Abstract
Saccharomyces interspecies hybrids are critical biocatalysts in the fermented beverage industry, including in the production of lager beers, Belgian ales, ciders, and cold-fermented wines. Current methods for making synthetic interspecies hybrids are cumbersome and/or require genome modifications. We have developed a simple, robust, and efficient method for generating allotetraploid strains of prototrophic Saccharomyces without sporulation or nuclear genome manipulation. S. cerevisiae × S. eubayanus, S. cerevisiae × S. kudriavzevii, and S. cerevisiae × S. uvarum designer hybrid strains were created as synthetic lager, Belgian, and cider strains, respectively. The ploidy and hybrid nature of the strains were confirmed using flow cytometry and PCR-RFLP analysis, respectively. This method provides an efficient means for producing novel synthetic hybrids for beverage and biofuel production, as well as for constructing tetraploids to be used for basic research in evolutionary genetics and genome stability.
Keywords: Saccharomyces, hybrids, biofuels, brewing, synthetic zymurgy, prototrophic
Graphical abstract
1. Introduction
Many eukaryotic organisms are able to reproduce sexually, wherein meiosis serves to both increase genetic diversity and to repair genetic material (Kohl and Sekelsky, 2013). As with other eukaryotes, fungal meiosis generally initiates in diploid cells (John, 1990). Regulation of this process involves specialized genetic loci called the mating-type (MAT) loci (Kronstad and Staben, 1997). While basidiomycetes may possess thousands of different mating types, ascomycetes possess only two (Casselton and Olesnicky, 1998). Ascomycete MAT loci contain between one and three genes. The mating-type genes are not recently diverged homologs, and are thus described as idiomorphs, rather than alleles. These mating-type genes encode transcription factors that regulate the expression of meiosis-specific genes (Van Heeckeren et al., 1998), as well as other genes that function in self recognition (Glass et al., 2000), pheromone production (Bardwell, 2004), and non-homologous end joining (Frank-Vaillant and Marcand, 2001). Heterothallic ascomycetes, such as Neurospora crassa, require two individuals of different mating types to contribute haploid nuclei towards a transient diploid meiotic precursor cell (Glass et al., 1990; Staben and Yanofsky, 1990).
The genus Saccharomyces is composed of unicellular diploid ascomycete fungi (Hittinger, 2013). The S. cerevisiae MAT idiomorphs are termed MATa and MATα, and both possess two genes (Haber, 2012). Unlike many other ascomycetes where vegetative haploid nuclei give rise to a transient diploid cell, Saccharomyces haploid spores of a single mating type rapidly give rise to stable diploid cells possessing both mating-type loci by way of mating-type switching (Haber, 2012). When a newly germinated haploid cell divides, the mother cell expresses HO, producing an endonuclease that specifically targets the mating-type locus. The resulting DNA double-strand break (DSB) is repaired using information from silenced copies of either mating-type idiomorph found on the same chromosome as the MAT locus (designated HML and HMR). Repairing the DSB lesion occurs by replacing the existing MAT idiomorph with the silenced copy of the other MAT idiomorph, resulting in the mother cell switching to the opposite mating type. As the daughter cell retains the initial idiomorph, mating-type switching ensures two haploid cells of opposite mating-types are adjacent to one another for schmooing and the formation of a diploid zygote. Deletion of HO prevents mating types from being switched, forming stable haploid strains, such as those typically used in laboratory research (Walker et al., 2003). Indeed, the plasticity of Saccharomyces ploidy is a major component of the awesome power of yeast genetics. Induced HO expression from plasmids in hoΔ strains triggers mating-type switching, resulting in diploidization of otherwise stable haploid strains (Herskowitz and Jensen, 1991; Jensen and Herskowitz, 1984). This diploid yeast cell can then be sporulated by standard methods, dissected, and the progeny screened for their mating type. The resulting haploids are isogenic to the original haploid strain save for their mating types, which can then be used in downstream genetic crosses. In addition to long-standing use to control mating type and ploidy in lab strains of S. cerevisiae, induction of HO expression has also been used to enable crosses between a fertile strain of S. uvarum and a sterile strain whose genome was predominantly S. uvarum, allowing a trait to be mapped using standard meiotic techniques (Schwartz et al., 2012).
All seven species of Saccharomyces yeasts possess the same mating-type locus organization and a predominantly diploid or diplontic lifestyle. Only limited pre-zygotic speciation barriers exist between Saccharomyces species (Maclean and Greig, 2008), making hybridization a trivial process with marked heterothallic haploids (Bullard et al., 2010; Hebly et al., 2015; Hittinger, 2013; Piotrowski et al., 2012; Scannell et al., 2011; Swain Lenz et al., 2014; Tirosh et al., 2009). Hybridization events also happen in the wild, though at a very low frequency (Mortimer, 2000). These hybrids arise when a cell of one species mates with a cell from another. Interspecies hybridization can either occur between two haploids, as is typically done in the lab, or through "rare mating" when one or both parents are diploid. Rare mating diploids can gain mating competency by simply inactivating one idiomorph to become hemizygous or undergoing spontaneous gene conversion at the MAT locus and becoming MATa/MATa or MATα/MATα diploids (Gunge and Nakatomi, 1972).
Interspecies hybrids spontaneously arising in this manner have found purchase in the conditions created by humans during industrial fermentations, such as brewing; indeed, hybrids produce many commercially important fermented beverages. For example, Saccharomyces cerevisiae × Saccharomyces eubayanus hybrids are used to produce lager beer, the most common fermented beverage on the planet (Corran, 1975; Libkind et al., 2011). Two major lineages are used in lager production (Dunn and Sherlock, 2008; Nakao et al., 2009; Walther et al., 2014), and recent evidence indicates that these two lineages arose from independent hybridization events, suggesting that genetic diversity from the parental populations may be one contributor to the phenotypic differences seen in modern industrial strains (Baker et al., 2015). Many other fermented beverages also make use of Saccharomyces hybrids: S. cerevisiae × Saccharomyces uvarum hybrids are used in production of some ciders and cold-fermented wines (Le Jeune et al., 2007; Masneuf et al., 1998; Pérez-Través et al., 2014b), while many Belgian ale and some European wine yeasts are S. cerevisiae × Saccharomyces kudriavzevii hybrids (Peris et al., 2012a, 2012b).
