Abstract
The personal care product Triclosan, 5-chloro-2(2,4-dichlorophenoxy)-phenol, is widely used in consumer products as an antibacterial agent and is increasingly found in the environment as a contaminant of sewage sludge and wastewater. This compound has been identified in plasma and urine of people in the United States, Sweden and Australia. Triclosan is known to inhibit sulfonation of phenolic xenobiotics and is structurally related to inhibitors of estrogen sulfotransferase, such as polychlorobiphenylols. In pregnancy, the placenta is an important source of estrogen, which is needed for normal fetal development and successful parturition, and estrogen sulfotransferase is thought to play an important role in regulation of estrogen availability. In this study, we examined the effect of Triclosan on sheep placental cytosolic sulfotransferase activity with 17-beta-estradiol and estrone as substrates. For comparison, we studied the effects of 4-hydroxy-3,3′,4′,5-tetrachlorobiphenyl and 2′-hydroxytriclocarban on estradiol sulfonation. The apparent Km for placental cytosolic sulfotransferase activity with estradiol as substrate was 0.27±0.06 nM (mean±S.D., n=3 individuals) and with estrone as substrate was 1.86±0.22 nM. Partial substrate inhibition was observed with estradiol at concentrations higher than 10–20 nM, as is typical of estrogen sulfotransferases (SULT1E1) in other species. Studies of the effect of Triclosan on estrogen sulfotransferase activity were conducted with several concentrations (0.1–6 nM) of estradiol and with 2 nM estrone. Triclosan was a very potent inhibitor of both estradiol and estrone sulfonation. For estradiol the inhibition was shown to be mixed competitive/uncompetitive, with Kic of 0.09±0.01 nM and Kiu of 5.2±2.9 nM. The IC50 for inhibition of estrone sulfonation was 0.60±0.06 nM. At an environmentally relevant concentration of 1 μM, Triclosan was not a substrate for glucuronidation in sheep placental microsomes. Triclosan could be sulfonated in placental cytosol with Km 1.14±0.18 μM and Vmax 160±26 pmol/min/mg protein, however the calculated rates of Triclosan sulfonation were negligible at the low nM concentrations that potently inhibit estrogen sulfonation. The high potency of Triclosan as an inhibitor of estrogen sulfotransferase activity raises concern about its possible effects on the ability of the placenta to supply estrogen to the fetus, and in turn on fetal growth and development.
Keywords: Triclosan, Polychlorobiphenylol, Placental estrogen sulfotransferase inhibition
1. Introduction
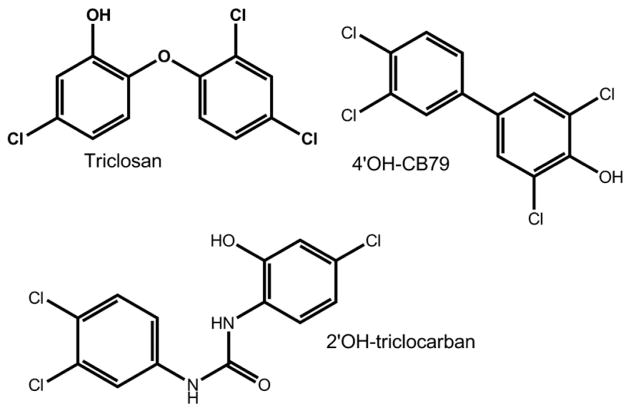
Triclosan, 5-chloro-2(2,4-dichlorophenoxy)-phenol, also known as Irgasan, is an antibacterial agent that is widely used in soaps, toothpastes, first-aid products, fabrics and plastic goods. The structure of Triclosan is shown in Fig. 1. Exposure of people to Triclosan has been documented in several recent studies by analyzing biological samples after enzymatic or acid hydrolysis to liberate free Triclosan from glucuronide or sulfate conjugates that are likely to be present in these samples (Wang et al., 2004). Triclosan was detected in mother’s milk (Adolfsson-Erici et al., 2002; Allmyr et al., 2006a), in plasma of people in Sweden and Australia (Allmyr et al., 2006b; Hovander et al., 2002) and in urine of people in the United States (Calafat et al., 2008). Because of its widespread use in consumer products and its chemical stability, Triclosan is found in sewage and is incompletely removed by wastewater treatment plants, so that Triclosan remains in effluent water as well as sewage sludge biosolids (Coogan et al., 2007; Heidler and Halden, 2007; Hua et al., 2005; Kinney et al., 2008; Ying and Kookana, 2007). Through the use of biosolids as fertilizers of agricultural land, and due to its incomplete removal during waste-water treatment, Triclosan is ubiquitous in the environment.
Fig. 1.
Structures of the chemicals investigated as inhibitors of sheep placental estrogen sulfotransferase activity.
Although it is acutely non-toxic to mammals, recent studies have shown that Triclosan has several biological activities that are unrelated to its antibacterial action. Triclosan was shown to affect thyroxine homeostasis in weanling rats (Zorrilla et al., 2008) and to exhibit estrogenic and androgenic activity in breast cancer cells (Gee et al., 2008). Triclosan inhibited sulfotransferase activity with several phenolic xenobiotics, namely 3-hydroxybenzo(a)pyrene, bis-phenol A, acetaminophen and p-nitrophenol in human liver cytosol and with expressed sulfotransferases SULT1A1, SULT1E1, SULT1B1 (Wang et al., 2004). Triclosan shows structural similarity to polychlorobiphenylols such as 4-hydroxy-3,3′,4′5-tetrachlorobiphenyl (4′OH-CB79, see Fig. 1), which are known potent inhibitors of human and animal estrogen sulfotransferase activity (Jurgella et al., 2006; Kester et al., 2000; Wang and James, 2007). Thus it was of interest to examine the effect of Triclosan on estrogen sulfotransferase in a physiologically important tissue, the placenta. For comparison, we included the known potent inhibitor, 4′OH-CB79 and the hydroxylated metabolite of another widely used antibacterial agent, 2′-hydroxy-triclocarban (2′-OH-TCC, see Fig. 1).
The pregnant sheep animal model has been used to examine the physiological functions of the placenta in providing estrogen to the developing fetus (Wood, 2005). During pregnancy, estrogen is known to modulate or control several critical processes. Uterine blood flow is increased by estrogen, allowing supply of blood gases and nutrients to the developing fetus (Magness et al., 1993, 2005; Vagnoni et al., 1998). Myometrial activity is increased at the end of pregnancy by an increase in estrogen and in some species a decrease in progesterone (Challis, 2000). Estrogen potently stimulates fetal ACTH secretion (Saoud and Wood, 1997; Wood and Saoud, 1997): the increase in fetal ACTH and cortisol concentrations augment fetal stress responsiveness, accelerate fetal maturation, and hasten the timing of parturition. The most potent estrogen is 17-beta-estradiol. Placenta, known to express estrogen sulfotransferase (Hoffmann et al., 2001; Stanley et al., 2001), provides nearly all of the estrogen that circulates in fetal blood, where it is mostly in the forms of sulfoconjugated estradiol and estrone, which are present at much higher concentrations than the unconjugated estrogens. Indeed, the circulating concentrations of estrone sulfate are as high as 4 and 2 ng/mL in fetal and maternal plasma, respectively (Carnegie and Robertson, 1978; Tsang, 1974). We have recently reported the presence of estradiol-3-sulfate in the plasma of fetal sheep at concentrations of around 1 ng/mL (Wood et al., 2003), similar to that previously reported for estradiol sulfate in fetal plasma (Carnegie and Robertson, 1978). The estrogen-responsive tissues co-express estrogen receptor and steroid sulfatase, and are therefore able to hydrolyze the sulfoconjugated estrogen into the active steroid in the target tissues (Purinton and Wood, 1998). Thus, inhibition of placental estrogen sulfotransferase is likely to affect the delivery of estrogens from the placenta to the fetus.
