Abstract
The African malaria mosquito, Anopheles gambiae, is widespread south of the Sahara including in dry savannahs and semi-arid environments where no surface water exists for several months a year. Adults of the M form of An. gambiae persist through the long dry season, when no surface waters are available, by increasing their maximal survival from 4 weeks to 7 months. Dry season diapause (aestivation) presumably underlies this extended survival. Diapause in adult insects is intrinsically linked to depressed reproduction. To determine if reproduction of the Sahelian M form is depressed during the dry season, we assessed seasonal changes in oviposition, egg batch size, and egg development, as well as insemination rate and blood feeding in wild caught mosquitoes. Results from xeric Sahelian and riparian populations were compared. Oviposition response in the Sahelian M form dropped from 70% during the wet season to 20% during the dry season while the mean egg batch size among those that laid eggs fell from 173 to 101. Correspondingly, the fraction of females that exhibited gonotrophic dissociation increased over the dry season from 5% to 45%, while a similar fraction of the population retained developed eggs despite having access to water. This depression in reproduction the Sahelian M form was not caused by a reduced insemination rate. Seasonal variation in these reproductive parameters of the riparian M form population was less extreme and the duration of reproductive depression was shorter. Blood feeding responses did not change with the season in either population. Depressed reproduction during the dry season in the Sahelian M form of An. gambiae provides additional evidence for aestivation and illuminates the physiological processes involved. The differences between the Sahelian and riparian population suggest an adaptive cline in aestivation phenotypes between populations only 130 km apart.
Keywords: aestivation, diapause, dormancy, gonotrophic dissociation, malaria, seasonality, reproduction
1. Introduction
Malaria remains one of the worst human diseases, causing hundreds of millions of clinical cases and approximately one million deaths annually, most of which occur in sub-Saharan Africa (WHO, 2008), where transmission depends on Anopheles gambiae, Anopheles arabiensis, and Anopheles funestus (Coluzzi, 1964; Davidson, 1964; Gillies and De Meillon, 1968). An. gambiae s.s., the primary vector, is divided into sub-populations (Coluzzi et al., 1979; Toure et al., 1998) believed by many to represent incipient species, known as the M and S molecular forms (Coluzzi M. et al., 1985; della Torre A. et al., 2001; Favia G. et al., 2001; Gentile G. et al., 2001; Krzywinski and Besansky, 2003; Lehmann et al., 2003; Mukabayire et al., 2001; Touré Y.T. et al., 1998; Turner et al., 2005). Prevalence of malaria varies seasonally and spatially (e.g., by latitude) largely following vector density, which in turn depends on the availability of larval sites, and hence on rainfall (Bayoh et al., 2001; Coetzee et al., 2000; Koenraadt et al., 2004; Lindblade et al., 1999; Lindsay et al., 1998). Because eggs, larvae, and pupae cannot withstand desiccation for more than a few days (Beier et al., 1990) and adults usually survive only a few weeks (Gillies, 1961; Gillies and De Meillon, 1968; Gillies and Wilkes, 1965; Lehmann and Diabate, 2008), persistence of malaria in areas where no surface waters exist for over two months a year has been long-debated (Coluzzi, 1993; Donnelly et al., 2002; Minakawa et al., 2001; Simard et al., 2000; Taylor et al., 1993; Toure et al., 1994). Evidence for dry season diapause (aestivation) of adults (Holstein, 1954; Omer and Cloudsley-Thomson, 1968), was met by contradictory evidence showing that migration from nearby areas where surface water persisted, was the main mechanism of population persistence (Charlwood et al., 2000; Jawara et al., 2008; Minakawa et al., 2001; Ramsdale and Fontaine, 1970a, b). Recent studies, however, have demonstrated that the M form of An. gambiae aestivates naturally (Lehmann et al., 2010) and that aestivation is a major mechanism that allows persistence of the M form and thus malaria in the Sahel (Adamou et al., 2011). Further, the latter study suggested that unlike the M form, the S form and An. arabiensis did not persist by aestivation but by migration from distant location(s). Understanding the mechanisms that allow persistence of anopheline vectors throughout the dry season may have important implications for malaria control because without breeding opportunities, the population is believed to be at its most vulnerable state (Coluzzi, 1992; Donnelly et al., 2002; Holstein, 1954; Lehmann et al., 2010; Lehmann and Diabate, 2008; Lehmann et al., 1998; Minakawa et al., 2001; Omer and Cloudsley-Thomson, 1968; Ribeiro et al., 1996; Simard et al., 2000; Taylor et al., 1993).
Past studies on An. gambiae did not distinguish between different forms of seasonal dormancy, namely diapause and quiescence, both typically are manifested by drastic reductions in activity, development and growth of immature stages, and reproduction in adults during inhospitable conditions (Denlinger, 1986; Kostal, 2006; Tauber and Tauber, 1976). Unlike quiescence, which is a direct response to adverse conditions (e.g. desiccation, high salinity, cold or hot temperatures) and includes extreme inhibition of life processes, diapause is programmed far in advance of the adverse conditions via responses to token stimuli such as change in photoperiod (Denlinger, 1986, 2002; Kostal, 2006; Tauber and Tauber, 1976). Additional information will be required to separate these forms of dormancy, e.g., to determine whether the dormant state is induced prior to onset of inhospitable conditions. For consistency with the previous literature and because the distinction is beyond the scope of this study, the term aestivation (summer diapause) will be used hereafter.
Physiological and behavioral changes associated with diapause are used to identify insects in this state more than their capacity to withstand inhospitable conditions (Tauber 1986). Like other insects in temperate climates, different mosquito species overwinter in diapause as eggs, larvae, or adults. Overwintering adult mosquitoes undergoing diapause shelter in protected environments such as culverts and cellars after they accumulate nutritional reserves, manifested as fat body hypertrophy. During diapause, flight activity, feeding, and reproduction are suppressed whereas tolerance to desiccation and cold are enhanced (Benoit, 2010; Benoit and Denlinger, 2007; Danks, 2007; Denlinger, 1986, 2002). Recent evidence suggests higher desiccation tolerance in the M form of An. gambiae compared with that of the S form (Gray et al., 2009; Lee et al., 2009). The term ‘reproductive diapause’ has been used frequently in studies of overwintering mosquitoes (Reisen et al., 2010; Spielman and Wong, 1973; Washino, 1977) in reference to reproductive arrest which may not involve suppression of flight, feeding, or overall metabolism. Nonetheless, ‘reproductive diapause’ is considered a form of diapause and applies equally to aestivation. The phenomenon of gonotrophic dissociation pertains to diapausing mosquitoes that blood feed but do not develop eggs, e.g., An. maculipennis atroparvus and An. hyrcanus, whereas gonotrophic concordance pertains to mosquitoes that do not take blood meals nor develop eggs e.g. An. maculipennis messae, An.superpictus and Culiseta inornata (Vinogradova 1960, (Washino, 1977)). Importantly, cold alone, is conducive for reduced feeding and suppressed reproduction in ectotherms. An. gambiae in the Sahel may exhibit either phenomenon, and it is not yet known which. Aestivating insects share much the same repertoire of phenotypes despite the high prevailing temperatures (Denlinger, 1986; Tauber et al., 1986). To the best of our knowledge, Culiseta inornata is the only mosquito known to aestivate besides An. gambiae. During aestivation in southern California, Cs. inornata accumulate fat reserves and reduce both reproduction and blood feeding (Washino, 1977) Breland et al. 1964, Washino et al. 1962, Barnard and Mulla 1977). Except during diapause, longevity of most mosquito species is considerably shorter in duration than the winter in temperate regions or the dry season in the subtropics. Thus extended survival, long enough to traverse the inhospitable season and reproduce when conditions become favorable once again, is the ultimate outcome of their diapause.
Studies of life history evolution have amassed evidence in support of its theoretical prediction that diapause and extension of life span are costly in terms of reproduction e.g., (Dao et al., 2010; Huestis and Marshall, 2006; Stearns, 1992). The reproductive costs associated with diapause refer mostly to the periods preceding and following diapause; however, here we focus on the period of diapause exclusively. Accordingly, we expected that the M form reduces its reproduction during the dry season. To determine if reproduction is depressed during the dry season (November-May) in Sahelian populations of An. gambiae, we bioassayed wild-caught female mosquitoes every month from October 2009 through August 2010 in a Sahelian village where no surface water exists from December to May. In that area, the last rains typically fall by mid October, yet surface water may remain until December. The first rain typically falls in early June, although most rains fall between July and September. Measures of reproduction included (1) oviposition response, (2) egg batch size, and (3) gonotrophic dissociation. We evaluated seasonal variation in these parameters and specifically tested if they were depressed during the dry season in comparison to the wet season. To better understand the underlying causes of possible changes in reproduction, we determined the concomitant seasonal changes in insemination rate and in blood-feeding response. To further assess how unique the results from Sahelian populations are, identical experiments were conducted every month at another village located near the Niger River (~130 km away), where aestivation may not occur because of the availability of some larval sites year on the river’s banks. Accordingly, we expected stable reproduction in the riparian population through the dry season.