The discovery of the wild genetic stocks related to the constituent species of industrial interspecies hybrids (Almeida et al., 2014; Libkind et al., 2011; Sampaio and Gonçalves, 2008), as well as the discovery of several more divergent lineages that do not seem to have contributed to the production of fermented beverages (Bing et al., 2014; Hittinger et al., 2010; Leducq et al., 2014; Liti et al., 2009, 2005; Peris et al., 2014; Wang et al., 2012), has raised interest in synthetic interspecies hybrids that may possess novel properties and allow for strain improvement. New hybrid brewing strains have been generated by a laborious process of isolating auxotrophic mutants, which arise spontaneously at low frequency, followed by crossing to obtain hybrids (Krogerus et al., 2015; Pérez-Través et al., 2012). Although this method lacks markers, which would likely streamline approval for food and beverage applications, the strains contain mutations in important biosynthetic pathways. An easier method is to first generate stable heterothallic haploids for one or both parents, for example by replacing HO with drug markers, followed by interspecies crosses (Bullard et al., 2010; Swain Lenz et al., 2014; Tirosh et al., 2009). Variations of this strategy have used complementary drug markers and auxotrophic mutants in one species and spore dissection of wild-type diploids from another (Hebly et al., 2015; Piatkowska et al., 2013). However, the persistence of drug markers in the latter hybrids raises legitimate concerns about their safety that would need to be addressed prior to introducing them into the food and beverage industry.
Here we describe a generalized method for the efficient production of designer hybrid strains of Saccharomyces based on a series of inducible expression plasmids named HyPr (for Hybrid Production). These plasmids contain complementary dominant drug-resistance markers, a doxycycline-inducible HO cassette, and a generalized replication origin that provides functionality across Saccharomyces and many other yeasts. HyPr efficiently produces allotetraploid and autotetraploid strains of Saccharomyces. The resulting strains can be rapidly screened for plasmid loss, providing an efficient route towards meeting the standards that the United States Department of Agriculture and Food and Drug Administration have previously applied to a wine strain of S. cerevisiae, which is Generally Recognized As Safe (GRAS) (Husnik et al., 2006). These techniques also provide a valuable and general research tool for basic and applied research on prototrophic hybrids and polyploids of Saccharomyces.
2. Materials and Methods
2.1 Strains, culture conditions, and media
Strains used in this work are found in Table 1. S. cerevisiae strains were cultured at 30 °C, except when they were being co-cultured with another species. All other Saccharomyces species were cultured at room temperature (22–23 °C). Routine cultures were maintained in YPD (1% yeast extract, 2% peptone, 2% glucose). Hygromycin was added to YPD at a concentration of 200 mg/L to make YPD +hyg. Nourseothricin was added to YPD at a concentration of 100 mg/L to make YPD +nat. All liquid media was solidified when needed by the addition of 1.8% agar.
Table 1.
Strains used in this study.
| Identifier | Species | Genotype | Source |
|---|---|---|---|
| RM11-1a | Saccharomyces cerevisiae | MATa leu2-Δ ura3-Δ hoΔ::KanMX | Brem et al., 2002 |
| Ethanol Red | Saccharomyces cerevisiae | MATa/MATα | Fermentis |
| NRRL YB-210 | Saccharomyces cerevisiae | MATa/MATα | Mortimer and Johnston, 1986 |
| Wyeast #1007 German Ale | Saccharomyces cerevisiae | MATa/MATα | Wyeast |
| ZP 591 | Saccharomyces kudriavzevii | MATa/MATα | Sampaio and Gonçalves, 2008 |
| CBS 7001 | Saccharomyces uvarum | MATa/MATα | Scannell et al., 2011 |
| yHKS210 | Saccharomyces eubayanus | MATa/MATα | Peris et al., 2014 |
| White Labs WLP830, German Lager Weihenstephan 34/70 | Saccharomyces cerevisiae × Saccharomyces eubayanus | MATa/MATa/MATα/MATα | White Labs |
| yHWA338 | Saccharomyces cerevisiae | RM11-1a [pHCT2] | this work |
| yHWA340 | Saccharomyces cerevisiae | Ethanol Red [pHCT2] | this work |
| yHWA341 | Saccharomyces cerevisiae | NRRL YB-210 [pHMK34] | this work |
| yHWA348 | Saccharomyces uvarum | CBS 7001 [pHMK34] | this work |
| yHWA350 | Saccharomyces cerevisiae | Wyeast #1007 [pHCT2] | this work |
| yHWA352 | Saccharomyces eubayanus | yHKS210 [pHMK34] | this work |
| yHWA354 | Saccharomyces kudriavzevii | ZP 591 [pHMK34] | this work |
| yHWA358 | Saccharomyces cerevisiae | Ethanol Red [pHCT2] × NRRL YB-210 [pHMK34] | this work |
| yHWA375 | Saccharomyces cerevisiae × Saccharomyces kudriavzevii | Wyeast #1007 [pHCT2] × ZP 591 [pHMK34] | this work |
| yHWA377 | Saccharomyces cerevisiae × Saccharomyces uvarum | Wyeast #1007 [pHCT2] × CBS 7001 [pHMK34] | this work |
| yHWA425 | Saccharomyces cerevisiae × Saccharomyces eubayanus | Wyeast #1007 [pHCT2] × yHKS210 [pHMK34] | this work |
| yHWA439 | Saccharomyces cerevisiae × Saccharomyces eubayanus | Wyeast #1007×yHKS210 | this work |
2.2 Saccharomyces transformation
Transformation of yeast strains was done using the lithium acetate/PEG-4000/carrier DNA method as previously described (Gietz and Woods, 2002) with the following modifications: S. cerevisiae was heat shocked at 42 °C for 30 minutes, S. uvarum and S. eubayanus were heat shocked at 37 °C for 30 minutes, and S. kudriavzevii was heat shocked at 34° C for 30 minutes. Cells were suspended in YPD, followed by incubation at optimal temperature for three hours before being plated to selective media.
2.3 PCR
Primers used in this work are found in Table S1. Plasmid components were amplified using the Phusion PCR Kit (New England Biolabs, Ipswich, MA) as directed by the product insert. Mating-type screening and PCR-RFLP analysis used the Standard Taq Polymerase (New England Biolabs, Ipswich, MA) system as directed by the product insert for amplification.
