Abstract
Carbon-based nanomaterials including single- and multi-walled carbon nanotubes, graphene oxide, fullerenes and nanodiamonds are potential candidates for various applications in medicine such as drug delivery and imaging. However, the successful translation of nanomaterials for biomedical applications is predicated on a detailed understanding of the biological interactions of these materials. Indeed, the potential impact of the so-called bio-corona of proteins, lipids, and other biomolecules on the fate of nanomaterials in the body should not be ignored. Enzymatic degradation of carbon-based nanomaterials by immune-competent cells serves as a special case of bio-corona interactions with important implications for the medical use of such nanomaterials. In the present review, we highlight emerging biomedical applications of carbon-based nanomaterials. We discuss recent studies on nanomaterial ‘coronation’ and how this impacts on biodistribution and targeting along with studies on the enzymatic degradation of carbon-based nanomaterials, and the role of surface modification of nanomaterials for these biological interactions.
Keywords: carbon nanotubes, graphene oxide, fullerenes, nanodiamonds, biodegradation, bio-corona
Graphical abstract
The present review discusses biological interactions of carbon-based nanomaterials, focusing on bio-corona formation, biodegradation, and biodistribution and targeting for various biomedical applications.
Introduction
Engineered nanomaterials provide unique advantages and opportunities in several areas of medicine including therapeutics, diagnostics, imaging, and regenerative medicine [1,2]. Carbon-based nanomaterials such as fullerenes, carbon nanotubes, carbon nanohorns, carbon nanodots, nanodiamonds, and graphene and its derivatives have unique electronic, optical, thermal, and mechanical properties and have attracted considerable attention in recent years in nanomedicine [3–5]. Hence, many studies have attempted to exploit these materials for drug delivery or imaging, or both. As pointed out in a recent editorial, the successful commercialization of nanomedicines ultimately depends on demonstrating their superiority over existing approaches and on documenting their safety [2]. Indeed, a detailed understanding of the biological interactions of nanomaterials, not least the interactions with cellular and other components of the immune system (Figure 1) is important both from an efficacy and safety point of view, as is the understanding of the ultimate fate of the nanomaterial – accumulation, degradation, and/or excretion – in the human body [6]. To this end, particular attention should be devoted to the role of adsorbed biomolecules which may confer a new biological ‘identity’ to nanomaterials [7], and is likely to play an important role for cellular uptake and in vivo biodistribution of nanomaterials [8].
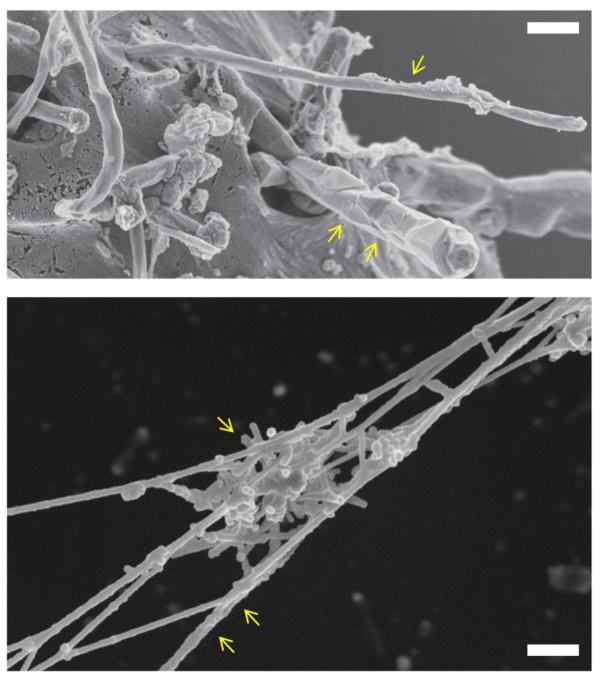
Figure 1.
Cellular and extracellular interactions of carbon nanotubes. The upper panel shows an SEM image of isolated MWCNTs (single arrow) or a bundle of MWCNTs (two arrows) entering human mesothelial cells. Reprinted from: Shi X, von dem Bussche A, Hurt RH, Kane AB, Gao H. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat Nanotechnol. 2011;6(11):714–9, with permission from Nature Publishing Group. The lower panel shows a cluster of short-cut SWCNTs (single arrow) entrapped in chromatin fibers (two arrows) of purified neutrophil extracellular traps [see 159 for further details]. SEM courtesy of K. Hultenby, Karolinska Institutet.
Detailed accounts of the routes of synthesis and the physicochemical properties of carbon-based nanomaterials are beyond the scope of the present review, but a brief introduction is provided here. Fullerenes are entirely composed of carbon and have the form of spheres, ellipsoids or tubules. Spherical and cylindrical fullerenes are also referred to as buckyballs and buckytubes (or carbon nanotubes), respectively. The first representative of the buckyball family, referred to as buckminsterfullerene, is composed of 60 carbon atoms (C60) and has the shape of a truncated icosahedron with 20 hexagons and 12 pentagons and a diameter of approximately 1 nm, thus resembling a football (in the United States, a soccer ball); indeed, a picture of a football was included in the very first publication, and the authors even contemplated the alternative name, soccerene [9]. Iijima is credited with the discovery of carbon nanotubes (CNTs) [10] although some claim that these structures (“graphitic carbon needles”) had been observed decades earlier [11]. CNTs are graphitic tubules, which can be capped with hemifullerenes at the ends, consisting of a single graphene sheet (single-walled carbon nanotubes, SWCNTs) or several concentric and nested sheets (multi-walled carbon nanotubes, MWCNTs). Both types of CNTs have nano-scale dimensions and display a very high aspect ratio, i.e., the ratio between the length and the diameter of the material. Hence, SWCNTs have a diameter of approximately 1 nm and lengths up to a few microns or more, whereas MWCNTs have diameters of several tens of nanometers and lengths up to several tens of microns or more. All of the aforementioned nanomaterials can be related to a parent material known as graphene consisting of a single atomically thin sheet of hexagonally bound sp2 carbon atoms [12]. For a comprehensive overview of the structural, electronic, and biological properties and applications of graphene and other 2-D materials, see 13. Nanodiamonds represent yet another class of nanoparticles in the carbon family, with highly versatile physical and chemical properties [14]. They are mainly composed of carbon sp3 structures in the core, with sp2 and disorder/defect carbons on the surface, and display single-digit nm sizes.
In the present review, we will highlight emerging biomedical applications of various carbon-based nanomaterials. We will also discuss bio-corona formation and the propensity for enzymatic degradation, especially with regards to CNTs and graphene oxide (GO), which are the most intensively investigated carbon-based nanomaterials to date in the field of nanomedicine, along with fullerenes and nanodiamonds. The impact of surface modifications, including grafting of polymers, on the biological interactions of these materials is also highlighted.
Biocompatibility of carbon-based nanomaterials
Being small confers advantages in terms of negotiating biological barriers, which may be desirable, but nanoscale size per se is not sufficient to qualify as a nanotechnology [15]. Carbon-based nanomaterials, however, possess intrinsic physicochemical properties that can potentially be exploited. For instance, CNTs display strong optical absorption in the near infrared, Raman scattering as well as photo-acoustic properties that widen the scope of in vivo applications as they can potentially have bio-imaging and tracing functions coupled with drug delivery [4]. Graphene is another material with many promising areas of application as a result of its large surface area and possibility of easy functionalization, providing opportunities for drug delivery [5]. Moreover, its unique mechanical properties suggest tissue engineering and regenerative medicine applications [16]. Other carbon-based nanomaterials such as fullerenes and nanodiamonds have also received much attention in recent years, with emphasis mainly in the area of cancer medicine [4]. In the present review, we will highlight some illustrative, pre-clinical examples from recent literature.
However: safety first. The potential toxicity of carbon-based nanomaterials has been the subject of much concern in the past decade and much skepticism initially surrounded the notion of using, in particular, CNTs as drug delivery systems due to the fact that these fibre-like materials were presumed to be biopersistent, and, therefore, to possess asbestos-like pathogenicity [17–19]. However, more recent research has suggested strategies to improve the biocompatibility of CNTs through surface modification of the materials and has also demonstrated the susceptibility for enzymatic degradation of these nanomaterials (discussed below). Indeed, it is important to distinguish potentially harmful CNTs [20] from more biocompatible ones. Moreover, there are important lessons to be learned from these extensive toxicological investigations [21,22]. Categorization or grouping of nanomaterials according to their risk potential, taking into account indicators of both hazard and exposure, is needed to identify ‘nanomaterials of concern’ [23]. Overall, it is necessary to avoid generalizations about the toxicity of ‘carbon nanotubes’ or, for that matter, of ‘graphene’, as these are not single nanomaterials, but classes of nanomaterials with important differences in terms of their physicochemical properties (such as, aspect ratio or lateral dimensions, purity, surface functionalization, and so on) and, hence, in their toxicological profile. Thus, with careful evaluation of the biological interactions of each nanomaterial, a more favorable scenario for their exploitation in medicine presents itself. Of key importance for any biocompatibility assessment of nanomaterials is the evaluation of potential effects on the immune system [8].
Indeed, the immune system has evolved to protect us from pathogens and other foreign intrusion. In brief, the immune system can be divided into the innate and the adaptive (or acquired) immune system. The innate immune system is comprised of inflammatory cells or ‘sensors’ and soluble ‘mediators’ (i.e., complement factors, chemokines, and cytokines) [19]. It is this arm of the immune system that nanomaterials first encounter following either deliberate or accidental (occupational/non-occupational) exposures. The inflammatory cells encompass macrophages, professional phagocytic cells that differentiate from monocytes that migrate from the circulation and extravagate into tissues. The main functions of monocytes are phagocytosis, antigen presentation and cytokine production. Functionalized CNTs have been reported to activate immune-related pathways in monocytes suggesting that such carbon-based nanomaterials may function as immunostimulatory agents [24]. CNTs were also found to trigger so-called inflammasome activation in monocytes [25] as well as in primary human monocyte-derived macrophages [26] leading to the secretion of the pro-inflammatory cytokine, interleukin (IL)-1β [for a review, see 19]. In a related study, surface functionalization of CNTs or carbon nano-onions (CNOs) attenuated the inflammatory properties of these nanomaterials, with a reduction in the recruitment of inflammatory neutrophils and monocytes in vivo and reduced IL-1β production [27]. Strategically located macrophages act as sentinels against foreign materials and can be divided into various subpopulations based upon their anatomical location and functional phenotypes. The granulocytes, including neutrophils, basophils, and eosinophils, also form part of the innate immune system, along with mast cells, a tissue-resident granulocytic cell that is closely related to basophils. Natural killer (NK) cells are a component of the innate immune system which does not directly attack invading microbes. Instead, these cells destroy tumor cells or virus-infected cells. The interaction between immune cells and tumors, and the role of immune cells as sentinels in eliminating continuously arising transformed cells, is of particular importance for nanomedicine. Dendritic cells (DCs) are antigen-presenting cells that serve as a ‘bridge’ between the innate and adaptive arms of the immune system. The adaptive immune system, in turn, is comprised of B cells and T cells, and these cells are responsible for immunological ‘memory’ which is ‘adaptive’ because it occurs during the lifetime of an individual as an adaptation to encounters with a specific pathogen. Nanomaterials have been shown to interact with cells of the innate immune system, while effects on the adaptive immune system occur, in most but not all cases, via the innate immune system [see 8, 28 for a review]. To give one recent example, GO was shown to trigger a typical ‘foreign body’ reaction in mice upon subcutaneous implantation, with recruitment of neutrophils, followed by monocytes; these cells secreted a variety of soluble mediators resulting in the establishment of an inflammatory microenvironment [29]. GO and CNTs have both been reported to act directly on macrophages and DCs ex vivo and in animal models [30–32]. As we shall discuss in the present review, the interactions between carbon-based nanomaterials and the immune system can be reciprocal in the sense that immune-competent cells such as macrophages and neutrophils can ‘strike back’ and digest nanomaterials [19].
