by a stromal–epithelial or autocrine interaction. Elevated MCP-1 levels in the prostate may also further attract inflammatory cells and contribute to a positive-feedback loop to stimulate BPH. Therefore, MCP-1 and its receptor molecule CCR2b in the prostate may present novel therapeutic targets for the treatment or prevention of BPH. Interestingly, BPH specimens have low macrophage inhibitory cytokine-1 (MIC-1) expression [31]. MIC-1 is an inhibitory factor for macrophage activation and thus acts in opposition to MCP-1 [32]. Thus, macrophages may be a key player in BPH development alongside T lymphocytes and other immunoeffectors.
High levels of MCP-1 exist in EPS, suggesting that MCP-1 levels in body fluids (prostatic secretions, urine, and serum) may potentially serve as markers for BPH, CPPS, and LUTS and help distinguish these conditions. MCP-1 and another monocyte/macrophage chemoattractant molecule macrophage inflammatory protein-1α (MIP-1α) have been proposed as possible biomarkers for CPPS, with intriguing data to support this [10]. In a careful study of EPS obtained in the clinic from a cohort of young male controls and CPPS patients, as well as from older men with BPH, elevated MCP-1 and MIP-1α levels correlated with the CPPS diagnosis and were also slightly elevated in BPH. The MCP-1 levels in the EPS of men in our study were some 10-fold higher in those with large prostate glands, but this might be explained by different sampling methodologies (ours represented EPS collected directly from ex vivo prostates rather than from clinic after digital rectal examination), different patient sets (our patients had prostates of known volume but unknown LUTS score, whereas in the Desireddi study neither prostate size nor LUTS score were not mentioned), and the use of different commercial ELISA kits. Since there has been no biologic marker for BPH in clinical use to date, a molecule such as MCP-1 presents a novel, potentially important prognostic marker for prostate enlargement/BPH, and/or LUTS and associated conditions such as CPPS.
A limitation of our study is that the prostate glands from which prostatic secretions were expressed all contained small volume, low-grade prostate cancers. We chose radical prostatectomy specimens for study because they provide an excellent source of prostatic secretions uncontaminated by the remainder of the male urologic tract and they provide us with accurate pathologic weight, a reliable surrogate for prostate volume; these specific specimens were chosen because their predominant pathology was indeed BPH or benign prostatic tissue rather than cancer. Small, low-grade prostate cancers are in any case known to be present in the prostates of most men by their 7th decade, including those diagnosed only with clinical BPH [33]. Cancerous prostate glands have lower MCP-1 expression than benign prostate glands at the mRNA level [34]; thus, it appears unlikely that the presence of prostate cancer significantly elevated the MCP-1 levels in the EPS we studied.
CONCLUSIONS
In conclusion, increasing MCP-1 levels in EPS correlate with prostate volume as well as with the macrophage marker CD68. MCP-1 is expressed by prostatic stromal cells in vitro and stimulates the growth of prostatic epithelial cells. The inhibition of MCP-1 or of its receptor (CCR2) suppresses these stromal–epithelial interactions and results in the inhibition of prostate epithelial cell proliferation. Taken together, these data suggest that MCP-1 and macrophages may play a role in the pathogenesis of prostate enlargement, and that MCP-1 may be a potentially useful biomarker and therapeutic target for BPH.
Acknowledgments
Grant sponsor: NIH/NIDDK; Grant number: 1K23DK071262; Grant sponsor: Department of Defense; Grant number: W81XWH-05-0167.