2. Material and Methods
2.1. Study sites and mosquito collection
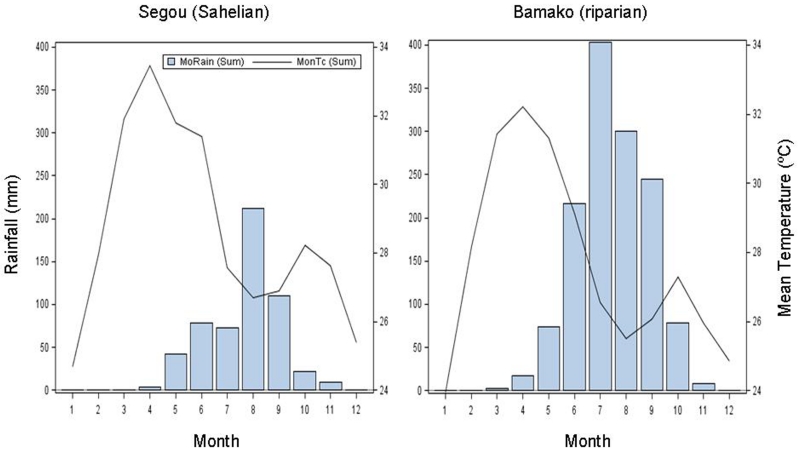
Indoor resting mosquitoes were collected (using mouth aspirators) between 08:00-10:00 from the villages of M’Piabougou (13.59°N, 7.19°W) and N’Gabakoro Droit (12.667°N, 7.86°W) from September 2009 to August 2010. M’Piabougou is located in the southern Sahel (hereafter referred to as the Sahelian locality), where the last rains fall in October and no surface water can be found over 30 km around from December to the end of May (Lehmann et al. 2010). N’Gabakoro Droit is located in the savannah ~130 km SSW from M’Piabougou, only ~100 m from the Niger River (hereafter referred to as the riparian locality), where puddles form as the river recedes. During the dry season (December-May), collections were done daily for 14 days/month in the Sahelian village to maximize sample sizes and were also done 3-5 days/month in the riparian village. The average annual temperature of both villages is similar (27-30°C) as is the annual relative humidity (RH, 50-58%). The average rainfall and temperature (from 2007 until 2011) that were recorded in the nearest national weather stations of Mali, Segou and Bamako for Mpiabougou and N’Gabakoro, respectively, are shown in Figure 1. After collection, mosquitoes were provided with water and transported to a nearby field laboratory (6 km away) where they were processed as described below.
Fig. 1.
Rainfall and temperature variation over months during the study period in nearest weather stations to M’Piabougou (Segou) and N’Gabakoro (Bamako). Data were obtained from the National Oceanic and Atmospheric Adminstration (NOAA: http://hurricane.ncdc.noaa.gov/pls/plclimprod/cdomain.subqueryRouter).
2.2. Mosquito processing
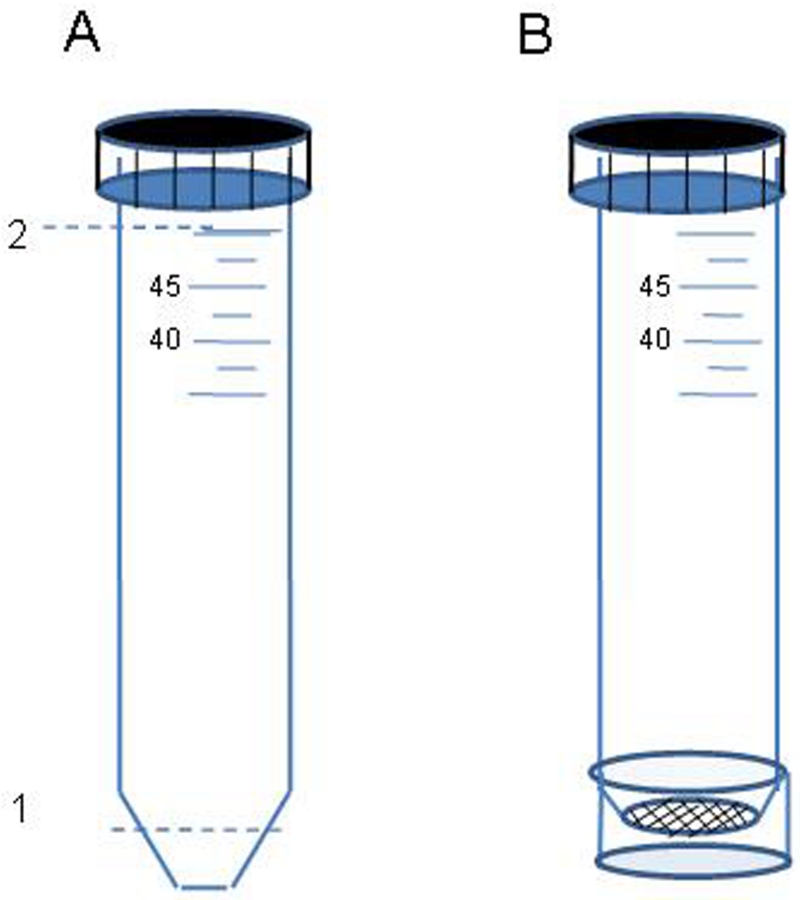
Immediately after collection, mosquitoes were provided with water and their gonotrophic status was determined as they were placed individually into modified 50 ml tubes (Fig. 2). Mosquitoes had access to water until sunset (typically around 18:00-19:00), when the water-soaked cotton balls were removed. Unfed and gravid mosquitoes were subjected to blood feeding assay (below) between 19:00 and 23:00, after which all mosquitoes received water. Gravid females were next subjected to the oviposition assay (below). Mosquitoes collected as half gravid (HG) or freshly blood-fed (fed) were held for 1-2 d in a field insectary (a typical village house with thatch and mud roof) and provided with fresh water daily until they were gravid, at which time they were subjected to the oviposition assay. After the assays, mosquitoes were preserved (below). To identify the species and molecular form, PCR and restriction-enzyme assays were conducted on 2 legs (Fanello et al., 2002). Body size was estimated using wing length (WL) as described previously (Huestis et al. 2011).
Fig. 2.
A schematic showing the modified tube used for the blood feeding (probing) assay. (A) The main 50 ml tube was cut at is tapering bottom end (1). Another tube was cut at its top (2). (B) The top segment removed from the second tube was placed over a net fitted on the lower opening, fixing the net over the opening and forming an edge which protrudes ~1 cm over the net.
2.3. Oviposition assay and insemination rate
Gravid females were individually transferred into 50ml tubes containing 15 ml water for oviposition. A strip of filter paper (2 cm in width) surrounded the water edge, providing a wet surface to collect the eggs. During the next 3 days, all tubes were examined twice daily for eggs. Once eggs were found, they were removed and counted under a dissecting microscope and the female was preserved by desiccation in tubes with silica gel. Half of the females that did not lay eggs by three days after they became gravid (and a smaller subset of those that laid eggs) were dissected to determine their insemination status and the number of developed eggs that were not laid before they were preserved in 80% ethanol. This assay yielded several indices of the female’s reproductive state: (i) oviposition response, defined by whether a female laid eggs or not, (ii) the female’s egg batch size (EBS), determined with respect to the number of eggs she laid and the number of developed eggs found during dissection, and (iii) whether a female was in a state of gonotrophic dissociation, defined for inseminated females that did not lay eggs nor develop eggs. Because females lay eggs only if they are inseminated, insemination status of females was determined by examination of the spermatheca as previously described (Dao et al., 2010) in approximately half the females that did not lay eggs (and only in a few of those that laid eggs). Because females that laid eggs were inseminated, the unbiased insemination rate was calculated as
Where:
Ov is the fraction of females that laid eggs,
NOv is the fraction of females that did not lay eggs, and
InsemNOv is the fraction of inseminated females among those that did not lay eggs.