Biomedical applications of carbon-based nanomaterials
Carbon-based nanomaterials display excellent mechanical, thermal and optical properties making them potentially useful and attractive in medicine, including for therapeutics and/or diagnostics, as well as in regenerative medicine. In the following section, we shall discuss examples of each of these broad areas beginning with therapeutics, which in turn may be divided into: carbon-based nanomaterials as drug or gene delivery vehicles, or carbon-based nanomaterials as drugs per se.
CNTs have been studied intensively as drug carriers, with doxorubicin being the most common model drug [see 4 for a recent review]. To this end, drugs may be loaded onto CNTs through noncovalent interactions, eg., π-π stacking as shown for doxorubicin [4], although covalent binding has also been explored for hydrophilic drugs [33]. In the latter study, the authors covalently attached not only the drug, cisplatin but also the targeting ligand, epidermal growth factor (EGF), and demonstrated that these targeted vectors were selectively taken up by head and neck squamous carcinoma cells overexpressing EGF receptors [33]. Moreover, regression of tumor growth was rapid in mice treated with targeted SWCNT-cisplatin conjugates relative to the non-targeted ones. CNTs may also serve as multi-functional devices for selective cancer cell destruction, by virtue of their intrinsic physicochemical properties. For instance, Kam et al. [34] reported that SWCNTs can be deployed for targeted delivery of oligonucleotides to cancer cells with near-infrared light-mediated killing of cancer cells due to the excessive local heating of the CNTs.
CNTs are known to interact with DNA and much interest has been devoted to the potential use of CNTs for gene delivery or delivery of small interfering RNA (siRNA). Some authors have claimed a passive, “needle-like” mechanism of cellular entry for CNTs [35], which could be exploited for gene delivery, if proven to be specific for the intended target cells. In this context, the formation of a so-called bio-corona on the surface of the CNTs and its potential impact on cellular recognition and uptake needs to be taken into account (discussed below). Al-Jamal et al. [36] provided evidence for efficient delivery of siRNA directly to the CNS through stereotactic administration of MWCNTs, resulting in neuroprotection in mice and rats. We recently demonstrated that PEG-modified SWCNTs can be deployed as carriers for intra-articular delivery of antisense oligonucleotides to chondrocytes in mice without affecting cartilage homeostasis or eliciting systemic side-effects [37]. In another recent study, we developed a novel strategy for delivery of microRNAs to endothelial cells to regulate angiogenesis, using polymer functionalized MWCNTs (submitted for publication). We found that endothelial cells displayed efficient uptake of miR-503 following administration of miR-503 bound to the functionalized CNTs, and a decrease of vessel formation was observed in a mouse model of angiogenesis. Moreover, the polymer-coated CNTs displayed a reduced toxicity when compared to the pristine CNTs.
Graphene is another promising material for drug delivery. Indeed, as pointed out by Novoselov et al. [38], graphene derivatives can solubilize and bind drug molecules as a result of their large surface area and delocalized л electrons, and thus have the potential to act as drug delivery vehicles if sufficiently high drug loading and suitable in vivo drug distribution and release profiles can be achieved. In one of the earliest studies on the potential biomedical uses of graphene, Yang et al. [39] showed that intravenous administration of PEG-modified GO labeled with a near-infrared fluorescence dye, but not carrying any drug, displayed significant passive tumor targeting in several mouse xenograft models and relatively low retention in the reticuloendothelial system. The authors utilized the strong optical absorbance of the nanomaterial in the near-infrared region for in vivo photothermal therapy, achieving efficient tumor ablation. Moreover, a reduced GO-iron oxide nanoparticle complex functionalized with PEG was found to display excellent physiological stability, strong near-infrared optical absorbance, and superparamagnetic properties [40]. Using this novel theranostic probe, in vivo tri-modal fluorescence, photoacoustic, and magnetic resonance imaging was carried out, uncovering high passive tumor targeting, and this was further used for photothermal ablation of tumors in mice [40]. Furthermore, loading of doxorubicin onto the PEG-modified GO-iron oxide nanoparticle complex enabled magnetically targeted drug delivery [41]. In the latter study, magnetic resonance imaging of breast tumor-bearing mice was also demonstrated using GO–iron oxide NP–PEG as contrast agent. In a recent study, doxorubicin was chemically conjugated to polymer (i.e., PEI-PEG) grafted GO via a matrix metalloproteinase 2 (MMP2)-cleavable peptide linker [42]. MMPs are a family of enzymes predominantly secreted by tumor cells. Under normal conditions the intrinsic fluorescence property of doxorubicin is quenched by GO; upon incubation with MMP2, the peptide is cleaved thereby permitting the unloading of doxorubicin for tumor cell killing and concurrent fluorescence recovery of doxorubicin for tumor cell imaging [42], making this a versatile system, if not ‘theranostic’ in the conventional sense. Further studies are warranted to evaluate this approach using relevant in vivo tumor models, and to ascertain whether the adsorption of biomolecules leading to a bio-corona (discussed below) would obscure the peptide linker.
Fullerenes, especially C60, have received widespread attention as drug and gene delivery vehicles [43]. In one pertinent example, gene delivery in vivo using water-soluble fullerenes was demonstrated [44]. The in vivo biodistribution of the fullerene-DNA complexes and a lipid-based system (Lipofectin) showed similar patterns; however, levels of reporter gene expression varied insofar as the fullerene-based system achieved up to 10-fold higher gene expression than Lipofectin in the liver and spleen, and no gene expression in the lung. The differences in organ selectivity of the fullerene-based system could be exploited for diseases of the liver and spleen [44]. Furthermore, as proof-of-principle, the authors demonstrated that the delivery of an insulin gene using fullerenes increased plasma insulin levels and reduced blood glucose concentrations in mice.
The metallofullerenol nanoparticles are fullerene derivatives consisting of a metal atom inside a fullerene cage and are currently investigated for their unique mechanical, thermal and electrochemical properties. In particular, gadolinium (Gd) based metallofullerenes are developed as innovative contrast agents, and may also act as anti-cancer agents [45]. For example, the multi-hydroxylated metallofullerenol Gd@C82(OH)22 was recently shown to inhibit tumor metastasis through MMP inhibition rather than through direct killing of the cancer cells [46], thus suggesting a new, nanomedicine-based approach in the management of tumor metastasis [47]. In subsequent studies, based on computational and experimental approaches, the authors proposed that Gd@C82(OH)22 suppress pancreatic cancer metastasis by inhibiting the interaction of histone deacetylase 1 (HDAC1) and metastasis-associated protein 1 (MTA1), thus acting as a novel HDAC inhibitor [48]. These fullerene derivatives were also shown to possess intrinsic inhibitory activity against breast cancer cells blocking epithelial-to-mesenchymal transition with efficient elimination of so-called breast cancer stem cells resulting in abrogation of tumor initiation and metastasis [49]. Taken together, these studies thus exemplify the use of nanoparticles as drugs per se [50].
Chemoresistance is the main cause of treatment failure in advanced, metastatic cancer. Drug efflux from tumor cells by drug transporter proteins including multi-drug resistance protein 1 (MDR1), also known as P-glycoprotein, is the most common mechanism of chemoresistance [51]. Doxorubicin is a standard treatment for many cancers; however, its clinical use is limited by its known dose-dependent toxicity (cardiotoxicity and myelosuppression, i.e., decreased bone marrow activity), the emergence of so-called multi-drug resistance – which is explained by drug efflux by transporter proteins – and its low specificity against cancer cells [52]. Nano-based delivery systems, with or without targeting ligands, could potentially overcome these limitations, by reducing the side-effects and increasing the therapeutic effectiveness of the drug [53]. Interestingly, novel approaches to circumvent chemoresistance using nanodiamonds were recently reported. Chow et al. [54] showed that a complex of nanodiamonds and doxorubicin (NDX) overcame drug efflux and significantly increased tumor growth inhibition in mice bearing chemoresistant tumors. The authors found that nanodiamond conjugation resulted in sustained drug release. To measure drug retention in cells, the authors used cells overexpressing the drug transporter MDR1, and found that treatment with NDX resulted in a 10-fold increase in retained doxorubicin when compared to the free drug [54]. Moreover, NDX displayed less toxicity in mice (no myelosuppression, with no mortality at the highest doses) when compared to standard treatment with free doxorubicin [54]. In a subsequent study, nanodiamonds were used to deliver the related chemotherapeutic drug, epirubicin to cancer cells. Epirubicin is favored over doxorubicin for its lower cardiotoxicity, but can also be effluxed by cancer cells via drug transporters. Wang et al. [55] reported that nanodiamond-epirubicin complexes displayed higher efficacy compared to unmodified standard treatment in killing both normal cancer cells and cancer stem cells in vitro and in vivo, in a model of hepatic cancer enriched for chemoresistant cancer stem cells. The authors also documented that the association of epirubicin to nanodiamonds prevented efflux of the drug by drug transporters [55]. Notably, this was a function specific to nanodiamond-mediated drug delivery as epirubicin delivery by liposomes failed to enhance drug retention. Together, these studies suggest novel approaches for overcoming chemoresistance using nanodiamonds. It will be of interest to learn whether nanodiamonds are susceptible to degradation, as shown for other carbon-based nanomaterials (below).
A second major area in nanomedicine is imaging and diagnostics and carbon-based nanomaterials have received much attention also in this regard. Moreover, as already alluded to previously, therapeutic and diagnostic modalities can be combined in multi-functional theranostic devices [4]. Here, we will touch briefly on this topic [for a more comprehensive discussion, refer to 4, 56].
CNTs have been studied intensively for multiple imaging modalities including fluorescence imaging, photoacoustic and Raman imaging, and so on; some examples are provided here. De La Zerda et al [57] demonstrated that SWCNTs conjugated with cyclic Arg-Gly-Asp (RGD) peptides can be used as a contrast agent for photoacoustic imaging of malignant glioma tumors in mice. Intravenous administration of these targeted nanotubes to mice bearing tumors showed eight times greater photoacoustic signal in the tumor than mice injected with non-targeted nanotubes. Ghosh et al. [58] reported on the use of SWCNTs to visualize deep, disseminated tumors in vivo which could facilitate surgical excision of model ovarian cancers with submillimeter precision. Delogu et al. [59] provided evidence for the use of MWCNTs as ultrasound contrast agents, in a large animal model (pig). The authors could demonstrate that the ultrasound signal of functionalized MWCNTs was higher than GO, pristine MWCNTs, and functionalized SWCNTs. Similarly, graphene and its derivatives are also investigated as optical or non-optical imaging agents [56]. For instance, as already mentioned previously, novel, PEG-functionalized GO-iron oxide nanoparticle hybrid materials were recently developed for in vivo tri-modal fluorescence, photoacoustic, and magnetic resonance imaging [40]. In another related example, Shi et al. [60] reported on the application of multi-functional sensors based on GO decorated with both iron oxide and gold nanoparticles and functionalized with PEG molecules. Additionally, graphene quantum dots, an emerging fluorescent material, were shown to act as photodynamic therapy agents, with a quantum yield that is higher than for any other known PDT agent [61].
Fullerenes such as C60 have been functionalized using metals for use as contrast agents and radiotracers. Indeed, metallofullerenes have been explored as contrast agent for MRI for more than a decade [45]. Moreover, Shi et al [62] recently developed a hybrid nanoplatform with multi-functional properties for combined cancer diagnosis, photodynamic therapy, radiofrequency thermal therapy, and magnetic targeting. Hence, the authors produced a C60-iron oxide nanoparticle composite functionalized by PEG and decorated with folic acid, a widely used tumor targeting molecule, and were able to achieve synergistic, multi-modal ablation of tumors in sarcoma-bearing mice [62]. More information on the biodistribution and long-term toxicity is needed, but the approach aptly demonstrates the theranostic potential of carbon-based nanomaterials. Nanodiamonds presenting nitrogen-vacancy centers have intrinsic fluorescence properties and nanodiamonds, as well as the metal hybrid nanodiamonds, therefore present themselves as interesting tools for imaging and diagnostics [see 14 for a review]. For instance, Fu et al. [63] reported on the use of fluorescent nanodiamonds as single-particle biomarkers for in vitro studies.