2.4 Construction of pBM5155, pHCT2, and pHMK34
pBM5155 (Figure S1A) has been previously published and shown to facilitate the doxycycline-inducible gene expression in S. cerevisiae (Sabina and Johnston, 2009), but its sequence and the details of its construction by co-author CTH were not previously documented. pBM5155 was built using the backbone of pCM186 (Garí et al., 1997), which is a CEN plasmid containing all of the heterologous machinery for doxycycline-inducible expression of genes that are cloned into its NotI site, through several consecutive rounds of gap repair cloning in S. cerevisiae, followed by recovery and amplification in Escherichia coli by selection on LB +carbenicillin (or +ampicillin). Modifications of parts were introduced during PCR using oligonucleotides. The original rtTA transcription factor of pCM186, which is driven by a cytomegalovirus promoter, was excised and replaced with rtTA-M2 (Urlinger et al., 2000). The URA3 marker was replaced with a natMX4 marker (Goldstein and McCusker, 1999). An 8× glycine linker was added immediately downstream of the doxycline-inducible promoter and immediately upstream of a 16× myc tag created from a 13× myc tag (Longtine et al., 1998) plus a 3× myc duplication created during gap repair. To improve stability in other yeast species, KARS101 from Kluyveromyces lactis (Fabiani et al., 2001) was added in addition to the S. cerevisiae CEN and ARS sequences already present. Modifications were made sequentially and confirmed by Sanger sequencing. The complete sequence of pBM5155 has been deposited in GenBank under accession KT725394.
pHCT2 (Figure S1B) was built by PCR-amplifying S. cerevisiae HO using YCp50-HO-D6 (Russell et al., 1986) as a template, co-transforming that fragment with NotI-digested pBM5155 into S. cerevisiae, selecting on YPD +nat media, and recovering the resulting plasmid into E. coli. The natMX gene in pHCT2 was switched to the hphMX gene (Goldstein and McCusker, 1999) by amplifying the hph gene with oHWA230 and oHWA231, co-transforming the PCR product with AgeI-digested pHCT2 into S. cerevisiae, and selecting on YPD +hyg media. The resulting plasmid was recovered from yeast into bacteria, yielding pHMK34 (Figure S1C). The sequence of pHCT2 was confirmed by 36-bp single-end Illumina sequencing, followed by assembly with VELVET (Zerbino and Birney, 2008). The manipulated region of pHMK34 was confirmed by PCR analysis and drug resistance. pHCT2 and pHMK34 sequences were deposited in GenBank under the accession numbers KT725395 and KT781077, respectively.
2.5 Induction of HO expression and mating
Plasmid-bearing cells were grown to saturation over 12–36 hours in 3 mL liquid YPD +drug. 1.5 mL of culture was discarded and replaced with 1.5 mL fresh liquid YPD +drug, and 3 µL of 10 mg/mL of filter-sterilized doxycycline dissolved in water was added. The culture was incubated for four hours to induce HO expression. Induced cells were pelleted, washed with YPD, and 5 µL each of two separate induced cultures were mixed in an Eppendorf tube and patched to a small area of an YPD agar plate to allow mating between newly formed MATa/MATa or MATα/MATα diploids. After 12–36 hours of incubation, a small amount of the patch was struck to or plated on YPD +hyg +nat agar.
2.6 Determination of ploidy via flow cytometry
Overnight cultures of putative tetraploids were used to inoculate fresh YPD. These cultures were grown until cultures reached exponential phase, then fixed with 70% ethanol and stained with SYBR Green (Thermo Fisher Scientific, Waltham, MA) as previously described (Fortuna et al., 1997); due to possible degradation issues encountered with the normal fixation protocol, the NRRL YB-210 and Wyeast #1007 strains were not heated, nor were they treated with Proteinase K. DNA content was determined with a Guava easyCyte (EMD Millipore, Darmstadt, Germany). Data were processed, analyzed, and visualized in R 2.14.
2.7 Confirmation of hybridization with PCR-based RFLP
Putative tetraploid hybrids had their genomic DNA extracted, and BRE5 was amplified using primers oHDP022 (González et al., 2008), oHDP023, and oHDP024. Resulting PCR products were digested with HaeIII (New England Biolabs, Ipswich, MA). Undigested PCR products were visualized on a 1.5% agarose gel, while digested PCR products were visualized on a 3% agarose gel.
3. Results
3.1 Doxycycline-inducible expression of HO in Saccharomyces
pHCT2 was transformed into the stable haploid S. cerevisiae strain RM11-1a, and HO expression was induced with doxycycline. The induced culture was struck onto YPD +nat plates, and 47 colonies were screened by PCR of the mating-type locus (Gerstein et al., 2006); the Wyeast #1007 German Ale strain was included as a diploid control. 35 colonies produced bands consistent with the presence of both mating-type loci, while 12 colonies only produced one band from primers targeting MATa, the original mating type. The presence of both MATa and MATα are characteristic of a diploid cell, indicating that successful induction of HO by doxycycline, followed by mother-daughter or clonemate selfing, had occurred in the cell lineage to give rise to approximately 75% of the colonies (Figure S2). Since HO was applied for an extended period of time during this experiment, rather than the normal tightly regulated process, it is formally possible (although highly unlikely) that a fraction of the remaining 25% of the cells could be MATa/MATa diploids rather than haploids.
3.2 Intraspecies tetraploids are selectable from co-cultured, HO-induced diploids
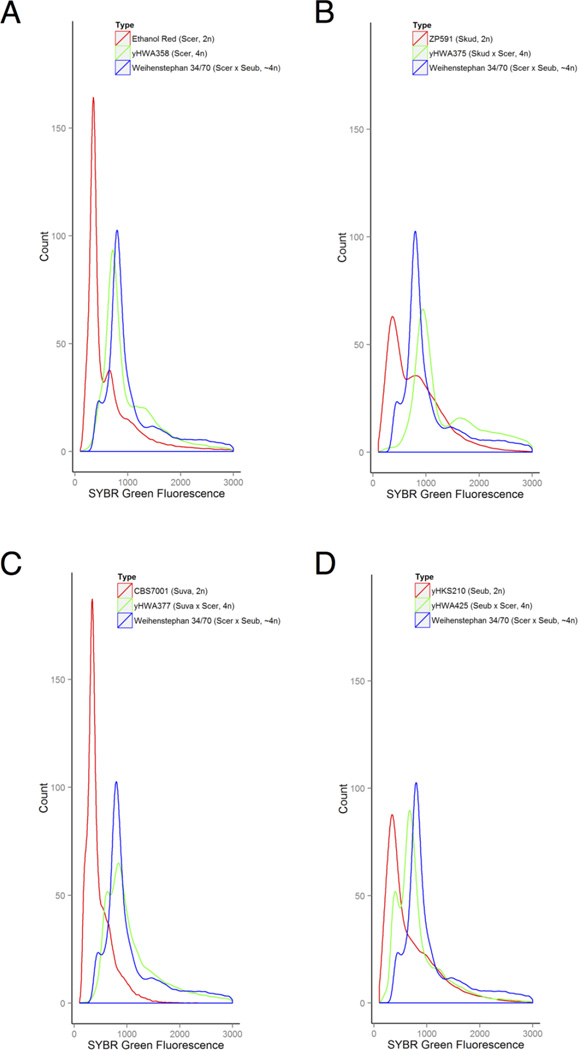
S. cerevisiae strains NRRL YB-210 and Ethanol Red are of biofuel interest due to their natural stress tolerance and routine use in ethanol production, respectively (Wohlbach et al., 2014). Strains containing pHMK34 and pHCT2 (Table 1), respectively, had HO expression induced with doxycycline and were co-cultured on YPD overnight at 30 °C. The co-cultured patch was suspended in liquid YPD, and a 10−4 dilution was made with fresh YPD. 200 µL of dilution was plated onto each of three YPD +hyg +nat plates, while 2 µL was plated onto each of three YPD plates to estimate the frequency of hybridization (Table 2). We recovered an average of 9.67 double-drug-resistant tetraploids out of a calculated 6100 average viable cells per plate, or 0.158%. Flow cytometry of representative putative hybrids indicates DNA content consistent with tetraploidy when compared against known standards (Figure 1A, Figure S3). We note that induction of HO in diploids produces several possible genotypes by gene conversion, including MATa/MATa, MATα/MATα, and MATa/MATα diploids, but the HyPr method is expected to only select for those progeny that result from the mating of MATa/MATa diploids with MATα/MATα diploids that contain complementary marked plasmids. The HyPr method does not control which mating type is contributed by which parent.