As with other hydroxylated xenobiotics, the bioavailability and tissue distribution of Triclosan is likely to be reduced by glucuronidation, sulfonation or both pathways. In human liver subcellular fractions, Triclosan is efficiently glucuronidated and somewhat less efficiently sulfonated (Wang et al., 2004), suggesting that first-pass hepatic conjugation will reduce the systemic bioavailability of ingested Triclosan. The ability of placenta to glucuronidate or sulfonate Triclosan is unknown, but could potentially reduce the concentration of free Triclosan. Human placenta was shown to express UGT2B family mRNAs and proteins, but not UGT1A family isoforms and activity was demonstrated with 4-methylumbelliferone as substrate (Collier et al., 2002a), however sheep placenta was not able to glucuronidate or sulfonate acetaminophen (Wang et al., 1986).
The objectives of this study were to determine if Triclosan inhibited sheep placental estrogen sulfotransferase with two physiologically important estrogens, estradiol and estrone; to determine the mechanism of inhibition; to compare the activity of Triclosan with that of the known human SULT1E1 inhibitor, 4′OH-CB79 and a related compound 2′OH-TCC; and to examine the possibility that Triclosan could be detoxified by glucuronidation or sulfonation in the placenta.
2. Materials and methods
2.1. Chemicals
Triclosan was purchased from Sigma-Aldrich chemical company (St. Louis, Mo.) and shown to be >99% pure by HPLC analysis with UV detection at 280 nm. The 4-hydroxy-3,3′,4′,5-tetrachlorobiphenyl (4′OH-CB79) was a gift from L.W. Robertson, University of Iowa Superfund Basic Research Program, and the 2′-hydroxy-triclocarban (2′OH-TCC) a gift from B. D. Hammock, University of California, Davis, Superfund Basic Research Program. The 3H-estrone, 68 Ci/mmol was purchased from Sigma-Aldrich, while the 3H-estradiol, 44.9 Ci/mmol, 14C-UDPGA, 180 mCi/mmole and 35S-3′-phosphoadenosine-5′-phosphosulfate (PAPS), 1.85 Ci/mmol were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). The 14C-UDPGA was diluted with unlabeled UDPGA (Sigma-Aldrich) to a specific radioactivity of 3.5 mCi/mmol and the 35S-PAPS was diluted with unlabeled PAPS to 1 Ci/mmol for use in assays. The unlabeled PAPS (>97% pure) was obtained from Dr. S.S. Singer, University of Dayton, OH. Buffer chemicals and solvents were obtained from Sigma-Aldrich or Fisher scientific (Orlando, FL).
2.2. Placental tissue
The placental tissue was harvested from 3 time-dated fetal sheep that were 126–130 days gestation at the time of tissue collection (term is approximately 147 days in the sheep). The pregnant ewes were euthanized using an overdose of sodium pentobarbital. After cardiac arrest was confirmed, the placental tissue was removed and rapidly frozen in liquid nitrogen. The tissue was stored at −80 °C until use for these experiments. Samples of placental cotyledon (3 g) from three individual sheep were homogenized in 12 ml of 0.25 M sucrose, 0.05 M Tris–Cl, pH 7.4, 0.1 mM EDTA. The homogenate was subjected to differential centrifugation to isolate washed microsomes and cytosol fractions (James and Little, 1983). The protein content of each fraction was determined by the Lowry method with bovine serum albumin as standard.
2.3. Sulfotransferase assays
To determine the position of sulfonation of estradiol, an HPLC method was used as described previously (Wang and James, 2005). After demonstrating that estradiol was sulfonated only at the 3-hydroxyl group, for all other studies, estradiol and estrone sulfotransferase activities were measured with a radiochemical extraction assay (Wang and James, 2005). All assays were conducted in duplicate or triplicate. The assay conditions were optimized with sheep placental cytosol fractions (n=3) so that product formation was linear with time and protein concentrations, the concentration of PAPS was saturating, and less than 15% of the substrate was consumed over the reaction time. For initial studies of IC50 values with 3H-estradiol and 3H-estrone, the final steroid concentrations in assay tubes were 1 nM (estradiol) or 2 nM (estrone). The steroids were added to assay tubes from ethanol solution and the ethanol removed under nitrogen before adding other assay components. The final reaction volume of 0.5 ml contained 100 mM Tris–Cl pH 7.4, 10 mM MgCl2, 20 μM PAPS and varying concentrations of Triclosan or other inhibitors in DMSO, such that the concentration of DMSO was 0.5% by volume. Vehicle controls contained DMSO alone. Tubes were placed in a water bath at 37°C and the reaction started by adding placental cytosolic protein, usually 0.6 μg. Blanks were incubated without added protein. After incubation for 5 min, the reaction was terminated by adding 0.2 ml of an ice-cold solution of trichloroacetic acid, 3% w/v and vortex-mixing. Tubes were extracted three times with 1 ml dichloromethane to remove unreacted estrogen. Samples of the aqueous phase, 0.2 ml, were assayed for [3H] content by liquid scintillation counting. For studies of the kinetics of sulfonation, the range of substrate concentrations used was 0.1 to 160 nM (estradiol) or 0.25 to 6 nM (estrone). At concentrations below 1 nM, less cytosolic protein was used to ensure a linear rate of estradiol or estrone sulfonation. To study the mechanism of inhibition, varying concentrations of estradiol between 0.1 and 6 nM were incubated as described above in the presence of vehicle or Triclosan concentrations between 0.1 and 2.5 nM. To assess inhibition by 4′OH-CB79, concentrations between 1 and 50 nM were added to assay tubes, and for 2′OH-TCC the concentration range was 0.05 to 5 μM.