Biosensors are important tools in biomedical research and are becoming an essential part of modern healthcare [64]. By taking advantage of their unique electrical and optical properties, CNTs can be integrated into highly sensitive sensors and probes [65]. For instance, Iverson et al. [66] showed that single-stranded DNA oligonucleotide-functionalized SWCNTs can be used for the selective detection of local nitric oxide (NO) concentrations in vivo in mice following intravenous injection. NO is an important signaling molecule involved in many physiological and pathological processes. The authors also found that the SWCNTs can function as implantable inflammation sensors for NO detection, with no intrinsic immune reactivity or other adverse responses. Due to the absence of photobleaching, the SWCNT-based sensors are highly stable (no negligible change of activity was noted after 400 days) [66]. In a recent study, biocompatible GO biosensors for detecting blood glucose levels over a broad concentration range were developed by covalently attaching the amine groups of glucose oxidase to the carboxyl acid groups of GO [67]. Furthermore, Jiang et al. [68] reported a novel approach for electrical sensing of NO using hemin-functionalized graphene. The graphene-hemin sensors could respond rapidly to NO in physiological environments with sub-nanomolar sensitivity. Additionally, in vitro studies showed that the sensors could be used for the detection of NO released from macrophages and endothelial cells [68].
Finally, carbon-based nanomaterials are emerging as potential candidates for the development of synthetic scaffolds in tissue engineering [see 69 for a comprehensive review]. CNTs offer several characteristics similar to those of the extracellular matrix, the environment in which cells physiologically migrate and proliferate to form tissues and organs. Cellot et al. [70] provided theoretical and experimental evidence that CNTs might improve neuronal performance by favoring electrical ‘shortcuts’ between the soma or cell body of neurons and the dendrites. Bosi et al. [71] recently reported a biocompatible, synthetic polymer based-scaffold that allowed the development of 3-dimensional hippocampal cultures. Furthermore, the authors endowed the scaffold with nano-topographies by incorporating MWCNTs which enabled the nanotubes to interface and boost cultured neuronal circuits [71]. In addition, nanodiamonds have been reported to act as a platform for neuronal growth [72] while hybrid structures of GO and silica nanoparticles promoted growth and alignment of human neural stem cells [73]. Graphene is also envisioned for artificial retinas, i.e., prosthetic devices that interface with the optical nerve; see the Science and Technology roadmap of the Graphene Flagship Project [13]. However, for such applications to be realized – indeed, for any biomedical applications of nanomaterials – a detailed understanding of the biological interactions of the nanomaterial, including bio-corona formation, is needed, and the propensity for degradation and/or clearance in vivo should also be evaluated.
Bio-corona formation on carbon-based nanomaterials
In biological environments, nanomaterials are rapidly coated with proteins, lipids and other biomolecules [74]. This so-called bio-corona formation confers a new biological ‘identity’ to the nanomaterial, and this is of key importance for the subsequent biological (and toxicological) interactions of nanomaterials in living systems [7,75]. Moreover, it is important to consider the ‘shifting identities’ of a nanomaterial as it translocates from one biological compartment to another (for instance, from the lungs or the gastrointestinal tract to the systemic circulation) and from the extracellular environment to intracellular locations (cytoplasm, lysosomes, etc) [76]. The bio-corona could also exhibit dynamic changes when passing through these different environments, for instance as a result of enzymatic processing of the corona constituents [74]. For targeted nanomedicines, it is important to consider whether the acquired bio-corona could ‘mask’ the ligands and thereby prevent targeting to the desired location, for instance, to a tumor [77]. On the other hand, it also remains possible that the bio-corona could display functional epitopes that may engage specific cellular receptors [78]; indeed, nanomaterials could undergo ‘functionalization’ in vivo and an important challenge is thus to decipher and to control this phenomenon [79]. In the following sections, we discuss bio-corona formation in relation to the biological behavior of nanomaterials, and more specifically in relation to targeting of nano-carriers.
There are several experimental and theoretical studies on bio-corona formation on CNTs, and also some recent studies on GO. Dutta et al. [80] identified albumin as the major fetal bovine or human serum/plasma protein adsorbed onto SWCNTs and noted that the bio-corona plays an important role in modulating cellular uptake of SWCNTs in murine RAW264.7 macrophage-like cells, presumably through interactions with scavenger receptors. Ge et al. [81] employed experimental and theoretical approaches to study the interaction of four major serum proteins – bovine fibrinogen (BFG), immunoglobulin, transferrin, and bovine serum albumin (BSA) – with SWCNTs and found that serum protein-coated SWCNTs caused less cytotoxicity than uncoated SWCNTs in human leukemia cell line (THP-1) and human umbilical vein endothelial cells (HUVECs), with BFG showing the most pronounced effect. Notably, BFG were found to rearrange themselves on the SWCNT surface in the most compact manner and the most layers (five layers as compared to two or three layers for other proteins), which may potentially explain why this protein was more effective at protecting cells from the exposure of SWCNTs [81]. Using an 80-member combinatorial MWCNT library, Gao et al. [82] found that surface chemistry modification reduced the immune perturbations of MWCNTs both in vitro and in vivo. Furthermore, these authors demonstrated that the modified MWCNTs changed their preferred binding pattern from mannose receptor to scavenger receptor, in the THP-1 macrophage model [82]. While the role of the bio-corona was not investigated in the latter study, it is more than likely that the surface modifications altered the binding of serum proteins both in vivo and in cell culture which in turn mediated the ‘switch’ from mannose receptor to scavenger receptor-mediated uptake. We recently noted that serum proteins are accountable for the Toll-like receptor (TLR)-dependent signaling of SWCNTs in primary monocyte-derived macrophages, while GO did not display such effects (manuscript in preparation). Taken together, macrophage recognition of CNTs seems to depend critically on the bio-corona and different CNT surface properties may impart critical changes in the composition of the bio-corona and hence affect the biological outcomes.
GO has an extremely high protein adsorption capacity. Hu et al. [83] noted that the cytotoxicity of GO towards human A549 lung carcinoma cells was greatly mitigated in presence of 10% fetal bovine serum, the concentration usually employed in cell culture medium. The authors noted that GO had a much higher capacity for protein loading when compared to both SWCNTs and MWCNTs. Similarly, Chong et al. [84] also found that adsorption of serum proteins onto GO drastically reduced their cytotoxicity towards A549 lung carcinoma cells and found that GO exhibits a dramatic enhancement of adsorption capacity compared to SWCNTs. In a subsequent study, coating of GO with BSA was suggested to reduce cytotoxicity towards A549 cells by reducing the physical interaction of GO with the cell membrane [85]. It is noted, however, that A549 is a notoriously robust carcinoma cell line not reflective of normal cell physiology. It will therefore be of interest to perform similar studies using professional phagocytic cells (macrophages) or other primary immune-competent cells. Using molecular dynamics (MD) simulations, aromatic residues were found to contribute significantly to the protein adsorption due to the strong π-π stacking interactions between their aromatic rings and the graphene sp2-carbons [86]. In addition, basic residues like arginine played an equally or even stronger role during this process. Furthermore, in another MD study, the dependence on surface curvature was investigated for adsorption of BSA onto CNTs of increasing radius versus a flat graphene sheet, and the results confirmed that protein adsorption capacity is indeed enhanced on flatter surfaces [87].
Most studies to date on the bio-corona have been conducted using human plasma or bovine serum as a source of biomolecules reflective of the conditions in the blood or in cell culture, respectively [74]. However, following the introduction of nanomaterials into other compartments, such as the lung or the gastrointestinal tract, nanomaterials may encounter a different environment leading to the formation of a distinct bio-corona. In the first study on the potential in vivo formation of a bio-corona in the lungs, Kapralov et al. [88] found that pharyngeal aspiration of SWCNTs in mice resulted in adsorption of lung surfactant proteins and surfactant lipids and, furthermore, that this protein-lipid bio-corona facilitated uptake of SWCNTs by murine RAW264.7 macrophage-like cells. In a related in vitro study using amino- and carboxyl-modified MWCNTs, Gasser et al. [89] found that surfactant lipids (derived from Curosurf) bind unspecifically to the different functionalized MWCNTs, in contrast to plasma proteins which showed characteristic binding patterns. They also noted that the pattern of plasma protein binding was altered when MWCNTs had been previously coated with pulmonary surfactant. This could be interpreted to suggest that nanomaterials retain a ‘memory’ of previous biological environments or compartments in vivo, for instance, upon translocation across the lung-blood barrier.
As we have discussed, the adsorption of proteins and other biomolecules onto the surface of nanomaterials can influence the ‘identity’ and biological behavior of the nanomaterials. Conversely, the interactions between biomolecules and nanomaterials can also lead to altered conformational and orientational changes of the biomolecules, potentially revealing cryptic epitopes that could trigger immune responses via specific cell surface receptors [78]. Indeed, one may view the altered proteins on the surface of nanomaterials as ‘nanomaterial-associated molecular patterns’ or NAMPs analogous to the pathogen-associated molecular patterns (PAMPs) displayed by microbes [8]. Moreover, protein adsorption by nanomaterials, not least by GO, which presents a vast surface for protein binding, can lead to inhibition of enzyme activity. Hence, recent studies have shown that carbon-based nanomaterials can inhibit the bacterial enzyme, VIM-2 belonging to the clinically relevant class of metallo-β-lactamases that provide resistance to a broad spectrum of antibiotics including penicillin; the inhibition was noncompetitive and was attributed to hydrophobic interactions with the enzyme [90]. Moreover, adsorption of VIM-2 was further probed using protein displacement assays and it could not displace or be displaced by BSA. We recently found that both SWCNTs and GO inhibit CYP3A1, a major drug-metabolizing enzyme and that this was mitigated when the nanomaterials were pre-coated with BSA (submitted for publication). In addition, previous studies have shown that GO is an inhibitor of α-chymotrypsin [91] and β-galactosidase [92] while, on the other hand, PEGylated GO can apparently boost the activity of trypsin, but has no effect on chymotrypsin or proteinase K, which are also serine proteases [93]. Shurin et al. [32] reported that GO can trigger alveolar macrophage production of chitinases, enzymes whose expression is associated with asthma, in mice and theoretical and experimental data suggested that GO could directly interact with and inhibit chitinase activity. Whether inhibition of chitinases also occurs in a complex biological environment, in the presence of lung surfactant or other biomolecules, remains to be understood.
The complement system is a part of the innate immune system that helps or complements other humoral (antibodies) or cellular (phagocytes) components of the immune system to clear pathogens. Importantly, carbon-based nanomaterials, not least CNTs, have been shown to bind complement factors and this phenomenon thus represents a special case of bio-corona formation which is of considerable relevance as complement-mediated toxicity is a major limiting factor for nanomedicine applications following intravenous administration of the nano-carrier [reviewed in 94]. There are three established pathways of complement activation: the so-called classical, lectin and alternative pathways. The majority of complement activation studies with nanomaterials – including CNTs and GO with or without polyethylene glycol (PEG) modification on the surface – have focused mostly on the classical and alternative pathways [95–97]. However, as recently pointed out by Moghimi et al. [98], there is now evidence to suggest that many nanoparticles may trigger complement activation through the lectin pathway, which involves carbohydrate recognition, even though these nanoparticles do not per se express surface-exposed sugars. Instead, according to Moghimi et al. [89, and see references therein], functionalized nanoparticles may ‘mimic’ pathogens by virtue of the projected polymeric surface architecture that resembles structural motifs of peptidoglycan constituents of pathogens which then triggers the lectin pathway.