Table 2.
Recovery of double-drug-resistant colonies from induced, co-cultured S. cerevisiae strains.
| Replicate | YPD +hyg +nat | YPD1 |
|---|---|---|
| A | 12 | 5200 |
| B | 7 | 6900 |
| C | 10 | 6200 |
| Mean ± SD | 9.67 ± 2.52 | 6100 ± 850 |
Total YPD colonies were calculated from actual colony counts using dilution factors.
Figure 1.
Double-drug-resistant colonies arising from HO-induced, co-cultured diploids are tetraploid (4n). Flow cytometry was used to measure the DNA content of fixed, SYBR Green-stained cells harvested during exponential growth. The approximately tetraploid Weihenstephan strain is included on all graphs as a standard (Walther et al., 2014). Strains NRRL YB-210 and Wyeast #1007 German Ale did not survive the standard fixation and staining procedure intact, but genome sequencing and tetrad dissection have shown that NRRL YB-210 is approximately diploid (Wohlbach et al., 2014). These strains were stained by removing heating and protease treatments, and their comparative ploidy was determined (Figure S3). A) yHWA358 and its diploid (2n) Ethanol Red parent, B) yHWA375 and its diploid S. kudravzevii parent, C) yHWA377 and its diploid S. uvarum parent, D) yHWA425 and its diploid S. eubayanus parent. Scer = S. cerevisiae, Skud = S. kudriavzevii, Suva = S. uvarum, Seub = S. eubayanus.
3.3 Interspecies tetraploids are selectable from co-cultured, HO-induced diploids
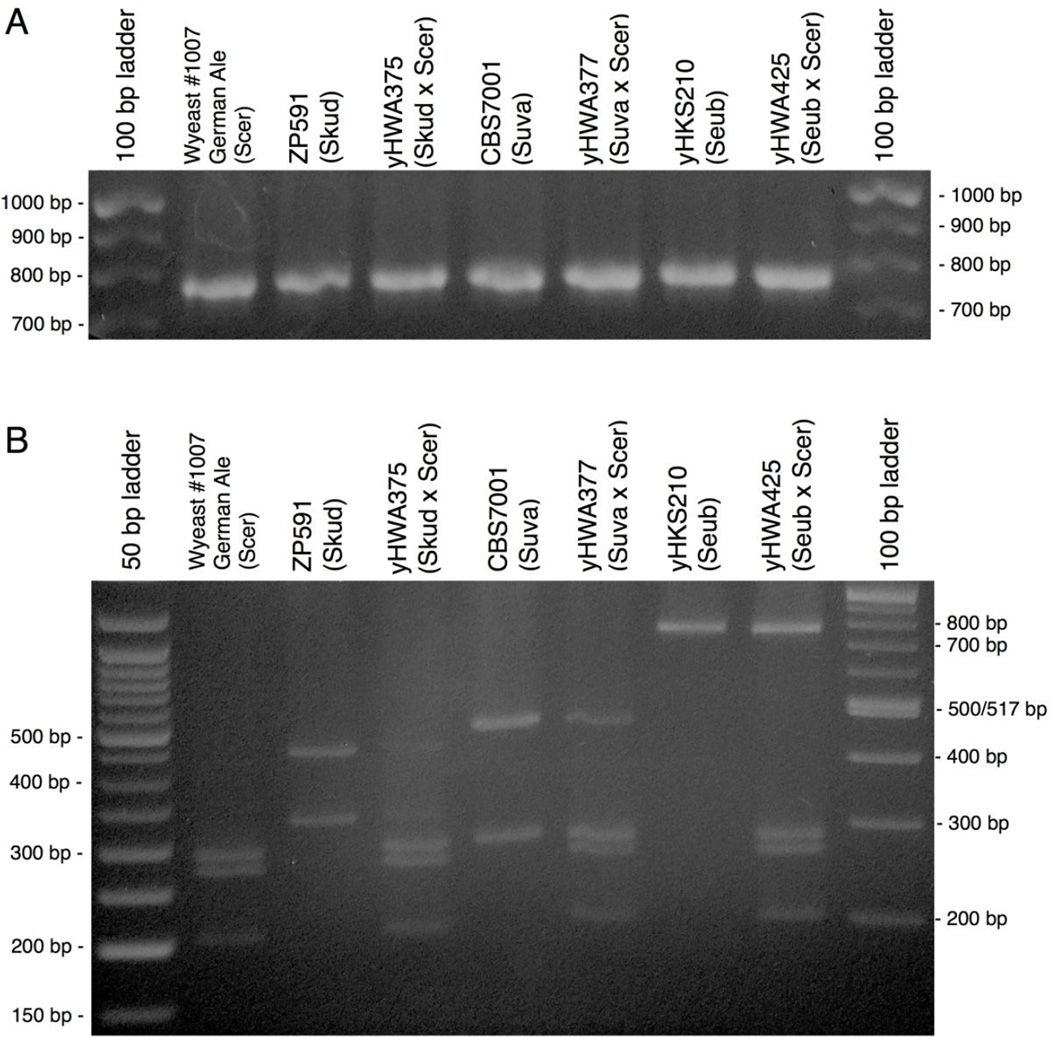
A German Ale strain of S. cerevisiae was transformed with pHCT2, while strains of S. kudrivzevii, S. uvarum, and S. eubayanus were transformed with pHMK34 (Table 1). HO expression was induced with doxycycline, and strains possessing complementary plasmids were co-cultured on YPD for up to 36 hours at room temperature. Patches were struck to YPD +hyg +nat, and resulting single double-drug-resistant colonies were grown in liquid YPD. Flow cytometry indicated a DNA content consistent with tetraploidy (Figure 1B–D, Figure S3), and PCR-RFLP analysis confirmed these strains were hybrids between S. cerevisiae and the intended Saccharomyces species (Figure 2).