Triclosan sulfonation in placental cytosol was assayed using radiolabeled 35S-PAPS and the method described previously (Wang et al., 2004). Assay conditions were optimized so that Triclosan sulfate formation was linear with time and sheep placental cytosolic protein, and the concentration of 35S-PAPS was saturating. To determine the kinetics of sulfonation, Triclosan at final concentrations of 0.1 to 10 μM was added to tubes from ethanol solution. The ethanol was removed under nitrogen and 100 mM Tris–Cl pH 7.4, 0.1 mg cytosolic protein, 0.4% BSA, 5 mM MgCl2 and water were added. After pre-incubation at 37 °C for 1 min, the reaction was started by adding 35 S-PAPS, 20 μM, with a total assay volume of 0.1 ml. Tubes were incubated for 15 min at 37 °C with gentle shaking, then the reaction was terminated by adding 0.1 ml of a 1:1 ice-cold mixture of 2.5% acetic acid and 0.2 mM tetrabutylammonium sulfate (PIC®A), 0.3 ml water, and 2.0 ml water-saturated ethyl acetate. Blanks were prepared as for samples, but did not contain Triclosan. Tubes were vortex-mixed, centrifuged to separate the phases and the ethyl acetate phase containing the ion-pair of PIC®A with Triclosan sulfate was transferred to a scintillation vial. Assay tubes were extracted a second time with water-saturated ethyl acetate and the ethyl acetate extracts were combined and evaporated under air to less than 0.5 ml. Scintillation cocktail was added to measure the extracted sulfate conjugates.
2.4. Glucuronosyltransferase assay
Triclosan glucuronidation was assayed using 14C-UDPGA as described previously (Wang et al., 2004). Sheep placental microsomes from 3 individuals were treated with Brij 58 at a ratio of 0.25 mg Brij 58 per mg of microsomal protein. Ethanol solutions of Triclosan were added into each tube to give a final assay concentration of 1 μM. The ethanol was removed under a stream of nitrogen. Next, the mixture of Brij 58 and microsomes containing 0.2 mg protein was added into each tube, vortex-mixed, and incubated on ice for 20 min. Then 0.1 M Tris–Cl pH 7.6 and 5 mM MgCl2 were added, the mixture was preincubated for 1 min at 37 °C in a water bath, and the reaction was started by addition of 14C-UDPGA, 1 mM in a total assay volume of 0.1 ml. Incubated blanks were identical to samples but did not contain the Triclosan substrate. After incubation for 60 min at 37 °C with shaking, the reaction was terminated by adding 0.1 ml of 1:1 ice-cold mixture of 2.5% acetic acid and PIC®A, 0.3 ml water. Tubes were extracted twice with 2 ml water-saturated ethyl acetate and the extracts combined. The ethyl acetate was concentrated to about 0.5 ml under a stream of air, scintillation cocktail was added and the samples were counted for radioactivity.
2.5. Data analysis
Enzyme kinetic data was fit to the Michaelis–Menten equation at concentrations of Triclosan, estrone or estradiol where no substrate inhibition was observed, using the software Graphpad Prism 4.0 (San Diego, CA). Over the whole range of estradiol concentrations studied, data were fit to Eq. (1), a model for partial inhibition of estrogen sulfotransferase in which binding of a second molecule of substrate to the enzyme reduces but does not abolish activity (Zhang et al., 1998).
| (1) |
This equation denotes the constant for binding of the first substrate (S) molecule as and the second substrate molecule as Ki. is the maximum rate for the non-inhibitory substrate concentration range, and Vmin is the minimum rate in the inhibitory substrate concentration range.
The kinetic mechanism of inhibition of estradiol sulfotransferase activity by Triclosan, studied in the range of estradiol concentrations where substrate inhibition was not observed, was determined by calculating the apparent Michaelis constants appKm and appVmax at each of several concentrations of Triclosan, then plotting appKm/appVmax and 1/appVmax against the concentration of Triclosan to obtain values for the parameters of mixed inhibition, Kic and Kiu, signifying binding of inhibitor to enzyme, Kic, and to the enzyme-substrate complex, Kiu (Cornish-Bowden, 2004). The IC50 values for inhibition of estrone sulfonation by Triclosan and estradiol sulfonation by 4′OH-CB79 and 2′OH-TCC were obtained by fitting the fraction of control uninhibited activity versus log inhibitor concentration to a sigmoidal dose–response curve (Graphpad software). The Ki values were calculated from IC50 values, assuming competitive inhibition, using the formula in Eq. (2) (Cheng and Prusoff, 1973).
| (2) |
3. Results
3.1. Sulfotransferase activity with estradiol and estrone in sheep placental cytosol
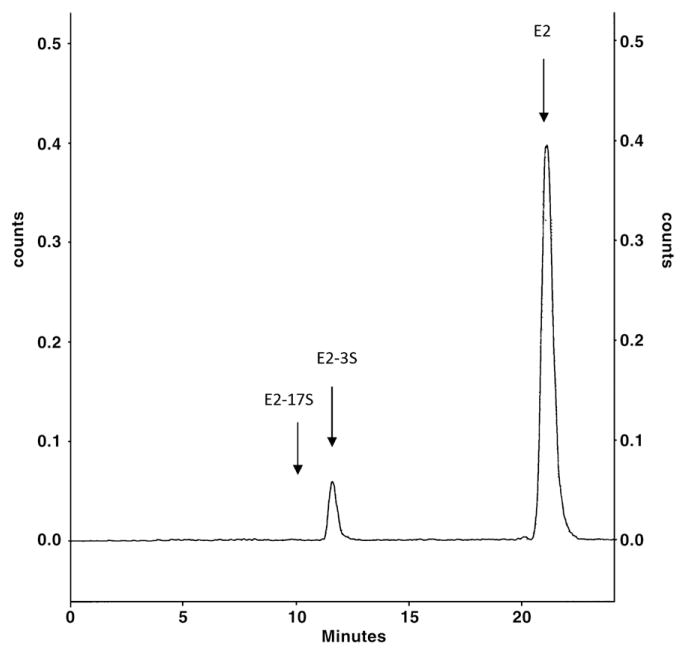
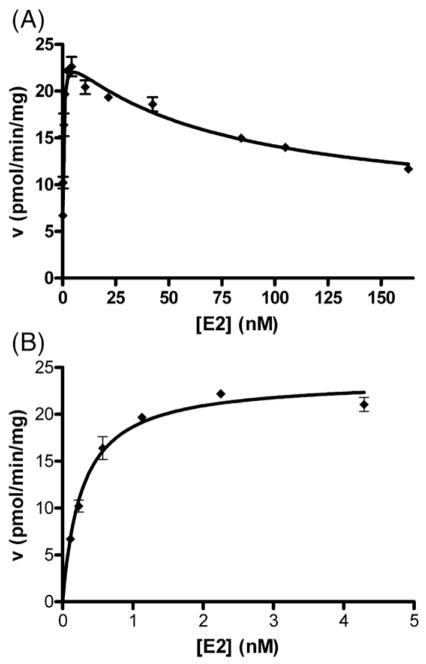
HPLC analysis showed that the only product of sulfonation of estradiol formed in the presence of sheep placental cytosol was estradiol-3-sulfate, with no estradiol-17-sulfate (Fig. 2). The sheep placental cytosol had high estrogen sulfotransferase activity at low, physiologically relevant, concentrations of estradiol and estrone. The kinetics of estradiol sulfonation, studied under linear conditions of estradiol-3-sulfate formation, showed substrate inhibition at concentrations above 6–10 nM (Fig. 3). The data fit the equation for partial substrate inhibition with kinetic constants as shown in Table 1. Estrone sulfonation was studied only at low nM substrate concentrations. The data fit the Michaelis–Menten equation at these estrone concentrations and the kinetic constants are shown in Table 1.
