Abstract
Plasmalemmal caveolae were first identified as an endocytic compartment in endothelial cells, where they appear to move molecules across the cell by transcytosis. More recently, they have been found to be sites where small molecules are concentrated and internalized by a process called potocytosis. A growing body of biochemical and morphological evidence indicates that a variety of molecules known to function directly or indirectly in signal transduction are enriched in caveolae. This raises the possibility that a third function for caveolae is to process hormonal and mechanical signals for the cell. Insights gained from studying potocytosis suggest several different ways that this membrane specialization might function to integrate incoming and outgoing cellular messages.
Full text
PDF
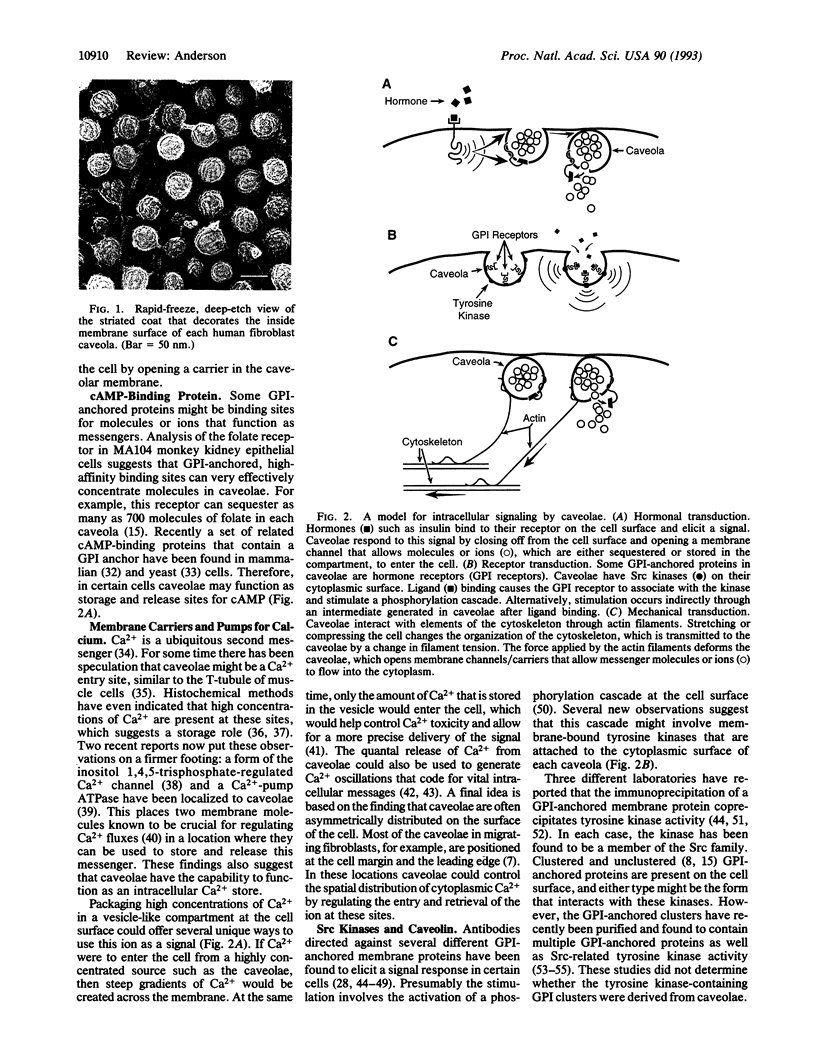
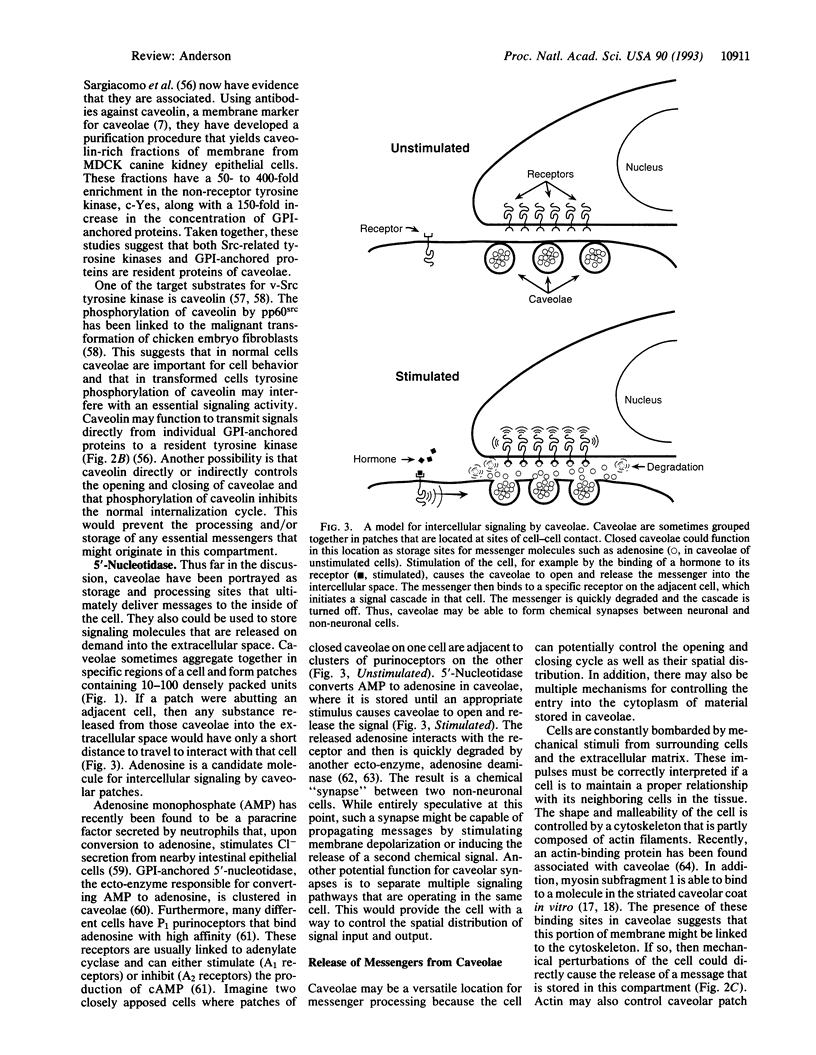


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Kamen B. A., Rothberg K. G., Lacey S. W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992 Jan 24;255(5043):410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- Anderson R. G. Potocytosis of small molecules and ions by caveolae. Trends Cell Biol. 1993 Mar;3(3):69–72. doi: 10.1016/0962-8924(93)90065-9. [DOI] [PubMed] [Google Scholar]
- Andersson Forsman C., Gustafsson L. E. Cytochemical localization of 5'-nucleotidase in the enteric ganglia and in smooth muscle cells of the guinea-pig. J Neurocytol. 1985 Aug;14(4):551–562. doi: 10.1007/BF01200797. [DOI] [PubMed] [Google Scholar]
- Andy R. J., Kornfeld R. The adenosine deaminase binding protein of human skin fibroblasts is located on the cell surface. J Biol Chem. 1982 Jul 25;257(14):7922–7925. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium oscillations. Adv Second Messenger Phosphoprotein Res. 1992;26:211–223. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bohuslav J., Cinek T., Horejsí V. Large, detergent-resistant complexes containing murine antigens Thy-1 and Ly-6 and protein tyrosine kinase p56lck. Eur J Immunol. 1993 Apr;23(4):825–831. doi: 10.1002/eji.1830230409. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Bundgaard M. The three-dimensional organization of smooth endoplasmic reticulum in capillary endothelia: its possible role in regulation of free cytosolic calcium. J Struct Biol. 1991 Aug;107(1):76–85. doi: 10.1016/1047-8477(91)90033-s. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Receptor tyrosine kinase substrates: src homology domains and signal transduction. FASEB J. 1992 Nov;6(14):3283–3289. doi: 10.1096/fasebj.6.14.1385243. [DOI] [PubMed] [Google Scholar]
- Casadó V., Lluis C., Canela E., Franco R., Mallol J. The distribution of A1 adenosine receptor and 5'-nucleotidase in pig brain cortex subcellular fractions. Neurochem Res. 1992 Feb;17(2):129–139. doi: 10.1007/BF00966790. [DOI] [PubMed] [Google Scholar]
