Abstract
The primary cilium, a microtubule-based organelle found in most cells, is a centre for mechano-sensing fluid movement and cellular signalling, notably through the Hedgehog pathway. We recently found that each lens fibre cell has an apically situated primary cilium that is polarised to the side of the cell facing the anterior pole of the lens. The direction of polarity is similar in neighbouring cells so that in the global view, lens fibres exhibit planar cell polarity (PCP) along the equatorial-anterior polar axis. Ciliogenesis has been associated with the establishment of PCP, although the exact relationship between PCP and the role of cilia is still controversial. To test the hypothesis that the primary cilia have a role in coordinating the precise alignment/orientation of the fibre cells, IFT88, a key component of the intraflagellar transport (IFT) complex, was removed specifically from the lens at different developmental stages using several lens-specific Cre-expressing mouse lines (MLR10- and LR-Cre). Irrespective of which Cre-line was adopted, both demonstrated that in IFT88-depleted cells, the ciliary axoneme was absent or substantially shortened, confirming the disruption of primary cilia formation. However no obvious histological defects were detected even when IFT88 was removed from the lens placode as early as E9.5. Specifically, the lens fibres aligned/oriented towards the poles to form the characteristic Y-shaped sutures as normal. Consistent with this, in primary lens epithelial explants prepared from these conditional knockout mouse lenses, the basal bodies still showed polarised localisation at the apical surface of elongating cells upon FGF-induced fibre differentiation. We further investigated the lens phenotype in knockouts of Bardet–Biedl Syndrome (BBS) proteins 4 and 8, the components of the BBSome complex which modulate ciliary function. In these BBS4 and 8 knockout lenses, again we found the pattern of the anterior sutures formed by the apical tips of elongating/migrating fibres were comparable to the control lenses. Taken together, these results indicate that primary cilia do not play an essential role in the precise cellular alignment/orientation of fibre cells. Thus, it appears that in the lens cilia are not required to establish PCP.
Keywords: lens, primary cilium, IFT88, Bardet, Biedl Syndrome (BBS), planar cell polarity (PCP)
1. Introduction
The primary cilium is a microtubule-based cell membrane projection that emanates from a modified centriole, the basal body. The ciliary membrane contains various receptors or channels so that the cilium can act as a sensor or an antenna to detect diverged signals from extracellular space (Berbari et al., 2009; Satir et al., 2010). Among several signalling pathways that are known to be associated with cilia the most intensively examined is the Hedgehog pathway (Hh); indeed, cilia are the dominant domain for enabling the Hh pathway to initiate its signalling cascade (Briscoe and Therond, 2013). An emerging new pathway that has also been linked to the cilium is the Wnt/Frizzled planar cell polarity (PCP) pathway that provides a mechanism for orienting cells relative to the axis along the plane of the tissue (Jones et al., 2008; Ross et al., 2005). However, in contrast to the consensus on the role of cilia in the Hh pathway, the link between cilia and PCP is still controversial (Wallingford and Mitchell, 2011). Primary cilia are found in almost all vertebrate cells and in several tissues cilia are indispensable for their development and maintenance. Indeed disrupted ciliary function is related to a broad range of diseases including polydactyly, obesity, retinal degeneration and renal disease. These are collectively known as ciliopathies, and many syndromes are linked to genetic mutations in cilia-related genes (Waters and Beales, 2011).
The eye lens is comprised of two types of cells, the highly elongated fibre cells that constitute the bulk of the lens and the epithelial cells that form a thin layer on the anterior surface of the fibres (Fig. 1A). The lens epithelial cells are proliferative and are the progenitors for additional fibre cells that are progressively added to the fibre mass as the lens grows throughout life. The lens fibre cells align and pack regularly as they elongate and undergo terminal differentiation. During this process they also undergo directed migration towards the poles and eventually meet up with equivalent fibres from other segments of the lens. Because this behaviour is highly coordinated, the points of contact at their tips form distinctive Y-shaped suture lines (Fig. 1B, Kuszak et al., 2006). Primary cilia/basal bodies have been detected in both lens epithelial and fibre cells and interestingly, they show asymmetric localisation on the apical surfaces (Sugiyama et al., 2010). This is most pronounced in the fibre cells of the lens cortex; each fibre has a hexagonal apical surface and the cilium/basal body is localised towards the anterior side (the side facing towards the anterior pole, Fig. 1C). Since the direction of this polarity is similar in neighbouring cells, in the global view the lens fibres show planar polarity along the equatorial-anterior polar axis (Sugiyama et al., 2010). Given the role of primary cilia as antenna to detect extracellular signals and also their involvement in PCP formation, we hypothesised that the primary cilia on the fibre cells detect a global guidance signal (presumably coming from the anterior pole) to promote directed fibre cell migration to form lens PCP (Sugiyama et al., 2011).
Fig. 1. Lens structure (A–C) and development (D–H).