Fig. 2.
Reverse-phase C18 HPLC with radiochemical detection following incubation of sheep placental cytosol with [3H]-17-β-estradiol,100 nM and PAPS, 20 μM. The retention positions of standard estradiol-17-sulfate (E2-17S), estradiol-3-sulfate (E2-3S) and estradiol (E2) are indicated. The chromatographic system was as described in Wang and James, 2005.
Fig. 3.
Kinetics of estradiol sulfonation in sheep placental cytosol. Results shown are from one sample. Similar results were obtained with two other individuals. Part A shows rates of estradiol-3-sulfate formation over a wide range of estradiol concentrations: the line indicates the fit of the data to a model of partial substrate inhibition (see Materials and methods section). Part B shows an expanded view of the low substrate concentration range: the line was obtained by fitting the data to the Michaelis–Menten equation.
Table 1.
Kinetic parameters for estradiol and estrone sulfonation in sheep placental cytosol.
| Substrate | ApparentKm nM | ApparentVmax pmol/min/mg protein | Ki nM | Vmin pmol/min/mg protein |
|---|---|---|---|---|
| Estradiol | 0.27±0.06 | 32.7±7.5 | 54.7±25.3 | 12.1±7.7 |
| Estrone | 1.86±0.22 | 48.1±9.9 |
Values shown are mean±S.D., n=3 individuals.
Apparent: Km and apparentVmax are the Michaelis constants, Ki is the constant for partial inhibition of estradiol sulfotransferase activity by substrate and Vmin is the plateau low rate at high estradiol concentrations (Zhang et al., 1998).
Estrone sulfonation was only studied in the range 0.3 to 6 nM estrone, not in the range where substrate inhibition would be observed.
3.2. Inhibition by Triclosan
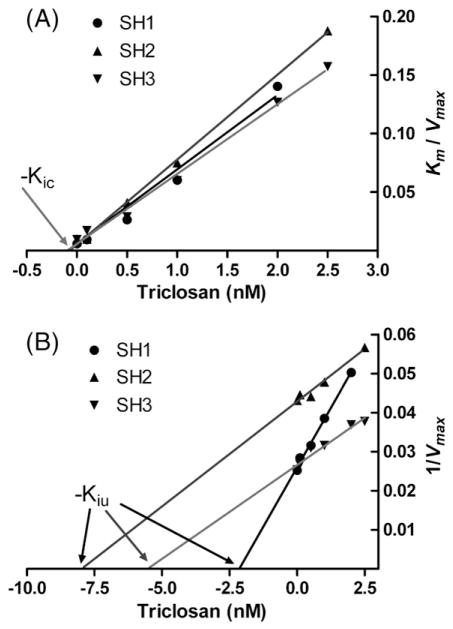
Triclosan potently inhibited estradiol sulfonation. Addition of Triclosan markedly increased the apparent Km for estradiol and somewhat reduced the apparent Vmax, indicating mixed inhibition, as shown in Table 2 for each individual. The values for the inhibitory constants for the effects of Triclosan on estradiol kinetics were calculated from linear regression plots of appKm/appVmax against the concentration of Triclosan (Kic) and 1/appVmax against the concentration of Triclosan (Kiu) as shown in Fig. 4A and B respectively. The competitive inhibitory constant, Kic was 0.09±0.01 nM (mean±S.D., n =3 individuals) and the uncompetitive inhibitory constant Kiu was 5.2±2.9 nM. The 50-fold difference in inhibitory potency indicated a strong competitive component to the inhibition.
Table 2.
Effect of Triclosan on the kinetic constants for estrogen sulfotransferase.
| Triclosan (nM) | SH1
|
SH2
|
SH3
|
|||
|---|---|---|---|---|---|---|
| Km (nM) | Vmax (pmol/ min/mg) | Km (nM) | Vmax (pmol/ min/mg) | Km (nM) | Vmax (pmol/ min/mg) | |
| 0 | 0.24 | 39.7 | 0.23 | 23.2 | 0.37 | 39.5 |
| 0.1 | 0.33 | 35.2 | 0.22 | 22.4 | 0.64 | 37.4 |
| 0.5 | 0.84 | 31.6 | 0.93 | 22.7 | 0.94 | 32.4 |
| 1.0 | 1.57 | 26.0 | 1.58 | 20.9 | 1.89 | 31.6 |
| 2.0 | 2.79 | 19.9 | – | – | 3.44 | 27.1 |
| 2.5 | – | – | 3.31 | 17.6 | 4.17 | 26.5 |
Values shown are the means of duplicate or triplicate determinations of estradiol sulfonation kinetics at several concentrations of Triclosan.
Data are shown for each individual placental sample.
Fig. 4.
Inhibition plots for the effect of Triclosan on estradiol sulfonation in 3 individual sheep placental cytosol samples. Part (A) shows the plot of appKm/appVmax against the concentration of Triclosan present. The intercepts on the x axis show the values for −Kic, the constant for competitive inhibition. Part (B) shows the plot of 1/appVmax against the concentration of Triclosan. The intercepts on the x axis show the values for −Kiu, the constant for inhibitor binding to the enzyme-substrate complex.
Triclosan was also a potent inhibitor of estrone sulfonation, as shown in Fig. 5. Only one concentration of estrone (2 nM) was studied and the calculated IC50 was 0.60± 0.06 nM, mean±S.D., n=3 individuals. Assuming the inhibition is mainly competitive, as our results for estradiol show, the calculated Ki values were 0.29±0.05 nM. These values were calculated from the IC50 values, the apparent Km for estrone in each individual placental sample and the estrone concentration, according to Eq. (2).
Fig. 5.
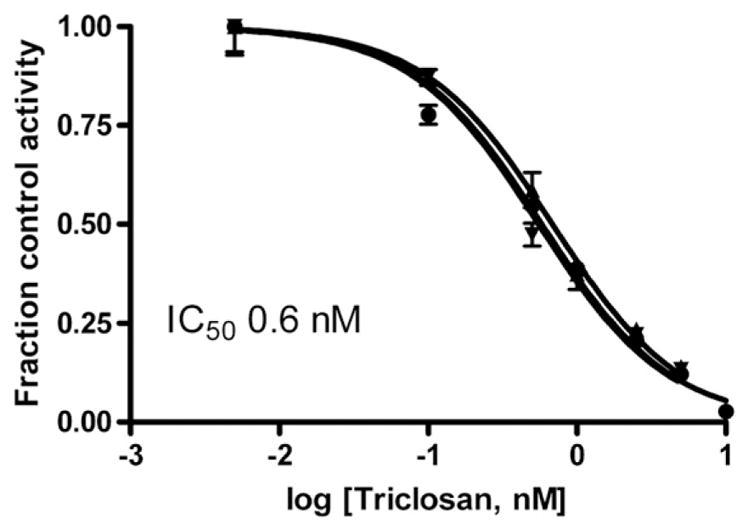
Inhibition of estrone sulfonation in sheep placental samples, measured at 2 nM estrone, by various concentrations of Triclosan. The lines shown are the fits of fraction control activity versus log [Triclosan] to sigmoidal dose–response curves for each of 3 individuals. Error bars on each line indicate the S.D. of assay replicates at each concentration of Triclosan. The mean IC50 value is indicated on the graph.