Nanomaterials intended for use as drug delivery vehicles are commonly functionalized using long hydrophilic polymers such as poly(acrylic acid), chitosan or PEG, as this increases the biocompatibility of these systems and is thought to reduce non-specific protein adsorption and clearance by phagocytic cells of the reticuloendothelial system (RES), thereby promoting passive targeting to the desired location, such as a tumor [99]. However, as we shall discuss in more detail below, PEG functionalization does not completely prevent protein adsorption. Moreover, to effectively counter the non-specific uptake by phagocytic cells, PEG molecules typically need to have a molecular weight in excess of 2 kDa, which adds considerably to the overall hydrodynamic diameter of the nanoparticles. In recent years, zwitterionic coatings have been explored as an alternative strategy to endow nanoparticles with “stealth” properties [see 100 for an excellent review]. Because such coatings can be constructed from low-molecular weight materials they provide an opportunity to develop ultra-small, excretable nanoparticles for biomedical applications [100]. Choi et al. [101] demonstrated renal filtration and urinary excretion of inorganic, metal-containing nanoparticles with zwitterionic or neutral organic coatings. Notably, zwitterionic coating using the amino acid cysteine prevented protein adsorption while yielding the highest solubility and the smallest hydrodynamic diameter. In comparison, although neutral, PEGylated nanoparticles did not bind serum protein, it was not possible to synthesize such particles with a hydrodynamic diameter < 10 nm; shorter PEG chains resulted in insoluble particles [101].
Active targeting of nanoparticles is also frequently deployed. For instance, folic acid (FA) or transferrin, recognized by the folate receptor and transferrin receptor, respectively, are commonly used in an attempt to increase cellular uptake of drug-loaded carriers in cancer cells overexpressing these receptors [102]. However, if it is true that all nanoparticles are rapidly coated with biomolecules in a living organism, then it is also possible that the additional layer(s) of proteins (and other biomolecules) could obscure the targeting ligands that have been grafted onto the surface of the nanoparticles. Indeed, in a recent in vitro study using transferrin-conjugated nanoparticles, Salvati et al. [77] found that proteins in the cell culture medium can shield transferrin from binding to its cognate receptors on cells. Thus, although nanoparticles continued to enter the cells, the targeting specificity of transferrin was lost. In contrast, we previously observed that specific, i.e., FA-dependent uptake of FA-conjugated iron oxide nanoparticles by human ovarian cancer cells expressing the corresponding receptor was observed only in the presence of serum proteins, possibly due to a stabilizing effect of the serum proteins on the functionalized nanoparticles in vitro [103]. However, while Fe3O4-SiO2-FA particles were specifically internalized, Fe3O4-PEG-FA nanoparticles did not undergo specific (targeted) uptake in the same model cell line; it is conceivable that the targeting ligand (FA) on the PEGylated particles was embedded in a matrix of polymers and therefore not accessible for binding to its receptor [103]. Indeed, Dai et al. [104] reported that backfilling the surface of a targeted nanoparticle with PEG molecules reduces protein corona formation and noted that the length of the PEG molecules must be less than the length of the ligand linker; otherwise, PEG interferes with the binding of the targeting ligand to its cellular receptor. More recently, it was demonstrated that the formation of a protein corona does not significantly influence the targeting ability of antibody-functionalized polymeric particles towards human colon cancer cells [105]. In another recent study. Hadjidemetriou et al. [106] reported on the formation of a bio-corona on clinically relevant, antibody-functionalized nanoparticles (liposomes) in mice. The authors found that both in vitro and in vivo formed protein coronas significantly reduced cellular internalization of the antibody-conjugated liposomes, using human cervix or breast cancer cell lines (however, in vivo targeting was not evaluated); notably, the in vivo corona formation did not completely prevent the targeting capability [106]. Thus, it appears that the bio-corona may impact on targetability of nanomedicines, but it is unlikely to be the sole critical factor determining their behavior.
Turning now to targeting of carbon-based nanomaterials, we previously reported on in vivo targeting of intratumoral regulatory T cells (Treg) using PEG-modified SWCNTs [107]. We focused our attention on the glucocorticoid-induced TNFR-related receptor (GITR), as it showed higher expression on intratumoral versus peripheral (i.e., splenic) Treg compared to other reported Treg-specific markers. Our in vivo investigations showed that PEG-SWCNTs armed with GITR ligands targeted Treg residing in a melanoma xenograft more efficiently then intratumoral non-Treg or splenic Treg [107]. The latter result was likely accomplished through a combination of passive tumor targeting (i.e., enhanced permeability and retention effect, EPR) due to enhanced tumor vascular permeability and active targeting of markers enriched in intratumoral Treg. This example of intratumoral immune cell targeting thus points towards novel, nano-based immunotherapies against cancer. Further examples of targeted SWCNTs are discussed in the following section. Hong et al. [108] demonstrated that GO can be specifically directed to the tumor neovasculature in vivo through targeting of CD105, a vascular marker for tumor angiogenesis (Figure 2). Notably, incorporation of an active targeting ligand (TRC105, a monoclonal antibody that binds to CD105) led to significantly improved tumor uptake of functionalized GO, which was specific for the neovasculature [108]. The administration of a blocking dose of TRC105 before injection of the nano-graphene significantly reduced the tumor uptake which demonstrated CD105 specificity. Hence, although one might assume that the masking of targeting ligands would be of particular concern for graphene-based materials, as the potential for protein adsorption is considerable, this study suggests that relevant targeting can in fact be achieved in vivo.
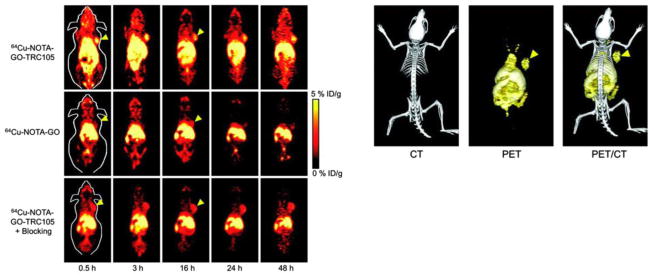
Figure 2.
Targeting of tumor vasculature with graphene oxide. In vivo PET/CT imaging of 64Cu-labeled GO conjugates in breast tumor-bearing mice. Left panel shows serial coronal PET images of tumor-bearing mice at different time points post-injection of 64Cu-NOTA-GO-TRC105, 64Cu-NOTA-GO, or 64Cu-NOTA-GO-TRC105 after a pre-injected blocking dose of TRC105. Tumors are indicated by arrowheads. Right panel displays representative PET/CT images of 64Cu-NOTA-GO-TRC105 in tumor-bearing mice. Reprinted from: Hong H, Yang K, Zhang Y, Engle JW, Feng L, Yang Y, Nayak TR, Goel S, Bean J, Theuer CP, Barnhart TE, Liu Z, Cai W. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano. 2012;6(3):2361–70, with permission from American Chemical Society.
Finally, it is pertinent to note that the molecular composition of biological fluids in patients suffering from cancer or other diseases is unlikely to resemble the normal situation. Indeed, recent studies on cellular uptake of GO have suggested that attention should be focused on the ‘personalized bio-corona’ resulting from differences in protein content in human plasma from various types of disease [109]. This is a potentially important challenge for the nanomedicine community.
Biodistribution of carbon-based nanomaterials
Understanding the fate and behavior of carbon-based nanomaterials in vivo is imperative for the clinical translation of these materials. Pharmakokinetic (PK) profiling addresses the adsorption, distribution, metabolism, and excretion (ADME) of a drug or nanomaterial in vivo [see 110 for a review]. Metabolism (or, degradation) of carbon-based nanomaterials is discussed in a subsequent section. Here, we focus on other aspects of in vivo biodistribution of nanomaterials.
Drug molecules diffuse and distribute freely throughout the body, causing unpredictable or undesirable effects in bystander tissues while also limiting the achievement of doses needed for a therapeutic response. One of the great promises of nanomedicine is the local or targeted delivery of drugs. Efficient targeting would allow for a reduced systemic dosage meaning also a reduced toxicity while resulting in relatively higher or more efficient dosage at the desired target site [111]. In an excellent and very recent review, Ferrari and co-workers highlighted the biological barriers that drug-loaded nanoparticles encounter upon intravenous administration [112]. These barriers include, for instance, opsonization and subsequent sequestration by the RES, as discussed at length in the present review, as well as hemorheological/blood vessel flow limitations, and they prevent efficacious, site-specific delivery to tumors, as well as in other clinical conditions. Tasciotti et al. [113] developed a multi-stage delivery system designed specifically to circumvent several biological barriers after intravascular delivery. In this paradigm, stage-1 mesoporous particles were loaded with stage-2 nanoparticles, i.e., quantum dots (QDs) or SWCNTs, which in turn could carry active agents or higher-stage particles. The authors reasoned that by loading the stage-2 nanoparticles inside the pores of the stage-1 particles, RES uptake would be prevented. In this manner, the mesoporous particles would transport and protect a payload of nanoparticles and bioactive agents throughout their journey in the circulatory system [113]. To escape circulation, as in the case of drug delivery to a solid tumor, the size of the nanoparticles is obviously critical, but it is also important to note that the EPR phenomenon may vary dramatically with regards to the degree of tumor vascularity [114]. Moreover, while the presence of a targeting ligand (see previous section for a discussion on active targeting) does not seem to significantly affect extravasation of nanoparticles, inefficient extravasation could significantly affect targeted delivery [111]. This means that both passive and active targeting mechanisms are likely to play a role.
In order to study the biodistribution of carbon-based nanomaterials, appropriately labeled nanomaterials are needed, or one may capitalize on their intrinsic physicochemical properties [115]. In an early effort to monitor the fate of CNTs, Singh et al. [116] examined the PK behavior of water-soluble, SWCNTs functionalized with the chelating molecule DTPA and labeled with 111In for imaging purposes. The authors noted that the CNTs were not retained in the liver or spleen upon intravenous administration in mice, and that the functionalized CNTs were rapidly cleared from systemic blood circulation through the renal excretion route with a blood circulation half-life of 3.5 h. This ‘paradoxical’ glomerular filtration of SWCNTs was also reported by others [117]. Subsequent studies on the retention of functionalized MWCNTs in the organs of mice showed that the degree of chemical functionalization determines tissue distribution and excretion profile; hence, increasing the degree of functionalization enhanced renal clearance, while lower functionalization promoted RES accumulation (i.e., liver and spleen) [118]. Additionally, using similarly radiolabeled MWCNTs, the authors could show that the diameter of the functionalized MWCNTs also affects their organ distribution in vivo in mice [119]. Using, 125I-labeled nanographene sheets (i.e., GO) functionalized with PEG, Yang et al. [120] demonstrated that the nanomaterial mainly accumulated in the liver and spleen after intravenous administration; substantial bone uptake was also noted at early time points, possibly owing to macrophage uptake in the bone marrow. However, the PEGylated GO was gradually cleared (and/or degraded), without appreciable toxicity up to 3 months post-exposure [120]. In a very recent study, Jasim et al. [121] studied the tissue distribution of radiolabeled and chemically functionalized GO and found that the injected material accumulated predominantly in the liver and spleen while evidence for renal excretion was also provided. As discussed by the authors, the biological fate of graphene-based materials is likely to depend both on lateral dimension and thickness (i.e., layer number) as well as on the degree of functionalization, which may play an important role for subsequent biological interactions in vivo including bio-corona formation [122]. Cherukuri et al. [123] investigated the distribution of chemically pristine, non-labeled SWCNTs upon intravenous administration. The authors made use of the intrinsic near-infrared fluorescence, a property of individualized or debundled SWCNTs, to measure the blood elimination kinetics and to identify the target organs in rabbits exposed to the nanomaterials. First, as CNTs are hydrophobic and tend to form aggregates, the SWCNTs were ultrasonically dispersed in artificial surfactant, Pluronic F108. The results showed that the SWCNT concentration in the blood decreased exponentially with a half-life of 1 h [123]. Twenty-four hours after administration, significant concentrations of SWCNTs were found only in the liver. Notably, in separate in vitro experiments, the authors determined that the surfactant was displaced within seconds by serum proteins suggesting that the PK results obtained are reflective of the fate of SWCNTs with a bio-corona of endogenous (serum) proteins rather than a synthetic surfactant [123]. Nonetheless, the retention of the near-IR fluorescence implied that the SWCNTs remained disaggregated in vivo. In another study using pristine, 13C-labeled SWCNTs, major accumulations were seen in liver, spleen, and lung following intravenous injection [124]. Thus, it is clear that the biodistribution of pristine versus functionalized CNTs differs greatly, with the former being predominantly trapped in the RES organs, while the latter favor a renal excretion route [115].
