Abstract
Multiple epidemiologic studies have documented an association between the anti-diabetic agent metformin and reduced cancer incidence and mortality. However, this effect has not been consistently demonstrated in animal models or more recent epidemiological studies. The purpose of this paper is to examine metformin's chemopreventive potential by reviewing relevant mechanisms of action, preclinical evidence of efficacy, updated epidemiologic evidence after correction for potential biases and confounders, and recently completed and ongoing clinical trials. Although repurposing drugs with well described mechanisms of action and safety profiles is an appealing strategy for cancer prevention, there is no substitute for well executed late phase clinical trials to define efficacy and populations that are most likely to benefit from an intervention.
Introduction
There is intense interest in the cancer prevention research community regarding the potential of metformin, the front-line oral treatment for type II diabetes (T2D), to prevent a wide variety of cancers. The increased risk of cancer in hyper-insulinemic states such as metabolic syndrome or T2D is well recognized 1. Meta-analyses have estimated relative risks (RR) of 1.1-2.5 for cancer risk at various organ sites in patients with T2D 2. These sites include liver, breast, bladder, endometrium, pancreas, colorectum, kidney, and non-Hodgkin lymphomas, among others. Metformin (1,1-dimethylbiguanide) is a cheap generic drug with a well understood safety profile and track record of tolerability that has been shown to decrease the progression from prediabetes to overt diabetes 3. Numerous epidemiologic studies and meta-analyses have documented an association between metformin use and reduced cancer incidence and mortality (fully discussed below), making metformin an ideal candidate for drug re-purposing. However, recent recognition of time-related biases as a major potential source of error in some epidemiologic studies calls for a re-evaluation of the literature linking metformin use to reduced cancer incidence 4, 5. In this paper we examine the current state of understanding of the potential of metformin use to reduce cancer incidence and mortality, from putative mechanisms of action through the limited data on current and ongoing clinical trials.
Molecular mechanisms of metformin in cancer and diabetes
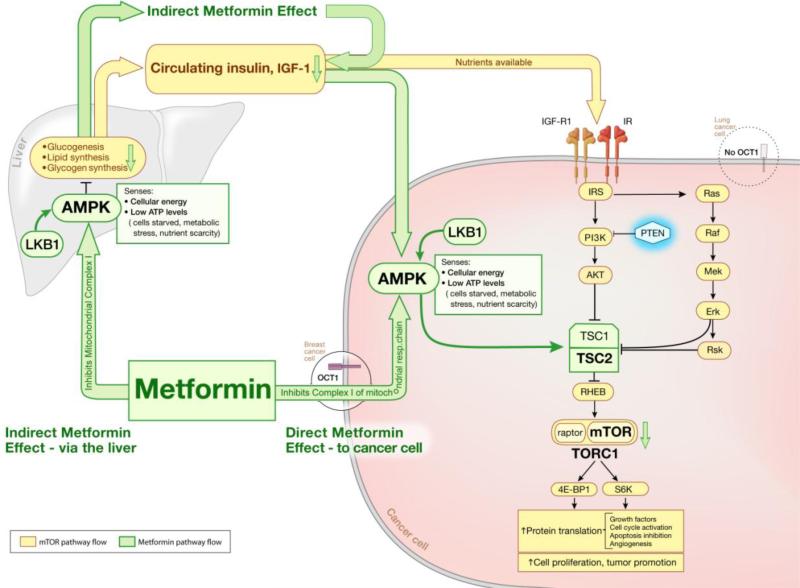
Despite its long history of clinical use in the diabetes setting, the precise mechanisms underlying the anti-hyperglycemic and anti-hyperinsulinemic effects, as well as the anti-cancer properties, of metformin are still incompletely understood. Two basic routes of metformin action have potential to contribute to its anti-neoplastic activity (Figure 1)6, 7: (1) an indirect route involving “endocrine-type effects” related to its insulin-lowering activity which may slow tumor proliferation in hyperinsulinemic patients; and (2) direct actions in target cells resulting from its suppression of ATP production due to its inhibition of mitochondrial complex I 8. Both routes of metformin activity converge on the mTOR (mammalian target of rapamycin) pathway resulting in inhibition of its pro-proliferative activity. An important caveat to understanding the anti-proliferative effects of metformin is that they are context-dependent. As seen below, “context” here refers to the nutritional, energetic/oxidative state of the cell as well as to pharmacokinetic factors related to individual cell types 8, 9.
Figure 1.
Two basic routes of metformin action have potential to contribute to its anti-neoplastic activity. (1) The indirect route involves “endocrine-type effects” related to its insulin-lowering activity (left arrow from “Metformin” box); this action may slow tumor proliferation in hyperinsulinemic patients. (2) Direct actions in target cells result from metformin's suppression of ATP production due to its inhibition of mitochondrial complex I (right arrow from “Metformin” box). Both routes of metformin activity converge on the mTOR (mammalian target of rapamycin) pathway resulting in inhibition of mTOR's pro-proliferative activity.
Inhibition of the mTOR pathway
Key components of the mTOR pathway include LKB1 (serine-threonine liver kinase B1), AMPK (5’-adenosine monophosphate-activated protein kinase), TSC (tuberous sclerosis complex/tuberin), and mTOR (Figure 1), whereas upstream activators of mTOR include PI3K (phosphatidyl inositol-3-kinase) and AKT (protein kinase B). In the setting of nutrient availability, growth factors such as insulin activate PI3K which, in turn, activates AKT which then phosphorylates and inactivates TSC2, counteracting the inhibitory effect that this complex has on mTOR. Thus, when amino acids and glucose are available, mTOR activation occurs. The mTOR molecule functions as part of a larger complex, mTORC1 (mTOR complex 1), which has several other components. These regulate mTOR's response to nutrients as well as to known mTOR inhibitors, such as rapamycin 10. Together the players in mTOR activation constitute a linear pathway that is responsive to activation by growth factors 10, promoting protein translation in part and leading to cell growth and proliferation, all in a setting of nutrient and energy availability (Figure 1).
Inhibition of mTOR activation takes place at several points along this linear pathway. The PTEN (phosphatase and tensin homolog) tumor suppressor protein restrains mTOR activity upstream by counteracting PI3K activity (Figure 1) 10. Further downstream activation of AMPK serves a major role in mTOR inhibition 11. As a central cellular energy sensor and the key player in metformin-mediated direct inhibition of the mTOR pathway 6, 11, AMPK is activated by metformin and then phosphorylates and activates the mTOR inhibitor, TSC2. AMPK activation is dependent on phosphorylation of its catalytic subunit by its upstream activator, LKB17, 12, which is encoded by a tumor suppressor gene that is mutated in many nonsmall cell lung cancers and in the germline of cancer-prone patients with Peutz-Jeghers syndrome (Figure 1) 12-14. Although LKB1 is required for this activation of AMPK by metformin, the LKB1 molecule itself is not the direct target of these drugs 12, 15. The LKB1/AMPK system functions as a sensor of ATP levels 9. Furthermore, this LKB1/AMPK/TSC2 pathway is only one route by which metformin exerts its inhibitory effect on mTOR. In the absence of functional TSC2, metformin-activated AMPK can still inhibit mTOR/mTORC1 by directly phosphorylating the raptor component of the mTORC1 complex 6, 16. In addition, metformin has also been shown to inhibit mTORC1 in an AMPK-independent manner 17, 18.
In the indirect route of inhibiting neoplastic progression (referred to as route (1) above), pro-growth processes involving the insulin/insulin-like growth factor-1 (IGF-1) pathway are activated in a setting of nutrient availability 6. In normal cells, following binding to its ligand, the IGF-1 receptor (IGF-1R) is phosphorylated, leading to a series of downstream phosphorylations that activate (a) the PI3K/Akt/mTOR and (b) the RAS/RAF/mitogen-activated protein kinase (MAPK) pathways (Figure 1). By activating both pathways, IGF-1/IGF-1R activation, stimulated by circulating insulin, contributes to increased cell growth and proliferation, ultimately pro-carcinogenic processes. Metformin acts on this pathway by suppressing IGF-1 signaling via inhibition of phosphorylation of IGF-1R. This decreases hepatic glucose output, increases muscle uptake of glucose, and reduces plasma insulin levels, with a concomitant antiproliferative effect that is “indirect” in relation to the ultimate cellular target, the cancer cell.