3.3. Inhibition of estradiol sulfotransferase by 4′OH-CB79 and 2′OH-TCC
The known potent inhibitor of human SULT1E1, 4′-OH-CB79 was also a potent inhibitor of sheep placental cytosolic sulfotransferase, measured at 1 nM estradiol, with IC50 values of 4.1±0.4 nM (mean±S.D., n =3), as shown in Fig. 6. Conversion of the IC50 values to Ki, assuming the inhibition is competitive, gives Ki values of 0.89± 0.08 nM. A hydroxylated metabolite of triclocarban, 2′OH-TCC, did inhibit estradiol sulfonation, but was two orders of magnitude less potent than Triclosan or 4′OH-CB79, with IC50 values of 0.31±0.04 μM (Fig. 7). Again assuming that the inhibition is primarily competitive, the calculated Ki for 2′OH-TCC is 0.06±0.02 μM.
Fig. 6.
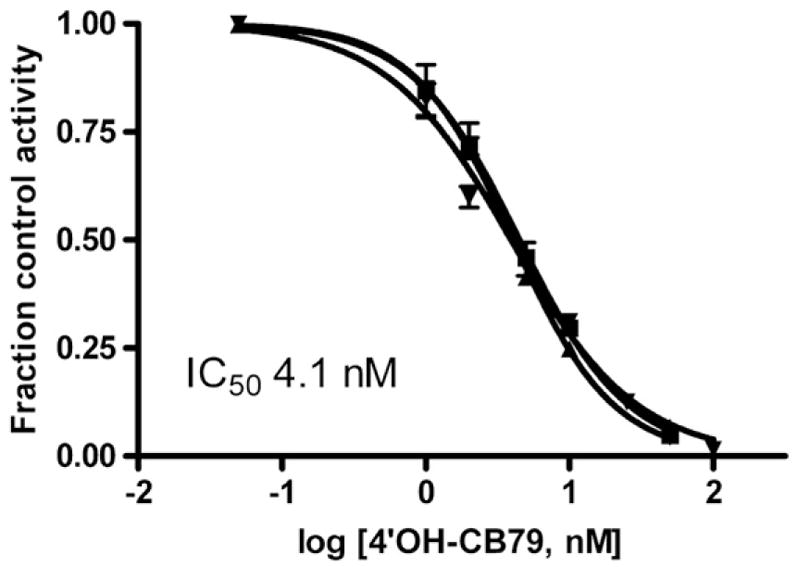
Inhibition of estradiol sulfonation in sheep placental samples, measured at 1 nM estradiol, by various concentrations of 4′OH-CB79. The lines shown are the fits of fraction control activity versus log [4′OH-CB79] to sigmoidal dose–response curves for each of 3 individuals. Error bars on each line indicate the S.D. of assay replicates at each concentration of 4′OH-CB79. The mean IC50 value is indicated on the graph.
Fig. 7.
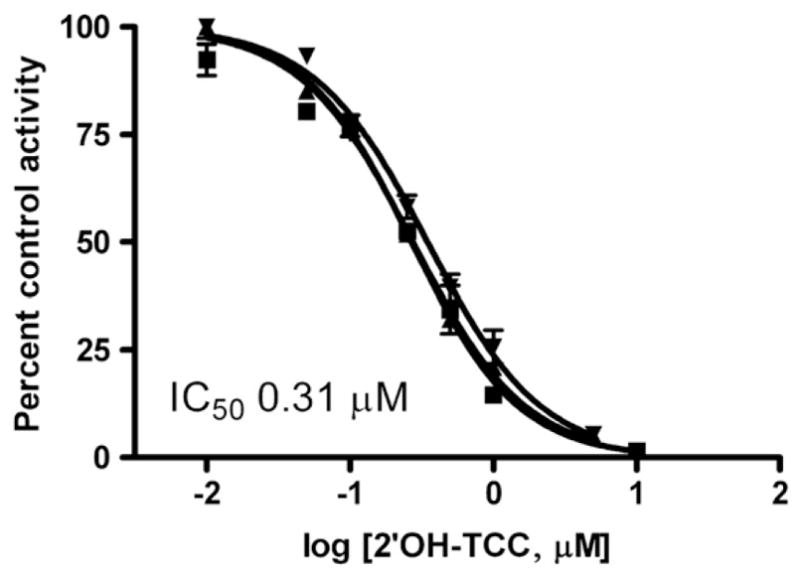
Inhibition of estradiol sulfonation in sheep placenta, measured at 1 nM estradiol, by various concentrations of 2′hydroxy-triclocarban (2′OH-TCC). The lines shown are the fits of fraction control activity versus log [2′OH-TCC] to sigmoidal dose–response curves for each of 3 individuals. Error bars on each line indicate the S.D. of assay replicates at each concentration of 2′OH-TCC. The mean IC50 value is indicated on the graph.
3.4. Sulfonation and glucuronidation of Triclosan by placental fractions
To assess the likelihood that environmentally relevant concentrations of Triclosan will be conjugated in the placenta, cytosolic sulfonation and microsomal glucuronidation of Triclosan was examined, using established methods (Wang et al., 2004). There was no detectable glucuronidation of Triclosan in the sheep placental microsomes. The limit of detection was 0.5 pmol Triclosan glucuronide/min/mg protein. Triclosan was a substrate for sulfonation in sheep placental cytosol. In the absence of substrate but presence of 20 μM PAPS (incubated substrate blank), components present in the cytosol were sulfonated at a rate of 5.5±0.8 pmol/min/mg protein (mean±S.D., n=3 individuals). For each individual placental cytosol sample, the rate of sulfonation of endogenous components was subtracted from sulfonation rates in the presence of each concentration of Triclosan. The rates of Triclosan sulfate formation followed Michaelis–Menten kinetics in the substrate range of 0.1 to 10 μM studied. The apparent kinetic constants are shown in Table 3.
Table 3.
Sulfation and glucuronidation of Triclosan in sheep placental cytosol and microsomes respectively.
| Sulfonation
|
Glucuronidation (pmol/min/mg) | |
|---|---|---|
| ApparentKm μM | ApparentVmax (pmol/min/mg protein) | |
| 1.14±0.18 | 160±26 | N.D. |
N.D. not detected. Triclosan glucuronidation was examined in the presence of 1 μM Triclosan. The limit of detection of Triclosan glucuronidation under the conditions used was <0.5 pmol/min/mg protein.