Figure 2.
PCR-RFLP confirms the presence of two different genomes in the synthetic allotetraploids. A) The BRE5 PCR product is approximately the same size in all species shown. B) Digestion of the BRE5 PCR product with HaeIII produces a unique pattern for each species. Double-drug-resistant allotetraploids produce banding patterns consistent with those expected from a hybrid strain. Scer = S. cerevisiae, Skud = S. kudriavzevii, Suva = S. uvarum, Seub = S. eubayanus.
3.4 Plasmids are rapidly lost in non-selective media
A saturated liquid non-selective culture of yHWA425, the synthetic S. cerevisiae × S. eubayanus hybrid (Table 1), was diluted 10−4, and 1 µL was plated onto each of three YPD plates. After incubation at room temperature for two days, these plates were replicated to YPD +hyg and YPD +nat plates. 54.9% of colonies lost pHMK34, while 63.8% of colonies lost pHCT2. Some colonies grew on YPD and were sensitive to both drugs (Figure S4). These colonies were harvested from the YPD plate and were successfully struck for single colonies on YPD but not on YPD +hyg and YPD +nat (yHWA439), indicating that plasmid-free interspecies hybrids containing unmarked nuclear genomes can be readily obtained by this method.
4. Discussion
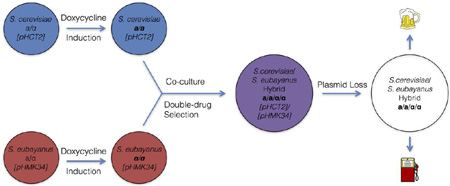
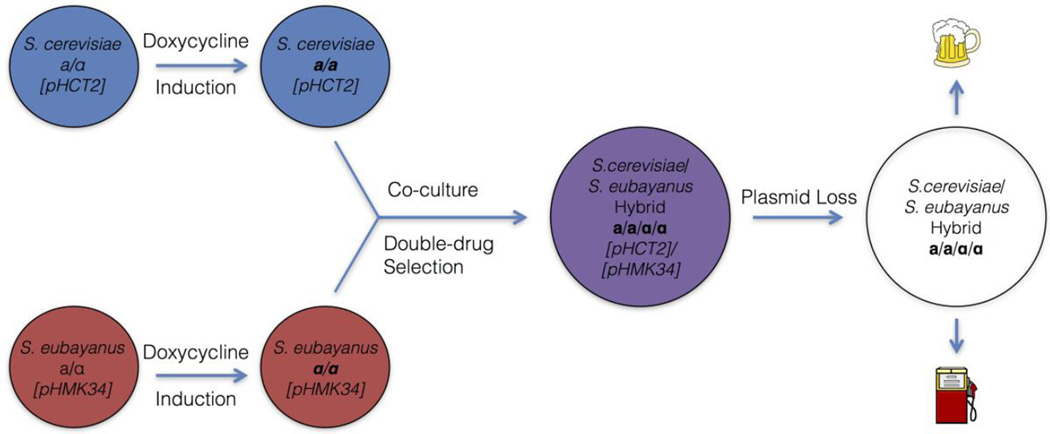
pHCT2 and pHMK34 function similarly to previously constructed vectors that drive HO expression with the galactose-inducible promoter of GAL1 (Jensen and Herskowitz, 1984) and are valuable tools for researchers working with stable haploid prototrophic strains of Saccharomyces. More interestingly, pHCT2 and pHMK34 can be used together in a method called HyPr, which produces allotetraploid and autotetraploid Saccharomyces yeasts (Figure 3). The HyPr success rate is high (around 1 out of 1000 plated cells) relative to the corresponding rate of rare mating between two diploids in a population, which has been estimated to be between 1 out of 1 million to 100 million cells (Gunge and Nakatomi, 1972). Moreover, the complementary markers on the plasmids themselves also provide the means to easily select allotetraploids. The high success rate, the ease of use in prototrophs, the lack of permanent genomic modification, and the capability of plasmid loss together make HyPr the ideal method for production of new designer hybrid strains for industrial fermentations, such as beer, wine, cider, and biofuel production. To introduce the utility of this approach, we have created novel S. cerevisiae × S. eubayanus, S. cerevisiae × S. kudriavzevii, and S. cerevisiae × S. uvarum strains, which are designed as synthetic lager, Belgian, and cider strains, respectively.
Figure 3.
An overview of the HyPr method. Induction of HO expression by a doxycycline-inducible promoter in two diploid cultures, followed by co-culture and subsequent double-drug selection, will produce hybrids at a rate approaching 1 out of 1000 cells plated. Plasmids can then be easily cured to produce strains without genome modifications.
HyPr may also provide an alternative method for optimization via hybridization of Saccharomyces chassis strains to be used in a variety of synthetic biology applications. Selection of an appropriate chassis strain prior to the installation of genetic and metabolic modifications is a sound strategy, but prior work has often focused on screening existing strain libraries to find a chassis strain that fit a set of criteria (Jin et al., 2013). The meiotic sterility of many industrial strains of interest complicates the production of new Saccharomyces chassis strains because many strains do not produce viable spores. HyPr can be used to create chassis strains de novo from two strains with desirable traits, producing a new chassis strain with both desired characteristics without sporulation. As with a previous case where HO was induced in a sterile strain whose genome was predominantly S. uvarum (Schwartz et al., 2012), HyPr is expected to even enable hybridization of strains whose defect is in sporulation or chromosome segregation, rather than mating per se. This new chassis strain could then undergo optimization via selective conditions, further enhancing desired phenotypes of the chassis strain through aneuploidy and other mutations or through modification using various genome-editing techniques (Alexander et al., 2014; DiCarlo et al., 2013; Ryan et al., 2014).
Chassis strains made by HyPr lack drug markers and auxotrophies, both of which are desirable qualities in beverage and biofuel strains. Note that, while integration of any portion of the plasmid into the Saccharomyces genome is highly unlikely, it is formally possible that fragments of the plasmid could have integrated and conferred no detectable phenotype. Depending on the desired applications and safety regulations, any hybrids made using HyPr could have their genomes sequenced to ensure that all plasmid DNA had been eliminated from the strain, or other routine molecular approaches, such as PCR or Southern blots, could be taken to verify the absence of specific parts of the plasmid that were of concern.