The second scenario, referred to as route (2) above, occurs when energy sources/glucose are not available and ATP levels are low, relying on AMPK, which is a keen sensor of cellular energy status 11, specifically the intracellular ratio of AMP to ATP. AMPK is directly activated when intracellular ATP concentrations decrease and intracellular AMP concentrations increase 12, leading to inhibition of the mTOR pathway. In cancer cells, the consequences of this process include decreased protein synthesis, which correlates with reduced energy expenditure, and a decrease in cell proliferation7, 19. Metformin implements this second mechanism by acting as a mitochondrial poison, inhibiting complex I in the electron transport chain 20. This impairs production of mitochondrial adenosine-5'-triphosphate (ATP) 19, 21 and creates a state of energy restriction, mimicking the naturally occurring state described for scenario (2). The resulting rise in the AMP:ATP ratio, with an increase in AMP, leads to increased binding of AMP to AMPK, which makes the AMPK a better substrate for LKB1 15. Activation of LKB1-AMPK signaling results, leading to downregulation of phospho-AKT, mTOR, S6 kinase and 4EBP1 (4E binding protein 1) and the reprogramming of the metabolism of glucose and lipids. Of note, this effect of metformin on the mitochondrion elicits AMPK activation 13; metformin has not been shown to activate AMPK directly 15. Metformin has been coined an “energy restriction mimetic agent” because of this ability to inhibit mitochondrial generation of ATP 19.
In summary, the insulin-mediated pathway leads to synthesis of lipids, proteins and glycogen, in contrast to the AMPK pathway which inhibits these biosynthetic activities; yet, both mechanisms hone in on mTOR as a common target. The targeting of mTOR is funnelled through the TSC2/tuberin protein, which integrates the effects of the two pathways, insulin-generated growth and energy restriction (Figure 1) 6.
Other anti-carcinogenic mechanisms of metformin
The pharmacokinetic and pharmacodynamic properties of metformin contribute to its anti-carcinogenic impact on specific cell types. Metformin can only directly affect tissues capable of taking up the drug. The organic cation transporter 1 (OCT1) protein is critical for uptake into hepatocytes 7, 9, 22. The 17-fold lower expression of OCT1 in lung tissue in a mouse lung cancer model has been invoked as a possible explanation for the inability of metformin to induce activation of AMPK in lung tissue in this model 23. Despite this, metformin has been shown to reduce the burden of lung cancer in mice, which it does by a mechanism that is independent of AMPK activation but instead involves the insulin pathway 17, 23.
The context dependence of metformin's anti-cancer activity is evident from studies suggesting selective targeting of cancer stem cells 24. Metformin has also been shown to inhibit cell cycle progression by preventing increases in cyclin D and E2F1 protein levels and phosphorylation of pRb in prostate cancer cells 25, resulting in G0/G1 arrest. Metformin induces cell death in cancer cells by both caspase-dependent, apoptotic and caspase-independent, nonapoptotic pathways 23, 26. In addition, metformin blocks migration and invasion of tumor cells, in part by inhibiting matrix metalloproteinase-9 activation 27. Metformin also exhibits anti-inflammatory and anti-oxidant properties, targeting mechanisms known to play roles in both obesity/diabetes and cancer 28. Both metformin and the mTOR inhibitor rapamycin have been shown to affect the immune system, enhancing memory T-cell development following resolution of acute infection (or cancer) 29. Finally, metformin has been shown to inhibit transcription of the aromatase gene, CYP19A1, in a manner that is selective for expression of this estrogen-synthesizing enzyme in the breast 30.
Animal carcinogenesis studies
The evidence for a cancer preventive effect for metformin, however, has not been consistently demonstrated in vivo in animal studies. Multiple studies examining the effect of metformin on breast tumors occurring spontaneously in outbred mouse strains or in transgenic Her2/neu mice or carcinogen-treated rodents have shown results ranging from no effect to minor, albeit statistically significant, decrease in tumor multiplicity and increase in latency when given early and at high doses 19, 31-35. Two of these studies showed an increase in female animal life span independent of effect on tumor incidence 31, 33, although in a study by Anisimov et al., the 4.4% increase in mean life span in females was counterbalanced by a 13.4% decrease in male animal mean life spans 34. A recent study using both an ER-positive rat carcinogenesis model and Neu+/p53 knockout mice showed no effect of metformin administered at doses achievable in human beings 36. Of note, the tumor burden and proliferation index trended toward increases with metformin treatment, the latter reaching statistical significance.
Studies in colon cancer models showed modest to no effect on polyp number in azoxymethane-treated or ApcMin/+ mice, respectively, although large polyps were completely eliminated in the former model and 50% decreased in the latter 37, 38. Inhibition of mTOR phosphorylation was demonstrated in both models. Lung adenoma multiplicity and tumor volume in A/J mice treated with the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) were mildly inhibited by oral metformin treatment, with little evidence for an effect on AMPK activation in the lung and minimal mTOR inhibition 23. Instead, circulating insulin and IGF-1 levels were reduced, suggesting an indirect effect on tumorigenesis. Intraperitoneal metformin treatment was more effective in reducing tumor burden and demonstrated enhanced mTOR inhibition, but still without effect on AMPK activation in the lung. On the other hand, studies using carcinogen-induced pancreas, oral, liver, skin, and prostate cancer rodent model systems showed much more profound suppression of tumor formation, which was associated with mTOR inhibition in some cases but independent of AMPK activation in other instances 39-44. It should be noted that in the case of high-fat diet induced liver carcinogenesis, metformin treatment was effective during the early stages only, not by the time mice developed non-alcoholic fatty liver disease 43. Of note, the positive studies in pancreas, liver, and skin model systems were performed under high-fat conditions, which may have optimized the opportunities for metformin efficacy.
Epidemiologic studies
The epidemiologic literature regarding metformin use and cancer incidence and mortality, both overall and linked to specific organ sites, has exploded over the past decade. Multiple meta-analyses have now reported a decrease in cancer incidence of approximately 14-40%, along with a decrease in mortality in the same range (Table 1) 45-50. Organ sites reported to be thus protected include breast, colon, liver, pancreas, prostate, endometrium, and lung, among others. However, these studies represent a multitude of trial designs, from cohort and case-control studies through randomized controlled trials. As the stringency of the study design increases, metformin's effect on cancer incidence appears to decrease. For example, Thakkar et al. found that while cancer incidence was decreased by 30% in cohort studies and 10% in case-control trials, there was no discernable effect in randomized controlled trials 49. We similarly performed a meta-analysis with particular attention to biases and confounders, and also found that the risk reduction for cancer incidence decreased from 31% (summary relative risk (SRR) =0.69; 95% Confidence Interval (CI), 0.52-0.90) for all studies to 29% (SRR=0.71; 95%CI, 0.47-1.07) for prospective studies to 5% (SRR=0.95; 95%CI, 0.69-1.30) for randomized clinical trials 5.
Table 1.