2.4. Feeding Assay
Wild unfed or gravid females collected the morning were assayed for feeding response between 19:00 and 23:30 of the same day. Typically only unfed mosquitoes will blood feed, but it was suspected that before and during the dry season, gravid females may bite to obtain additional nutritional resources. Half gravid mosquitoes were not subjected to the test because of the greater variation in their status. The mosquito’s response to a person’s arm was determined when contained in a modified 50 ml tube (Fig. 2B). Mosquitoes either ignored the arm or landed on the screen separating it from the skin and inserted its proboscis through the net in repeated probing motions. The mosquito responded “positively” (hereafter “probed”) if she probed at least 3 times consecutively within 1-3 seconds. Otherwise, she responded negatively. The mosquito could not reach the skin because the net was raised 5-10 mm above the skin by a rim of plastic (see Fig. 2B). The tapering end of the tube was removed leaving ~3 mm “edge” of the tapered end. This edge was covered with a net which was fastened in place by a “ring” made from the top end of another 50 ml tube cut just above the 50 ml line (Fig. 2). The ring protruded 5-10mm beyond the net surface. The assay started when the tube was placed on a person’s arm and lasted for 5 minutes or until the mosquito probed.
2.5. Statistical Analysis
Despite extensive collection efforts spread over 14 days/month in the Sahel and 4 days/month in the riparian area, respectively (except March), available sample sizes were sometimes small, especially after subdividing each sample among taxa (An. arabiensis, M and S molecular forms of An. gambiae) and gonotrophic states. Analyses of seasonal variation, therefore, required pooling small samples across adjacent time periods. Seasonal periods included: October-November, reflecting the late wet season after the rains ended, but when larval sites are still available; December, the transition into the dry season, when the last larval sites desiccate and both aestivators and non-aestivators may be found; January-February, representing the early dry season; April-May, representing the late dry season; June, representing the emergence of aestivators from their hidden shelters after the first rains (Lehmann 2010; Adamou 2011); and July-August, representing the early wet season when rains are most intense. If a sample size was small (N<5) for a particular analysis, samples of the same taxon and gonotrophic state from adjacent periods were pooled together provided that there was no evidence that the samples were heterogeneous, e.g. October-November was pooled with December.
Populations were analyzed separately in most cases when the periods of sampling were not balanced (N<5 for a particular period in a particular population). Continuous traits such as egg batch size were analyzed by ANOVA or analysis of covariance (ANCOVA) using Proc GLM (SAS, 2010). Discrete traits, such as oviposition, were analyzed by loglinear models using Proc CATMOD (SAS, 2010). In either case, contrasts were used to compare the dry season with the wet season and the homogeneity across periods in the dry season.
3. Results
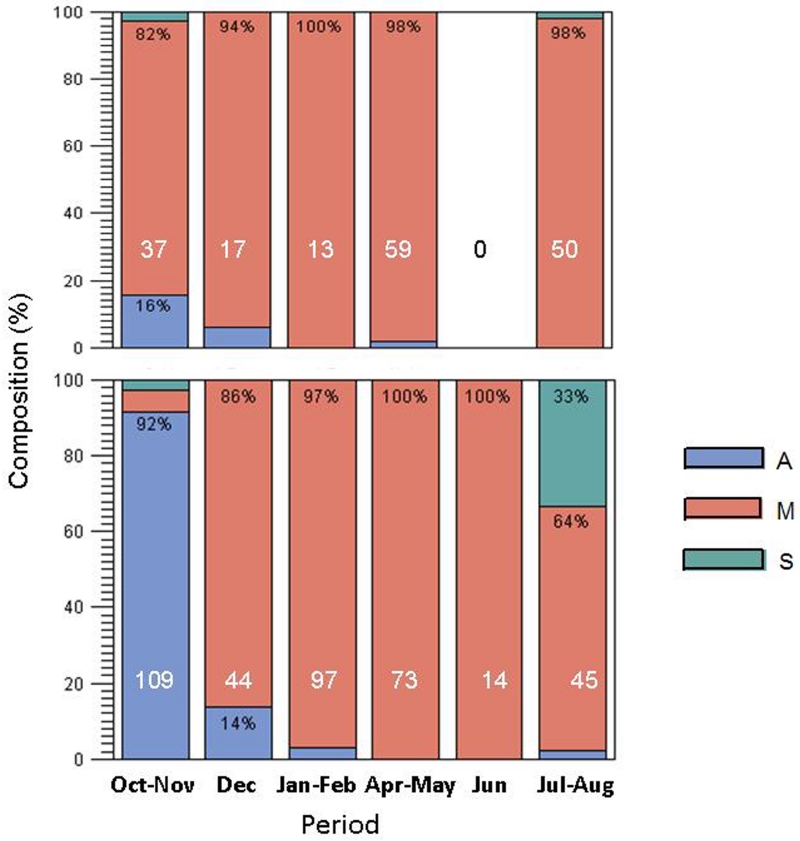
A total of 711 An. gambiae s.l. females were bioassayed, but only 559 (79%) were successfully genotyped to species and molecular form. The high failure rate was attributed to poor DNA preservation because most of the females that failed to be genotyped died in their oviposition water. The M form predominated in the dry season (December-May) and the early wet season (June-Aug) in both the Sahelian and riparian populations (Fig. 3). During the late wet season (October-November) in the Sahel, the M form was outnumbered by An. arabiensis (Fig. 3).
Fig. 3.
Composition of species and molecular form in the Sahelian (bottom) and riparian (top) populations among phenotyped mosquitoes. Sample size for each period is shown in white at the bottom of each bar and the percentage of each taxon is shown at the top in black (where space permits). Blue, pink, and green fractions represent An. arabiensis, M-form An. gambiae, and S-form An. gambiae, respectively. The riparian location was not sampled in June.
3.1. Reproductive components
Oviposition response was measured as the proportion of gravid females that laid eggs within 3 d after water was provided. Species comparisons were performed exclusively within the Sahelian population, where sufficient sample sizes of An. arabiensis were obtained during October-November and the S form during July-August. During October-November, a similar oviposition response was observed between An. arabiensis (91%) and the M form (100%; P>0.5, χ2=0.4, df=1, N=69). Likewise, similar rates of oviposition was observed during July-August between the S (73%) and the M forms (69%; P>0.7, χ2=0.1, df=1, N=44).
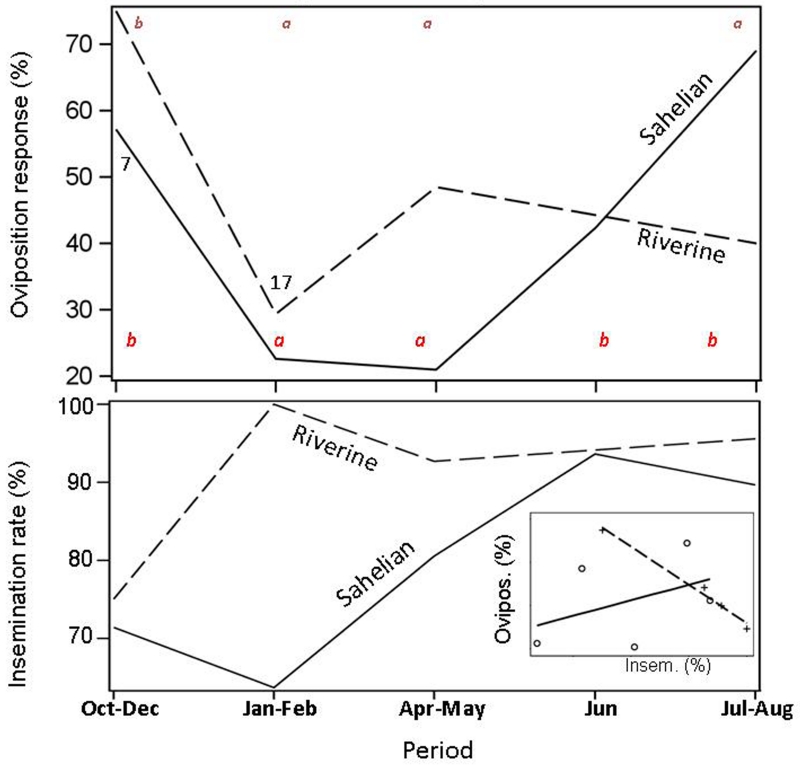
Only the M form was sufficiently represented in our samples to compare across periods (Fig. 3). A marked seasonal change in oviposition response was evident in the Sahelian population (Fig. 4, P<0.001 df=4, n=207, ML χ2 test), where it fell from 60% in October-December to 20% in January-May, before it increased to 50% in June, and then to 70% in July-August. Oviposition response during the whole dry season (January-May) was significantly lower (P<0.001) than during the periods when larval sites were found (June-December), whilst no difference (P>0.65) was found between the early and late dry seasons. Likewise, significant differences were found between the whole dry season and each of the other periods separately (P<0.04, Fig. 4). In the riparian population, less extreme seasonal variation was revealed (Fig. 4, n=140, P<0.015, df=3, ML χ2 test). Importantly, seasonal change in oviposition response in the riparian population did not follow the expected pattern of aestivation because there was no difference between the whole dry and the whole wet seasons (P>0.68). During the dry season, oviposition response in the riparian populations was lower than that of the late wet season (October-December, P<0.02) but not lower than that of the early wet season (P>0.29, July-August, Fig. 4A). The relatively low oviposition response of the riparian population in the early wet season (July-August) was possibly due to high recruitment of newly-emerged pre-gravid females, many of which require two blood meals prior to the maturation of the first egg batch (Charlwood et al., 2003; Hogg et al., 1996; Yaro et al., 2006).