- Chang W. J., Rothberg K. G., Kamen B. A., Anderson R. G. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992 Jul;118(1):63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinek T., Horejsí V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992 Oct 1;149(7):2262–2270. [PubMed] [Google Scholar]
- Davis L. S., Patel S. S., Atkinson J. P., Lipsky P. E. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J Immunol. 1988 Oct 1;141(7):2246–2252. [PubMed] [Google Scholar]
- Dráberová L., Dráber P. Thy-1 glycoprotein and src-like protein-tyrosine kinase p53/p56lyn are associated in large detergent-resistant complexes in rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3611–3615. doi: 10.1073/pnas.90.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Koshland M. E. Glycosylphosphatidylinositol: a candidate system for interleukin-2 signal transduction. Science. 1991 Jan 4;251(4989):78–81. doi: 10.1126/science.1824727. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993 Mar;120(5):1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992 Dec;119(6):1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989 Dec 5;264(34):20163–20166. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989 Jun;108(6):2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A. B., Soloski M. J. Anti-Qa-2-induced T cell activation. The parameters of activation, the definition of mitogenic and nonmitogenic antibodies, and the differential effects on CD4+ vs CD8+ T cells. J Immunol. 1989 Jul 15;143(2):407–413. [PubMed] [Google Scholar]
- Izumi T., Shibata Y., Yamamoto T. Quick-freeze, deep-etch studies of endothelial components, with special reference to cytoskeletons and vesicle structures. J Electron Microsc Tech. 1991 Nov;19(3):316–326. doi: 10.1002/jemt.1060190307. [DOI] [PubMed] [Google Scholar]
- Izumi T., Shibata Y., Yamamoto T. Striped structures on the cytoplasmic surface membranes of the endothelial vesicles of the rat aorta revealed by quick-freeze, deep-etching replicas. Anat Rec. 1988 Mar;220(3):225–232. doi: 10.1002/ar.1092200302. [DOI] [PubMed] [Google Scholar]
- Izumi T., Shibata Y., Yamamoto T. The cytoplasmic surface structures of uncoated vesicles in various tissues of rat as revealed by quick-freeze, deep-etching replicas. J Electron Microsc (Tokyo) 1989;38(1):47–53. [PubMed] [Google Scholar]
- Jacquemin C. Glycosyl phosphatidylinositol in thyroid: cell signalling or protein anchor? Biochimie. 1991 Jan;73(1):37–40. doi: 10.1016/0300-9084(91)90071-8. [DOI] [PubMed] [Google Scholar]
- Kamen B. A., Wang M. T., Streckfuss A. J., Peryea X., Anderson R. G. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem. 1988 Sep 25;263(27):13602–13609. [PubMed] [Google Scholar]
- Korty P. E., Brando C., Shevach E. M. CD59 functions as a signal-transducing molecule for human T cell activation. J Immunol. 1991 Jun 15;146(12):4092–4098. [PubMed] [Google Scholar]