Meta-analyses of Trials Examining Effect of Metformin on Cancer Incidence and Mortality
| Study | Cancer Incidence Summary Relative Risk (95%CI) | Cancer Mortality Relative Risk (95%CI) |
|---|---|---|
| DeCensi et al., 201045 | 0.68 (95%CI, 0.52-0.88) | 0.70 (95%CI, 0.51-0.96) |
| Noto et al., 201247 | 0.67 (95%CI, 0.53-0.85) | 0.66 (95%CI, 0.49-0.88) |
| Soranna et al., 201248 | 0.61 (95%CI, 0.54-0.70) | NR |
| Thakkar et al., 201349 | 0.70 (95%CI, 0.67-0.73) Coh | NR |
| 0.90 (95%CI, 0.84-0.98) CC | ||
| 1.01 (95%CI, 0.81-1.26) RCT | ||
| Franciosi et al., 201346 | 0.73 (95%CI, 0.61-0.88) | 0.65 (95%CI, 0.53-0.80) |
| 0.98 (95%CI, 0.81-1.19) RCT | ||
| Gandini et al., 20145 | 0.69 (95%CI, 0.52-0.90) | 0.66 (95%CI, 0.54-0.81) |
| 0.95 (95%CI, 0.69-1.30) RCT | ||
| Wu et al., 201550 | 0.86 (95%CI, 0.83-0.90) | 0.70 (95%CI, 0.53-0.94) |
| 0.88 (95%CI, 0.83-0.92) Coh | 0.66 (95%CI, 0.49-0.89) Coh | |
| 0.71 (95%CI, 0.63-0.80) CC | 0.91 (95%CI, 0.37-2.23) RCT | |
| 1.05 (95%CI, 0.94-1.18) RCT |
95%CI=95% Confidence Interval; NR=not reported; Coh=cohort study; CC=case-control study; RCT=randomized control trial
It has been pointed out recently that the existing metformin literature is subject to important sources of time-related bias, which potentially exaggerate cancer-reducing effects 4. Time-related bias is most often a form of differential misclassification bias that can generally be avoided by appropriate accounting of follow-up time and exposure status in the design and analysis of such studies. The different types of time-related biases include immortal time bias (unexposed time is misclassified as drug-exposed time), time-window bias (differential exposure opportunity time windows between exposed and unexposed subjects), and time-lag bias (comparison of treatment given during different stages of the disease). Immortal time bias refers to a period of cohort follow-up time during which a cancer event (that determines end of follow-up) cannot occur. Immortal time bias, for example, can arise when the period between cohort entry and date of first exposure to metformin, during which cancer has not occurred, is either misclassified or simply excluded and not accounted for in the analysis. This is frequently found in studies that compare ‘users’ against ‘non-users’. In cohort studies where a first-line therapy with metformin is compared with second- or third-line therapies, patients are unlikely to be at the same stage of diabetes, which can induce confounding of the association with an outcome (e.g., cancer incidence) by disease duration. An outcome related to the first-line therapy may also be attributed to the second-line therapy if it occurs after a long period of exposure. Such a situation requires matching on disease duration and consideration of latency time windows in the analysis.
In our meta-analysis, we found that adjustment for time-related biases resulted in a diminution of the protective effect on cancer incidence 5. The risk reduction in cancer incidence at all sites decreased from 31% for all studies (19 studies, SRR=0.69; 95% CI, 0.52-0.90) to 10% for studies adjusted for time-related biases (8 studies, SRR=0.90; 95% CI, 0.89-0.91), albeit still statistically significant. With regard to specific organ sites where at least 3 studies could be appropriately evaluated, metformin use was significantly associated with decreased breast cancer incidence after adjustment for time-related biases (SRR= 0.94; 95%CI, 0.90-0.99) compared with a 12% non-significant decreased incidence in all studies (SRR=0.88; 95%CI, 0.75-1.03), but the magnitude of the effect was rather small. For colon cancer, a similarly small 8% decrease in incidence was noted (SRR=0.92; 95%CI, 0.85-0.98) that compared with a 20% marginally significant decrease when all studies were considered (SRR=0.80; 95%CI, 0.64-1.00). For lung cancer, examination of all studies showed an 18% decrease in cancer incidence (SRR=0.82; 95%CI, 0.67- 0.99). Adjustment for time-related biases decreased this protective effect to a statistically significant 12%, but adjustment for smoking, which is by far the most relevant lung cancer risk factor, resulted in no association between metformin use and cancer incidence. Similarly, the strong protective effect of metformin use against liver cancer (9 studies, SRR=0.47; 95%CI, 0.28-0.79) became non-significant when only the 3 non-time biased studies were examined (SRR=0.65; 95%CI, 0.39-1.08). It should be emphasized, however, that the total number of studies and patients was rather small for the liver cancer associations and thus these results should be interpreted with caution. Finally, for prostate and pancreatic cancers, metformin use was not associated with a statistically significant protective effect.
Another potential source of confounding that is often not adequately explored in the existing literature is the effect of obesity and its surrogate, body mass index (BMI). Obesity is intimately linked to increased risk of multiple cancer types 2. Potential mechanisms include both direct and indirect effects of obesity on insulin, IGF-1, sex hormones, adipokines, and inflammation, many of which are directly impacted by metformin. Metformin, unlike several other anti-diabetic agents, is associated with weight loss. Furthermore, a recent clinical trial showed that metformin affected breast cancer proliferation differentially according to insulin resistance status and BMI, with a trend toward inhibiting proliferation only in women with insulin resistance or high BMI (see below)51. Therefore, we examined BMI as a potential confounder in our meta-analysis and found that adjustment for BMI decreased the protective effect of metformin for all-cancer incidence from 31% to 18%, which was still statistically significant 5. For breast cancer, adjustment for BMI revealed a borderline association of metformin use with cancer incidence (SRR=0.82; 95%CI, 0.67-1.00). For colon and prostate cancer, there was no statistically significant association between metformin use and cancer incidence. Other organ sites could not be assessed due to a small number of reported studies. It should be noted, however, that BMI is dynamic and therefore adjusting for a single BMI value might be inadequate to account for confounding by BMI dynamics over time. Furthermore, we were unable to assess the effect of BMI and time-related biases simultaneously due to inadequate numbers of studies that controlled for both factors.
The effect of metformin on cancer mortality is informed by a much smaller literature and thus we were unable to determine the effect on isolated cancer sites 5. With regard to all-cancer mortality, metformin use was associated with a 34% decrease (7 studies, SRR=0.66; 95%CI, 0.54-0.81) that remained significant when the analysis was limited to only prospective trials (4 studies, SRR=0.48; 95%CI, 0.23-0.97). Adjustment for BMI maintained the magnitude of the effect (5 studies, SRR=0.60; 95%CI, 0.45-0.80), although adjustment for time-related biases, limited to only 3 studies, resulted in loss of statistical significance (SRR=0.45; 95%CI, 0.16-1.26). Different mechanisms may be responsible for metformin's effect on cancer mortality compared with cancer incidence. Several retrospective analyses have suggested that diabetics treated with metformin during chemotherapy have longer survival than individuals treated with other anti-diabetic agents 52, 53. A previous mouse xenograft study showed that metformin targets breast cancer stem cells and synergizes with doxorubicin to prevent relapse 54. Increasing the effectiveness of chemotherapy could result in improved survival.
Taken together, these results point out the limitations of the current literature regarding the association between metformin use and cancer incidence and mortality. While all the studies, including our own meta-analysis, suggest that metformin use is associated with a reduced risk of cancer and death from cancer, the effect may be far smaller than previously believed.
Review of Clinical Trials
Although the literature reviewed above suggests a potential role for metformin in cancer prevention, well-crafted clinical trials addressing specific research questions in uniform cohorts will be needed to eventually determine if metformin is, indeed, effective in reducing cancer incidence. Here we review the status of completed and ongoing clinical trials that address the potential efficacy of metformin to prevent cancer in a variety of clinical settings. Eleven early phase studies of metformin in individuals at risk for or with cancer have been published (Table 2). Fifty-one additional studies are registered in clinical trial registries. Fifteen trials are being conducted in various high cancer risk populations (Table 3). Seventeen studies are being conducted in the presurgical setting (Table 4) and 20 studies are registered in the adjuvant setting (Table 5).
Table 2.