Fig 4.
Seasonal variation in oviposition response (bottom panel) and unbiased insemination rate (top panel) of An. gambiae M-form females. Different letters denote significantly different means for the Sahelian (lower) and riparian (higher) populations. Sample sizes smaller than 20 are indicated below (Sahelian) and above (riparian) the lines. Inset (top panel) shows the relationship between oviposition response (Ovipos.) and unbiased insemination rate (Insem.). Sahelian values are depicted by circles and continuous line whereas the riparian population by pluses and broken line (see text for details).
Insemination status was measured in females that did not lay eggs. Although the un-adjusted insemination rate underestimated the overall insemination rate (see Methods), it was relatively high throughout the year (>65%) in the Sahelian M form (Fig. 4). Contrary to the hypothesis that a lower insemination rate contributed to the lower oviposition response, the insemination rate was higher in the dry season (82% in April-May) than in the mid rainy season (67% July-August), although the difference was not significant (P>0.28, df=4, χ2 test). Likewise, the unbiased inseminated rate (see Methods) was not correlated with oviposition rate of the Sahelian M form (Inset-Fig. 4B, r=0.42 P>0.4, n=5) and was negative in the riparian population (Inset-Fig. 4B, r=−0.9 P<0.01, n=4). Therefore, the seasonal variation in oviposition response could not be caused by variation in insemination rates.
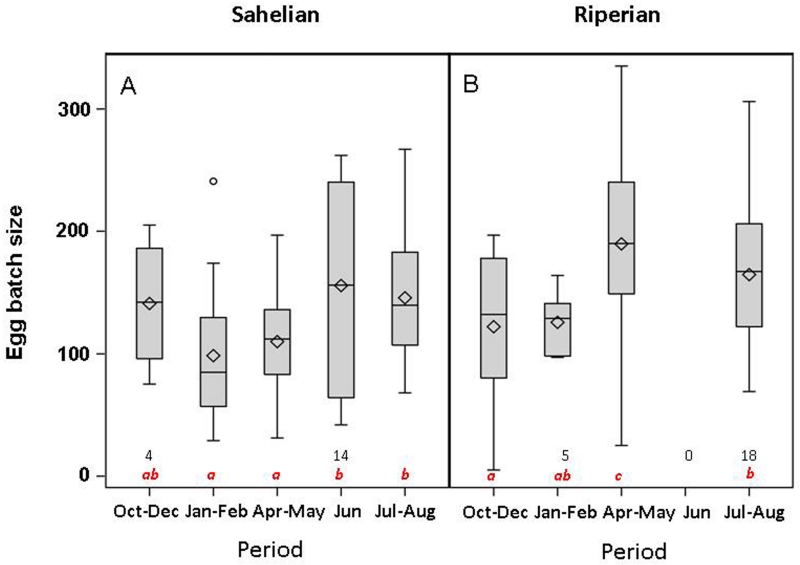
To minimize the correlation between oviposition response and egg batch size (EBS), the latter was analyzed including only those that laid eggs (69 of 207 and 71 of 140 in the Sahelian and Riparian populations, respectively). The interaction between season and body size measured as wing length (WL) was not significant (P>0.2) in both populations and was removed. The main effect of WL on EBS in the Sahelian population was significant and was kept in the model (Table 1) as was previously found in numerous studies e.g., (Charlwood et al., 2003; Dao et al., 2010; Hogg et al., 1996; Lyimo and Takken, 1993; Yaro et al., 2006). Remarkable seasonal variation was evident in EBS (P<0.001, Fig 4A) after accommodating the variation in WL. Contrasts (and least square means) revealed that the early and late dry season were not different from each other, nor from October-December, but were lower than June and July-August together and separately (Table 1). In the riparian population, on the other hand, the pattern of seasonal change in EBS did not fit the expectations under aestivation because the early dry season was lowest and the late dry season the highest (P<0.001, Fig. 5B, Table 1). EBS (adjusted to WL by ANCOVA) in the late dry season was higher than the other three periods (P<0.03, Table 1) but no significant differences were found between early dry season (January-February) and either early or late wet season (July-August and October-December, respectively). Thus, although seasonal variation in EBS was apparent in both populations, only the pattern of the Sahelian population agreed with expectations based on aestivation.
Table 1.
Seasonal variation in reproductive parameters of the M form of An. gambiae.
| Sahelian | Riparian | ||||||
|---|---|---|---|---|---|---|---|
| Dependent | Source | DF | χ2//F (MS)f | P | DF | χ2//F(MS)e | P |
| Oviposition | Intercept/Nc | 1 | 3.2 / 275 | 0.074 | 1 | 0.1 / 162 | 0.76 |
| [Catmod]a | Season | 4 | 27.83 | 0.001 | 3 | 11.69 | 0.01 |
| Cont:Dry<Wetd | 1(1) | 27.0 (18.9) | 0.001 (0.001) | 0.63 (4.4) | 0.43 (0.036) | ||
| Cont:Early<Latee | 1(1) | 0.08 (0.34) | 0.76 (0.88) | 1.95 (--) | 0.16 (--) | ||
|
| |||||||
| EBS | Model | 5 | 4.38 (11640) | 0.002 | 4 | 5.8(20555) | 0.001 |
| [GLM]b | Error | 59 | -- (2655) | -- | 69 | -- (3523) | -- |
| Season | 4 | 4.4(14409) | 0.003 | 3 | 7.4 | 0.001 | |
| WL | 1 | 4.2 (11028) | 0.046 | 1 | 7.3 | 0.009 | |
| Cont:Dry<Wetd | 1(1) | 12.1 (15.9) | 0.001(0.001) | 1(1) | 0.4 (1.03) | 0.85 (0.31) | |
| Cont:Early-Latee | 1(1) | 0.52 (1.5) | 0.47 (0.13) | 1(1) | 8.03 (--) | 0.006 (--) | |
Categorical variables were analyzed using loglinear models estimated by maximum likelihood.
Quantitative variables were analyzed using ANOVA or ANCOVA models estimated by least squares.
Denotes total sample size used in this analysis
Contrasts dry season (January-May) vs. wet season (July-August); in parenthesis: January-May vs. rest of the year. When contrast is carried out in an ANOVA, F statistic is given without mean square (MS).
Contrasts within-season variation: January-February vs. April-May; in parenthesis June vs. July-August (when applicable). When contrast is carried out in an ANOVA, F statistic is given without mean square (MS).
χ2 value given for tests using the log linear models and comparable statistics (F and MS) are given for ANOVA models.
Fig 5.
Seasonal variation in egg batch size (EBS, excluding zeros) of M-form females from the Sahelian (A) and riparian (B) populations. Different letters denote significantly different means. Numbers denote sample size if it was smaller than 20. Lines denote medians, diamonds means, boxes mark quartiles, and whiskers extend up to 1.5 times the inter-quartile range.