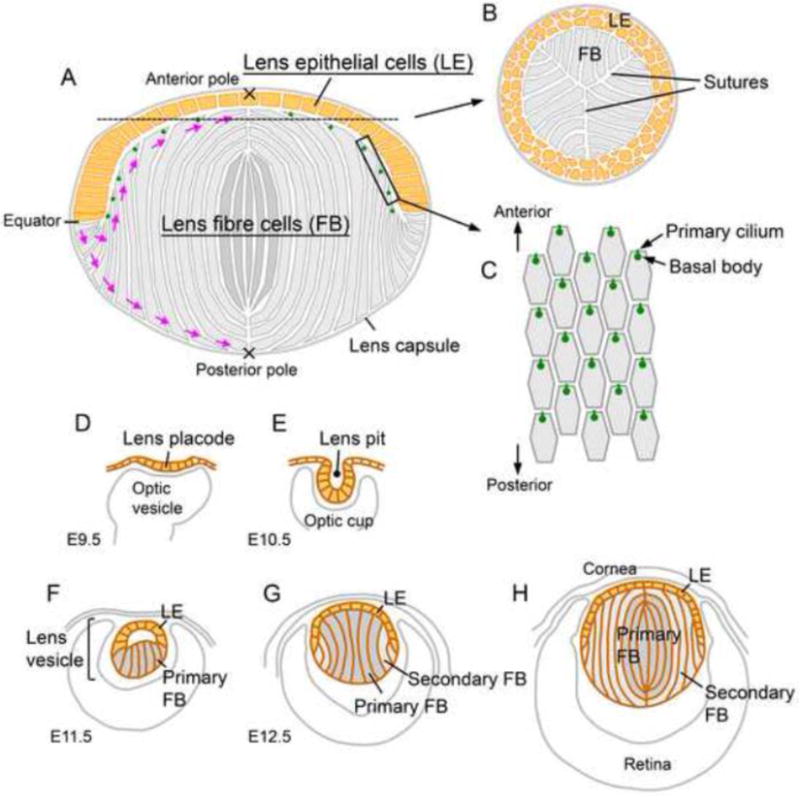
(A) Most of the proliferative activity in the lens epithelium (yellow) is found in the region anterior to the equator. Cells that shift posterior to the equator exit the cell cycle and initiate terminal differentiation into lens fibre cells (grey). The apical and basal tips of the elongating fibres undergo directed migration (purple arrows) to the anterior and the posterior poles, respectively. At the poles, each fibre tip meets the tip from an equivalent fibre from another segment and collectively these aligned junctions form the lens sutures. The epithelial and fibre cells are contained within a thick extracellular matrix, the lens capsule. (B) A cross section around the anterior pole shows Y-shaped sutures formed by the aligned apical tips of the fibre cells. (C) A superficial view shows the hexagonal apical surfaces of the fibres with anteriorly polarised primary cilia/basal bodies. (D–H) Lens morphogenesis begins with the thickening of the surface ectoderm in the head region adjacent to the optic cup to form the lens placode (D). Following invagination to form the lens pit (E), the cells round up and separate from the surface ectoderm to form the lens vesicle (F). The cells in the posterior half of the lens vesicle elongate and undergo terminal differentiation to form the primary fibre cells that fill the vesicle lumen (G). The cells in the anterior half of the vesicle form an epithelial layer and this establishes the characteristic anterior-posterior polarity of the lens (G). Secondary fibre cells differentiate from the lens epithelial cells at the lens equator and progressively accumulate around the primary fibre cells so that the primary fibres are internalised and comprise the lens nucleus (H).
Hh signalling plays critical roles during eye development. During eye formation Hh is essential to separate the two presumptive eye regions and its failure leads to cyclopia (Gongal et al., 2011). Hh is also required for closure of the choroid fissure during eyecup formation and its defective signalling causes coloboma (Gongal et al., 2011). It is also known that Hh mediates a switch between lens and anterior pituitary placodal cell identity; Hh signal suppresses lens fate and promotes the specification of anterior pituitary cells (Gunhaga, 2011). In accord with this, reduction of Hh activity leads to development of ectopic lenses whereas over-expression blocks lens formation (Gunhaga, 2011). Hh has also been reported to play an essential role during early lens development since conditional knockout (cKO) of Smo, the essential transducer of Hh signalling, in lens with Le-Cre [which expresses Cre in the lens placode from embryonic day 9.5 (E9.5)] induces lens defects (Choi et al., 2014). However the Hh pathway appears to be dispensable during later lens development since cKO of Smo with MLR10 which expresses Cre in the lens vesicle after E11.5 did not show any obvious lens defects (Choi et al., 2014). Thus, given the evidence that the primary cilium is a major site for Hh signalling, primary cilia may be essential during early lens development.
Extension and maintenance of the cilia requires intraflagellar transport (IFT), a kinesin/dynein motor-based trafficking system, by which components required to build up cilia are transported along the ciliary axoneme (Sung and Leroux, 2013). IFT proteins are the specialised components for this system and they form multi-protein complexes to mediate binding between the motors and cargoes. One of the IFT proteins, IFT88, is involved in anterograde (i.e. base to tip) transport and its disruption blocks cilia formation and suppresses Hh pathway signalling (Lehman et al., 2008). The IFT88 KO mice also showed orientation defects of the sensory hair cells in cochlea, suggesting a link to PCP (Jones et al., 2008). IFT88 KO mice are mid-gestation lethal with multiple defects including situs inversus, neural tube defects and a heart looping defect (Haycraft et al., 2007; Murcia et al., 2000). To test our hypothesis that cilia have a role in fibre cell organisation, we generated mice with IFT88 specifically inactivated in the lens. We also analysed lens phenotypes of Bardet-Biedl syndrome (BBS) protein mutants, which are also involved in cilia-mediated functions (Sung and Leroux, 2013).
2. Results
2.1 IFT88 is required for cilia formation in lens but not essential for lens development
Lens morphogenesis in mice begins by E9.5 with the appearance of the lens placode, a thickened region of surface ectoderm adjacent to the optic cup (Fig. 1D). This region invaginates to form the lens pit at E10.5 (Fig. 1E) and then eventually separates from the surface ectoderm to form the lens vesicle at E11.5 (Fig. 1F). The anterior cells of the lens vesicle develop an epithelial character whilst the posterior cells exit from the cell cycle and differentiate into elongated primary lens fibres. Thus by E12.5, the lens has generated its characteristic anterior-posterior polarity and spheroidal shape (Fig. 1G). After this, only epithelial cells proliferate and those that shift below the lens equator differentiate into secondary fibres that are progressively added to the primary fibre mass (Fig. 1H).