Published Metformin Clinical Trials
| Organ Site; Phase; ClinicalTrials.gov, EudraCT or ICTRP Number | Study Design | Study Population | Planned Accrual/Evaluable | Primary Outcome |
|---|---|---|---|---|
| Barrett's Esophagus Phase II NCT0144792763 | 500-2000mg qd over time metformin vs. placebo for 12 weeks | Barrett's esophagus ≥ 2cm in length, daily PPI for ≥ 4wks, excluding high-grade dysplasia | 74 | No significant change in pS6K1 |
| Breast Phase II55 | 500mg tid for 6 months | Women with IBC who completed therapy with fasting insulin of ≥45 pmol/L and glucose < 7.0 mmol/L | 40/22 | Change in insulin levels 22.4% decrease (p=0.024) |
| Breast Phase I NCT0089788459 | 500mg bid for 2-3 weeks | Women <70 Presurgical- IBC T1-4 | 48/39 | 2.97% decrease in Ki-67 (p=0.016) |
| Breast Phase II 2008-004912-1051 | 850mg/d for 3 days followed by 850mg bid day 4-28 Vs. Placebo for 4 weeks prior to surgery | Women Presurgical-Stage I-III IBC patient not suitable for neoadjuvant therapy | 200/196 | No overall change in Ki-67 10.5% decrease in Ki-67 if HOMA >2.8 (p for interaction =0.045) |
| Breast Phase II 2007-000306-7058 | 500mg/d for 1 week followed by 1000mg/d for 1 week Vs. Placebo | Stage 1-2 IBC, >1cm, no history of diabetes | 47/39 | 3.4% decrease in Ki-67 (p=0.02) |
| Breast Phase 0 NCT0198082360 | 500mg am and 1000mg pm metformin with 80mg atorvastatin for at least 2 weeks prior to surgery | Histologically confirmed DCIS or IBC who undergo CNB followed by surgery | 40 | No reduction in Ki-67 |
| Breast Phase II 2006-006236-2257 | 1000 vs. 1500 mg/d for 3 months | Postmenopausal with history of IBC and 6 mos post-surgery, on tamoxifen for at least 6 mos and plan to continue, or at least 6 mos post chemo | 125/96 | 1500mg/d decreased testosterone by 23% (p<0.01) |
| Colon Phase II64 | 250 mg qd vs. control for 4 weeks | Presence of ACFs, no concurrent use of NSAIDs | 26/23 | Number of ACFs decreased from 8.78 ± 6.45 to 5.11 ± 4.99 P = 0.007 for metformin arm |
| Endometrial Phase 0 NCT0191124761 | 850mg qd for 1-4 weeks | BMI 30 or greater with confirmed type 1 endometrial cancer | 28/20 | 11.75% reduction in Ki-67 (p=0.008) |
| Endometrial Phase I JPRN-UMIN00000485262 | 750mg/d increased to 2250mg/d if no side effects prior to surgery | Women age 20-70 with grade 1-2 histologically confirmed EA without myometrium invasion | 40/31 | 44.2% decrease in Ki-67 (p<0.001) |
| Prostate Phase I NCT0088172565 | 500mg tid for 4-12 weeks prior to surgery | Confirmed PC scheduled for prostatectomy | 40/22 | 29.5% decrease in Ki-67 (p=0.006) |
IBC-invasive breast cancer, DCIS- ductal carcinoma in situ, qd- one a day, bid- twice a day, tid- three times a day, EIO- European Institute of Oncology, Tam-Tamoxifen, T- Testosterone, ACFs- aberrant crypt foci, NSAIDs- non-steroidal anti-inflammatory drugs, BMI- body mass index, PC- prostate cancer, PPI-proton pump inhibitor, HOMA- Homeostasis Model Assessment, CNB- core needle biopsy, EA- Endometrial adenocarcinoma
Table 3.
Metformin Cancer Prevention Trials – High Risk Populations (N=15)
| Organ Site; Phase; ClinicalTrials.gov, EudraCT or ICTRP Number, Status | Study Design | Study Population | Target Accrual | Primary Endpoint |
|---|---|---|---|---|
| All Phase III NCT00038727, O | 1850mg/d metformin, intensive lifestyle vs. placebo | Pre-diabetes | 3234 | Development of diabetes Secondary: Development of cancer |
| Barrett's Esophagus Phase I NCT01312467, C | 500-2000mg/d over time metformin for 12 weeks | Barrett's esophagus ≥ 2cm in length, daily PPI for ≥ 4weeks, excluding high-grade dysplasia | 50 | % Change in pS6K |
| Barrett's Esophagus Phase 0 NCT01465113, R | 50,000IU weekly Vitamin D3 with 1000mg bid metformin (dose escalated over 4 weeks) vs. vitamin D alone for 12 weeks | Barrett's esophagus ≥ 2cm in length, daily PPI for ≥ 4weeks, excluding high-grade dysplasia | 66 | 15-prostaglandin expression |
| Breast Phase I ACTRN12610000219088 | 500mg/d for 1 week followed by 1000mg/d for 4 weeks prior to reduction mammoplasty | Women age 40-60 | 60 | AMPK signaling and aromatase expression |
| Breast Phase II NCT02028221, R | 850mg for 1 month followed by 850mg bid for an additional 11 months vs. placebo | Premenopausal women age 30-45 with BMI of 25 or greater and have metabolic syndrome | 150 | Change in breast density from baseline at 6 and 12 months |
| Breast Phase II NCT01793948, R | 850mg qd for 30 days and bid for 11 months vs. placebo | Postmenopausal and high risk for breast cancer with BMI 25 or greater and high breast density | 24 | Changes in phosphorylated proteins by RPPA |
| Breast Phase III NCT01905046, R | 850mg qd for 4 weeks followed by 850mg bid vs. placebo for 24 months. Placebo group may cross over to metformin for months 13-24. | Premenopausal, prior AH, LCIS or DCIS, >1.66% Gail or known BRCA carrier, MD >25% and cytological atypia | 400 | Regression of atypia at 12 and 24 mos |
| Colon Phase II NCT01312467, C | 500mg d1-7, 1000mg d8-14; 1500mg d15-21, 2000mg d22-end of study endoscopy | Age 35-80; history of adenoma in previous 3 yrs; BMI≥30 | 50 | Reduction in pS6K-S235 |
| Colon Phase II NCT01725490, R | 500mg qd vs 500mg tid vs. placebo 2:2:1 for 7 mos | FAP, age 20-65 excluding patients who use NSAIDs | 100 | Mean percentage change in the number of polyps |
| Endometrial Phase 0 NCT01685762, R | 850mg qd for 4 weeks then 850mg BID for 8 weeks | EH without atypia | 15 | Response rate |
| Endometrial Phase II NCT02035787, R | 850mg bid with Levonorgestrel-releasing IUD for 12 months | Non-surgical candidate for histologically confirmed CAH or grade 1 endometrial cancer | 30 | Response rate – compared to base rate of 50% |
| Endometrial Phase II NCT01686126, R | Levonorgestrel-releasing IUD vs Levonorgestrel-releasing IUD plus metformin 500mg bid, vs. Mirena plus Weight Watchers for 6 months | Non-surgical candidate for histologically confirmed CAH or grade 1 endometrial cancer | 165 | pCR |
| Endometrial Phase II NCT01697566, R | Exercise, exercise + 850mg bid, 850mg bid, vs placebo for 4 months (weekly dose escalation) | Women BMI≥30 | 100 | Change in Ki-67 |
| Liver Phase III NCT02319200, NO | 1000mg bid metformin vs. placebo for 36 months | Patients with viral Hepatitis C cirrhosis | 444 | Rate of HCC occurrence and liver related death or transplantation |
| Prostate Phase III NCT01864096, R | 850mg once daily for 1 month followed by 850mg bid for 35 months | Biopsy-proven, low risk localized prostate cancer, undergoing active surveillance | 408 | Time to progression |
R- recruiting, NO- not open yet, C- completed, O- ongoing, not recruiting, qd- one a day, bid- twice a day, tid- three times a day, BMI- body mass index, LCIS- lobular carcinoma in situ, DCIS- ductal carcinoma in situ, PPI- proton pump inhibitor, RPPA, reverse phase protein array, MD- mammographic density, NSAIDs- non-steroidal anti-inflammatory drugs, FAP – familial adenomatous polyposis, EH- endometrial hyperplasia, CAH- complex atypical hyperplasia, pCR- pathologic complete response, IUD- intrauterine device, HCC- hepatocellular carcinoma, pS6K-S235- phosopho-serine 6 kinase -serine 235
Table 4.