Data for sulfonation and glucuronidation represent mean±S.D. of three individual samples.
From these kinetics constants, rates of sulfonation of Triclosan under the conditions of the estrogen sulfonation assays were calculated, to determine if there would be significant loss of Triclosan during assays to study the inhibition of estrogen sulfonation by Triclosan. At a concentration of 1 nM, the rate of formation of Triclosan sulfate was 0.141±0.003 pmol/min/mg protein (mean±S.D., n=3 individuals). Thus, under the conditions of assay of estradiol or estrone sulfonation in the presence of 1 nM Triclosan, less than 0.1% of the Triclosan would be sulfonated.
4. Discussion
The results demonstrate that sheep placental cytosol contains a sulfotransferase enzyme (or enzymes) with very high affinity for estradiol, which forms only estradiol-3-sulfate, but not estradiol-17β-sulfate at the low estradiol concentrations used. This site specificity for sulfonation suggests that the low concentrations of estradiol employed in this study were metabolized in sheep placenta by phenol sulfotransferases in the SULT1 family, not hydroxysteroid sulfotransferases in the SULT2 family, which also metabolize estradiol at the 17-position (Wang and James, 2005). The finding that estradiol sulfotransferase activity is partially inhibited by estradiol concentrations higher than 10 nM further suggests that the estradiol is predominantly sulfonated by a sheep homolog of human SULT1E1, which displays a similar partial substrate inhibition at concentrations of estradiol above 20 nM (Zhang et al.,1998). The presence of a SULT1E1 homolog in the sheep placenta is in accord with the hypothesis that sulfated estrone and estradiol formed in the placenta are transferred to the fetus, as a major source of estrogen (Wood, 2005). Estrogen sulfotransferase activity and SULT1E1 protein expression have been found in placenta of humans, cows and mice (Alnouti and Klaassen, 2006; Hirayama et al., 2008; Stanley et al., 2001; Ushizawa et al., 2007). It is possible that other SULT1 and SULT2 family sulfotransferases are expressed in sheep placenta, as has been shown for SULT1A1 and SULT2A1 in human placenta (Stanley et al., 2001), however although these sulfotransferases can metabolize estrogens, they do not exhibit as high an affinity as SULT1E1 and are unlikely to play a major role in the sulfonation of low nM concentrations of estradiol or estrone (Falany et al., 1994; Wang and James, 2005).
This study demonstrated that Triclosan was a very potent inhibitor of placental estrogen sulfotransferase activity, with sub-nM Ki values for inhibition of estradiol and estrone sulfonation. Investigation of the kinetics of inhibition with estradiol as substrate showed that the inhibition was primarily competitive, which assumes that Triclosan occupies the substrate binding site and prevents estradiol from binding. This mechanism has been demonstrated through protein crystallography of a complex of human SULT1E1 with another potent inhibitor, 4,4′-dihydroxy-3,3′,5,5′-tetrachlorobiphenyl: the inhibitor was shown to occupy the estradiol-binding site (Shevtsov et al., 2003). The small uncompetitive component of inhibition may be analogous to the described mechanism of partial substrate inhibition, in which a second molecule of substrate (or inhibitor) binds to the enzyme-substrate complex and partially inhibits product formation (Gamage et al., 2006; Zhang et al., 1998). The toxicological implications of potent inhibition of estrogen sulfotransferase in placenta by Triclosan have yet to be determined. The role of placental estrogen sulfotransferase in maintenance of pregnancy is still being elucidated, however changes in estrogen sulfotransferase expression have been associated with spontaneous abortion and fetal loss in SULT1E1 knockout mice and in cows that overexpress placental estrogen sulfotransferase (Hirayama et al., 2008; Tong et al., 2005), suggesting a delicate balance in the requirement for sulfonation of estrogens in the placenta. Secretion of sulfated estrogen by placenta is thought to be an important source of estrogen for the fetus (Wood, 2005). Sulfoconjugated estrogens are the predominant form of circulating estrogens in both fetal and maternal plasma (Carnegie and Robertson, 1978; Wood et al., 2003). It is very clear that estrogen is required not only for maintenance of pregnancy but also for normal fetal development (Magness et al., 1993, 2005; Vagnoni et al., 1998). Although the role of placental estrogen sulfotransferase is not completely understood, it is likely that inhibition of this enzyme by Triclosan will be deleterious to the successful maintenance or outcome of pregnancy.
Sulfotransferases are known to exhibit species differences, so it is possible that inhibitors of sulfotransferase in one species may not have the same effect on homologous proteins in other species. To compare the potency of Triclosan with that of a known potent inhibitor of human SULT1E1 (Kester et al., 2000), we examined the ability of 4′OH-CB79 to inhibit the sheep placental enzyme. This polychlorobiphenylol was reported to give an IC50 value of 0.2–0.6 nM with expressed human SULT1E1, and was also highly potent as an inhibitor of sheep placental estrogen sulfotransferase, with an IC50 of 4.1±0.4 nM. The finding that 4′OH-CB79 is a very potent inhibitor of both human and sheep estrogen sulfotransferase demonstrates that the sheep and human enzymes respond similarly to inhibitors, and suggests that Triclosan is likely to inhibit SULT1E1 in human placenta.
Several other environmental chemicals with structural similarity to Triclosan have the potential to inhibit estrogen sulfotransferase. We postulated that the hydroxylated metabolite of another widely used antibacterial compound, triclocarban, could be an inhibitor of sheep placental estrogen sulfotransferase. Although 2′OH-triclocarban did inhibit the sheep placental enzyme, it was of much lower potency than Triclosan, with an IC50 of 310±40 nM. The relatively low potency suggests that this triclocarban metabolite will not be present in tissues at a sufficient concentration to affect activity.