To generate lenses devoid of cilia we first crossed IFT88 cKO mice with the MLR10-Cre line which initiates Cre expression during the lens vesicle stage around E11.5 (Zhao et al., 2004). In this line, Cre is expressed in both the lens epithelial and the fibre cells (Zhao et al., 2004). We first examined IFT88 expression in the lens at E16.5. IFT88 was detected at the apical surface of the lens epithelial and fibre cells as small dots (Fig. 2Aa, a′). Acetylated tubulin is a marker for cilia and basal bodies (Fig. 2Ab, b′) and it showed extensive co-localisation with IFT88 signal (Fig. 2Ac, c′), confirming IFT88 localisation on the cilia/basal bodies. This IFT88 signal was absent from most cells in the cKO lenses (Fig. 2Ad, d′, f, f′). The structures detected by acetylated tubulin appeared as round spots (Fig. 2Ae, e′), indicating that basal bodies had lost the cilium extension in the cKO lenses (without cilia it is clearly seen that most of the basal bodies exist as twin spots, i.e. mother and daughter centrioles, Fig. 2Ae, e′). Cilia appeared to be shortened also in the region where IFT88 remnants were detected (Fig. 2Ad′–f′, arrowhead). This indicates formation of cilia was largely blocked in these cKO lenses.
Fig. 2. Depletion of IFT88 disrupts cilium formation but does not affect cellular alignment/orientation of lens fibre cells.
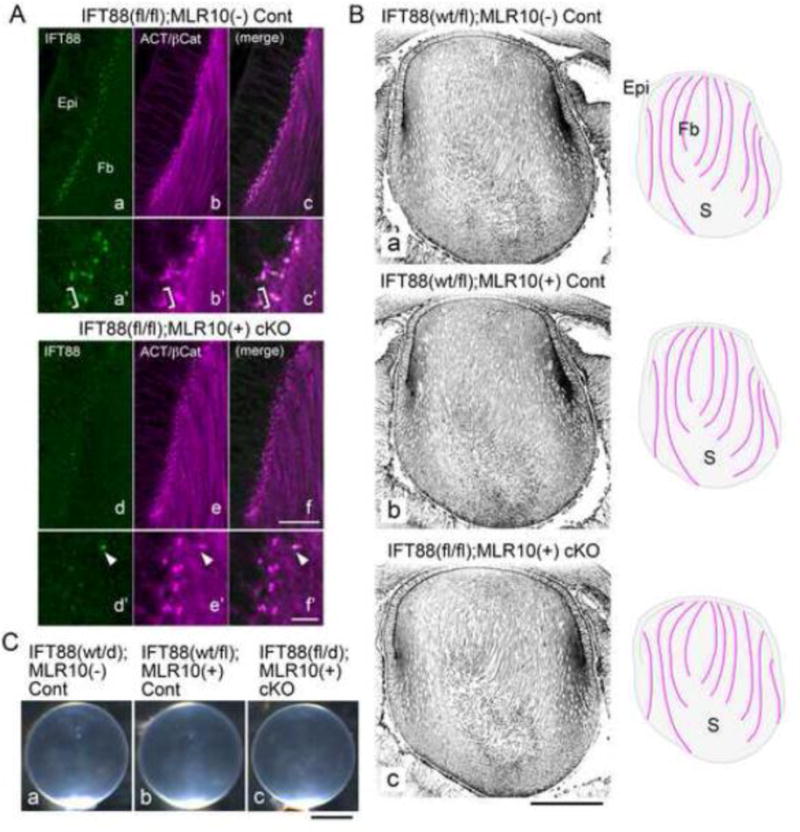
(A) Immunofluorescent staining of paraffin sections for IFT88 (green) and acetylated α–tubulin + β-catenin (purple) (E16.5). This shows lens epithelial (Epi) and fibre (Fb) cells in a region anterior to the equator of control [IFT88(fl/fl);MLR10(−)] and cKO [IFT88(fl/fl);MLR10(+)] lenses. IFT88 signals found at the apical surfaces of the epithelial and fibre cells in the control lenses were mostly depleted in the cKO lenses, although some remnants were still detectable (e.g. arrowhead, d′, e′, f′). Acetylated α–tubulin detected cilia, basal bodies and filamentous tubulin bundles in the elongating fibres, whereas the signal of β-catenin was visible on the lateral membrane of the epithelial cells (the other signals of β-catenin were obscured by the strong acetylated α-tubulin signals and barely detectable in these images). IFT88 signals were detected as dots at the bases and the tips of cilia (bracket in a′, b′ and c′ highlights a cilium which has two spots of IFT88 at both ends. Note the primary cilium is absent on the next basal body, ie. the daughter centriole). (B) Immunofluorescent staining of paraffin sections against β-catenin to show cellular alignment/orientation of lens fibres (E16.5). Cre-negative [IFT88(wt/fl);MLR10(−)] and Cre allele-positive [IFT88(wt/fl);MLR10(+)] controls and cKO [IFT88(fl/fl);MLR10(+)] lenses show a similar pattern of fibre cell alignment/orientation. Schematic drawings on the right side of each image outline overall fibre cell alignment. Note that around the posterior region of the lenses where fibre tips had begun to form sutures (S) they are cut transversely rather than along their length. (C) In 8-month-old cKO mice the lenses maintained normal transparency and appeared identical to the lenses of control mice of the same age. Abbreviations for IFT88 alleles, wt, wild-type; fl, floxed; d, deleted. For MLR10 Cre allele, +, positive; −, negative. Scale bars: A 20 μm (upper panels) and 5 μm (lower panels), B 200 μm, C 1mm.