Metformin Presurgical Trials (N=16)
| Organ Site; Phase; ClinicalTrials.gov, EudraCT or ICTRP Number, Status | Study Design | Study Population | Target Accrual | Primary Endpoint |
|---|---|---|---|---|
| Breast Pilot NCT00930579, O | 500mg in morning and 1000 mg in the evening for 2 weeks | Women Presurgical- DCIS or operable IBC with BMI>25 | 35 | AMPK/mTOR signaling |
| Breast Phase 0 NCT01302002, T | 500mg bid for 3 weeks | Women Presurgical- IBC T1 or T2 | 30 | Ki67, TUNEL, pAKT |
| Breast Phase I NCT00984490, T | 850mg bid for 7-21 days | Women Presurgical- IBC Stage I-IIIA | 5 | % Change in Ki-67 |
| Breast Phase II NCT01266486, C | 1500mg/d for 14-21 days prior to neoadjuvant chemotherapy | Women with IBC with no prior treatment | 41 | Change in pS6K, p4E-BP-1 and pAMPK |
| Breast Phase II NCT01589367, R | Letrozole 2.5mg with metformin 500mg bid for 1 week, 1000mg morning, 500mg evening for 1 week then 1000mg bid for 22 weeks or placebo | ER-positive breast cancer with no evidence of metastasis | 208 | CRR |
| Breast Phase II NCT01929811, R | 500mg tid (500mg qd in cycle 1) with TEC vs. TEC alone until disease progression | Stage IIb or III with BMI 25 or greater | 200 | pCR |
| Bladder Phase II NCT02360618, R | 850mg qd metformin and 20mg simvastatin for 12 weeks prior to surgery | Biopsy proven invasive bladder cancer | 44 | Change in Ki-67 |
| Colon Phase II NCT01632020, O | 1700mg bid vs. placebo 10-21 days prior to surgery | Newly diagnosed colon or rectal adenocarcinoma | 40 | Change in proliferation and apoptosis of tumor and surrounding normal |
| Colon Phase I NCT01440127, T | 500mg bid 7-14 days prior to surgery | Colorectal cancer diagnosis undergoing surgery or biopsy | 9 | Expression of CD133 in tumors metformin vs. control |
| Colon Phase I NCT01816659, T | 500mg qd for 1 week with escalation to 200mg qd for up to 30 days prior to surgery | Colorectal cancer or FAP with planned surgery | 3 | Ki-67 |
| Endometrial Phase I NCT01205672, O | 850mg/d for 7-30 days | Women age 18-80 with histologically confirmed endometrial adenocarcinoma | 30 | Change in S6K expression |
| Endometrial Phase II NCT01877564, R | Metformin vs. no treatment prior to surgery | Histological confirmed diagnosis of grade I or II endometrial adenocarcinoma with BMI 30 or greater | 40 | Ki-67 |
| Endometrial Phase II NCT02042495, NO | 500mg tid with possible dose reduction to 500mg bid for 4-6 wks | Confirmed diagnosis of endometrial cancer with planned surgical staging | 80 | pS6K |
| Head and Neck Phase 0 NCT02402348, R | 850mg/d for 3 days, 850mg bid day 4 until surgery | Newly diagnosed HNSCC Stage II-IV | 30 | % Tumor cell death |
| Head and Neck Phase 0 NCT02083692, R | 500mg qd days 1-3, 500mg bid days 4-6, 1000mg bid starting day 6 for 9-28 days | Biopsy proven head and neck cancer with planned definitive resection | 40 | Change in TOMM20 and MCT4 |
| Prostate Phase I NCT01433913, C | 500mg/d d1-7, 1000mg/d d8-14, 1500mg/d d22- surgery; total study length 4-12 weeks | Men scheduled for prostatectomy | 21 | Change in Ki-67 |
R- recruiting, NO- not open yet, C- completed, O- ongoing, not recruiting, T- terminated, qd- one a day, bid- twice a day, tid- three times a day, TEC- docetaxel, epirubicin and cyclophosphamide, BMI- body mass index, IBC-invasive breast cancer, DCIS- ductal carcinoma in situ, FAP- familial adenomatous polyposis, pCR- pathologic complete response, ER- estrogen receptor, AMPK- AMP-activated protein kinase, mTOR- mammalian target of rapamycin, TUNEL- terminal deoxynucleotidyl transferase dUTP nick end labeling (apoptosis), pS6K- phosopho-serine 6 kinase, p4E-BP- phosphor-eukaryotic translation initiation factor 4E binding protein, CD133- cancer stem cell marker, CRR- complete response rate, HNSCC- head and neck squamous cell carcinoma, TOMM20- translocase of outer mitochondrial membrane 20, MCT4- monocarboxylate transporter 4
Table 5.
Metformin Adjuvant Trials (N=20)
| Organ Site; Phase; ClinicalTrials.gov, EudraCT or ICTRP Number, Status | Study Design | Study Population | Target Accrual | Primary Endpoint |
|---|---|---|---|---|
| All Phase I NCT01981525, R | 500mg qd for 1week, 500mg bid for 1 wk, 500mg tid for 1 week and 1000mg bid for 1 wk if tolerated continued treatment for 14 weeks | TP53 germline mutation at least 18 yrs old. If prior cancer at least 6 months from surgery and 1 year from chemotherapy | 36 | Tolerability and effect on IGF-1, insulin, IGFBP-3 |
| Breast Phase II NCT01302379, R | Exercise, exercise + metformin 500mg at dinner (1 weeks) 1000mg at dinner (2-4 weeks) 500mg in morning and 1000mg at dinner, metformin + standard dietary guidelines, Vs. dietary guidelines for 6 months | Postmenopausal IBC survivor Stage 1-3A within past 4 yrs, completed chemotherapy, BMI ≥ 25 | 340 | Biological markers associated with breast cancer survival |
| Breast Phase II NCT00909506, U | 500mg/d vs 1000mg/d vs. Placebo for 6 months | BC survivor with BMI ≥ 23 6-24 months since surgery and 4 weeks since chemotherapy or radiation | 105 | Weight loss |
| Breast Phase III (MA.32) NCT01101438, O | 850mg bid vs. Placebo for 5 yrs (850mg qd for first 4 weeks) | IBC Survivor 4 weeks post therapy | 3649 | Invasive disease-free survival |
| Breast Phase III NCT01666171, U | Ancillary study to MA.32 | ER-negative IBC with 25% or greater MD with contralateral breast available for analysis | 458 | Change in % mammographic density |
| Breast Phase III NCT01286233, O | Ancillary Study to MA.32 | DNA available | 394 | QoL |
| Breast Pilot NCT02278965, R | 850mg bid metformin and 1120mg bid omega-3 fatty acids | History of Stage 0-III breast cancer, 6 months since completion of chemotherapy, biologic therapy, and tamoxifen | 20 | Number of participants completing 1 year intervention |
| Colon/ Breast Phase II NCT01340300, R | Exercise, exercise + metformin 850mg qd for 2weeks then 850mg bid metformin, vs. educational information for 1 year | Stage 1-3 breast or colorectal cancer survivor completed all adjuvant therapy within 2-24 months prior to enrollment | 200 | Change in fasting insulin levels at 6 months |
| Endometrial Pilot JPRN-UMIN000002210, C | 400mg/d MPA, 100mg/d aspirin for 24 weeks with 750mg/d metformin continuously for 4 years (increased to 1500mg/d if no side effects) | Histologically well differentiated EA at presumed stage IA and atypical endometrial hyperplasia | 30 | Recurrence free interval |
| Lung Phase II NCT01717482, R | 850mg qd for 4 weeks followed by 850mg BID for a total of 6 months | Suspected or biopsy proven stage IB-IIIA NSSLC-squamous with coincident bronchial dysplasia or carcinoma in situ in a non-resected region | 24 | Feasibility |
| Prostate Phase II NCT01215032, O | 1000mg bid for 1 year | Castrate resistant prostate cancer minimum PSA of 2.0 ng/mL | 106 | PSA response and PSA difference |