In a recent study, a novel approach was developed to monitor the distribution of carbon-based nanomaterials at the organ and sub-organ level. Chen et al. [125] thus reported on label-free mass spectrometry imaging to detect MWCNTs, single-layer GO, and carbon nanodots (CDs) in mice based on their intrinsic carbon cluster fingerprint signal. With this approach, it was observed that MWCNTs and CDs were predominantly distributed in the kidneys, whereas all three nanomaterials were detected in the red pulp of the spleen, following intravenous administration. Evidence for clearance of the CNTs and CDs via the renal excretion route was also provided, in line with previous studies [116,126]. The highest concentration of MWCNTs was found in the marginal zone of the spleen (the interface between the non-lymphoid red pulp and the lymphoid white pulp), where particulate antigens from the circulation are trapped and presented to the lymphocytes in the spleen [125] (Figure 3). This level of detail is difficult to achieve by other means. Overall, this new mass spectrometry method has the potential to be used as a general approach for the detection of carbon-based nanomaterials in tissue samples. As pointed out [127], the method does not indicate whether the nanomaterials have been transformed in vivo. Nevertheless, other methods, such as Raman confocal imaging, could provide such information [128].
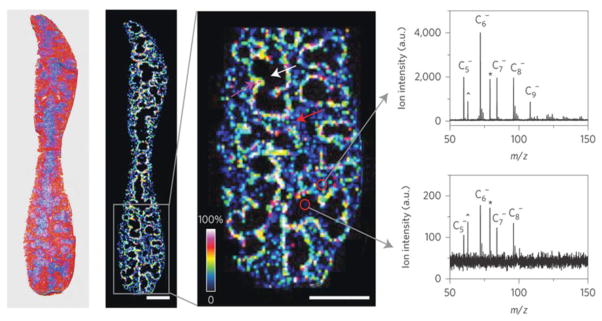
Figure 3.
Sub-organ biodistribution of carbon nanotubes. Laser desorption/ionization (LDI) mass spectrometry imaging (MSI) is an emerging label-free technique that can map chemical compounds in biological samples. From left to right: Optical image of a spleen tissue slice from mice following administration of MWCNTs; heat map showing the ion intensity distribution (m/z 72.0) of MWCNTs in a spleen tissue slice; magnified view showing the distribution of MWCNTs in the red pulp (red arrow), white pulp (white arrow) and marginal zone (purple arrow) of the spleen. Scale bars, 2 mm. Finally, representative LDI mass spectra of red and white pulp regions are depicted to the far right. Reprinted from: Chen S, Xiong C, Liu H, Wan Q, Hou J, He Q, Badu-Tawiah A, Nie Z. Mass spectrometry imaging reveals the sub-organ distribution of carbon nanomaterials. Nat Nanotechnol. 2015;10(2):176–82, with permission from Nature Publishing Group.
In nanomedicine, it is common to modify the surface of the nanomaterial with polymers such as PEG in order to avoid rapid clearance by the immune system. This has been shown to increase the circulation half-life of the nanomaterial. Liu et al. [129] reported on the biodistribution of radiolabeled SWCNTs in mice and determined the effect of PEG chain length on the biodistribution and circulation of the SCWNTs. They noted that effectively PEGylated SWCNTs exhibited relatively long blood circulation times and low uptake by RES organs. Moreover, efficient targeting of tumors in mice was achieved with SWCNTs coated with PEG chains linked to an arginine-glycine-aspartic acid (RGD) peptide [129]. Notably, injection of a blocking dose of RGD into mice bearing αvβ3-positive tumors significantly reduced the uptake of SWCNT-PEG-RGD in the tumor. However, while PEGylation has been shown to reduce protein adsorption, this surface modification does not entirely prevent bio-corona formation. The question, therefore, is whether and to what extent the bio-corona influences the biodistribution of PEGylated nanomaterials. In a recent study, we investigated the protein corona adsorbed onto SWCNTs modified with 2 kDa PEG chains by using large-scale gel-based proteomics [130]. We identified more than 500 proteins in the bio-corona; a subset of these plasma proteins were selected and grouped according to their physiological function. Coagulation proteins, immunoglobulins, apolipoproteins, and complement factors were among the proteins bound by the PEGylated SWCNTs [130]. Interestingly, PEG conformation had a stronger influence on the protein corona repertoire than nanotube surface charge. Moreover, the bio-corona affected the biodistribution of the SWCNTs in mice. Hence, a change in PEG conformation from mushroom to mushroom-brush transition affected the competitive adsorption of the major constituents of the protein corona and promoted shorter blood circulation time, faster renal excretion, and higher relative spleen versus liver uptake of PEG-SWCNTs [130]. Our data thus suggest that the bio-corona, along with steric stabilization, may mediate the action of PEG conformation on the PK profile of PEG-modified SWCNTs.
As discussed above, the PEG chains have to be of high molecular weight (>2 kDa) in order to avoid RES clearance [100]. Zwitterions on the other hand provide a highly stable coating on the surface of nanomaterials with little change in the hydrodynamic diameter. In order to both circumvent non-specific protein adsorption and promote cellular uptake by tumor cells, Yuan et al. [131] synthesized surface charge switchable nanoparticles based on zwitterionic polymers for drug delivery. The authors noted that, in physiological conditions the nanoparticles showed prolonged circulation time as a result of the reduced protein absorption afforded by the zwitterionic polymer. After accumulating in the relatively acidic tumor tissue, the zwitterionic polymer-based nanoparticle switched its surface to a positive charge, which facilitated tumor cell uptake and delivery of the anti-cancer drug, doxorubicin. Thus, zwitterionic coatings present an alternative to PEG and offer opportunities for the design of “smart” nanoparticles for biomedical applications [100].
Finally, in an intriguing twist on tumor targeting, Smith et al. [132] recently reported that specific immune cell populations in the blood may act as Trojan horses to deliver CNTs to tumors. In general, as pointed out by the authors, tumor targeting of nanoparticles may transpire both via passive and active mechanisms, including extravasation from the blood stream into the tumor (i.e., the EPR effect) and ligand-mediated targeting to tumor cells or to the tumor vasculature, examples of which were provided in previous sections of the present review. Smith et al. [132] hypothesized that circulating cells in the blood take up nanoparticles and deposit them in the tumor, thus serving to complement the other mechanisms. Indeed, using severe combined immunodeficient (SCID) mice lacking functional B and T cells, the authors discovered that PEG-modified SWCNTs are selectively taken up by a single subset of circulating immune cells, the Ly-6Chi monocytes [132]. The mechanism for this cellular uptake was not disclosed, but it is noted that several known opsonins (i.e., phagocytosis-promoting factors) are found in the bio-corona formed on PEGylated SWCNTs [130]. Notably, these monocytes are known to differentiate into so-called tumor-associated macrophages (TAMs) and are attracted to hypoxic regions of the tumor, which may be of particular relevance in cancer treatment [133]. The authors found that the uptake of SWCNTs in circulating monocytes promoted the delivery to tumors and, remarkably, that the conjugation of a targeting ligand (RGD) to the CNTs promoted the homing of SWCNT-loaded monocytes to tumors when compared to non-conjugated and control peptide-conjugated SWCNTs [132]. These results suggest a novel mechanism for tumor targeting and demonstrate that PEGylation does not necessarily prevent immune cell recognition. Several questions arise: what is the mechanism of the selective (receptor-mediated) uptake of the SWCNTs? How does RGD functionalization of SWCNTs promote homing of these cells to tumors? Furthermore, following infiltration of this subset of monocytes into the tumor, are the SWCNTs (and their cargo) released? Finally, do the PEG-SWCNTs undergo biodegradation in TAMs?
Biodegradation of carbon-based nanomaterials
As discussed in preceding sections, there has been a widespread concern that certain CNTs, in particular, may exhibit asbestos-like pathogenicity, in part due to the fiber-like morphology, but also based on the assumption that CNTs are biopersistent, like asbestos fibers. However, several groups have reported in recent years that carbon-based nanomaterials are susceptible to biodegradation [134]. Importantly, these studies have highlighted an important role for the innate immune system in the enzymatic ‘digestion’ of carbon-based nanomaterials [135]. These observations suggest that the potentially detrimental effects of such materials may be mitigated thereby allowing the materials to be more widely applied in nanomedicine. The fact that our immune system is capable of ‘sensing’ and destroying carbon-based nanomaterials through oxidative reactions may not come as a surprise given that such nanomaterials have been shown to occur abundantly in nature [136,137]. Moreover, diamond, graphitic, fullerenic and amorphous carbon particles can form in the flame of a candle [138]. Thus, this suggests that mankind has been exposed to carbon-based nanomaterials since the dawn of time and it is conceivable that defense mechanisms have evolved to protect us not only from microbes but also from other foreign particles.
While chemical degradation of carbon-based nanomaterials was demonstrated through either harsh chemical treatment with concentrated mineral acids [139] or destruction of graphitic lattices through high temperature treatment [140], neither of these processes are relevant once these nanomaterials find their way into a living organism. However, peroxidases which have strong redox potentials, are known to catalyze oxidation of foreign particles and pathogens with hydrogen peroxide in biological systems. Allen et al. [141] initially demonstrated the degradation of SWCNTs using a plant-derived enzyme, horseradish peroxidase (HRP). HRP contains a single protoporphyrin IX heme group which in its inactive form exists in its ferric (Fe3+) oxidation state and upon reaction with hydrogen peroxide forms a ferryl oxo iron (Fe4+=O) known as Compound I [142]. The high redox potential of Compound I enables degradation of carboxylated SWCNTs due to the close proximity of SWCNTs to the heme catalytic active site [134]. We and others have documented the degradation of single- and multi-walled CNTs and GO by HRP and H2O2 [141,143–146]. Moreover, lignin peroxidase produced by white rot fungus also has the capacity to induce oxidative biodegradation of fullerenes, SWCNTs, and graphene nanoribbons [147–149] while recent studies have demonstrated biodegradation of MWCNTs and GO by various bacteria [150,151].