However we did not detect any morphological abnormality of the lens epithelial or fibre cells in the cKO lenses (Fig. 2B). At E16.5, the control lenses [without and with Cre allele, i.e. IFT88(wt/fl);MLR10(−) and IFT88(wt/fl);MLR10(+), respectively (Fig. 2Ba, b)] showed complicated but characteristically-patterned alignment of the lens fibres. This fibre cell alignment was faithfully maintained in the cKO lenses [IFT88(fl/fl);MLR10(+) (Fig. 2Bc)]. The lens epithelial cells also maintained their characteristic columnar structure both in the control and the cKO lenses (Fig. 2Ba–c). Any morphological abnormality or metabolism defect that arises in lens cells can eventually lead to development of cataract. However, even after 8 months, the cKO lenses maintained their characteristic clarity and showed no signs of cataract formation (Fig. 2Cc); histologically they were indistinguishable from control lenses (Fig. 2Ca, b).
2.2 Directed migration and polarised localisation of the basal bodies was not disrupted by depletion of cilia in the elongating lens fibres
We next prepared lens epithelial explants from IFT88 control and cKO neonates (Fig. 3). The lens epithelial cells maintained their original cobblestone-like appearance when they were cultured for two days without growth factor supplement (Fig. 3A). In control cultures (Fig. 3Aa–c) each lens epithelial cell developed a cilium on the apical surface that was detected by acetylated α–tubulin staining (Fig. 3Ab). IFT88 signal was also detected on these cilia (Fig. 3Aa, c), confirming expression and localisation of endogenous IFT88 on the cilia in control cells. The number of cilia was greatly reduced in the epithelial cells prepared from IFT88 cKO lenses (Fig. 3Ad–f); remnants of cilia, as shown by IFT88 staining, were found in just a few cells (Fig. 3Ad–f, arrow), again confirming requirement of IFT88 for cilia formation in the lens cells.
Fig. 3. Depletion of IFT88 blocks cilia formation in lens epithelial cell explants but does not prevent polarised localisation of basal bodies during fibre differentiation.
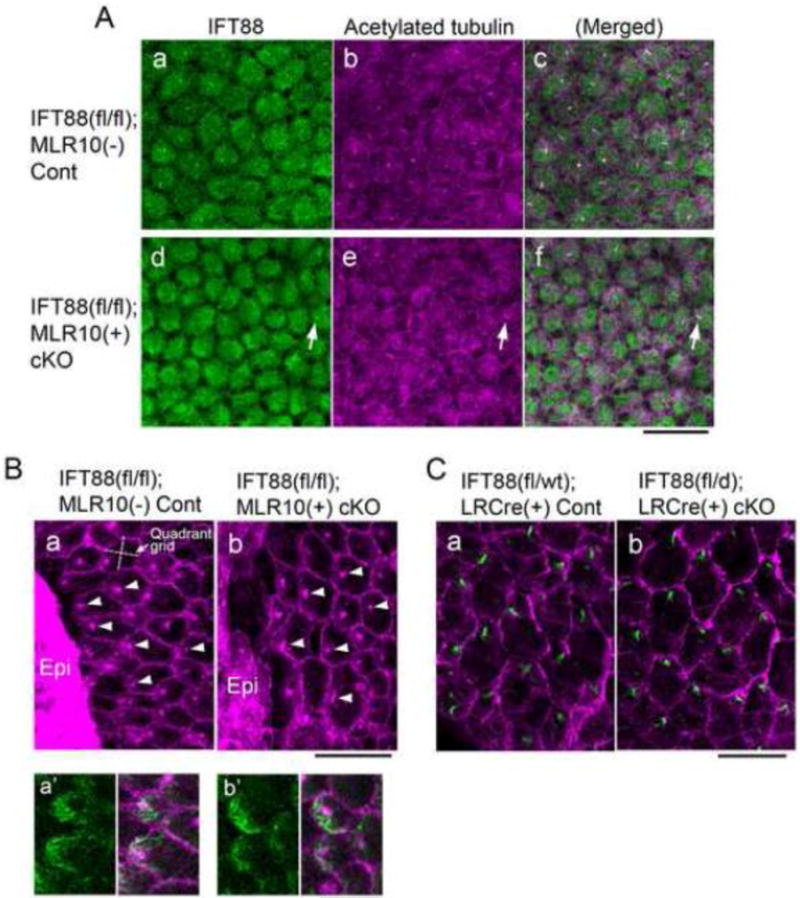
(A) Immunostaining of IFT88 (a, d, green) and acetylated α-tubulin (b, e, purple) of lens epithelial explants prepared from control (a–c) and IFT88 cKO (d–f) neonates (c and f show merged image of a and b, and d and e, respectively). In cKO explants most of the epithelial cells were devoid of cilia and only a few cells were positive for IFT88 and acetylated α-tubulin staining (d–f, arrows). Note that the low level of cytoplasmic staining for IFT88 was non-specific since it was also detected in cKO cells. (B) Immunostaining for β-catenin (signal was localised to cell boundaries) and pericentrin (signal localised basal bodies) in the epithelial explants prepared from lenses of neonates that were cultured for 8 days with 50 ng/mL FGF to induce fibre differentiation. Most cells differentiated into fibre cells with characteristic hexagonal profiles but some cells remained undifferentiated and formed epithelial islands (Epi). The basal bodies at the apical surfaces of the elongating fibres were polarised towards an epithelial island in most cells of control (a) and IFT88 cKO (b) explants. The orientation of the quadrant grid in each cell was related to the direction of the nearest epithelial island (an example gird is shown in a). In these elongating fibres polarised accumulation of acetylated α-tubulin (green in a′, control and b′, cKO) was located at the migrating front of the apical surface of each fibre. (C) Immunostaining for rootletin (green) localised the ciliary rootlet that extends from the basal body and forms part of the basal body at the apical surface of each of the fibres of control (a) and IFT88 cKO (b) lenses (5-week-old). β-catenin staining (purple) delineates the hexagonal boundaries of fibre cells. The anterior pole and the equator of each lens locate to the top and the bottom of the images, respectively. The cilia/basal bodies in both control and cKO lenses showed polarised localisation to the anterior side of the hexagonal apical surface of the fibres. Scale bars: A–C 20 μm; Ba′, b′ 10 μm.