| Prostate Phase II NCT01561482, T | 500mg bid escalated to 1000mg bid metformin plus 20mg simvastatin qd for 6 months | Biochemical recurrence of prostate cancer with at least 3 PSA rises | 37 | Improvement in PSA doubling time |
| Prostate Phase II NCT01243385, O | 1000mg bid until disease progression, unacceptable toxicity, or refusal | Biochemical recurrence of locally-advanced or metastatic prostate cancer with at least 3 PSA rises | 44 | PFS at 12 weeks |
| Prostate Phase II NCT01620593, R | 500mg tid vs. placebo for 1 year | Men with locally-advanced or metastatic prostate cancer planning on castration therapy | 94 | Metabolic Syndrome PSA response, progression, pathway inhibition in PBMCs |
| Prostate Phase II NCT02176161, R | 750mg bid for 9 months | Prostate cancer patients who have received treatment with radiation or surgery | 70 | PSA Doubling time |
| Prostate Phase II NCT01996696, R | 500mg tid vs placebo for 3 years | Pathologically confirmed prostate adenocarcinoma with at least 1 high risk feature | 104 | Mean body weight at 12 months |
| Prostate Phase II NCT02420652, NO | Metformin bid and aspirin qd vs. placebo for 6 months | Histologically proven prostate cancer treated with local therapy | 66 | Change in PSA rates |
| Prostate Pilot NCT02376166, R | 850mg bid for 4 weeks | Histologically proven prostate cancer with biochemical progression | 15 | Completion of telemedicine visits |
| Solid Tumors Phase II NCT02431676, NO | Self-directed weight loss, coach directed weight loss Vs. up to 2000mg /d metformin | Previous diagnosis of a solid tumor at least 3 months from therapy | 120 | IGF-1 and IGF-1:IGFBP3 ratio |
| Thyroid Phase II NCT01341886, C | Levothyroxine with 30% dose reduction with either metformin or placebo for 3 months | Papillary or Follicular thyroid cancer with surgery with levothyroxine treatment | 51 | TSH |
R- recruiting, NO- not open yet, C- completed, O- ongoing, not recruiting, T- terminated, U- unknown, qd- one a day, bid- twice a day, tid- three times a day, BMI- body mass index, IBC-invasive breast cancer, ER- estrogen receptor, EA- endometrial adenocarcinoma, NSSLC- non-small cell lung cancer, PSA- prostate specific antigen, PFS- progression free survival, MD- mammographic density, IGF-I- insulin-like growth factor 1, IGF-BP3- insulin-like growth factor binding protein 3, QoL- quality of life, MPA- medroxyprogesterone acetate, PBMCs- peripheral blood mononuclear cells, TSH- thyroid stimulating hormone
Completed Clinical Trials
Two studies examined various doses of metformin given for 3-6 months to women after therapy for breast cancer (Table 2). The first published study examined women with breast cancer who completed adjuvant therapy and whose plasma levels of insulin were at least 45 pmol/L (Table 2) 55, based on positive results from a prior cohort study 56. Metformin decreased circulating insulin levels by 22.4% (p.0024), although 25% of participants dropped out prematurely. A second study in women with breast cancer who had completed adjuvant therapy but had elevated testosterone compared two different doses of metformin, demonstrating that the higher dose significantly reduced serum testosterone levels and free androgen index compared to the lower dose 57.
Five trials examined the short-term effects (1-4 weeks) of various doses of metformin on cell proliferation (Ki-67) in women awaiting surgery for breast cancer (pre-surgical trials). The first trial found a 3.4% reduction in Ki-67 (p=0.027) in the metformin arm, but 29% of the patients on metformin withdrew due to gastrointestinal side effects 58. In a second larger double-blind, placebo-controlled study, the metformin effect on Ki-67 change relative to placebo was not statistically significant, with a mean proportional increase of 4.0% 51. However, women with a HOMA index (Homeostasis Model Assessment, used to quantify insulin resistance) >2.8 had a non-significant decrease of 10.5% while women with a HOMA index <2.8 had a non-significant increase of 11.1%. The interaction between HOMA index and metformin on Ki-67 was statistically significant (p=0.045); a similar trend was seen with BMI, although it was not statistically significant. With dose escalation of metformin, patient drop-out was not problematic 51. A third single arm trial reported a 3% decrease in Ki-67 (p=0.016) after a median of 18 days of treatment 59 and a recently completed study showed no reduction in Ki-67 60.
Endometrial cancer prevention is of particular interest because of its relationship with obesity and the use of metformin for treatment of polycystic ovary syndrome, a condition associated with increased endometrial cancer risk. Two studies have been reported in women with early stage endometrial cancer. With a dose of 850mg QD, a single arm study of stage 1-2 endometrioid endometrial cancer showed an 11.75% reduction in Ki-67 61. In a study that increased the dosage from 750mg QD to 1500mg or 2250 mg, 4 weeks of metformin resulted in a 44.2% decrease in Ki-67 62.
Three additional studies in other organ systems (Barrett's esophagus, colon, and prostate) have been reported. A study in Barrett's esophagus showed no significant change in phosphor-serine 6 kinase (pS6K), cell proliferation, or apoptosis 63. Another study examined change in the number of aberrant crypt foci (ACF, a putative precursor of colon cancer) in individuals with pre-existing ACF, randomizing participants to a very low dose of metformin (250 mg/day) or no treatment for one month 64. A significant decrease in ACF from 8.78 ± 6.45 to 5.11 ± 4.99 (p=0.007) was observed in the metformin arm but not in the control group. Additionally, proliferation was significantly decreased in the metformin group (p<0.001). Finally, men with prostate cancer were treated with metformin for 4-12 weeks prior to surgery. The primary endpoint, change in Ki-67, was statistically significant, with an absolute decrease of 1.44% and a relative decrease of 29.5% 65.
Ongoing Clinical Trials
The ongoing studies (Tables 3-5) examine similar endpoints, including pharmacodynamic markers such as tumor/tissue levels of pS6K or other measures of AMPK-activating kinase (AMPK) signalling. Many of the studies examine surrogate endpoints for cancer such as atypia for breast cancer or change in prostate specific antigen (PSA) for prostate cancer. Over 5,000 participants are expected to enroll in these trials examining the effect of metformin on tumorigenesis in multiple organ systems. Not all trials are discussed in detail in the text, which discusses the studies by organ site, but a complete list is available in the tables based on disease setting (prevention, presurgical, or adjuvant).