Extensive prior work has shown that S. cerevisiae autotetraploid strains are relatively unstable, rapidly losing chromosomes to form aneuploid strains (Storchova, 2014). This loss occurs rapidly with genome content reduction to near-diploid levels in 200 to 800 generations(Gerstein et al., 2006). We expect autotetraploids made via HyPr to behave in a similar manner. This could, in fact, be a desirable trait for many applications, as placing an unstable tetraploid strain in a selective condition will influence which components of the genome are retained or lost from which parent, allowing for more rapid adaptation to that condition (Selmecki et al., 2015). Allopolyploids between Saccharomyces species have been identified from many different sources, including the isolation of lager beer strains (Dunn and Sherlock, 2008; Nakao et al., 2009; Walther et al., 2014). The reduction of genomic components has been observed in many cases. For example, S. cerevisiae × S. kudriavzevii hybrids isolated from brewing environments have selectively lost components of the S. kudriavzevii genome (Peris et al., 2012c), while certain S. cerevisiae × S. eubayanus hybrids used in lager beer production have lost whole S. cerevisiae chromosomes (Walther et al., 2014). We expect that allotetraploids made by HyPr will also evolve aneuploidy given enough time, but again, many of these aneuploidies may be advantageous in the conditions where they are evolved. A recent evolution experiment using interspecies hybrids demonstrated that genomic stability was reached within 30–50 generations (Pérez-Través et al., 2014a). Even though allopolyploid strains are less stable than diploid Saccharomyces strains, we note that the allopolyploid strains that form the backbone of the brewing industry are sufficiently stable for routine application in large-scale fermentations.
Finally, interspecies hybrids are also useful to address a variety of basic research questions in genetics and evolutionary biology. For example, interspecies hybrids have been especially useful for examining the relative effects of cis and trans variation on gene expression (Bullard et al., 2010; Swain Lenz et al., 2014; Tirosh et al., 2009; Wittkopp et al., 2004). In Saccharomyces, these studies have traditionally mated haploids with integrated complementary markers to make F1 diploid hybrids. Our plasmid-based strategy may be preferable in strain backgrounds where gene targeting is inefficient or for high-throughput experiments. When diploidy is required, allotetraploid Saccharomyces that have not yet evolved aneuploidy or sterility can readily be sporulated readily to recover diploids (Greig et al., 2002; Gunge, 1966). In conclusion, this straightforward, robust approach allows the efficient construction of designer hybrids of Saccharomyces allotetraploids and autotetraploids for numerous basic and applied uses.
Supplementary Material
Highlights.
We developed a simple method to generate tetraploids in Saccharomyces called HyPr.
HyPr produces tetraploids in the same or between different Saccharomyces species.
HyPr tetraploids are produced at a rate up to 1 out of 1000 cells plated.
We created synthetic hybrids for brewing lager beers, Belgian beers, and ciders.
Acknowledgements
We would like to thank James Hose and Audrey Gasch for the training with and use of their Guava easyCyte flow cytometer; Carol Newlon and Lucia Fabiani for the KARS101 plasmid; and Trey K. Sato for the NRRL YB-210 strain. This material is based upon work supported by the National Science Foundation under Grant Nos. DEB-1253634 and DEB-1442148 to CTH and funded in part by the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494). CTH is an Alfred Toepfer Faculty Fellow, supported by the Alexander von Humboldt Foundation. CTH is a Pew Scholar in the Biomedical Sciences, supported by the Pew Charitable Trusts. BP was supported by the Predoctoral Training Program in Genetics, funded by the National Institutes of Health (5 T32 GM007133-40).
Abbreviations
- PCR
polymerase chain reaction
- RFLP
restriction fragment length polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander WG, Doering DT, Hittinger CT. High-efficiency genome editing and allele replacement in prototrophic and wild strains of Saccharomyces. Genetics. 2014;198:859–866. doi: 10.1534/genetics.114.170118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P, Gonçalves C, Teixeira S, Libkind D, Bontrager M, Masneuf-Pomarède I, Albertin W, Durrens P, Sherman DJ, Marullo P, Hittinger CT, Gonçalves P, Sampaio JP. A Gondwanan imprint on global diversity and domestication of wine and cider yeast Saccharomyces uvarum. Nat. Commun. 2014;5:4044. doi: 10.1038/ncomms5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E, Wang B, Bellora N, Peris D, Hulfachor AB, Koshalek JA, Adams M, Libkind D, Hittinger CT. The genome sequence of Saccharomyces eubayanus and the domestication of lager-brewing yeasts. Mol. Biol. Evol. msv. 2015 doi: 10.1093/molbev/msv168. 168–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides. 2004;25:1465–1476. doi: 10.1016/j.peptides.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Bing J, Han P-J, Liu W-Q, Wang Q-M, Bai F-Y. Evidence for a Far East Asian origin of lager beer yeast. Curr. Biol. 2014;24:R380–R381. doi: 10.1016/j.cub.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Brem RB, Yvert G, Clinton R, Kruglyak L. Genetic dissection of transcriptional regulation in budding yeast. Science. 2002;296:752–755. doi: 10.1126/science.1069516. [DOI] [PubMed] [Google Scholar]
- Bullard JH, Mostovoy Y, Dudoit S, Brem RB. Polygenic and directional regulatory evolution across pathways in Saccharomyces. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5058–5063. doi: 10.1073/pnas.0912959107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselton LA, Olesnicky NS. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 1998;62:55–70. doi: 10.1128/mmbr.62.1.55-70.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corran HS. A history of brewing. David & Charles; 1975. [Google Scholar]
- DiCarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013;41:4336–4343. doi: 10.1093/nar/gkt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B, Sherlock G. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 2008;18:1610–1623. doi: 10.1101/gr.076075.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani L, Irene C, Aragona M, Newlon CS. A DNA replication origin and a replication fork barrier used in vivo in the circular plasmid pKD1. Mol. Genet. Genomics. 2001;266:326–335. doi: 10.1007/s004380100562. [DOI] [PubMed] [Google Scholar]