The catalytic heme active site is also characteristic of mammalian peroxidases including neutrophil myeloperoxidase (MPO), eosinophil peroxidase (EPO), and lactoperoxidase (LPO), suggesting avenues for biological degradation of carbon-based nanomaterials. Indeed, MPO, EPO, and LPO have all been shown to catalyze the degradation of carboxylated SWCNTs in vitro in the presence of H2O2 and halide ions through the production of reactive radical intermediates in addition to hypohalous acids [152–154] (Figure 4). Degradation of oxidized SWCNTs has been demonstrated upon incubation with MPO and H2O2, in addition to incubation only with sodium hypochlorite (NaOCl). However, only the combined effects of MPO, H2O2, and NaCl (resulting in the production of hypochlorous acid, HOCl) allowed for rapid degradation of oxidized SWCNTs [152]. In addition to electron microscopy-based evidence (SEM and TEM), degradation has further been proven by tracking decreases in the intrinsic Raman peaks of SWCNTs, specifically the D-band (disorder in sp2 hybridized carbon ~ 1350 cm−1) and the G-band (graphitic C-C bond stretching ~ 1600 cm−1) with complete degradation occurring at 24 h of incubation with MPO, H2O2, and NaCl [152]. The peroxidase-catalyzed degradation of SWCNTs has been shown to proceed efficiently with oxidized SWCNTs where incorporated functionalities create defect sites for docking of the respective enzymes [142]. The higher the degree of the incorporated defects the higher the rate of degradation, with pristine SWCNTs remaining unaffected by the degradation cycle [142]. The degradation of SWCNTs results in shortening of nanotube length, leading to the production of oxidized polyaromatic hydrocarbons and, ultimately, CO2 [142]. However, detailed determination of degradation intermediates has proven difficult as the system contains multiple complex molecular ions and fragments according to mass spectrometry (MS). In order to better understand the degradation products an enzyme-free system of GO degradation by the photo-Fenton reaction was investigated in which the products were identified via multiple analytical techniques (FTIR, MS, and NMR) [155]. The degradation was found to proceed through an oxidation and decarboxylation mechanism ultimately resulting in oxidized hydrocarbons, specifically aromatic rings functionalized with carboxylic acid groups [155]. These findings suggest that partially degraded GO or CNTs could trigger genotoxicity, as shown for extracts from HRP-degraded SWCNTs [156]. Further studies using mammalian peroxidases, in relevant in vitro and in vivo settings, are needed to address whether partial biodegradation of carbon-based nanomaterials elicits more or less genotoxicity when compared to the undigested, as-delivered nanomaterials. Complete degradation, however, is not expected to do so.
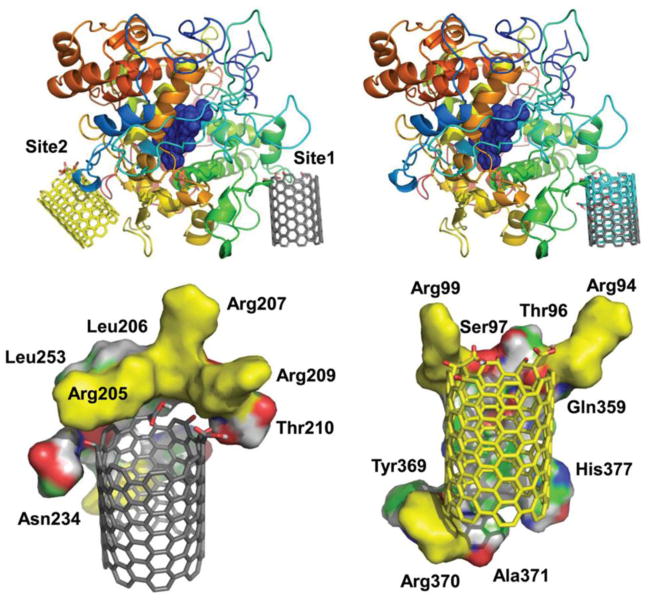
Figure 4.
Enzymatic degradation of carbon nanotubes. Molecular modeling demonstrating possible SWCNT interaction sites on eosinophil peroxidase, EPO. Upper left: The two predicted interaction sites, Site 1 and Site 2 of oxidized SWCNTs modified at the edge. Upper right: Overlay of the possible interaction Site 1 of SWCNTs oxidized at the edge (colored in grey) and in the middle (colored in cyan). Lower left and right: The residues that are in close proximity (within 4 Å), stabilizing the binding sites (left) Site 1 and (right) Site 2. Positively charged residues (arginines) that are predicted to stabilize the oxidized groups on SWCNTs are colored in yellow. Reprinted from: Andón FT, Kapralov AA, Yanamala N, Feng W, Baygan A, Chambers BJ, Hultenby K, Ye F, Toprak MS, Brandner BD, Fornara A, Klein-Seetharaman J, Kotchey GP, Star A, Shvedova AA, Fadeel B, Kagan VE. Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small. 2013;9(16):2721–9, 2720, with permission from John Wiley and Sons.
In addition to test-tube experiments of SWCNT degradation using recombinant peroxidases, we and others have shown that primary cells of the innate immune system are capable of enzymatic degradation of SWCNTs [152,153,157]. Notably, this degradation may take place both intracellularly and extracellularly. Kagan et al. [152] demonstrated that opsonization of SWCNTs with immunoglobulin (IgG) promotes neutrophil uptake ex vivo with subsequent degradation of the nanotubes. However, evidence for MPO-dependent degradation of oxidized SWCNTs in vivo in the lungs of mice was also obtained [158]. Importantly, activated neutrophils and eosinophils are known to release their granule contents including MPO and EPO, respectively, which enables extracellular destruction of microbes, and we cannot discount this pathway in the enzymatic degradation observed for SWCNTs [152,153]. Moreover, neutrophils can produce so-called neutrophil extracellular traps (NETs) consisting of nuclear chromatin fibers studded with granule proteins, and our studies have shown that purified NETs can ‘capture’ and digest SWCNTs, testifying to the versatility of these cells [159]. In addition to neutrophils and eosinophils, recent data also suggest that macrophages can ‘digest’ SWCNTs [160]. Neutrophils are short-lived with a life-span of days. In contrast, tissue-resident macrophages may persist for weeks in the context of chronic inflammation. However, in contrast to neutrophils, macrophages do not express significant amounts of MPO. Instead, the oxidative metabolism and destruction of foreign bodies including pathogens is driven by NADPH oxidase (producing superoxide) and the inducible isoform of nitric oxide synthase (iNOS) (producing nitric oxide), which conspire to produce a highly potent oxidant, peroxynitrite (ONOO−) [160]. Using a model of activated human THP-1 cells, Kagan et al. [144] recently demonstrated peroxynitrite-driven degradation of SWCNTs. Evidence for NADPH oxidase-dependent degradation of SWCNTs in vivo was also provided [160]. Moreover, Zhang et al. [161] reported that carbon nanohorns also undergo partial degradation in macrophage-like cell lines (RAW264.7 and THP-1) and degradation of MWCNTs has also been reported recently using differentiated THP-1 cells as a model [162]. Importantly, in the studies cited above, degradation was shown to occur in a complex biological environment, i.e., in cell culture in the presence of fetal bovine serum, or in the lungs of mice, suggesting that bio-corona formation does not prevent degradation of carbon-based nanomaterials. To probe this in further detail, we recently investigated whether LPO-mediated degradation could occur in the presence of lung surfactant [154]. LPO is a secreted enzyme that has been shown to be important for airway defense against infection. Indeed, efficient LPO-mediated degradation of carboxylated SWCNTs pre-coated with porcine lung surfactant (Curosurf) was documented by Raman spectroscopy, and we also observed biodegradation of SWCNTs in cell-free broncheoalveolar lavage fluid [154]. Thus, the presence of a protein-lipid corona [88] does not appear to interfere with degradation. One may postulate that several complementary pathways act together to ensure that foreign intruders – such as nanoparticles – are recognized and cleared from the lungs, including secreted enzymes as well as cell-based systems. Perhaps, as previously suggested, neutrophils are initially engaged while macrophages are called into action at a later stage [160]. Moreover, similar, macrophage-driven reactions may take place in other compartments; for instance, recent studies have suggested that MWCNTs stereotactically injected into the mouse brain cortex are internalized by microglia, the resident macrophages of the brain, and degraded [163].
As mentioned before, PEGylation is commonly applied in nanomedicine in order to reduce non-specific protein adsorption and extend the half-life of nanomaterials in systemic circulation. However, it is important to ask whether such modifications make the nanomaterials impervious to enzymatic degradation. In a recent study, we noted that the presence of PEG chains on the surface of SWCNTs may interfere with the degradation under in vitro conditions using recombinant MPO in a PEG chain molecular weight dependent manner, suggesting that there could be some steric hindrance [164]. However, when SWCNTs were incubated with activated human neutrophils undergoing degranulation, effective degradation of SWCNTS was observed irrespective of whether they were PEG-modified or not, and we provided evidence for a cooperative action of NE, a neutrophil protease, and MPO, suggesting that neutrophils release enzymes that can ‘strip’ the PEG chains of PEGylated SWCNTs allowing for efficient peroxidase-driven degradation [164]. Furthermore, other investigators have documented defunctionalization of PEGylated SWCNTs in vivo following intravenous administration in mice [165]. Combined, these results imply that PEGylated CNTs may undergo defunctionalization and degradation.
We previously developed so-called nitrogen-doped carbon nanotube cups [166]. In a recent study, we found that these hollow, cup-shaped nano-containers can be effectively ‘corked’ with gold nanoparticles and we have shown that MPO can ‘open’ the corked carbon nanotube cups through detachment of the gold nanoparticles, with subsequent enzymatic degradation of the graphitic shells [167]. Furthermore, the gold-corked carbon nanotube cups were demonstrated to function as drug delivery carriers, capable of delivery of the chemotherapeutic agent, paclitaxel to myeloid-derived suppressor cells (MDSC), with MPO-regulated release of the drug, resulting in the differentiation of MDSC into dendritic cells (DC), a property of MDSC that has been reported to be lost in cancer [167]. The findings indicate the potential of the gold-corked carbon nanotube cups in drug delivery applications. MDSC are known to overexpress both MPO and inducible nitric oxide synthase (iNOS), potentially providing a route for enzymatic degradation similar to the peroxidase-catalyzed and peroxynitrite-mediated degradation route of neutrophils and macrophages, respectively [160]. Notably, not only the nano-carrier, but also the payload (drug) may undergo degradation. In a recent study, we were able to demonstrate that SWCNTs externally functionalized with doxorubicin could be employed for drug delivery, and found that the nano-carriers improved the efficacy of the drug in an in vitro melanoma cell model due to the protection provided against oxidative degradation exerted by MDSC present in the cell culture [168]. Thus, on the basis of these studies, one may conclude that it is crucial to understand and control the degradation of carbon-based nanomaterials, not only from the perspective of their perceived toxicity, but also for the implementation of carbon-based drug delivery vehicles. Indeed, it is important to strike the right balance between degradation and resistance of the carrier and its payload against oxidants generated by inflammatory cells in the tumor microenvironment [168].
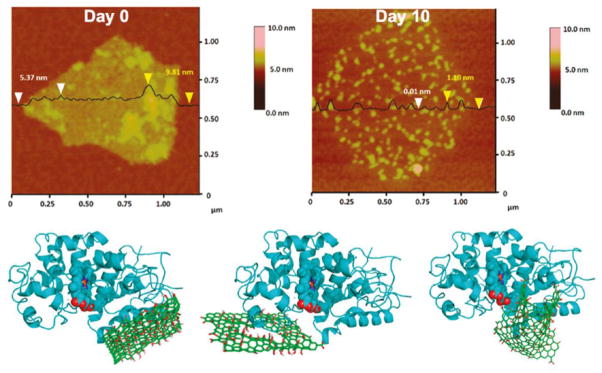
GO was also found to be degraded through the same peroxidase cycle as CNTs; however, the degradation occurs through a slightly different mechanism. Due to preferential binding of peroxidase enzymes such as HRP to the basal plane of GO, as opposed to the edges of GO flakes, the graphitic lattice is degraded from select points throughout the GO sheets resulting in the formation of holes [169] (Figure 5). Moreover, analogous to pristine, un-oxidized SWCNTs which are resistant to enzymatic degradation, reduced GO with an absence of oxygen functionalities resisted degradation and remained intact with no evidence of hole formation after 20 days of incubation with HRP and H2O2 [169]. More recent studies have shown that the level of oxidation of GO directly correlates with the efficiency of MPO-mediated degradation in vitro [170]. The oxygen functional groups provide better dispersibility of GO in aqueous media thus facilitating the degradation of GO [170]; additionally, it can be argued that the incorporated oxygen functionalities may allow for close proximity with the active site of MPO. Furthermore, we recently obtained evidence for degradation of GO by activated neutrophils (manuscript in preparation). Degradation of carboxylated graphene has been reported in vivo in mice following intravenous injection. Using Raman confocal imaging, Girish et al. [128] reported degradation of graphene from day 8 onwards, beginning from the edges and growing inwards. Spleen samples showed the most enhanced disorder leading to an almost complete amorphization of graphene over a period of 3 months. The authors provided evidence of phagocytosis of graphene by alveolar macrophages, Kupffer cells and spleen bound macrophages, implying that the degradation of graphene was mainly orchestrated by macrophages in the respective organs [128]. In addition, in vitro studies using murine RAW264.7 macrophage-like cells showed development of structural disorder in the engulfed graphene when monitored up to 7 days, supporting the role of macrophages in their biodegradation [128]. Together, the available data suggest that both neutrophil and macrophage mediated degradation of graphene and its derivatives is possible, as shown for CNTs.