Thirteen additional studies are examining the effect of metformin in the breast. These include a study in women scheduled for a reduction mammoplasty that compares changes in LKB1 and AMPK signaling in women treated with metformin 500 mg twice a day (dose escalated) versus no treatment (Table 3, ACTRN12610000219088)66 and two larger studies one in overweight and obese premenopausal women with high breast density (Table 3, NCT01793948) and one in high-risk premenopausal women with cytologic atypia (Table 3, NCT01905046) , four additional single-arm pre-surgical breast studies examining changes in Ki-67 and AMPK signaling following metformin 1000-1700 mg/ day for 2-3 weeks (Table 4, NCT00930579, NCT01266486, NCT01589367, NCT01929811) 67-70 and two presurgical studies that were terminated due to poor accrual (Table 4, NCT01302002 and NCT00984490), and four studies in the adjuvant setting. The largest of these is the NCIC MA.32 trial, a phase III adjuvant breast cancer trial randomizing 3649 women within 12 months of diagnosis to metformin 850 mg twice a day (850 mg/day during weeks 1-4) versus placebo for 5 years (Table 5, NCT01101438) 71. The primary endpoint is invasive disease free survival. The three other adjuvant studies include a trial examining the effects of metformin 500 mg/day, 500 mg twice a day, or placebo for 6 months on weight loss in breast cancer survivors with a BMI of ≥ 23 who have completed chemotherapy and radiation (Table 5, NCT00909506) 72, the “Reach for Health Study” that is randomizing postmenopausal breast cancer survivors with a BMI of ≥ 25 to metformin, exercise, the combination, or standard dietary guidelines for 6 months to determine the effects on biological markers associated with breast cancer survival (Table 5, NCT01302379) 73, and a pilot study examining the effect of metformin 850 mg twice daily in combination with omega-3 fatty acids in women with a history of stage 0-III breast cancer 6 months since completion of all therapy for 1 year to determine feasibility (Table 5, NCT02278965).
Five clinical trials are examining metformin in the colon. Two trials are studying individuals with a previous history of colorectal adenomas. One study treats subjects with BMI ≥ 30 with metformin dose escalated from 500 -2000 mg per day for 12 weeks and has a primary endpoint of change in pS6K-serine 235 (Table 3, NCT01312467) 74. A placebo-controlled, 2 dose trial studying individuals with familial adenomatous polyposis for 7 months examining mean change in number of polyps (Table 3, NCT01725490). One study is recruiting patients with newly diagnosed colon cancer who are scheduled to undergo surgery while 2 were terminated (Table 4, NCT01440127, NCT01816659). The active study is examining the effect of 2-3 weeks of metformin 850 mg twice a day versus placebo on proliferation and apoptosis in colon tumors and adjacent normal tissue (Table 4, NCT01632020) 75. The effect of combination of metformin plus exercise is also under study in a trial of colorectal cancer survivors (with the addition of breast cancer survivors as well due to slow accrual) who are randomized to metformin alone dose escalated to 850 mg twice a day, exercise, metformin plus exercise, or education for 1 year with a primary endpoint of change in fasting insulin at 6 months (Table 5, NCT01340300) 76.
Eight additional studies are being conducted in women with endometrial hyperplasia or early stage endometrial cancer. A pilot study is examining whether metformin dose escalated from 750 to 1500mg per day for 4 years in combination with 400 mg medroxyprogesterone acetate (MPA) and aspirin 100 mg/day for the first 24 weeks can affect the recurrence free interval in women diagnosed with stage 1A endometrial adenocarcinoma or atypical endometrial hyperplasia (Table 5, JPRN-UMIN000002210) 77. Three additional studies are examining the effect of biomarkers in women with newly diagnosed early stage endometrial cancer (Table 4, NCT01205672, NCT01877564, NCT02042495). Two studies are examining the combination of metformin with Mirena, a levonorgestrel-releasing intrauterine system, in women with endometrial hyperplasia (Table 3, NCT02035787, NCT01686126). One study is examining the change in endometrial proliferation comparing exercise, exercise plus metformin, metformin, and placebo in women with a BMI greater than or equal to 30 (Table 3, NCT01697566) and an additional study is examining the response rate of endometrial hyperplasia in a phase 0 pilot study (Table 3, NCT01685762).
Ten prostate cancer studies are ongoing. A pre-prostatectomy study is examining Ki-67 and drug concentrations in the prostate tissue after 4-12 weeks of metformin dose escalated to 1500 mg per day (Table 4, NCT01433913) 78. Four studies are examining the effect of metformin on PSA response in asymptomatic men with advanced or recurrent prostate cancer (Table 5, NCT01215032, NCT01243385, NCT01620593, NCT02176161). One of these studies is examining PSA doubling time in men after completion of initial therapy (Table 5, NCT02176161). A study comparing metformin to the combination of metformin and simvastatin on PSA doubling time was terminated due to poor enrollment (Table 5, NCT001561482). A Phase III randomized, placebo-controlled study in men with biopsy-proven, low-risk localized prostate cancer who are undergoing active surveillance is examining time to progression (Table 3, NCT01864096).
Two other Phase III studies are also ongoing. A phase III study examining the effect of metformin on progression of viral Hepatitis C cirrhosis to liver cancer is ongoing in France (Table 3, NCT02319200). In the US, participants in the Diabetes Prevention Program, a phase III study that started in 1996 to examine the influence of metformin and intensive lifestyle intervention on progression of pre-diabetes to diabetes, has retrospectively collected cancer outcomes and will continue to collect cancer outcomes as part of an extension to the original clinical trial (Table 3, NCT00038727).
Conclusions
The emerging data from preclinical, epidemiologic, and clinical studies suggest that there is a signal for cancer preventive potential with metformin use. There is biologic plausibility for a cancer preventive effect given the multiple ways that metformin can interfere with cancer promoting signalling pathways. However, both animal and epidemiologic studies have shown somewhat mixed effects. Further, the epidemiologic literature reviewed above relates only to individuals with diabetes and the effect is of lesser magnitude than previously reported once the appropriate adjustments to avoid bias and confounding are made. It remains to be determined whether a similar protective effect can be demonstrated in non-diabetic individuals. Multiple ongoing clinical trials are addressing which patient cohorts at risk for specific diseases are most likely to benefit from metformin. Although the long history and clinical experience with metformin make it a very attractive candidate for drug repurposing, general recommendations about its use, particularly in non-diabetic populations, need to await the results of the ongoing studies.
Acknowledgments
Funding/Support: The study was supported by grants from the Italian Association for Cancer Research AIRC (IG 12072), the Italian Ministry of Health (RF-2009-1532226), and the Italian League Against Cancer (14/08) to Andrea DeCensi. Andrea DeCensi's work was partially performed during a sabbatical at the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors do not report any potential financial conflicts
References
- 1.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher EJ, LeRoith D. Diabetes, antihyperglycemic medications and cancer risk: smoke or fire? Curr Opin Endocrinol Diabetes Obes. 2013;20:485–494. doi: 10.1097/01.med.0000433065.16918.83. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35:2665–2673. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16:1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res (Phila) 2010;3:1060–1065. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 9.Pollak M. Potential applications for biguanides in oncology. J Clin Invest. 2013;123:3693–3700. doi: 10.1172/JCI67232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 12.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 14.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie DG. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology. 2006;131:973. doi: 10.1053/j.gastro.2006.07.032. author reply 974-975. [DOI] [PubMed] [Google Scholar]
- 16.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelman JA, Cantley LC. Chemoprevention meets glucose control. Cancer Prev Res (Phila) 2010;3:1049–1052. doi: 10.1158/1940-6207.CAPR-10-0178. [DOI] [PubMed] [Google Scholar]