- Fortuna M, Joao Sousa M, Corte-Real M, Leao C. UNIT 11.13 Cell cycle analysis of yeasts. Current Protocols in Cytometry. 1997 doi: 10.1002/0471142956.cy1113s13. [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant M, Marcand S. NHEJ regulation by mating type is exercised through a novel protein, Lif2p, essential to the ligase IV pathway. Genes Dev. 2001;15:3005–3012. doi: 10.1101/gad.206801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garí E, Piedrafita L, Aldea M, Herrero E. A set of vectors with a tetracycline-regulatable promoter system for modulated gene expression in Saccharomyces cerevisiae. Yeast. 1997;13:837–848. doi: 10.1002/(SICI)1097-0061(199707)13:9<837::AID-YEA145>3.0.CO;2-T. doi:10.1002/(SICI)1097-0061(199707)13:9<837::AID-YEA145>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Gerstein AC, Chun H-JE, Grant A, Otto SP. Genomic convergence toward diploidy in Saccharomyces cerevisiae. PLoS Genet. 2006;2:e145. doi: 10.1371/journal.pgen.0020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Glass NL, Grotelueschen J, Metzenberg RL. Neurospora crassa A mating-type region. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4912–4916. doi: 10.1073/pnas.87.13.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass NL, Jacobson DJ, Shiu PKT. The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycete fungi. Annu. Rev. Genet. 2000;34:165–186. doi: 10.1146/annurev.genet.34.1.165. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–1553. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. doi:10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- González SS, Barrio E, Querol A. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl. Environ. Microbiol. 2008;74:2314–2320. doi: 10.1128/AEM.01867-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig D, Borts RH, Louis EJ, Travisano M. Epistasis and hybrid sterility in Saccharomyces. Proc. Biol. Sci. 2002;269:1167–1171. doi: 10.1098/rspb.2002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N. Breeding of bakers’ yeast-Determination of the ploidy and an attempt to improve practical properties. Japanese J. Genet. 1966;41:203–214. [Google Scholar]
- Gunge N, Nakatomi Y. Genetic mechanisms of rare matings of the yeast Saccharomyces cerevisiae heterozygous for mating type. Genetics. 1972;70:41–58. doi: 10.1093/genetics/70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JE. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics. 2012;191:33–64. doi: 10.1534/genetics.111.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebly M, Brickwedde A, Bolat I, Driessen MRM, de Hulster EAF, van den Broek M, Pronk JT, Geertman J-M, Daran J-M, Daran-Lapujade P. S. cerevisiae × S. eubayanus interspecific hybrid, the best of both worlds and beyond. FEMS Yeast Res. 2015;15 doi: 10.1093/femsyr/fov005. [DOI] [PubMed] [Google Scholar]
- Herskowitz I, Jensen RE. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 1991;194:132–146. doi: 10.1016/0076-6879(91)94011-z. [DOI] [PubMed] [Google Scholar]
- Hittinger CT. Saccharomyces diversity and evolution: a budding model genus. Trends Genet. 2013;29:309–317. doi: 10.1016/j.tig.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Hittinger CT, Gonçalves P, Sampaio JP, Dover J, Johnston M, Rokas A. Remarkably ancient balanced polymorphisms in a multi-locus gene network. Nature. 2010;464:54–58. doi: 10.1038/nature08791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husnik JI, Volschenk H, Bauer J, Colavizza D, Luo Z, van Vuuren HJJ. Metabolic engineering of malolactic wine yeast. Metab. Eng. 2006;8:315–323. doi: 10.1016/j.ymben.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Jensen RE, Herskowitz I. Directionality and regulation of cassette substitution in yeast. Cold Spring Harb. Symp. Quant. Biol. 1984;49:97–104. doi: 10.1101/sqb.1984.049.01.013. [DOI] [PubMed] [Google Scholar]
- Jin M, Sarks C, Gunawan C, Bice BD, Simonett SP, Avanasi Narasimhan R, Willis LB, Dale BE, Balan V, Sato TK. Phenotypic selection of a wild Saccharomyces cerevisiae strain for simultaneous saccharification and co-fermentation of AFEX™ pretreated corn stover. Biotechnol. Biofuels. 2013;6:108. doi: 10.1186/1754-6834-6-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B. Meiosis, Developmental and Cell Biology Series. 1990 [Google Scholar]
- Kohl KP, Sekelsky J. Meiotic and mitotic recombination in meiosis. Genetics. 2013;194:327–334. doi: 10.1534/genetics.113.150581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogerus K, Magalhães F, Vidgren V, Gibson B. New lager yeast strains generated by interspecific hybridization. J. Ind. Microbiol. Biotechnol. 2015;42:769–778. doi: 10.1007/s10295-015-1597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad JW, Staben C. Mating type in filamentous fungi. Annu. Rev. Genet. 1997;31:245–276. doi: 10.1146/annurev.genet.31.1.245. [DOI] [PubMed] [Google Scholar]
- Le Jeune C, Lollier M, Demuyter C, Erny C, Legras J-L, Aigle M, Masneuf-Pomarède I. Characterization of natural hybrids of Saccharomyces cerevisiae and Saccharomyces bayanus var. uvarum. FEMS Yeast Res. 2007;7:540–549. doi: 10.1111/j.1567-1364.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- Leducq J-B, Charron G, Samani P, Dubé AK, Sylvester K, James B, Almeida P, Sampaio JP, Hittinger CT, Bell G, Landry CR. Local climatic adaptation in a widespread microorganism. Proc. Biol. Sci. 2014;281:20132472. doi: 10.1098/rspb.2013.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libkind D, Hittinger CT, Valério E, Gonçalves C, Dover J, Johnston M, Gonçalves P, Sampaio JP. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14539–14544. doi: 10.1073/pnas.1105430108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G, Carter DM, Moses AM, Warringer J, Parts L, James Sa, Davey RP, Roberts IN, Burt A, Koufopanou V, Tsai IJ, Bergman CM, Bensasson D, O’Kelly MJT, van Oudenaarden A, Barton DBH, Bailes E, Nguyen AN, Jones M, Quail Ma, Goodhead I, Sims S, Smith F, Blomberg A, Durbin R, Louis EJ. Population genomics of domestic and wild yeasts. Nature. 2009;458:337–341. doi: 10.1038/nature07743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G, Peruffo A, James Sa, Roberts IN, Louis EJ. Inferences of evolutionary relationships from a population survey of LTR-retrotransposons and telomeric-associated sequences in the Saccharomyces sensu stricto complex. Yeast. 2005;22:177–192. doi: 10.1002/yea.1200. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Mckenzie A, III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Maclean CJ, Greig D. Prezygotic reproductive isolation between Saccharomyces cerevisiae and Saccharomyces paradoxus. BMC Evol. Biol. 2008;8:1. doi: 10.1186/1471-2148-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masneuf I, Hansen J, Groth C, Piskur J, Dubourdieu D. New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl. Envir. Microbiol. 1998;64:3887–3892. doi: 10.1128/aem.64.10.3887-3892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer RK. Evolution and variation of the yeast (Saccharomyces) genome. Genome Res. 2000;10:403–409. doi: 10.1101/gr.10.4.403. [DOI] [PubMed] [Google Scholar]