REFERENCES
- 1.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: Benign prostatic hyperplasia. J Urol. 2005;173(4):1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 2.Roehrborn CG, McConnell JD. In: Campbell's Urology. 8th edition Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Chapter 39. Saunders; Philadelphia, PA: 2002. pp. 1297–1397. [Google Scholar]
- 3.Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: New approaches to old problems. J Urol. 2007;178(2):395–401. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]
- 4.Roehrborn CG, McConnell . In: Campbell's Urology. 8th edition Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Chapter 39. Saunders; Philadelphia, PA: 2002. p. 1298. [Google Scholar]
- 5.Theyer G, Kramer G, Assmann I, Sherwood E, Preinfalk W, Marberger M, Zechner O, Steiner GE. Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest. 1992;66(1):96–107. [PubMed] [Google Scholar]
- 6.Di Silverio F, Gentile V, De Matteis A, Mariotti G, Giuseppe V, Luigi PA, Sciarra A. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: A retrospective analysis. Eur Urol. 2003;43(2):164–175. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 7.Anim JT, Udo C, John B. Characterisation of inflammatory cells in benign prostatic hyperplasia. Acta Histochem. 1998;100(4):439–449. doi: 10.1016/S0065-1281(98)80040-8. [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84(9):976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 9.Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9(1):9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 10.Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S, Thumbikat P, Pope RM, Landis JR, Koch AE, Schaeffer AJ. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179(5):1857–1861. doi: 10.1016/j.juro.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzucchelli L, Loetscher P, Kappeler A, Uguccioni M, Baggiolini M, Laissue JA, Mueller C. Monocyte chemoattractant protein-1 gene expression in prostatic hyperplasia and prostate adenocarcinoma. Am J Pathol. 1996;149(2):501–509. [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66(12):1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 13.Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, Neeley CK, Pienta KJ. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8(7):578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67(19):9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 15.Allan RW, Sanderson H, Epstein JI. Correlation of minute (0.5 MM or less) focus of prostate adenocarcinoma on needle biopsy with radical prostatectomy specimen: Role of prostate specific antigen density. J Urol. 2003;170(2 Pt 1):370–372. doi: 10.1097/01.ju.0000074747.72993.cb. [DOI] [PubMed] [Google Scholar]
- 16.Fujita K, Ewing CM, Sokoll LJ, Elliott DJ, Cunningham M, De Marzo AM, Isaacs WB, Pavlovich CP. Cytokine profiling of prostatic fluid from cancerous prostate glands identifies cytokines associated with extent of tumor and inflammation. Prostate. 2008;68(8):872–882. doi: 10.1002/pros.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer G, Steiner GE, Handisurya A, Stix U, Haitel A, Knerer B, Gessl A, Lee C, Marberger M. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002;52(1):43–58. doi: 10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69(4):1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91(7):2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartoli C, Civatte M, Pellissier JF, Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol. 2001;102(4):385–392. doi: 10.1007/s004010100394. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9(4):189–198. doi: 10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- 22.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taub DD, Proost P, Murphy WJ, Anver M, Longo DL, van Damme J, Oppenheim JJ. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest. 1995;95(3):1370–1376. doi: 10.1172/JCI117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51(5):1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2(2):102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 26.Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: Therapeutic opportunities and controversies. Microcirculation. 2003;10(3–4):247–257. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- 27.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadler RB, Koch AE, Calhoun EA, Campbell PL, Pruden DL, Bennett CL, Yarnold PR, Schaeffer AJ. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000;164(1):214–218. [PubMed] [Google Scholar]
- 29.Steiner GE, Stix U, Handisurya A, Willheim M, Haitel A, Reithmayr F, Paikl D, Ecker RC, Hrachowitz K, Kramer G, Lee C, Marberger M. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83(8):1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 30.Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol. 2006;16(1):25–29. [PubMed] [Google Scholar]
- 31.Kakehi Y, Segawa T, Wu XX, Kulkarni P, Dhir R, Getzenberg RH. Down-regulation of macrophage inhibitory cytokine-1/prostate derived factor in benign prostatic hyperplasia. Prostate. 2004;59(4):351–356. doi: 10.1002/pros.10365. [DOI] [PubMed] [Google Scholar]
- 32.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94(21):11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, Haas GP. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: An autopsy study of 249 cases. In Vivo. 1994;8(3):439–443. [PubMed] [Google Scholar]
- 34.Chetcuti A, Margan S, Mann S, Russell P, Handelsman D, Rogers J, Dong Q. Identification of differentially expressed genes in organ-confined prostate cancer by gene expression array. Prostate. 2001;47(2):132–140. doi: 10.1002/pros.1056. [DOI] [PubMed] [Google Scholar]