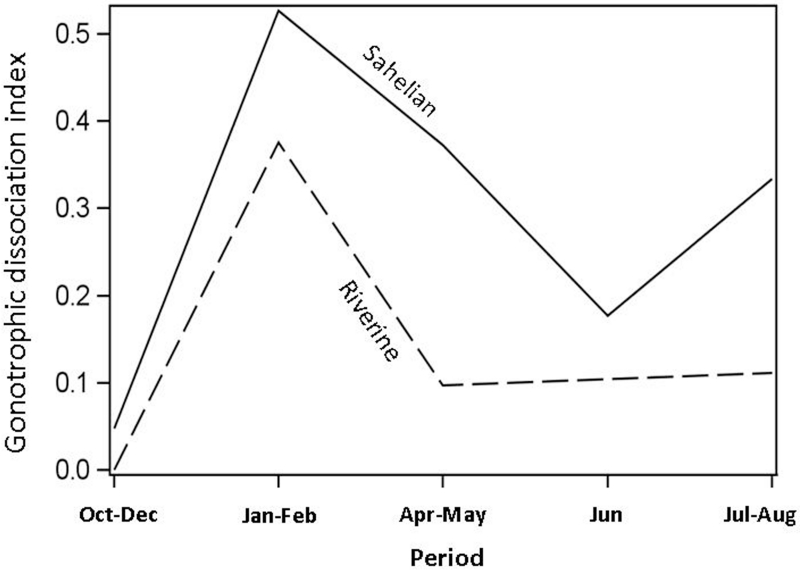
Gonotrophic dissociation was measured as the fraction of inseminated, blood-fed females that dissection revealed did not develop eggs. Marked seasonal variation in gonotrophic dissociation was found in the Sahelian M-form population (Fig.5; χ2 =17.3, df=4, P<0.002). In the early wet season, this index was probably overestimated because of the high frequency of newly-emerged females, many of which require two blood meals to develop their first egg batch (pre-gravids; (Hogg et al., 1996; Yaro et al., 2006). Although there appears to be a decline in gonotrophic dissociation between the early and late dry season (Fig. 6), the overall seasonal pattern fits the expected pattern of aestivation because the fraction of females exhibiting gonotrophic dissociation during the dry season (January-February: 53% and April-May: 37%) is higher than that of the wet season (the remaining of the year; 4-33%, Fig. 6). Thus, during the dry season in the Sahel, 35-50% of the females that did not lay eggs exhibited gonotrophic dissociation, whereas the remaining 50-65%, developed eggs but they did not lay them. In the riparian populations, gonotrophic dissociation occurred in 40% of the females in January-February, but dropped to near 10% in April-May, which is similar to the wet-season value. The differences among the three time points (excluding October-December due to small sample size, N=3) were not significant (P>0.12, χ2= 4.1712, df=2), however sample sizes were smaller than those of the Sahelian population.
Fig 6.
Seasonal variation in gonotrophic dissociation of M-form females from the Sahelian and riparian populations. Numbers denote sample size when it was smaller than 20.
3.2. Feeding response
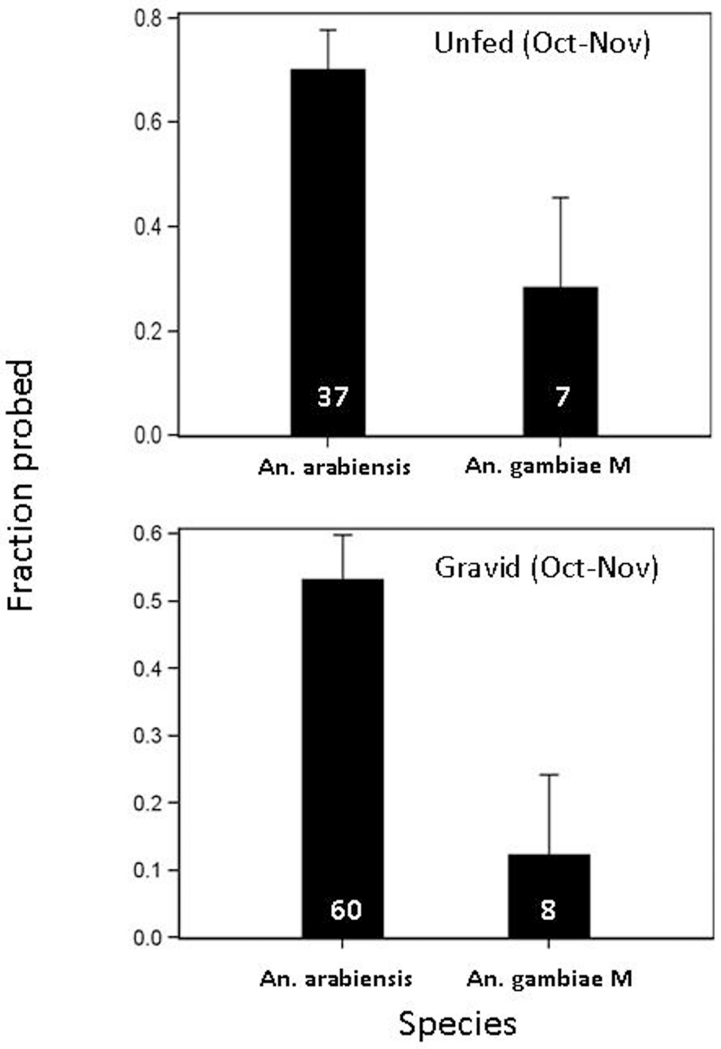
A total of 420 female mosquitoes that were subjected to the feeding response assays from October 2009 to August 2010 were successfully genotyped to species and molecular form. The S form was excluded from the analysis because of small sample size (total=19). A species comparison was performed on the Sahelian population, where most An. arabiensis were obtained during October-November (n=97 of 102, Fig. 3). During this time, the feeding response of the M form was lower than that of An. arabiensis (P<0.006, Table 2). The magnitude of the differences between species amounted to two- and three-fold between the unfed and gravid females, respectively (Fig. 7). The interaction between gonotrophic state and species was not significant (P>0.8, Table 2), nor was the main effect of gonotrophic state (P>0.07, Table 2). The lower blood feeding response of both unfed (Fig. 7A) and gravid (Fig. 7B) M-form females compared with the corresponding values of An. arabiensis may explain the scarcity of the M form indoors.
Table 2.
Variation in blood feeding response in relation to species, gonotrophic state, seasonality, and population. Log-linear categorical models (Proc Catmod, SAS) were used to assess the effects of various factors.
| Model | Source | DF/χ2 | P |
|---|---|---|---|
| Species & Gonotrophic state (Sahel: October-November) |
Species | 1/7.55 | 0.006 |
| Gono | 1/3.23 | 0.072 | |
| LR (interaction) | 1/0.05 | 0.83 | |
| Seasonality & Gono. (Sahelian M form) | Period | 4/13.1 | 0.011 |
| Gono. state | 1/1.44 | 0.23 | |
| LR (interaction) | 4/3.64 | 0.46 | |
| Seasonality & Sahel vs. river population | Period | 4/9.65 | 0.047 |
| Population | 1/0.05 | 0.82 | |
| LR (interaction) | 4/6.19 | 0.18 |
Fig. 7.
Blood feeding response (probing towards human skin) of Sahelian M form An. gambiae and An. arabiensis measured in October-November. The fraction of mosquitoes that responded (probed) within 5 min is shown. Standard error (line) for proportions was calculated based on the binomial distribution. Sample size is listed inside bars.
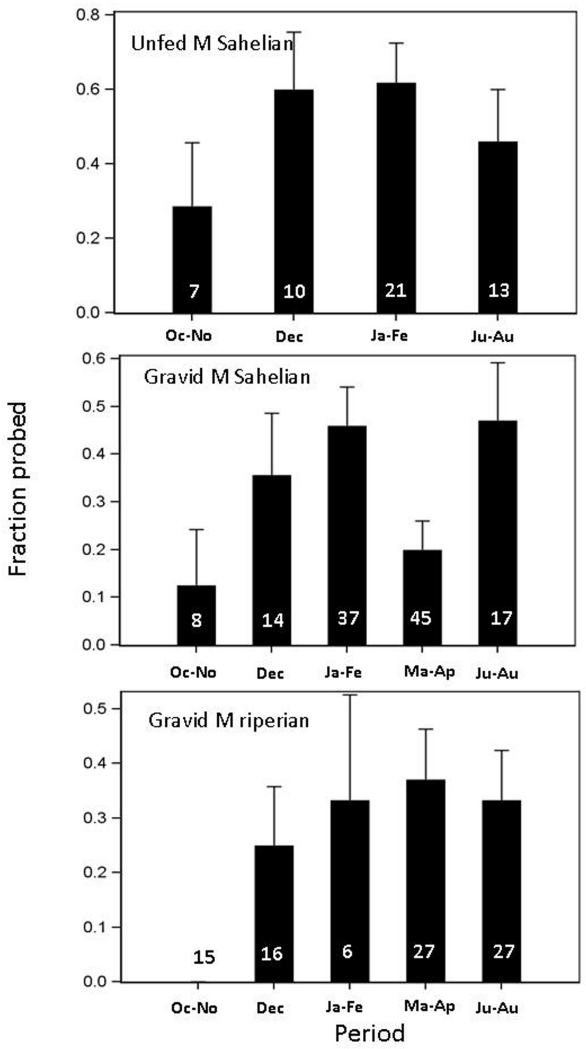
Seasonal variation in the feeding response of Sahelian M form was detected across both gonotrophic states (P<0.011, Table 2), with the lowest blood feeding response in October-November (Fig. 8). In the wet season, blood feeding response appeared to be intermediate in unfed females and high in gravid females. Comparing the seasonal variation of the M form between Sahelian and riparian populations was only possible for gravid females because unfed females were too scarce in the riparian population. A significant seasonal pattern was detected across populations (P<0.047, Table 2; Fig. 8), and no difference was detected between the Sahelian and riparian populations. Further, the interaction between season and population was not significant (Table 2).