- 18.Kalender A, Selvaraj A, Kim SY, et al. Metformin, Independent of AMPK, Inhibits mTORC1 in a Rag GTPase-Dependent Manner. Cell Metabolism. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Z, Jiang W, Thompson MD, McGinley JN, Thompson HJ. Metformin as an energy restriction mimetic agent for breast cancer prevention. J Carcinog. 2011;10:17. doi: 10.4103/1477-3163.83043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 21.Algire C, Moiseeva O, Deschenes-Simard X, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012;5:536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Shu Y, Liang X, et al. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc Natl Acad Sci U S A. 2014;111:9983–9988. doi: 10.1073/pnas.1314939111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci U S A. 2013;110:972–977. doi: 10.1073/pnas.1221055110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 26.Queiroz EA, Puukila S, Eichler R, et al. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One. 2014;9:e98207. doi: 10.1371/journal.pone.0098207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao B, Wang Z, Ali S, et al. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila) 2012;5:355–364. doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue W, Yang CS, DiPaola RS, Tan XL. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer Prev Res (Phila) 2014;7:388–397. doi: 10.1158/1940-6207.CAPR-13-0337. [DOI] [PubMed] [Google Scholar]
- 29.Prlic M, Bevan MJ. Immunology: A metabolic switch to memory. Nature. 2009;460:41–42. doi: 10.1038/460041a. [DOI] [PubMed] [Google Scholar]
- 30.Samarajeewa NU, Ham S, Yang F, Simpson ER, Brown KA. Promoter-specific effects of metformin on aromatase transcript expression. Steroids. 2011;76:768–771. doi: 10.1016/j.steroids.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 31.Anisimov VN, Berstein LM, Egormin PA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Anisimov VN, Berstein LM, Egormin PA, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 33.Anisimov VN, Berstein LM, Popovich IG, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anisimov VN, Piskunova TS, Popovich IG, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2010;2:945–958. doi: 10.18632/aging.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bojkova B, Orendas P, Garajova M, et al. Metformin in chemically-induced mammary carcinogenesis in rats. Neoplasma. 2009;56:269–274. doi: 10.4149/neo_2009_03_269. [DOI] [PubMed] [Google Scholar]
- 36.Thompson MD, Grubbs CJ, Bode AM, et al. Lack of effect of metformin on mammary carcinogenesis in nondiabetic rat and mouse models. Cancer Prev Res (Phila) 2015;8:231–239. doi: 10.1158/1940-6207.CAPR-14-0181-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49:662–671. doi: 10.1002/mc.20637. [DOI] [PubMed] [Google Scholar]
- 38.Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99:2136–2141. doi: 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akinyeke T, Matsumura S, Wang X, et al. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis. 2013;34:2823–2832. doi: 10.1093/carcin/bgt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhalla K, Hwang BJ, Dewi RE, et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 2012;5:544–552. doi: 10.1158/1940-6207.CAPR-11-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Checkley LA, Rho O, Angel JM, et al. Metformin inhibits skin tumor promotion in overweight and obese mice. Cancer Prev Res (Phila) 2014;7:54–64. doi: 10.1158/1940-6207.CAPR-13-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider MB, Matsuzaki H, Haorah J, et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120:1263–1270. doi: 10.1053/gast.2001.23258. [DOI] [PubMed] [Google Scholar]
- 43.Tajima K, Nakamura A, Shirakawa J, et al. Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. Am J Physiol Endocrinol Metab. 2013;305:E987–998. doi: 10.1152/ajpendo.00133.2013. [DOI] [PubMed] [Google Scholar]
- 44.Vitale-Cross L, Molinolo AA, Martin D, et al. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila) 2012;5:562–573. doi: 10.1158/1940-6207.CAPR-11-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 46.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soranna D, Scotti L, Zambon A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17:813–822. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thakkar B, Aronis KN, Vamvini MT, Shields K, Mantzoros CS. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metabolism. 2013;62:922–934. doi: 10.1016/j.metabol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Wu L, Zhu J, Prokop LJ, Hassan Murad M. Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies. Sci Rep. 2015;5:10147. doi: 10.1038/srep10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonanni B, Puntoni M, Cazzaniga M, et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol. 2012;30:2593–2600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 52.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23:1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan BX, Yao WX, Ge J, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117:5103–5111. doi: 10.1002/cncr.26151. [DOI] [PubMed] [Google Scholar]
- 54.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodwin PJ, Pritchard KI, Ennis M, Clemons M, Graham M, Fantus IG. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8:501–505. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]
- 56.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 57.Campagnoli C, Pasanisi P, Abba C, et al. Effect of different doses of metformin on serum testosterone and insulin in non-diabetic women with breast cancer: a randomized study. Clin Breast Cancer. 2012;12:175–182. doi: 10.1016/j.clbc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Hadad S, Iwamoto T, Jordan L, et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011;128:783–794. doi: 10.1007/s10549-011-1612-1. [DOI] [PubMed] [Google Scholar]
- 59.Niraula S, Dowling RO, Ennis M, et al. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat. 2012;135:821–830. doi: 10.1007/s10549-012-2223-1. [DOI] [PubMed] [Google Scholar]
- 60.Kalinsky K, Crew KD, Refice S, et al. Presurgical Trial of Metformin in Overweight and Obese Patients with Newly Diagnosed Breast Cancer. Cancer Investigation. 2014;32:150–157. doi: 10.3109/07357907.2014.889706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuler KM, Rambally BS, DiFurio MJ, et al. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer Medicine. 2015;4:161–173. doi: 10.1002/cam4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: A preoperative prospective trial. Cancer. 2014;120:2986–2995. doi: 10.1002/cncr.28853. [DOI] [PubMed] [Google Scholar]
- 63.Chak A, Buttar NS, Foster NR, et al. Metformin Does Not Reduce Markers of Cell Proliferation in Esophageal Tissues of Patients With Barrett‚Äôs Esophagus. Clinical Gastroenterology and Hepatology. 2015;13:665–672. e664. doi: 10.1016/j.cgh.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3:1077–1083. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 65.Joshua AM, Zannella VE, Downes MR, et al. A pilot /`window of opportunity/' neoadjuvant study of metformin in localised prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:252–258. doi: 10.1038/pcan.2014.20. [DOI] [PubMed] [Google Scholar]
- 66.Monash University [June 30, 2012];The effects of metformin on the LKB1/AMP-activated protein kinase (AMPK) pathway in breast tissue, a randomized clinical trial. Available at: http://apps.who.int/trialsearch/trial.aspx?trialid=ACTRN12610000219088.
- 67.Columbia University [June 30, 2012];Metformin Pre-Surgical Pilot Study. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00930579.
- 68.Instituto Nacional de Cancer Brazil [June 30, 2012];The Use of Metformin in Early Breast Cancer Patients Pre-Surgery. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01302002.
- 69.Vanderbilt-Ingram Cancer Center [June 30, 2012];Metformin Hydrochloride in Treating Women With Stage I or Stage II Breast Cancer That Can Be Removed By Surgery. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00984490.
- 70.Oxford University Hospitals NHS Trust [June 30, 2012];Effect of Metformin on Breast Cancer Metabolism. Available at: http://clinicaltrials.gov/ct2/show/NCT01266486.
- 71.NCIC Clinical Trials Group [June 30, 2012];A Phase III Randomized Trial of Metformin vs Placebo in Early Stage Breast Cancer. Available at: http://clinicaltrials.gov/ct2/show/study/NCT01101438.
- 72.Seoul National University Hospita [June 30, 2012];Efficacy and Safety of Adjuvant Metformin for Operable Breast Cancer Patients. Available at: http://clinicaltrials.gov/ct2/show/NCT00909506.
- 73.University of California- San Diego [June 30, 2012];Reach for Health Study: Obesity-related Mechanisms and Mortality in Breast Cancer Survivors. Available at: http://clinicaltrials.gov/ct2/show/NCT01302379.
- 74.University of California- Irvine [June 30, 2012];A Trial of Metformin for Colorectal Cancer Risk Reduction Among Patients With a History of Colorectal Adenomas and Elevated Body Mass Index. Available at: http://clinicaltrials.gov/ct2/show/NCT01312467.
- 75.University of Arkansas [June 30, 2012];Effect of Metformin on Biomarkers of Colorectal Tumor Cell Growth. Available at: http://clinicaltrials.gov/ct2/show/NCT01632020.
- 76.Dana-Farber Cancer Institute [June 30, 2012];Exercise and Metformin in Colorectal Cancer Survivors. Available at: http://clinicaltrials.gov/ct2/show/NCT01340300.
- 77.Chiba University [June 30, 2012];A pilot study of the medroxyprogesterone + metformin combination therapy for the endometrial cancer. Available at: http://apps.who.int/trialsearch/trial.aspx?trialid=JPRNUMIN000002210.
- 78.National Cancer Institute (NCI) [June 30, 2012];Metformin Hydrochloride in Treating Patients With Prostate Cancer Undergoing Surgery. Available at: http://clinicaltrials.gov/ct2/show/NCT01433913.