An important question that this work raises is whether or not Triclosan is likely to reach the placenta at a concentration that will inhibit the estrogen sulfotransferase. Triclosan is in widespread use in personal care products, and studies have shown that in particular, its use in toothpaste contributes to a measurable internal dose of this substance (Allmyr et al., 2006a). It is known that Triclosan is readily glucuronidated and sulfonated in human liver (Wang et al., 2004), however while hepatic biotransformation is likely to reduce the amount of free Triclosan transferred to the systemic circulation, it is unlikely that there would be complete conversion of Triclosan to conjugates during one pass through the liver. Indeed, it was shown that levels of unconjugated Triclosan in the plasma of people who ingested 4 mg Triclosan were between 22 and 47% of the total (conjugated+unconjugated) Triclosan present in the plasma (Sandborgh-Englund et al., 2006). Table 4 summarizes studies to date in which recent environmental or household exposure of people to Triclosan has been documented by its measurement in body fluids following work-up of samples to hydrolyze glucuronide or sulfate conjugates. Total Triclosan concentrations were in the low nM range. Although the concentration of unconjugated Triclosan in placenta of exposed mothers is not known, it is clear that the concentration of Triclosan that causes substantial inhibition of estrogen sulfotransferase is in the range of the environmental exposure level. Once Triclosan reaches the placenta, our results demonstrate that it will not undergo extensive phase II metabolism there. We saw no evidence for glucuronidation of Triclosan in the sheep placenta, and relatively low rates of sulfonation, especially at concentrations of Triclosan in the nM range. Thus, while Triclosan is readily conjugated in human liver, it is not readily conjugated in sheep placenta at environmentally relevant levels. Human placenta has been shown to express UGT2B isoforms, but not UGT1A isoforms (Collier et al., 2002a), however it is not known which UGT isoforms catalyze the glucuronidation of Triclosan, so the likelihood that human placenta could conjugate Triclosan is not known. It is possible that deconjugation of Triclosan conjugates present in blood could take place in the placenta. There is evidence for expression of sulfatase and glucuronidase in human and sheep placenta (Buchanan-Smith et al., 1969; Collier et al., 2002b; Gniot-Szulzycka, 1982; Mason et al.,1989). Thus if Triclosan glucuronide or sulfate are substrates for these hydrolytic enzymes, the availability of free Triclosan in placenta would be increased.
Table 4.
Concentrations of total Triclosan in human bodily fluids, compiled from the literature.
| Sample | Reported mean concentration
|
Reference | |
|---|---|---|---|
| μg/L (urine) or ng/g (serum and milk) | nM | ||
| Urine—United States | |||
| Female | 10.6 | 36.6 | (Calafat et al., 2008) |
| Male | 16.2 | 56.0 | |
| Serum—Australia | |||
| Female 2002 | 14 | 48.4 | (Allmyr et al., 2008) |
| Female 2004 | 7.2 | 24.9 | |
| Male 2004 | 12 | 41.5 | |
| Serum—Sweden | |||
| Control female | 0.07 | 0.25 | (Allmyr et al., 2006b) |
| Exposed female | 16 | 55.3 | |
| Breast milk—Sweden | |||
| Control female | 0.02 | 0.07 | (Allmyr et al., 2006b) |
| Exposed female | 0.54 | 1.87 | |
5. Conclusion
We conclude that Triclosan and 4′OH-CB79 are potent inhibitors of estrogen sulfotransferase in sheep placental tissue, and that Triclosan is a particularly potent inhibitor. The low Ki for Triclosan inhibition of estrogen sulfotransferase activity combined with the prevalence of Triclosan in the environment, suggest that sulfonation of estradiol and estrone in placenta is particularly vulnerable to this environmental contaminant. Ovine pregnancy is a good model of human pregnancy, especially with regard to the role of placental estrogen biosynthesis for maintenance of uterine blood flow and fetal development. Delivery of oxygen and nutrients to the developing fetus is highly dependent upon the maintenance of a high uterine blood flow which is, in turn, dependent upon placental estrogen secretion. At the same time, the majority of estrogen secreted by sheep and human placentae is sulfoconjugated. Sulfoconjugated estrogens, in turn, are made biologically available in target tissues by the action of steroid sulfatase. The present results suggest the possibility that exposure of pregnant sheep, and by analogy pregnant women, to Triclosan might endanger the pregnancy by reducing total placental estrogen secretion and thereby reducing estrogen action in target tissues critical for maintenance of the pregnancy.
References
- Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46:1485–9. doi: 10.1016/s0045-6535(01)00255-7. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ. 2006a;372:87–93. doi: 10.1016/j.scitotenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Allmyr M, McLachlan MS, Sandborgh-Englund G, Adolfsson-Erici M. Determination of triclosan as its pentafluorobenzoyl ester in human plasma and milk using electron capture negative ionization mass spectrometry. Anal Chem. 2006b;78:6542–6. doi: 10.1021/ac060666x. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Harden F, Toms LM, Mueller JF, McLachlan MS, Adolfsson-Erici M, et al. The influence of age and gender on triclosan concentrations in Australian human blood serum. Sci Total Environ. 2008;393:162–7. doi: 10.1016/j.scitotenv.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci. 2006;93:242–55. doi: 10.1093/toxsci/kfl050. [DOI] [PubMed] [Google Scholar]
- Buchanan-Smith JG, Nelson EC, Tillman AD. Effect of vitamin E and selenium deficiencies on lysosomal and cytoplasmic enzymes in sheep tissues. J Nutr. 1969;99:387–94. doi: 10.1093/jn/99.3.387. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–7. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie JA, Robertson HA. Conjugated and unconjugated estrogens in fetal and maternal fluids of the pregnant ewe: a possible role for estrone sulfate during early pregnancy. Biol Reprod. 1978;19:202–11. doi: 10.1095/biolreprod19.1.202. [DOI] [PubMed] [Google Scholar]
- Challis JRG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000;55:650–60. doi: 10.1097/00006254-200010000-00025. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Collier AC, Ganley NA, Tingle MD, Blumenstein M, Marvin KW, Paxton JW, et al. UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem Pharmacol. 2002a;63:409–19. doi: 10.1016/s0006-2952(01)00890-5. [DOI] [PubMed] [Google Scholar]
- Collier AC, Tingle MD, Paxton JW, Mitchell MD, Keelan JA. Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum Reprod. 2002b;17:2564–72. doi: 10.1093/humrep/17.10.2564. [DOI] [PubMed] [Google Scholar]
- Coogan MA, Edziyie RE, La Point TW, Venables BJ. Algal bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere. 2007;67:1911–8. doi: 10.1016/j.chemosphere.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. Fundamentals of enzyme kinetics. London: Portland Press; 2004. [Google Scholar]
- Falany CN, Wheeler J, Oh TS, Falany JL. Steroid sulfation by expressed human cytosolic sulfotransferases. J Steroid Biochem Mol Biol. 1994;48:369–75. doi: 10.1016/0960-0760(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, et al. Human sulfotransferases and their role in chemical metabolism. Toxicol Sci. 2006;90:5–22. doi: 10.1093/toxsci/kfj061. [DOI] [PubMed] [Google Scholar]