Fig. 8.
Seasonal variation in blood feeding response (as in Fig. 7) measured in Sahelian and riparian M-form An. gambiae. Note differences in sampling periods between populations. The fraction of mosquitoes that responded (probed) within 5 min is shown. Standard error (line) for proportions was calculated based on the binomial distribution. Sample size is listed inside bars.
4. Discussion
Despite their important implications for malaria control, the dry season ecology, physiology, and behavior of African anophelines are poorly understood. More than resolving the old debate, the recent evidence that aestivation is key to the persistence of the M form in arid environments (Adamou et al., 2011; Lehmann et al., 2010) highlighted unstudied facets of anopheline biology. Here we evaluated seasonal variation in reproductive physiology and blood feeding behavior of Sahelian and riparian populations of An. gambiae. Depressed reproduction is the most fundamental feature of diapause of adult insects (Denlinger, 1986; Schmidt and Paaby, 2008; Tatar and Yin, 2001; Tauber et al., 1986). Our results revealed marked seasonality in the reproductive physiology of the Sahelian M form that closely fits the aestivation predictions. During the dry season, oviposition response dropped more than threefold and EBS (among those that laid eggs) dropped to nearly half its wet-season value. These seasonal changes could not be attributed to insemination rate or to availability of a blood meal. Additionally, gonotrophic dissociation peaked during the early dry season and remained relatively high until the first rains, similar to An. arabiensis in the Sudan (Omer and Cloudsley-Thompson, 1970). These results support the hypothesis that the M form An. gambiae persists throughout the long dry season in the Sahel by diverting resources from reproduction and allocating them toward survival.
Seasonal variation in reproduction was also detected in the riparian population; however, this variation was weaker and did not fit the above predictions as well (Figs. 3-5, Tables 1 and 2). Both populations showed depressed reproduction during the early dry season (January-February, Figs. 3-5, Tables 1 and 2), but unlike the Sahelian population, the riparian population increased its reproductive output during the late dry season (April-May), as if the dry season in that area ends earlier. Although the riparian population is only 130 km SSW (~115 km S), the first rain arrives a few weeks earlier than at the Sahelian population (Figure 1). Moreover, the frequency of the light March rains typically falling every other year (“Mango rains”) is greater in the riparian area. Sometimes, these rains produce larval sites in stream beds, especially near the watershed’s ultimate artery, the Niger River, effectively shortening the dry season. The riparian M form population, thus, reacts to the general climatic conditions of shorter dry season and higher prospects for breeding rather than to the continued breeding opportunities dotting the edge of the Niger where larval sites can be found during the dry season (~30 m wide on either bank). Accordingly, the M-form An. gambiae in this dry savannah responds to the regional seasonal conditions rather than to the presence of larval sites in the confined local scale. Depressed reproductive activity in most females of the M form during the dry season even along the Niger River explains the low density of An. gambiae despite availability of larval sites (Lehmann et al., 2010; Toure et al., 1994; Toure et al., 1996). However, further (~60 km) south, mosquito populations inhabiting fishermen communities appear to respond primarily to the presence or absence of larval sites (Sogoba et al., 2007). This variation between populations may reflect local adaptive variation in aestivation phenotypes based on the expected length and severity of the dry season in each locale. Additional studies are required to further test this hypothesis.
Throughout the dry season, the Sahelian M-form exhibited several phenotypes. Provided with water for oviposition after blood feeding, some did not develop eggs (gonotrophic dissociation), others developed eggs but did not lay them, while others developed and laid eggs. Such heterogeneity may represent different strategies of aestivation with respect to its strength and duration. Heterogeneity along similar lines was noted in overwintering Cu. pipiens, Cu. tarsalis, and Cu. tritaeniorhynchus (Reisen et al., 2010; Spielman, 2001; Spielman and Wong, 1973; Tsuda and Kim, 2008), suggesting that several strategies coexist within populations. Possibly, “weak aestivators” develop eggs after blood feeding and will lay them if they are near water, whilst “strong aestivators” will not develop eggs before the “proper” termination of the dry season. The females that developed eggs but did not lay them without an additional “signal” may represent “intermediate aestivators,” although they may resorb their eggs and function as strong aestivators. Our riparian population may, therefore, consist of a larger fraction of weak (and intermediate) aestivators than the Sahelian population, explaining the shallower seasonal difference in overall reproductive parameters as well as the earlier “termination” of their reproductive depression/arrest. This perspective suggests that in some (weaker) aestivators, reproductive depression fades towards the end of the dry season, even before the first rain.
Seasonal variation in blood feeding was detected in unfed and gravid females of the M-form from both the Sahelian and riparian populations. The low blood feeding response in late October-November coincides with the apparent disappearance of the M form (Adamou et al., 2011; Lehmann et al., 2010) from indoor collections. Presumably these mosquitoes fly to unknown shelters and feed on plants (Muller et al., 2010) as proposed by (Huestis et al., 2011). On the other hand, the relative scarcity of unfed females during the dry season suggests that those females which do enter houses are highly intent on blood feeding. The blood feeding response was relatively stable and high during the rest of the year, suggesting that blood feeding does not decrease during aestivation. However, it is possible that the females found indoors during the dry season consist of weak aestivators (above), and thus convey a biased view of the population, the majority of is presumed to remain in their shelters and blood feed infrequently (Adamou et al., 2011; Lehmann et al., 2010). If correct, we may have underestimated the reproductive arrest of the population because all our females were collected indoors. Until we find and assay mosquitoes collected directly from their unknown shelters, this hypothesis cannot be tested.
The insemination rate in the Sahelian M-form varied over the year between 64% (January-February) to 94% (June). The increase in insemination rate during the dry season is not surprising because M-form males were found swarming throughout the dry season, albeit in small swarms (Yaro et al.: unpublished data). It appears that some females enter aestivation before they are inseminated. However, it is also possible that sperm may not survive in the female spermatheca over several months of aestivation and need to be replenished. The presence of males throughout the dry season is, to our knowledge, a unique feature of the aestivation of this mosquito species. Moreover, the continuation of their reproductive activity, i.e., swarming, albeit in much smaller swarms, may contradict the expectations of diapause. This probably reflects that unlike inhospitable conditions for activity (extreme temperatures, salinity, etc.) or scarcity of food (Muller et al., 2010), the underlying cause of this aestivation is merely the absence of surface water for larval sites, as is evident from the non-diapausing Sahelian M-form populations in large irrigated areas, e.g. Niono (Carnevale et al., 1999; Diabate et al., 2005; Diuk-Wasser et al., 2005; Dolo et al., 2004). This causal difference may have additional unique consequences for male and female aestivation strategies. In contrast to numerous studies on diapause of overwintering mosquitoes, aestivation has been scarcely studied. It is believed that the key phenotypes linked with winter diapause such as depressed reproduction and desiccation tolerance are also found in aestivation (Benoit, 2010). However, the Sahelian dry season poses unique constraints on diapausing mosquitoes. For example, the relatively high temperatures during aestivation (as oppose to those prevailing in the temperate winter) are not conducive for reduced metabolism and extended longevity; thus, aestivating mosquitoes may have evolved unique physiological and behavioral adaptations. Elucidating these adaptations and their implications for disease transmission and control are among the new frontiers in malariology.
Highlights.
-
▶
Reproductive activity of the M form Anopheles gambiae in the Sahel is suppressed during the dry season consistent with aestivation
-
▶
During the dry season oviposition rate and egg batch size decreased whereas the index of gonotrophic dissociation increased
-
▶
Insemination rate and blood feeding rate did not vary with season
-
▶
Seasonal variation in a riparian population was weaker and did not extend through the late dry season
-
▶
The variation between the Sahelian and riparian populations suggests a cline in aestivation based on the prospects to resume reproduction.