- Kurzchalia T. V., Dupree P., Parton R. G., Kellner R., Virta H., Lehnert M., Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992 Sep;118(5):1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner J., Huang L. C., Schwartz C. F., Oswald A. S., Shen T. Y., Kinter M., Tang G. Z., Zeller K. Rat liver insulin mediator which stimulates pyruvate dehydrogenase phosphate contains galactosamine and D-chiroinositol. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1416–1426. doi: 10.1016/s0006-291x(88)80520-5. [DOI] [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Patapoff T. W., Gillece-Castro B., Colgan S. P., Parkos C. A., Delp C., Mrsny R. J. 5'-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993 May;91(5):2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Ortega G., Chan C., Kroczek R. A., Shevach E. M. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986 Sep 1;164(3):709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalcu F., Ungureanu V., Gancevici G., Popescu C., Petrovici A., Burghele B., Hagiopol C. Study on some autochthonous polystyrene-latex products in view to obtain the streptococcal MAP latex antigen. Arch Roum Pathol Exp Microbiol. 1985 Apr-Jun;44(2):141–153. [PubMed] [Google Scholar]
- Misek D. E., Saltiel A. R. An inositol phosphate glycan derived from a Trypanosoma brucei glycosyl-phosphatidylinositol mimics some of the metabolic actions of insulin. J Biol Chem. 1992 Aug 15;267(23):16266–16273. [PubMed] [Google Scholar]
- Müller G., Dearey E. A., Pünter J. The sulphonylurea drug, glimepiride, stimulates release of glycosylphosphatidylinositol-anchored plasma-membrane proteins from 3T3 adipocytes. Biochem J. 1993 Jan 15;289(Pt 2):509–521. doi: 10.1042/bj2890509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Schubert K., Fiedler F., Bandlow W. The cAMP-binding ectoprotein from Saccharomyces cerevisiae is membrane-anchored by glycosyl-phosphatidylinositol. J Biol Chem. 1992 Dec 15;267(35):25337–25346. [PubMed] [Google Scholar]
- Ohmichi M., Decker S. J., Saltiel A. R. Nerve growth factor stimulates the tyrosine phosphorylation of a 38-kDa protein that specifically associates with the src homology domain of phospholipase C-gamma 1. J Biol Chem. 1992 Oct 25;267(30):21601–21606. [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Peters K. R., Carley W. W., Palade G. E. Endothelial plasmalemmal vesicles have a characteristic striped bipolar surface structure. J Cell Biol. 1985 Dec;101(6):2233–2238. doi: 10.1083/jcb.101.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Romero G., Gámez G., Huang L. C., Lilley K., Luttrell L. Anti-inositolglycan antibodies selectively block some of the actions of insulin in intact BC3H1 cells. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1476–1480. doi: 10.1073/pnas.87.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero G., Luttrell L., Rogol A., Zeller K., Hewlett E., Larner J. Phosphatidylinositol-glycan anchors of membrane proteins: potential precursors of insulin mediators. Science. 1988 Apr 22;240(4851):509–511. doi: 10.1126/science.3282305. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kamen B. A., Anderson R. G. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990 Dec;111(6 Pt 2):2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Sorbara-Cazan L. R. Inositol glycan mimics the action of insulin on glucose utilization in rat adipocytes. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1084–1092. doi: 10.1016/0006-291x(87)90519-5. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993 Jan 1;177(1):145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs N. J. Caveolae: static inpocketings of the plasma membrane, dynamic vesicles or plain artifact? J Cell Sci. 1988 Jul;90(Pt 3):341–348. doi: 10.1242/jcs.90.3.341. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Kwong J., Fujita T., Olszowy M. W., Shaw A. S., Lublin D. M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol. 1992 Dec 1;149(11):3535–3541. [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Differentiated microdomains on the luminal surface of capillary endothelium: distribution of lectin receptors. J Cell Biol. 1982 Aug;94(2):406–413. doi: 10.1083/jcb.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of intestinal capillaries. Pathway followed by dextrans and glycogens. J Cell Biol. 1972 May;53(2):365–392. doi: 10.1083/jcb.53.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Sugi H., Suzuki S., Daimon T. Intracellular calcium translocation during contraction in vertebrate and invertebrate smooth muscles as studied by the pyroantimonate method. Can J Physiol Pharmacol. 1982 Apr;60(4):576–587. doi: 10.1139/y82-077. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sugi H. Evidence for extracellular localization of activator calcium in dog coronary artery smooth muscle as studied by the pyroantimonate method. Cell Tissue Res. 1989 Aug;257(2):237–246. doi: 10.1007/BF00261826. [DOI] [PubMed] [Google Scholar]
- Thomas P. M., Samelson L. E. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J Biol Chem. 1992 Jun 15;267(17):12317–12322. [PubMed] [Google Scholar]
- Thompson L. F., Ruedi J. M., Glass A., Low M. G., Lucas A. H. Antibodies to 5'-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J Immunol. 1989 Sep 15;143(6):1815–1821. [PubMed] [Google Scholar]
- Vivien D., Petitfrère E., Martiny L., Sartelet H., Galéra P., Haye B., Pujol J. P. IPG (inositolphosphate glycan) as a cellular signal for TGF-beta 1 modulation of chondrocyte cell cycle. J Cell Physiol. 1993 Jun;155(3):437–444. doi: 10.1002/jcp.1041550302. [DOI] [PubMed] [Google Scholar]
- YAMADA E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955 Sep 25;1(5):445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y. S., Anderson R. G., Rothberg K. G. Each caveola contains multiple glycosyl-phosphatidylinositol-anchored membrane proteins. Cold Spring Harb Symp Quant Biol. 1992;57:593–604. doi: 10.1101/sqb.1992.057.01.065. [DOI] [PubMed] [Google Scholar]