- Gee RH, Charles A, Taylor N, Darbre PD. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol. 2008;28:78–91. doi: 10.1002/jat.1316. [DOI] [PubMed] [Google Scholar]
- Gniot-Szulzycka J. Arylsulphatase C and sterol sulphatase activities in microsomes from human placenta. Acta Biochim Pol. 1982;29:205–12. [PubMed] [Google Scholar]
- Heidler J, Halden RU. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere. 2007;66:362–9. doi: 10.1016/j.chemosphere.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Hirayama H, Sawai K, Moriyasu S, Hirayama M, Goto Y, Kaneko E, et al. Excess estrogen sulfoconjugation as the possible cause for a poor sign of parturition in pregnant cows carrying somatic cell clone fetuses. Reproduction. 2008;136:639–47. doi: 10.1530/REP-08-0157. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Falter K, Vielemeier A, Failing K, Schuler G. Investigations on the activity of bovine placental oestrogen sulfotransferase and -sulfatase from midgestation to parturition. Exp Clin Endocrinol Diabetes. 2001;109:294–301. doi: 10.1055/s-2001-16350. [DOI] [PubMed] [Google Scholar]
- Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, Bergman A, et al. Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Arch Environ Contam Toxicol. 2002;42:105–17. doi: 10.1007/s002440010298. [DOI] [PubMed] [Google Scholar]
- Hua W, Bennett ER, Letcher RJ. Triclosan in waste and surface waters from the upper Detroit River by liquid chromatography-electrospray-tandem quadrupole mass spectrometry. Environ Int. 2005;31:621–30. doi: 10.1016/j.envint.2004.10.019. [DOI] [PubMed] [Google Scholar]
- James MO, Little PJ. Modification of benzo(a)pyrene metabolism in hepatic microsomes from untreated and induced rats by imidazole derivatives which inhibit mono-oxygenase activity and enhance epoxide hydrolase activity. Drug Metab Dispos. 1983;11:350–4. [PubMed] [Google Scholar]
- Jurgella GF, Marwah A, Malison JA, Peterson R, Barry TP. Effects of xenobiotics and steroids on renal and hepatic estrogen metabolism in lake trout. Gen Comp Endocrinol. 2006;148:273–81. doi: 10.1016/j.ygcen.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, et al. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000;141:1897–900. doi: 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- Kinney CA, Furlong ET, Kolpin DW, Burkhardt MR, Zaugg SD, Werner SL, et al. Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ Sci Technol. 2008;42:1863–70. doi: 10.1021/es702304c. [DOI] [PubMed] [Google Scholar]
- Magness RR, Parker CR, Jr, Rosenfeld CR. Systemic and uterine responses to chronic infusion of estradiol-17 beta. Am J Physiol. 1993;265:E690–8. doi: 10.1152/ajpendo.1993.265.5.E690. [DOI] [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol. 2005;565:71–83. doi: 10.1113/jphysiol.2005.086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JI, France JT, Magness RR, Murry BA, Rosenfeld CR. Ovine placental steroid 17 alpha-hydroxylase/C-17,20-lyase, aromatase and sulphatase in dexamethasone-induced and natural parturition. J Endocrinol. 1989;122:351–9. doi: 10.1677/joe.0.1220351. [DOI] [PubMed] [Google Scholar]
- Purinton SC, Wood CE. Estrogen sulfatase and sulfotransferase are found in brain regions important for hypothalamus-pituitary-adrenal axis control. J Soc Gynecol Investig. 1998;5:153A. doi: 10.1159/000054541. Supplement. [DOI] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–73. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- Saoud CJ, Wood CE. Modulation of ovine fetal adrenocorticotropin secretion by androstenedione and 17beta-estradiol. Am J Physiol. 1997;272:R1128–34. doi: 10.1152/ajpregu.1997.272.4.R1128. [DOI] [PubMed] [Google Scholar]
- Shevtsov S, Petrotchenko EV, Pedersen LC, Negishi M. Crystallographic analysis of a hydroxylated polychlorinated biphenyl (OH-PCB) bound to the catalytic estrogen binding site of human estrogen sulfotransferase. Environ Health Perspect. 2003;111:884–8. doi: 10.1289/ehp.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EL, Hume R, Visser TJ, Coughtrie MW. Differential expression of sulfotransferase enzymes involved in thyroid hormone metabolism during human placental development. J Clin Endocrinol Metab. 2001;86:5944–55. doi: 10.1210/jcem.86.12.8081. [DOI] [PubMed] [Google Scholar]
- Tong MH, Jiang H, Liu P, Lawson JA, Brass LF, Song WC. Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat Med. 2005;11:153–9. doi: 10.1038/nm1184. [DOI] [PubMed] [Google Scholar]
- Tsang CP. Changes in plasma levels of estrone sulfate and estrone in the pregnant ewe around parturition. Steroids. 1974;23:855–68. doi: 10.1016/0039-128x(74)90059-2. [DOI] [PubMed] [Google Scholar]
- Ushizawa K, Takahashi T, Hosoe M, Ishiwata H, Kaneyama K, Kizaki K, et al. Global gene expression analysis and regulation of the principal genes expressed in bovine placenta in relation to the transcription factor AP-2 family. Reprod Biol Endocrinol. 2007;5:17. doi: 10.1186/1477-7827-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnoni KE, Shaw CE, Phernetton TM, Meglin BM, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. III. Ovarian and estrogen effects on NO synthase. Am J Physiol. 1998;275:H1845–56. doi: 10.1152/ajpheart.1998.275.5.H1845. [DOI] [PubMed] [Google Scholar]
- Wang LQ, James MO. Sulfotransferase 2A1 forms estradiol-17-sulfate and celecoxib switches the dominant product from estradiol-3-sulfate to estradiol-17-sulfate. J Steroid Biochem Mol Biol. 2005;96:367–74. doi: 10.1016/j.jsbmb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Wang LQ, James MO. Sulfonation of 17beta-estradiol and inhibition of sulfotransferase activity by polychlorobiphenylols and celecoxib in channel catfish, Ictalurus punctatus. Aquat Toxicol. 2007;81:286–92. doi: 10.1016/j.aquatox.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Rudolph AM, Benet LZ. Pharmacokinetic studies of the disposition of acetaminophen in the sheep maternal-placental-fetal unit. J Pharmacol Exp Ther. 1986;238:198–205. [PubMed] [Google Scholar]
- Wang LQ, Falany CN, James MO. Triclosan as a substrate and inhibitor of 3′-phosphoadenosine 5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos. 2004;32:1162–9. doi: 10.1124/dmd.104.000273. [DOI] [PubMed] [Google Scholar]
- Wood CE. Estrogen/hypothalamus-pituitary-adrenal axis interactions in the fetus: the interplay between placenta and fetal brain. J Soc Gynecol Investig. 2005;12:67–76. doi: 10.1016/j.jsgi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Wood CE, Saoud CJ. Influence of estradiol and androstenedione on ACTH and cortisol secretion in the ovine fetus. J Soc Gynecol Investig. 1997;4:279–83. [PubMed] [Google Scholar]
- Wood CE, Gridley KE, Keller-Wood M. Biological activity of 17beta-estradiol-3-sulfate in ovine fetal plasma and uptake in fetal brain. Endocrinology. 2003;144:599–604. doi: 10.1210/en.2002-220764. [DOI] [PubMed] [Google Scholar]
- Ying GG, Kookana RS. Triclosan in wastewaters and biosolids from Australian wastewater treatment plants. Environ Int. 2007;33:199–205. doi: 10.1016/j.envint.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang H, Varlamova O, Vargas FM, Falany CN, Leyh TS. Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. J Biol Chem. 1998;273:10888–92. doi: 10.1074/jbc.273.18.10888. [DOI] [PubMed] [Google Scholar]
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, et al. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2008 doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]