Acknowledgements
We are grateful to the residents of M’Piabougou, N’Gabakoro Droit and Thierola who accommodated our studies and went out of the way in their hospitality. We thank Drs. Martin Donnelly, Dia Elnaiem, Peter Billingsley, and Robert Gwadz for valuable suggestions on earlier versions of this manuscript. This study was supported by the Intramural Research Program in NIH, NIAID.
References
- Adamou A, Dao A, Timbine S, Kassogue Y, Yaro AS, Diallo M, Traore SF, Huestis DL, Lehmann T. The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar J. 2011;10:151. doi: 10.1186/1475-2875-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoh MN, Thomas CJ, Lindsay SW. Mapping distributions of chromosomal forms of Anopheles gambiae in West Africa using climate data. Med Vet Entomol. 2001;15:267–274. doi: 10.1046/j.0269-283x.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- Beier JC, Copeland RS, Oyaro C, Masinya A, Odago WO, Odour S, Koech DK, Roberts CR. Anopheles gambiae complex egg stage survival in dry soil from larval development sites in western Kenya. J. Am. Mosq. Control Assoc. 1990;6:105–109. [PubMed] [Google Scholar]
- Benoit JB. Water management by dormant insects: comparisons between dehydration resistance during summer aestivation and winter diapause. Progress in molecular and subcellular biology. 2010;49:209–229. doi: 10.1007/978-3-642-02421-4_10. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Denlinger DL. Suppression of water loss during adult diapause in the northern house mosquito, Culex pipiens. J Exp Biol. 2007;210:217–226. doi: 10.1242/jeb.02630. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Interaction between photoperiod, temperature, and chilling in dormant larvae of the tree-hole mosquito, Toxorhynchites rutilus Coq. Biol Bull. 1977;152:147–158. doi: 10.2307/1540555. [DOI] [PubMed] [Google Scholar]
- Carnevale P, Guillet P, Robert V, Fontenille D, Doannio J, Coosemans M, Mouchet J. Diversity of malaria in rice growing areas of the Afrotropical region. Parassitologia. 1999;41:273–276. [PubMed] [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Ferreira C, Petrarca V, Rosario VD. ’A mate or a meal′ - Pre-gravid behaviour of female Anopheles gambiae from the islands of Sao Tome and Principe, West Africa. Malaria Journal. 2003;2 doi: 10.1186/1475-2875-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD, Vij R, Billingsley PF. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am.J.Trop.Med.Hyg. 2000;62:726–732. doi: 10.4269/ajtmh.2000.62.726. [DOI] [PubMed] [Google Scholar]
- Coetzee M, Craig M, le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol.Today. 2000;16:74–77. doi: 10.1016/s0169-4758(99)01563-x. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Morphological Divergences in the Anopheles Gambiae Complex. Riv Malariol. 1964;43:197–232. [PubMed] [Google Scholar]
- Coluzzi M. Malaria vector analysis and control. Parasitology Today. 1992;8:113–118. doi: 10.1016/0169-4758(92)90277-9. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Advances in the study of Afrotropical malaria vectors. Parassitologia. 1993;35(Suppl):23–29. [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans.R.Soc.Trop.Med.Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Petrarca V, Di Deco MA. Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae Boll. Zool. 1985;52:45–63. [Google Scholar]
- Danks HV. How aquatic insects live in cold climates. Canadian Entomologist. 2007;139:443–471. [Google Scholar]
- Dao A, Kassogue Y, Adamou A, Diallo M, Yaro AS, Traore SF, Lehmann T. Reproduction-longevity trade-off in Anopheles gambiae (Diptera: Culicidae) Journal of Medical Entomology. 2010;47:769–777. doi: 10.1603/me10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G. Anopheles gambiae, a complex of species. Bull.World Health Organ. 1964;31:625–634. [PMC free article] [PubMed] [Google Scholar]
- della Torre A, Fanello C, Akogbeto M, Dossou-yovo J, Favia G, Petrarca V, Coluzzi M. Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol. Biol. 2001;10:9–18. doi: 10.1046/j.1365-2583.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- Denlinger DL. Dormancy in tropical insects. Annual Review of Entomology. 1986;31:239–264. doi: 10.1146/annurev.en.31.010186.001323. [DOI] [PubMed] [Google Scholar]
- Denlinger DL. Regulation of diapause. Annual Review of Entomology. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- Diabate A, Dabire RK, Kim EH, Dalton R, Millogo N, Baldet T, Simard F, Gimnig JE, Hawley WA, Lehmann T. Larval development of the molecular forms of Anopheles gambiae (Diptera: Culicidae) in different habitats: a transplantation experiment. J Med Entomol. 2005;42:548–553. doi: 10.1093/jmedent/42.4.548. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser M, Toure MB, Dolo G, Bagayoko M, Sogoba N, Traore SF, Manoukis N, Taylor CE. Vector abundance and malaria transmission in rice growing villages in Mali. Am. J. Trop. Med. Hyg. 2005;72:725–731. [PMC free article] [PubMed] [Google Scholar]
- Dolo G, Briet OJT, Dao A, Traore S, Bouare M, Sogoba N, Niare O, Bagayogo M, Sangare D, Teuscher T, Toure YT. Malaria transmission in relation to the rice cultivation in the irrigated Sahel of Mali, West Africa. Acta Tropica. 2004;89:147–159. doi: 10.1016/j.actatropica.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Donnelly MJ, Simard F, Lehmann T. Evolutionary studies of malaria vectors. Trends Parasitol. 2002;18:75–80. doi: 10.1016/s1471-4922(01)02198-5. [DOI] [PubMed] [Google Scholar]
- Fanello C, Santolamazza F, della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–464. doi: 10.1046/j.1365-2915.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- Favia G, Lanfrancotti A, Spanos L, Siden-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol. Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- Gentile G, Slotman M, Ketmaier V, Powell JR, Caccone A. Attempts to molecularly distinguish cryptic taxa in Anopheles gambiae s.s. Insect Mol. Biol. 2001;10:25–32. doi: 10.1046/j.1365-2583.2001.00237.x. [DOI] [PubMed] [Google Scholar]
- Gillies MT. Studies on the dispersion and survival of Anopheles gambiae in East Africa, by means of marking and release experiments. Bull.Entomol.Res. 1961;52:99–127. [Google Scholar]
- Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara. 2 edn. South African Institute for Medical Research; Johannesburg, South Africa: 1968. [Google Scholar]
- Gillies MT, Wilkes TJ. A study of the age-composition of populations of Anopheles gambiae Giles and A. funestus Giles in North-Easter Tanzania. Bull Entomol Res. 1965;56:237–262. doi: 10.1017/s0007485300056339. [DOI] [PubMed] [Google Scholar]
- Gray EM, Rocca KA, Costantini C, Besansky NJ. Inversion 2La is associated with enhanced desiccation resistance in Anopheles gambiae. Malaria Journal. 2009;8 doi: 10.1186/1475-2875-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA, Pumpuni CB, Brady RH, Craig GB., Jr. Overwintering survival of Aedes albopictus (Diptera: Culicidae) eggs in Indiana. J Med Entomol. 1989;26:122–129. doi: 10.1093/jmedent/26.2.122. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Thomson MC, Hurd H. Comparative fecundity and associated factors for two sibling species of the Anopheles gambiae complex occurring sympatrically in The Gambia. Med.Vet.Entomol. 1996;10:385–391. doi: 10.1111/j.1365-2915.1996.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Holstein MH. Biology of Anopheles gambiae. Research in French West Africa. World Health Organization; Geneva: 1954. [Google Scholar]
- Huestis DL, Marshall JL. Interaction between maternal effects and temperature affects diapause occurrence in the cricket Allonemobius socius. Oecologia. 2006;146:513–520. doi: 10.1007/s00442-005-0232-z. [DOI] [PubMed] [Google Scholar]