Figure 5.
Enzymatic degradation of graphene oxide. Upper panels show atomic force microscopy (AFM) images with section analysis of GO and horseradish peroxidase (HRP) at day 0 (left) and at day 10 (right). GO with HRP has a sheet height of 5.37 nm and 9.81 nm. Holey GO has a sheet height of 1.10 nm, and the holes were authentic at a height of 0.01 nm. Lower panels display binding poses of HRP on (from left to right) GO, holey GO, and a small sheet of GO calculated using molecular docking studies. Reprinted from: Kotchey GP, Allen BL, Vedala H, Yanamala N, Kapralov AA, Tyurina YY, Klein-Seetharaman J, Kagan VE, Star A. The enzymatic oxidation of graphene oxide. ACS Nano. 2011;5(3):2098–108, with permission from American Chemical Society.
Finally, Li et al. [171] reported that both PEG-coated and BSA-coated GO is fairly resistant to HRP-mediated biodegradation when compared to non-functionalized GO. In order to obtain functionalized GO that can still undergo enzymatic degradation, the authors conjugated PEG to GO via a cleavable disulfide bond, obtaining GO-SS-PEG with negligible toxicity and considerable degradability [171]. This study thus points towards a safe-by-design approach that also takes degradability into account. In fact, for drug delivery applications, it may be important to control the biodegradation of carbon-based nanomaterials both temporally and spatially (i.e., degradation at the right time and at the right place) and future efforts in this area should take into account the development of nanomedicine vectors that are degradable-on-demand [135]. For some other emerging biomedical applications, including in the field of regenerative medicine, rapid degradation of the nanomaterial in question may not be desirable; for instance in the case of CNTs as scaffolds for neurons in spinal cord injury or graphene as an artificial retina (discussed above).
Concluding remarks
In synopsis, one may conclude that carbon-based nanomaterials show tremendous promise as drug or gene delivery vehicles and/or imaging agents, and as biosensors, in diverse areas of medicine, although there is certainly a preponderance to date of studies on cancer. In addition, there is emerging evidence for potential applications of carbon-based nanomaterials in regenerative medicine. However, as we have discussed at length in the present review, studies on the biocompatibility, in vivo biodistribution, bio-corona formation, and biodegradation are necessary in order to translate these promising nanomaterials into the realm of clinical medicine. In fact, these various aspects of nanomaterial behavior in a living system are intimately entwined: the bio-corona has been shown to dictate biodistribution and may also impact on cellular recognition and biodegradation, which is relevant for the safety or biocompatibility of the material. Moreover, a detailed understanding of how these nanomaterials interact with the immune system is of critical importance. Indeed, coronation and degradation are directly connected to the role of the immune system in defending the body from foreign intrusion, and it can be argued that carbon-based nanomaterials are ‘sensed’ as pathogens by immune-competent cells [8]. Careful engineering of nanomaterials is needed to mitigate the potential toxicity of the material while retaining its useful properties, thereby allowing for the navigation of various compartments in the body and the timely execution of the intended function(s) at the desired location(s). In other words, one should take care not to throw out the baby with the bathwater. This is, in essence, the meaning of safe-by-design [172]. With this in mind, one may anticipate many exciting discoveries and novel applications of the carbon-based nanomaterials in the years to come.
Acknowledgments
Funding: This work was supported by grants from the European Commission (Flagship Project GRAPHENE, Grant No. 604391; FP7-NANOSOLUTIONS, Grant No. 309329; FP7-NANOREG, Grant No. 310584), the Swedish Research Council, and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) (to B.F.); National Institute of Environmental Health Sciences (NIEHS) Grant No. R01ES019304 (to A.S.); Arthritis National Research Foundation (John Vaughan Scholarship) (to M.B.); and SfP NATO Grant No. SfP-984537, and Italian Ministry of Health Grant No. PE-2011-02347026 (to S.B.).
Portions of the graphical abstract were reproduced with permission from Nature Publishing Group and John Wiley & Sons. We thank Dr. Kjell Hultenby, Karolinska Institutet, for assistance with SEM.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fadeel B. Nanosafety: towards safer design of nanomedicines. J Intern Med. 2013;274:578–80. doi: 10.1111/joim.12137. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Weissleder R. Nanotechnology. JAMA. 2015;313:135–6. doi: 10.1001/jama.2014.16315. [DOI] [PubMed] [Google Scholar]
- 3.Mundra RV, Wu X, Sauer J, Dordick JS, Kane RS. Nanotubes in biological applications. Curr Opin Biotechnol. 2014;28:25–32. doi: 10.1016/j.copbio.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Dougherty CA, Zhu K, Hong H. Theranostic applications of carbon nanomaterials in cancer: focus on imaging and cargo delivery. J Control Release. 2015;210:230–245. doi: 10.1016/j.jconrel.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli FM, Goulart VA, Gomes KN, Ladeira MS, Santos AK, Lorençon E, et al. Graphene-based nanomaterials: biological and medical applications and toxicity. Nanomedicine (Lond) 2015;10:2423–50. doi: 10.2217/nnm.15.65. [DOI] [PubMed] [Google Scholar]
- 6.Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta. 2011;1810:361–73. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Monopoli MP, Åberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–86. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 8.Farrera C, Fadeel B. It takes two to tango: understanding the interactions between engineered nanomaterials and the immune system. Eur J Pharm Biopharm. 2015;95:3–12. doi: 10.1016/j.ejpb.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–3. [Google Scholar]
- 10.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–8. [Google Scholar]
- 11.Monthioux M, Kuznetsov VL. Who should be given credit for the discovery of carbon nanotubes? Carbon. 2006;44:1621–3. [Google Scholar]
- 12.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–9. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari AC, Bonaccorso F, Fal’ko V, Novoselov KS, Roche S, Bøggild P, et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale. 2015;7:4598–810. doi: 10.1039/c4nr01600a. [DOI] [PubMed] [Google Scholar]
- 14.Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2011;7:11–23. doi: 10.1038/nnano.2011.209. [DOI] [PubMed] [Google Scholar]
- 15.Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442–7. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding X, Liu H, Fan Y. Graphene-based materials in regenerative medicine. Adv Healthc Mater. 2015;4:1451–68. doi: 10.1002/adhm.201500203. [DOI] [PubMed] [Google Scholar]
- 17.Kane AB, Hurt RH. Nanotoxicology: the asbestos analogy revisited. Nat Nanotechnol. 2008;3:378–9. doi: 10.1038/nnano.2008.182. [DOI] [PubMed] [Google Scholar]
- 18.Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol Appl Pharmacol. 2012;261:121–33. doi: 10.1016/j.taap.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya K, Andón FT, El-Sayed R, Fadeel B. Mechanisms of carbon nanotube-induced toxicity: focus on pulmonary inflammation. Adv Drug Deliv Rev. 2013;65:2087–97. doi: 10.1016/j.addr.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014;15:1427–8. doi: 10.1016/S1470-2045(14)71109-X. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez VC, Jachak A, Hurt RH, Kane AB. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol. 2012;25:15–34. doi: 10.1021/tx200339h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussy C, Ali-Boucetta H, Kostarelos K. Safety considerations for graphene: lessons learnt from carbon nanotubes. Acc Chem Res. 2013;46:692–701. doi: 10.1021/ar300199e. [DOI] [PubMed] [Google Scholar]
- 23.Godwin H, Nameth C, Avery D, Bergeson LL, Bernard D, Beryt E, et al. Nanomaterial categorization for assessing risk potential to facilitate regulatory decision-making. ACS Nano. 2015;9:3409–17. doi: 10.1021/acsnano.5b00941. [DOI] [PubMed] [Google Scholar]
- 24.Pescatori M, Bedognetti D, Venturelli E, Ménard-Moyon C, Bernardini C, Muresu E, et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials. 2013;34:4395–403. doi: 10.1016/j.biomaterials.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 25.Meunier E, Coste A, Olagnier D, Authier H, Lefèvre L, Dardenne C, et al. Double-walled carbon nanotubes trigger IL-1β release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine. 2012;8:987–95. doi: 10.1016/j.nano.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Palomäki J, Välimäki E, Sund J, Vippola M, Clausen PA, Jensen KA, et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–70. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Flavin K, Kopf I, Radics G, Hearnden CH, McManus GJ. Functionalization of carbon nanoparticles modulates inflammatory cell recruitment and NLRP3 inflammasome activation. Small. 2013;9:4194–206. doi: 10.1002/smll.201300481. [DOI] [PubMed] [Google Scholar]
- 28.Boraschi D, Costantino L, Italiani P. Interaction of nanoparticles with immunocompetent cells: nanosafety considerations. Nanomedicine (Lond) 2012;7:121–31. doi: 10.2217/nnm.11.169. [DOI] [PubMed] [Google Scholar]
- 29.Sydlik SA, Jhunjhunwala S, Webber MJ, Anderson DG, Langer R. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano. 2015;9:3866–74. doi: 10.1021/acsnano.5b01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tkach AV, Shurin GV, Shurin MR, Kisin ER, Murray AR, Young SH, et al. Direct effects of carbon nanotubes on dendritic cells induce immune suppression upon pulmonary exposure. ACS Nano. 2011;5:5755–62. doi: 10.1021/nn2014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tkach AV, Yanamala N, Stanley S, Shurin MR, Shurin GV, Kisin ER, et al. Graphene oxide, but not fullerenes, targets immunoproteasomes and suppresses antigen presentation by dendritic cells. Small. 2013;9:1686–90. doi: 10.1002/smll.201201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shurin MR, Yanamala N, Kisin ER, Tkach AV, Shurin GV, Murray AR, et al. Graphene oxide attenuates Th2-type immune responses, but augments airway remodeling and hyperresponsiveness in a murine model of asthma. ACS Nano. 2014;8:5585–99. doi: 10.1021/nn406454u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, et al. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3:307–16. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–5. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski S, Luangsivilay J, et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotechnol. 2007;2:108–13. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 36.Al-Jamal KT, Gherardini L, Bardi G, Nunes A, Guo C, Bussy C, et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci U S A. 2011;108:10952–7. doi: 10.1073/pnas.1100930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacchetti C, Liu-Bryan R, Magrini A, Rosato N, Bottini N, Bottini M. Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano. 2014;8:12280–91. doi: 10.1021/nn504537b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novoselov KS, Fal’ko VI, Colombo L, Gellert PR, Schwab MG, Kim K. A roadmap for graphene. Nature. 2012;490:192–200. doi: 10.1038/nature11458. [DOI] [PubMed] [Google Scholar]