- Mortimer RK, Johnston JR. Genealogy of principal strains of the yeast genetic stock center. Genetics. 1986;113:35–43. doi: 10.1093/genetics/113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao Y, Kanamori T, Itoh T, Kodama Y, Rainieri S, Nakamura N, Shimonaga T, Hattori M, Ashikari T. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 2009;16:115–129. doi: 10.1093/dnares/dsp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Través L, Lopes CA, Barrio E, Querol A. Stabilization process in Saccharomyces intra and interspecific hybrids in fermentative conditions. Int. Microbiol. 2014a;17:213–224. doi: 10.2436/20.1501.01.224. [DOI] [PubMed] [Google Scholar]
- Pérez-Través L, Lopes CA, Barrio E, Querol A. Evaluation of different genetic procedures for the generation of artificial hybrids in Saccharomyces genus for winemaking. Int. J. Food Microbiol. 2012;156:102–111. doi: 10.1016/j.ijfoodmicro.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Través L, Lopes CA, Querol A, Barrio E. On the complexity of the Saccharomyces bayanus taxon: hybridization and potential hybrid speciation. PLoS One. 2014b;9:e93729. doi: 10.1371/journal.pone.0093729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris D, Belloch C, Lopandić K, Álvarez-Pérez JM, Querol A, Barrio E. The molecular characterization of new types of Saccharomyces cerevisiae × S. kudriavzevii hybrid yeasts unveils a high genetic diversity. Yeast. 2012a;29:81–91. doi: 10.1002/yea.2891. [DOI] [PubMed] [Google Scholar]
- Peris D, Lopes CA, Arias A, Barrio E. Reconstruction of the evolutionary history of Saccharomyces cerevisiae × S. kudriavzevii hybrids based on multilocus sequence analysis. PLoS One. 2012b;7:e45527. doi: 10.1371/journal.pone.0045527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris D, Lopes CA, Belloch C, Querol A, Barrio E. Comparative genomics among Saccharomyces cerevisiae × Saccharomyces kudriavzevii natural hybrid strains isolated from wine and beer reveals different origins. BMC Genomics. 2012c doi: 10.1186/1471-2164-13-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris D, Sylvester K, Libkind D, Gonçalves P, Sampaio JP, Alexander WG, Hittinger CT. Population structure and reticulate evolution of Saccharomyces eubayanus and its lager-brewing hybrids. Mol. Ecol. 2014;23:2031–2045. doi: 10.1111/mec.12702. [DOI] [PubMed] [Google Scholar]
- Piatkowska EM, Naseeb S, Knight D, Delneri D. Chimeric protein complexes in hybrid species generate novel phenotypes. PLoS Genet. 2013;9:e1003836. doi: 10.1371/journal.pgen.1003836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski JS, Nagarajan S, Kroll E, Stanbery A, Chiotti KE, Kruckeberg AL, Dunn B, Sherlock G, Rosenzweig F. Different selective pressures lead to different genomic outcomes as newly-formed hybrid yeasts evolve. BMC Evol. Biol. 2012;12:46. doi: 10.1186/1471-2148-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW, Jensen R, Zoller MJ, Burke J, Errede B, Smith M, Herskowitz I. Structure of the Saccharomyces cerevisiae HO gene and analysis of its upstream regulatory region. Mol. Cell. Biol. 1986;6:4281–4294. doi: 10.1128/mcb.6.12.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan OW, Skerker JM, Maurer MJ, Li X, Tsai JC, Poddar S, Lee ME, DeLoache W, Dueber JE, Arkin AP, Cate JHD. Selection of chromosomal DNA libraries using a multiplex CRISPR system. Elife. 2014:e03703. doi: 10.7554/eLife.03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina J, Johnston M. Asymmetric signal transduction through paralogs that comprise a genetic switch for sugar sensing in Saccharomyces cerevisiae. J. Biol. Chem. 2009;284:29635–29643. doi: 10.1074/jbc.M109.032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio JP, Gonçalves P. Natural populations of Saccharomyces kudriavzevii in Portugal are associated with oak bark and are sympatric with S. cerevisiae and S. paradoxus. Appl. Environ. Microbiol. 2008;74:2144–2152. doi: 10.1128/AEM.02396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell DR, Zill OA, Rokas A, Payen C, Dunham MJ, Eisen MB, Rine J, Johnston M, Hittinger CT. The awesome power of yeast evolutionary genetics: new genome sequences and strain resources for the Saccharomyces sensu stricto genus. G3. 2011;1:11–25. doi: 10.1534/g3.111.000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Wenger JW, Dunn B, Sherlock G. APJ1 and GRE3 homologs work in concert to allow growth in xylose in a natural Saccharomyces sensu stricto hybrid yeast. Genetics. 2012;191:621–632. doi: 10.1534/genetics.112.140053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki AM, Maruvka YE, Richmond PA, Guillet M, Shoresh N, Sorenson AL, De S, Kishony R, Michor F, Dowell R, Pellman D. Polyploidy can drive rapid adaptation in yeast. Nature. 2015;519:349–352. doi: 10.1038/nature14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staben C, Yanofsky C. Neurospora crassa a mating-type region. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4917–4921. doi: 10.1073/pnas.87.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova Z. Ploidy changes and genome stability in yeast. Yeast. 2014;31:421–430. doi: 10.1002/yea.3037. [DOI] [PubMed] [Google Scholar]
- Swain Lenz D, Riles L, Fay JC. Heterochronic meiotic misexpression in an interspecific yeast hybrid. Mol. Biol. Evol. 2014;31:1333–1342. doi: 10.1093/molbev/msu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh I, Reikhav S, Levy AA, Barkai N. A yeast hybrid provides insight into the evolution of gene expression regulation. Science. 2009;324:659–662. doi: 10.1126/science.1169766. [DOI] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heeckeren WJ, Dorris DR, Struhl K. The mating-type proteins of fission yeast induce meiosis by directly activating mei3 transcription. Mol. Cell. Biol. 1998;18:7317–7326. doi: 10.1128/mcb.18.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ME, Gardner JM, Vystavelova A, McBryde C, Lopes MDB, Jiranek V. Application of the reuseable, KanMX selectable marker to industrial yeast: Construction and evaluation of heterothallic wine strains of Saccharomyces cerevisiae, possessing minimal foreign DNA sequences. FEMS Yeast Res. 2003;4:339–347. doi: 10.1016/S1567-1356(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Walther A, Hesselbart A, Wendland J. Genome sequence of Saccharomyces carlsbergensis, the world’s first pure culture lager yeast. G3. 2014;4:783–793. doi: 10.1534/g3.113.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q-M, Liu W-Q, Liti G, Wang S-A, Bai F-Y. Surprisingly diverged populations of Saccharomyces cerevisiae in natural environments remote from human activity. Mol. Ecol. 2012;21:5404–5417. doi: 10.1111/j.1365-294X.2012.05732.x. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Haerum BK, Clark AG. Evolutionary changes in cis and trans gene regulation. Nature. 2004;430:85–88. doi: 10.1038/nature02698. [DOI] [PubMed] [Google Scholar]
- Wohlbach DJ, Rovinskiy N, Lewis JA, Sardi M, Schackwitz WS, Martin JA, Deshpande S, Daum CG, Lipzen A, Sato TK, Gasch AP. Comparative genomics of Saccharomyces cerevisiae natural isolates for bioenergy production. Genome Biol. Evol. 2014;6:2557–2566. doi: 10.1093/gbe/evu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.