We have recently shown that FGF induces all features of fibre differentiation, including PCP formation (Dawes et al., 2014). FGF treatment induces groups of cells to elongate and differentiate into fibres but through a minor manipulation of the system (including the use of a sub-maximal fibre differentiation dose of 50 ng/ml FGF2), groups/islands of epithelial cells are retained. This co-existence of differentiated lens fibres and un-differentiated lens epithelial cells in close proximity has a profound effect; the elongating fibres become aligned/oriented towards the epithelial islands (Fig. 3Ba, Dawes et al., 2014). These fibres also show polarised localisation of cilia/basal bodies on their hexagonal apical surfaces and in this way recapitulate the PCP that is evident in the elongating fibres in vivo (Sugiyama et al., 2010). Here we showed that fibre cell differentiation and epithelial island formation were similarly induced in IFT88 cKO explants. In addition, and similar to controls, the basal bodies in IFT88 cKO explants at the apical tips of the elongating fibres also showed polarised localisation towards the epithelial cells (Fig. 3Bb, arrowheads). A superimposed quadrant grid (Fig. 3Ba) was used to quantify the position of the basal body within each cell that was located up to 50 μm from the epithelial islands. This showed that most of the basal bodies were located in the quadrant that was closest to the epithelial islands than in any of the other quadrants in both control (83.1%, n=65) and cKO (80.0%, n=30) explants. We also detected polarised accumulation of acetylated tubulin to the migrating front near the basal bodies both in control and cKO explants (Fig. 3Ba′, b′). Thus this observation indicates that IFT88 is not required for polarised localisation of cilia/basal bodies in the lens fibres and their directed migration in vitro.
2.3 Polarised localisation of basal bodies is maintained when cilia are depleted from the lens placode stage
Given that the timing of Cre expression in the MLR10 line was too late to see an influence of IFT88/cilia depletion on earlier events in lens development, we generated conditional IFT88 KOs with another Cre line. In this line (LR-Cre line) expression of Cre is detected in the lens placode from E9.5 (Kreslova et al., 2007). As before, we confirmed depletion of IFT88 expression by immunofluorescence staining; however, similar to the MLR10-Cre line, we did not detect any morphological abnormality in IFT88;LR-Cre cKO lenses (data not shown). Using these mice, we prepared whole mounts of epithelial cells from adult lenses and immunolabelled the apical surfaces of the fibres to localise the cilia/basal bodies as previously described (Sugiyama et al., 2010). We labelled for rootletin that is found in the ciliary rootlet originating from the basal body at the proximal end of the cilium (Yang et al., 2002). This showed that the basal bodies exhibited the same polarised localisation towards the anterior side of the hexagonal apical surfaces of the lens fibres in IFT88;LR-Cre cKO lenses, similar to that seen in the controls (Fig. 3Ca, b). Together, these observations indicate that IFT88 is not required for lens induction and development and that fibre cell polarity is established without any influence from cilia.
The LR-Cre KO mice were also useful for assessing the role of Hh signalling during lens development. Given cilia are essential for keeping the Hh pathway inactive, as well as transducing the activating signal, some developmental defects might have been expected in IFT88/cilia-depleted lenses since disruption of Hh signalling by early cKO of Smo (E9.5, using Le-Cre) in the lens has been reported to cause smaller lens formation with defective lens epithelium (Choi et al., 2014). Consistent with the observation that later depletion of Smo with MLR10 from E11.5 did not affect lens formation (Choi et al., 2014), in our study IFT88 depletion with MLR10-Cre also did not result in any lens abnormality (Fig. 2). Although we too observed small lens formation with cKO of IFT88 using Le-Cre (data not shown), the Le-Cre colony maintained in our facility consistently exhibited lens defects independent of the gene knockout [i.e. IFT88(fl/wt);LeCre(+) or even IFT88(wt/wt);LeCre(+) mice developed small and abnormal lenses]. This background trait was constantly segregated with the Cre-allele during backcross procedures from FVB to C57BL6 for 8 generations, suggesting the defect was closely linked to the exogenous Cre cassette or the insertion point of this cassette into the genome (data not shown). A similar defect, shown to be linked to the Le-Cre allele after a systematic analysis, was also reported recently (Dora et al., 2014). This prompted us to use the LR-Cre line which also expresses Cre from E9.5 in the lens placode (Kreslova et al., 2007). As with the MLR10-Cre, although the cilia were virtually completely removed from the lens, we did not see any abnormality during lens formation and maintenance in the LR-Cre-mediated IFT88 cKO lenses (data not shown; Fig. 3). Thus our current results do not support a role for cilia in Hh signalling regulation during lens formation and maintenance stages.