- Huestis DL, Yaro AS, Traore AI, Adamou A, Kassogue Y, Diallo M, Timbine S, Dao A, Lehmann T. Variation in metabolic rate of Anopheles gambiae and A. arabiensis in a Sahelian village. J Exp Biol. 2011;214:2345–2353. doi: 10.1242/jeb.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawara M, Pinder M, Drakeley CJ, Nwakanma DC, Jallow E, Bogh C, Lindsay SW, Conway DJ. Dry season ecology of Anopheles gambiae complex mosquitoes in The Gambia. Malar J. 2008;7:156. doi: 10.1186/1475-2875-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenraadt CJ, Githeko AK, Takken W. The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s. and Anopheles arabiensis in a Kenyan village. Acta Trop. 2004;90:141–153. doi: 10.1016/j.actatropica.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kostal V. Eco-physiological phases of insect diapause. Journal of Insect Physiology. 2006;52:113–127. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Krzywinski J, Besansky NJ. Molecular systematics of Anopheles: from subgenera to subpopulations. Annu Rev Entomol. 2003;48:111–139. doi: 10.1146/annurev.ento.48.091801.112647. [DOI] [PubMed] [Google Scholar]
- Lee Y, Meneses CR, Fofana A, Lanzaro GC. Desiccation resistance among subpopulations of anopheles gambiae s.s. from selinkenyi, Mali. Journal of Medical Entomology. 2009;46:316–320. doi: 10.1603/033.046.0216. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Dao A, Yaro AS, Adamou A, Kassogue Y, Diallo M, Sekou T, Coscaron-Arias C. Aestivation of the African Malaria Mosquito, Anopheles gambiae in the Sahel. American Journal of Tropical Medicine and Hygiene. 2010;83:601–606. doi: 10.4269/ajtmh.2010.09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, Diabate A. The molecular forms of Anopheles gambiae: A phenotypic perspective. Infection Genetics and Evolution. 2008;8:737–746. doi: 10.1016/j.meegid.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, Hawley WA, Grebert H, Collins FH. The effective population size of Anopheles gambiae in Kenya: implications for population structure. Mol.Biol.Evol. 1998;15:264–276. doi: 10.1093/oxfordjournals.molbev.a025923. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Licht M, Elissa N, Maega BT, Chimumbwa JM, Watsenga FT, Wondji CS, Simard F, Hawley WA. Population Structure of Anopheles gambiae in Africa. J Hered. 2003;94:133–147. doi: 10.1093/jhered/esg024. [DOI] [PubMed] [Google Scholar]
- Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Highland malaria in Uganda: prospective analysis of an epidemic associated with El Nino. Trans.R.Soc.Trop.Med.Hyg. 1999;93:480–487. doi: 10.1016/s0035-9203(99)90344-9. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Parson L, Thomas CJ. Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proc.R.Soc.Lond B Biol.Sci. 1998;265:847–854. doi: 10.1098/rspb.1998.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyimo EO, Takken W. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Med.Vet.Entomol. 1993;7:328–332. doi: 10.1111/j.1365-2915.1993.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Githure JI, Beier JC, Yan G. Anopheline mosquito survival strategies during the dry period in western Kenya. J.Med.Entomol. 2001;38:388–392. doi: 10.1603/0022-2585-38.3.388. [DOI] [PubMed] [Google Scholar]
- Mukabayire O, Caridi J, Wang X, Toure YT, Coluzzi M, Besansky NJ. Patterns of DNA sequence variation in chromosomally recognized taxa of Anopheles gambiae: evidence from rDNA and single-copy loci. Insect Mol.Biol. 2001;10:33–46. doi: 10.1046/j.1365-2583.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- Muller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J. 2010;9:262. doi: 10.1186/1475-2875-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer SM, Cloudsley-Thompson JL. Survival of female Anopheles gambiae Giles through a 9-month dry season in Sudan. Bull.World Health Organ. 1970;42:319–330. [PMC free article] [PubMed] [Google Scholar]
- Omer SM, Cloudsley-Thomson JL. Dry season biology of Anopheles gambiae Giles in the Sudan. Nature. 1968;217:879–880. [Google Scholar]
- Ramsdale CD, Fontaine RE. Ecological investigations of Anopheles gambiae and Anopheles funestus II. Dry season studies with colony-reared A. gambiae species B, Kaduna Nigeria. 1970a. pp. 1–8. WHO/VBC/70.249. [Google Scholar]
- Ramsdale CD, Fontaine RE. Ecological Investigations onf Anopheles gambiae and Anopheles funestus I. Dry season studies in villages near Kaduna Nigeria. 1970b. WHO/VBC/70.248. [Google Scholar]
- Reisen WK, Thiemann T, Barker CM, Lu H, Carroll B, Fang Y, Lothrop HD. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. J Med Entomol. 2010;47:230–237. doi: 10.1603/me09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Seulu F, Abose T, Kidane G, Teklehaimanot A. Temporal and spatial distribution of anopheline mosquitos in an Ethiopian village: implications for malaria control strategies. Bull.World Health Organ. 1996;74:299–305. [PMC free article] [PubMed] [Google Scholar]
- SAS, I., Inc. SAS for Windows. 1 ed. Sas Institute; Cary, NC: 2010. Version 9.2. [Google Scholar]
- Schmidt PS, Paaby AB. Reproductive diapause and life-history clines in North American populations of Drosophila melanogaster. Evolution. 2008;62:1204–1215. doi: 10.1111/j.1558-5646.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- Simard F, Lehmann T, Lemasson JJ, Diatta M, Fontenille D. Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Mol.Biol. 2000;9:467–479. doi: 10.1046/j.1365-2583.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Sogoba N, Doumbia S, Vounatsou P, Baber I, Keita M, Maiga M, Traore SF, Toure A, Dolo G, Smith T, Ribeiro JM. Monitoring of larval habitats and mosquito densities in the Sudan savanna of Mali: implications for malaria vector control. Am J Trop Med Hyg. 2007;77:82–88. [PubMed] [Google Scholar]
- Spielman A. Structure and seasonality of nearctic Culex pipiens populations. Ann N Y Acad Sci. 2001;951:220–234. doi: 10.1111/j.1749-6632.2001.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Spielman A, Wong J. Studies on autogeny in natural populations of Culex pipiens. 3. Midsummer preparation for hibernation in anautogenous populations. J Med Entomol. 1973;10:319–324. doi: 10.1093/jmedent/10.4.319. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford University Press; Oxford: 1992. [Google Scholar]
- Tatar M, Yin CM. Slow aging during insect reproductive diapause: Why butterflies, grasshoppers and flies are like worms. Experimental Gerontology. 2001;36:723–738. doi: 10.1016/s0531-5565(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Tauber MJ, Tauber CA. INSECT SEASONALITY - DIAPAUSE MAINTENANCE, TERMINATION, AND POSTDIAPAUSE DEVELOPMENT. Annual Review of Entomology. 1976;21:81–107. [Google Scholar]
- Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. Oxford University Press; New York: 1986. [Google Scholar]
- Taylor CE, Toure YT, Coluzzi M, Petrarca V. Effective population size and persistence of Anopheles arabiensis during the dry season in west Africa. Med.Vet.Entomol. 1993;7:351–357. doi: 10.1111/j.1365-2915.1993.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Touré YT, Petrarca V, Traoré SF, Coulibaly A, Maiga HM, Sankaré O, Sow M, Di Deco MA, Coluzzi M. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parasitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M. Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s.s. in Mali, West Africa. GENETICA. 1994;94:213–223. doi: 10.1007/BF01443435. [DOI] [PubMed] [Google Scholar]
- Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- Toure YT, Traore SF, Sankare O, Sow MY, Coulibaly A, Esposito F, Petrarca V. Perennial transmission of malaria by the Anopheles gambiae complex in a north Sudan Savanna area of Mali. Med Vet Entomol. 1996;10:197–199. doi: 10.1111/j.1365-2915.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Kim KS. Sudden autumnal appearance of adult Culex tritaeniorhynchus (Diptera: Culicidae) at a park in urban Tokyo: first field evidence for prediapause migration. J Med Entomol. 2008;45:610–616. doi: 10.1603/0022-2585(2008)45[610:saaoac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Turner TL, Hahn MW, Nuzhdin SV. Genomic islands of speciation in Anopheles gambiae. PLoS Biol. 2005;3:e285. doi: 10.1371/journal.pbio.0030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washino RK. The physiological ecology of gonotrophic dissociation and related phenomena in mosquitoes. Journal of Medical Entomology. 1977;13:381–388. doi: 10.1093/jmedent/13.4-5.381. [DOI] [PubMed] [Google Scholar]
- WHO . World malaria report 2008. 2008. [Google Scholar]
- Yaro AS, Dao A, Adamou A, Crawford JE, Traore SF, Toure AM, Gwadz R, Lehmann T. Reproductive output of female Anopheles gambiae (Diptera: Culicidae): comparison of molecular forms. J Med Entomol. 2006;43:833–839. doi: 10.1603/0022-2585(2006)43[833:roofag]2.0.co;2. [DOI] [PubMed] [Google Scholar]