2.4 Lens fibre cells form a normal suture pattern at the anterior pole in BBS4 and BBS8 KOs
We next examined lens phenotype in BBS4 and BBS8 KO mice (Fig. 4). These mice show several ciliopathy-related phenotypes including obesity and retinal degeneration (Kulaga et al., 2004; Mykytyn et al., 2004; Tadenev et al., 2011). They also show defective orientation of the sensory hair cells in the inner ear of the cochlea, a common failure found in PCP mutants (May-Simera et al., 2015; Ross et al., 2005). BBS4 null mice show partially penetrant embryonic lethality and all pups which develop to term are runts at birth (Kulaga et al., 2004; Mykytyn et al., 2004). BBS8 KOs develop to term but frequently die before weaning (Tadenev et al., 2011). To determine if there was any evidence of defective orientation of lens fibres we examined the suture pattern formed by the apical tips of the fibres at the anterior pole of each of the BBS KO lenses (Fig. 4). We detected the normal Y-shaped sutures in both BBS4 and BBS8 null lenses (Fig. 4B, D) as seen in their littermate controls (Fig. 4A, C). This observation indicates that the lens fibre cells align and orient towards the poles normally in the absence of BBS4 and BBS8.
Fig. 4. Lens fibre cells maintain normal cellular alignment in BBS4 and BBS8 KOs.
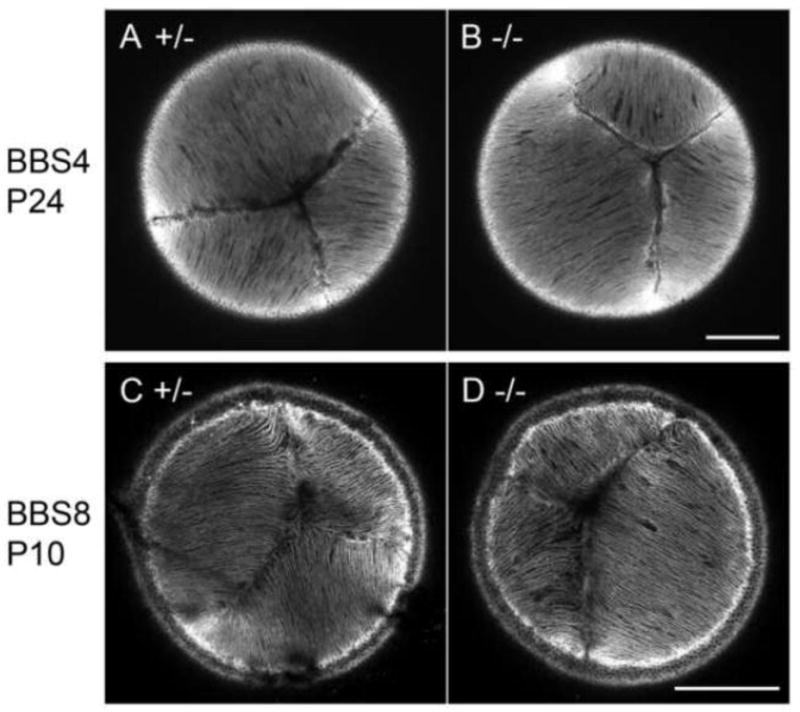
Confocal sections of the anterior aspect of the lenses stained with phalloidin. In control lenses (A, C) apical tips of the fibres formed the typical Y-shaped suture pattern. This pattern was also detected in both BBS4 (B) and BBS8 (D) KOs. Scale bars 200 μm.
3. Discussion
To test whether the primary cilia play essential roles in regulating early lens development and particularly in generating the characteristically highly ordered alignment/orientation of lens fibre cells, we generated lens specific IFT88 KOs and examined lens phenotypes. We observed disruption of cilia in the cKO lenses and also in the lens epithelial explants derived from the cKO lenses, confirming a requirement for IFT88 in primary cilia formation in the lens. However the lenses formed in the cKOs were morphologically normal with no indication of any abnormality caused by primary cilia depletion. This conclusion was obtained from the analysis of cKO mice generated with MLR10 (Cre expression starts from E11.5 at the lens vesicle stage) and LR-Cre (Cre expression starts from E9.5 at the lens placode stage). Thus the primary cilia are dispensable and appear to have non-essential roles during lens development and maintenance.
Further evidence that cilia do not have a functional role in alignment/orientation of fibres in the lens comes from the analysis of BBS4/8 KO mice. BBS is a pleiotropic genetic disorder characterised by retinal dystrophy, obesity, polydactyly, renal anomalies, cognitive deficits and hypogenitalism (Forsythe and Beales, 2013). So far more than 16 BBS genes are linked to this disease but all their products are associated with cilia/basal bodies. Generally BBS proteins are not essential for the ciliary axoneme formation but are indispensable for normal function of cilia in many tissues. Some of the BBS proteins form a multiple protein complex, the BBSome, and work as an adaptor for IFT machinery to transport various signalling molecules in the cilium. Retinal dystrophy is the most common indicator for BBS diagnosis and is found in more than 90% of patients. Obesity is also a common feature found in more than 70% of patients and some of this may be linked to the development of type 2 diabetes that is observed in 6–48% of patients. Cataract is reported in BBS patients but it is generally recognised as a secondary feature. BBS4 and BBS8 knockout mice show several of these ciliopathy-related phenotypes but their lenses had normal sutures at the anterior pole, indicating that normal fibre cell alignment/orientation takes place without these BBS proteins. Taken together with the fact that IFT88 KOs also did not develop cataract, some cataract observed in BBS patients may be induced as a side effect of other abnormalities such as retinal degradation and/or metabolic defects associated with diabetes and is not a direct cause of impairment of cilia function.
In the sensory hair cells of the cochlea and the ependymal cells in the cerebral ventricle of the brain, polarised localisation of the basal body at the apical surface of groups of cells depends on the presence of a primary cilium in their primordia (Jones et al., 2008; Mirzadeh et al., 2010). Although the loss of cilia in these cells disrupts polarised localisation of the basal bodies, planar-polarised distribution of the core PCP proteins is not affected (Guirao et al., 2010; Jones et al., 2008). Thus it has been suggested that cilia act independently of, or downstream from, the PCP signalling pathway during cellular polarisation (Wallingford and Mitchell, 2011). However this interpretation is still controversial as a recent report showed a possible upstream function of cilia-related proteins to target a core PCP protein to the specific apical region of the sensory hair cells of the cochlea (May-Simera et al., 2015). In the lens fibres the cilia show distinct polarisation and this prompted us to speculate that cilia have a role in cellular polarisation (Sugiyama et al., 2011). Given the evidence from our in vivo and in vitro studies that the basal bodies still become polarised to one side of each of the differentiating fibre cells in the absence of the cilium, this contrasts with the cilium-dependent polarisation in sensory hair cells and ependymal cells, and thus suggests different mechanisms may operate in these different contexts. In the lens fibres, PCP signalling may be solely required to establish polarised localisation of the cilia/basal body. Alternatively as the anterior localisation of the cilia/basal bodies corresponds to the direction of the fibre tip migration, this may relate to cytoskeletal rearrangement/polarisation induced at the leading edge of migrating cells that is regulated by another mechanism.
Although the cilia itself does not have a role in lens fibre differentiation, the importance of the basal bodies/centrioles in promoting alignment/orientation of the fibres should not be overlooked. In IFT88 cKO lenses the basal bodies retained their polarised localisation to the anterior side of the elongating fibre tips and at this region we also detected polarised accumulation of acetylated tubulin similar to that observed in control lenses (Fig. 3B). Given a potential role of the basal body/centrioles to serve as a microtubule organising centre, the polarised localisation of the basal bodies may be central and sufficient to induce polarisation of the cytoskeletal components and in turn the directed behaviour of the fibres. Further studies will be required to determine if the basal body/centrioles have a role in promoting the alignment/orientation of fibres that is critical for the formation of a functional lens with its characteristically highly ordered, three-dimensional cellular architecture.
4. Experimental Procedures
4.1 Animals
The animal study protocols and procedures have been approved by the Animal Ethics Committee of the University of Sydney. The conditional allele of IFT88 [IFT88(fl)] was designed to remove exons 4 to 6 by Cre-mediated loxP system (Haycraft et al., 2007). The germline null allele of IFT [IFT88(d)] was derived from this floxed allele (Haycraft et al., 2007). Cre expression of MLR10 transgenic line is driven by αA-crystallin promoter with consensus Pax6 binding site insertion (Zhao et al., 2004). Cre expression of LR-Cre transgenic line is driven by the Pax6 P0 minimum promoter with three copies of a lens-specific element originated from the upstream region of the Pax6 gene (Kreslova et al., 2007). We analysed the phenotype after IFT88 depletion in conditional KOs [IFT88(fl/fl);Cre(+) or IFT88(fl/d);Cre(+)] and compared with littermate Cre-negative controls [IFT88(wt/fl)Cre(−), IFT88(wt/d);Cre(−), IFT88(fl/fl);Cre(−) or IFT88(fl/d);Cre(−)] as well as Cre-positive controls [IFT88(wt/fl);Cre(+) or IFT88(wt/d);Cre(+)] to ensure that we detected any KO allele-independent effects caused by the Cre allele alone. BBS4 and BBS8 KO samples were provided by Philip Beales and Helen-May Simera, respectively. BBS4 KOs have a gene-trapping cassette insertion in intron 1, which resulted in aberrant splicing and complete loss of the mRNA (Kulaga et al., 2004). BBS8 KOs lack the first exons and have an insertion of a reporter construct that is expressed from the endogenous BBS8 start codon (Tadenev et al., 2011).
4.2 Antibodies
For immunostaining we used the following primary antibodies; rabbit antibodies against IFT88 (Haycraft et al., 2005), β-catenin (H102, sc7199, Santa Cruz), pericentrin (ab4448, Abcam); mouse antibodies against acetylated α–tubulin (T6793, Sigma), β-catenin (clone 14, 610154, Transduction Laboratories) and goat antibody against Rootletin (C20, sc-67824, Santa Cruz).
4.3 Histology
Embryonic heads were fixed with 10% neutral buffered formalin overnight at room temperature. Paraffin blocks were prepared by a standard method and 5 μm sections were used for histological analyses. For immunostaining the sections were boiled in 10 mM Na-citrate buffer (pH6.0) for 10 min at 120°C for antigen retrieval, followed by permeabilisation with 0.5% TritonX-100/PBS and aldehyde group-quenching with 100mM glycine/PBS before blocking with 10% normal donkey serum (NDS) in TBST-BSA [10 mM Tris–HCl (pH 8.0), 150 mM NaCl, 0.05% Tween20, 0.1% bovine serum albumin]. Primary antibodies were suspended in TBST-BSA with 1.5% NDS and reacted with tissues overnight at 4°C. Fluorescent secondary antibodies were suspended in TBST-BSA and applied to tissues for 45 minutes at room temperature. Aqua-Poly/Mount (18606, Polysciences, Inc.) was slightly diluted with 20% volume of dH2O and used for coverslip mounting. Fluorescent signals were detected by the Zeiss LSM5 Pascal or LSM700 confocal systems.
4.4 Lens epithelium explant culture
Each lens was dissected out from a neonatal mouse eye and the lens fibre mass was removed from the lens capsule, thereby leaving the lens epithelial sheet attached to the capsular surface. The epithelium-containing lens capsule was pinned onto the base of a 3.5cm plastic dish by either, (i) placing the epithelial cell side up (normal explant) for cilium growing experiments or (ii) placing the epithelial side face down on the dish surface (inverted explant) for FGF-induced differentiation experiments. Explants were cultured with M199 medium (Gibco) supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL of fungizone and 10 mM HEPES in a standard 5% CO2 chamber at 37°C. For the fibre cell differentiation assay, explants were cultured with 50 ng/mL FGF-2 (recombinant human FGF basic 146aa, R&D).
4.5 Whole mount and anterior suture observation
Detailed methods have been described previously (Sugiyama and McAvoy, 2012). Briefly, for whole mount preparations, dissected lenses were fixed with 100% methanol for 3 minutes and rinsed with PBS. Epithelial cell whole mounts were prepared and processed for immunostaining following the protocol described above after the blocking step. For anterior suture observation, lenses were fixed with 4% PFA overnight at room temperature. After rinsing with PBS, lenses were incubated with 4U/mL of Alexa Fluor 488 phalloidin (Molecular Probes) dissolved in PBS containing 1% TritonX-100 for 3 days at room temperature. Z-series images of confocal microscopy were collected around the anterior pole.
Highlights.
-
-
IFT88 is required for primary cilia formation in lens.
-
-
However IFT88 is not required for lens development.
-
-
BBS4 and BBS8 knockout mice develop morphologically normal lens.
Acknowledgments
We would like to thank Drs Carlo Iomini, Noriyuki Sugiyama and Takahiko Yokoyama for supplemental samples used in course of this study. IFT88 conditional mutants were provided through support from the UAB Heptorenal Fibrocystic Disease Core Center (NIH R01 DK074038). Funding for this work was provided by grants from the NIH (R01 EY03177), USA, the National Medical Research Council (NHMRC, Australia), the Sydney Foundation for Medical Research (now the National Foundation for Medical Research and Innovation) and the Ophthalmic Research Institute of Australia (ORIA). The work was also supported by the Grant Agency of the Czech Republic (15-23675S). Phil Beales is an NIHR Senior Investigator and is funded by the Dutch Kidney Foundation (Kouncil).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–35. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–29. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Ting CT, Trogrlic L, Milevski SV, Familari M, Martinez G, de Iongh RU. A role for smoothened during murine lens and cornea development. PLoS One. 2014;9:e108037. doi: 10.1371/journal.pone.0108037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Lovicu FJ, Harris CG, Shelley EJ, McAvoy JW. Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism. Dev Biol. 2014;385:291–303. doi: 10.1016/j.ydbio.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora NJ, Collinson JM, Hill RE, West JD. Hemizygous Le-Cre transgenic mice have severe eye abnormalities on some genetic backgrounds in the absence of LoxP sites. PLoS One. 2014;9:e109193. doi: 10.1371/journal.pone.0109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet. 2013;21:8–13. doi: 10.1038/ejhg.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongal PA, French CR, Waskiewicz AJ. Aberrant forebrain signaling during early development underlies the generation of holoprosencephaly and coloboma. Biochim Biophys Acta. 2011;1812:390–401. doi: 10.1016/j.bbadis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, Mirzadeh Z, Cremer H, Montcouquiol M, Sawamoto K, Spassky N. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–50. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- Gunhaga L. The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philos Trans R Soc Lond B Biol Sci. 2011;366:1193–203. doi: 10.1098/rstb.2010.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–16. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis. 2007;45:157–68. doi: 10.1002/dvg.20277. [DOI] [PubMed] [Google Scholar]
- Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–8. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Mazurkiewicz M, Zoltoski R. Computer modeling of secondary fiber development and growth: I. Nonprimate lenses. Mol Vis. 2006;12:251–70. [PubMed] [Google Scholar]
- Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Kidney mouse: modeling ciliopathies of mice and men. Dev Dyn. 2008;237:1960–71. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Simera HL, Petralia RS, Montcouquiol M, Wang YX, Szarama KB, Liu Y, Lin W, Deans MR, Pazour GJ, Kelley MW. Ciliary proteins Bbs8 and Ift20 promote planar cell polarity in the cochlea. Development. 2015;142:555–66. doi: 10.1242/dev.113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Han YG, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010;30:2600–10. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–55. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, Braun T, Casavant T, Stone EM, Sheffield VC. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci U S A. 2004;101:8664–9. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–40. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Lovicu FJ, McAvoy JW. Planar cell polarity in the mammalian eye lens. Organogenesis. 2011;7:191–201. doi: 10.4161/org.7.3.18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, McAvoy JW. Analysis of PCP defects in mammalian eye lens. Methods Mol Biol. 2012;839:147–56. doi: 10.1007/978-1-61779-510-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Stump RJ, Nguyen A, Wen L, Chen Y, Wang Y, Murdoch JN, Lovicu FJ, McAvoy JW. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev Biol. 2010;338:193–201. doi: 10.1016/j.ydbio.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CH, Leroux MR. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat Cell Biol. 2013;15:1387–97. doi: 10.1038/ncb2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadenev AL, Kulaga HM, May-Simera HL, Kelley MW, Katsanis N, Reed RR. Loss of Bardet-Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. Proc Natl Acad Sci U S A. 2011;108:10320–5. doi: 10.1073/pnas.1016531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–13. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–56. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Yue G, Adamian M, Bulgakov O, Li T. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J Cell Biol. 2002;159:431–40. doi: 10.1083/jcb.200207153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:1930–9. doi: 10.1167/iovs.03-0856. [DOI] [PubMed] [Google Scholar]