- 39.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–23. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 40.Yang K, Hu L, Ma X, Ye S, Cheng L, Shi X, et al. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv Mater. 2012;24:1868–72. doi: 10.1002/adma.201104964. [DOI] [PubMed] [Google Scholar]
- 41.Ma X, Tao H, Yang K, Feng L, Cheng L, Shi X, et al. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012;5:199–212. [Google Scholar]
- 42.Qin SY, Feng J, Rong L, Jia HZ, Chen S, Liu XJ, et al. Theranostic GO-based nanohybrid for tumor induced imaging and potential combinational tumor therapy. Small. 2014;10:599–608. doi: 10.1002/smll.201301613. [DOI] [PubMed] [Google Scholar]
- 43.Dellinger A, Zhou Z, Connor J, Madhankumar AB, Pamujula S, Sayes CM, et al. Application of fullerenes in nanomedicine: an update. Nanomedicine (Lond) 2013;8:1191–208. doi: 10.2217/nnm.13.99. [DOI] [PubMed] [Google Scholar]
- 44.Maeda-Mamiya R, Noiri E, Isobe H, Nakanishi W, Okamoto K, Doi K, et al. In vivo gene delivery by cationic tetraamino fullerene. Proc Natl Acad Sci U S A. 2010;107:5339–44. doi: 10.1073/pnas.0909223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu X, Feng L, Akasaka T, Nagase S. Current status and future developments of endohedral metallofullerenes. Chem Soc Rev. 2012;41:7723–60. doi: 10.1039/c2cs35214a. [DOI] [PubMed] [Google Scholar]
- 46.Kang SG, Zhou G, Yang P, Liu Y, Sun B, Huynh T, et al. Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82(OH)22 and its implication for de novo design of nanomedicine. Proc Natl Acad Sci U S A. 2012;109:15431–6. doi: 10.1073/pnas.1204600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balogh LP. Caging cancer. Nanomedicine. 2015;11:867–9. doi: 10.1016/j.nano.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Pan Y, Wang L, Kang SG, Lu Y, Yang Z, Huynh T, et al. Gd-metallofullerenol nanomaterial suppresses pancreatic cancer metastasis by inhibiting the interaction of histone deacetylase 1 and metastasis-associated protein 1. ACS Nano. 2015;9:6826–36. doi: 10.1021/nn506782f. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Chen C, Qian P, Lu X, Sun B, Zhang X, et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat Commun. 2015;6:5988. doi: 10.1038/ncomms6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallud A, Fadeel B. Keeping it small: towards a molecular definition of nanotoxicology. Eur J Nanomedicine. 2015;7:143–51. [Google Scholar]
- 51.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 52.Prados J, Melguizo C, Ortiz R, Vélez C, Alvarez PJ, Arias JL, et al. Doxorubicin-loaded nanoparticles: new advances in breast cancer therapy. Anticancer Agents Med Chem. 2012;12:1058–70. doi: 10.2174/187152012803529646. [DOI] [PubMed] [Google Scholar]
- 53.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 54.Chow EK, Zhang XQ, Chen M, Lam R, Robinson E, Huang H, et al. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med. 2011;3:73ra21. doi: 10.1126/scitranslmed.3001713. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Low XC, Hou W, Abdullah LN, Toh TB, Mohd Abdul Rashid M, et al. Epirubicin-adsorbed nanodiamonds kill chemoresistant hepatic cancer stem cells. ACS Nano. 2014;8:12151–66. doi: 10.1021/nn503491e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo JM, Kang JH, Hong BH. Graphene-based nanomaterials for versatile imaging studies. Chem Soc Rev. 2015;44:4835–52. doi: 10.1039/c5cs00072f. [DOI] [PubMed] [Google Scholar]
- 57.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3:557–62. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh D, Bagley AF, Na YJ, Birrer MJ, Bhatia SN, Belcher AM. Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes. Proc Natl Acad Sci U S A. 2014;111:13948–53. doi: 10.1073/pnas.1400821111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delogu LG, Vidili G, Venturelli E, Ménard-Moyon C, Zoroddu MA, Pilo G, et al. Functionalized multiwalled carbon nanotubes as ultrasound contrast agents. Proc Natl Acad Sci U S A. 2012;109:16612–7. doi: 10.1073/pnas.1208312109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi X, Gong H, Li Y, Wang C, Cheng L, Liu Z. Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials. 2013;34:4786–93. doi: 10.1016/j.biomaterials.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Ge J, Lan M, Zhou B, Liu W, Guo L, Wang H, et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat Commun. 2014;5:4596. doi: 10.1038/ncomms5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi J, Wang L, Gao J, Liu Y, Zhang J, Ma R, et al. A fullerene-based multi-functional nanoplatform for cancer theranostic applications. Biomaterials. 2014;35:5771–84. doi: 10.1016/j.biomaterials.2014.03.071. [DOI] [PubMed] [Google Scholar]
- 63.Fu CC, Lee HY, Chen K, Lim TS, Wu HY, Lin PK, et al. Characterization and application of single fluorescent nanodiamonds as cellular biomarkers. Proc Natl Acad Sci U S A. 2007;104:727–32. doi: 10.1073/pnas.0605409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bellucci S. Toxicology, Diagnostics, and Therapy Functions of Nanomaterials. In: Sattler KD, editor. HANDBOOK OF NANOPHYSICS: NANOMEDICINE AND NANOROBOTICS. CRC Press; Boca Raton, FL: 2010. [Google Scholar]
- 65.Wang Z, Dai Z. Carbon nanomaterial-based electrochemical biosensors: an overview. Nanoscale. 2015;7:6420–31. doi: 10.1039/c5nr00585j. [DOI] [PubMed] [Google Scholar]
- 66.Iverson NM, Barone PW, Shandell M, Trudel LJ, Sen S, Sen F, et al. In vivo biosensing via tissue-localizable near-infrared-fluorescent single-walled carbon nanotubes. Nat Nanotechnol. 2013;8:873–80. doi: 10.1038/nnano.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Yu D, Zeng C, Miao Z, Dai L. Biocompatible graphene oxide-based glucose biosensors. Langmuir. 2010;26:6158–60. doi: 10.1021/la100886x. [DOI] [PubMed] [Google Scholar]
- 68.Jiang S, Cheng R, Wang X, Xue T, Liu Y, Nel A, et al. Real-time electrical detection of nitric oxide in biological systems with sub-nanomolar sensitivity. Nat Commun. 2013;4:2225. doi: 10.1038/ncomms3225. [DOI] [PubMed] [Google Scholar]
- 69.Ku SH, Lee M, Park CB. Carbon-based nanomaterials for tissue engineering. Adv Healthc Mater. 2013;2:244–60. doi: 10.1002/adhm.201200307. [DOI] [PubMed] [Google Scholar]
- 70.Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 2009;4:126–33. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- 71.Bosi S, Rauti R, Laishram J, Turco A, Lonardoni D, Nieus T, et al. From 2D to 3D: novel nanostructured scaffolds to investigate signalling in reconstructed neuronal networks. Sci Rep. 2015;5:9562. doi: 10.1038/srep09562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thalhammer A, Edgington RJ, Cingolani LA, Schoepfer R, Jackman RB. The use of nanodiamond monolayer coatings to promote the formation of functional neuronal networks. Biomaterials. 2010;31:2097–104. doi: 10.1016/j.biomaterials.2009.11.109. [DOI] [PubMed] [Google Scholar]
- 73.Solanki A, Chueng ST, Yin PT, Kappera R, Chhowalla M, Lee KB. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv Mater. 2013;25:5477–82. doi: 10.1002/adma.201302219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Docter D, Westmeier D, Markiewicz M, Stolte S, Knauer SK, Stauber RH. The nanoparticle biomolecule corona: lessons learned - challenge accepted? Chem Soc Rev. 2015;44:6094–121. doi: 10.1039/c5cs00217f. [DOI] [PubMed] [Google Scholar]
- 75.Fadeel B, Feliu N, Vogt C, Abdelmonem AM, Parak WJ. Bridge over troubled waters: understanding the synthetic and biological identities of engineered nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:111–29. doi: 10.1002/wnan.1206. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharya K, Farcal LR, Fadeel B. Shifting identities of metal oxide nanoparticles: focus on inflammation. MRS Bull. 2014;39:970–5. [Google Scholar]
- 77.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–43. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 78.Kelly PM, Åberg C, Polo E, O’Connell A, Cookman J, Fallon J, et al. Mapping protein binding sites on the biomolecular corona of nanoparticles. Nat Nanotechnol. 2015;10:472–9. doi: 10.1038/nnano.2015.47. [DOI] [PubMed] [Google Scholar]
- 79.Hamad-Schifferli K. Exploiting the novel properties of protein coronas: emerging applications in nanomedicine. Nanomedicine (Lond) 2015;10:1663–74. doi: 10.2217/nnm.15.6. [DOI] [PubMed] [Google Scholar]
- 80.Dutta D, Sundaram SK, Teeguarden JG, Riley BJ, Fifield LS, Jacobs JM, et al. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol Sci. 2007;100:303–15. doi: 10.1093/toxsci/kfm217. [DOI] [PubMed] [Google Scholar]
- 81.Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci U S A. 2011;108:16968–73. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao N, Zhang Q, Mu Q, Bai Y, Li L, Zhou H, et al. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFκB activation and reduces its immunotoxicity. ACS Nano. 2011;5:4581–91. doi: 10.1021/nn200283g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu W, Peng C, Lv M, Li X, Zhang Y, Chen N, et al. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano. 2011;5:3693–700. doi: 10.1021/nn200021j. [DOI] [PubMed] [Google Scholar]
- 84.Chong Y, Ge C, Yang Z, Garate JA, Gu Z, Weber JK, et al. Reduced cytotoxicity of graphene nanosheets mediated by blood-protein coating. ACS Nano. 2015;9:5713–24. doi: 10.1021/nn5066606. [DOI] [PubMed] [Google Scholar]
- 85.Duan G, Kang SG, Tian X, Garate JA, Zhao L, Ge C, et al. Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane. Nanoscale. 2015;7:15214–24. doi: 10.1039/c5nr01839k. [DOI] [PubMed] [Google Scholar]
- 86.Gu Z, Yang Z, Wang L, Zhou H, Jimenez-Cruz CA, Zhou R. The role of basic residues in the adsorption of blood proteins onto the graphene surface. Sci Rep. 2015;5:10873. doi: 10.1038/srep10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu Z, Yang Z, Chong Y, Ge C, Weber JK, Bell DR, et al. Surface curvature relation to protein adsorption for carbon-based nanomaterials. Sci Rep. 2015;5:10886. doi: 10.1038/srep10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kapralov AA, Feng WH, Amoscato AA, Yanamala N, Balasubramanian K, Winnica DE, et al. Adsorption of surfactant lipids by single-walled carbon nanotubes in mouse lung upon pharyngeal aspiration. ACS Nano. 2012;6:4147–56. doi: 10.1021/nn300626q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gasser M, Rothen-Rutishauser B, Krug HF, Gehr P, Nelle M, Yan B, et al. The adsorption of biomolecules to multi-walled carbon nanotubes is influenced by both pulmonary surfactant lipids and surface chemistry. J Nanobiotechnology. 2010;8:31. doi: 10.1186/1477-3155-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang PJ, Pautler R, Shanmugaraj J, Labbé G, Liu J. Inhibiting the VIM-2 metallo-β-lactamase by graphene oxide and carbon nanotubes. ACS Appl Mater Interfaces. 2015;7:9898–903. doi: 10.1021/acsami.5b01954. [DOI] [PubMed] [Google Scholar]
- 91.De M, Chou SS, Dravid VP. Graphene oxide as an enzyme inhibitor: modulation of activity of α-chymotrypsin. J Am Chem Soc. 2011;133:17524–7. doi: 10.1021/ja208427j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J, Wu LJ, Guo SS, Fu HE, Chen GN, Yang HH. Simple colorimetric bacterial detection and high-throughput drug screening based on a graphene-enzyme complex. Nanoscale. 2013;5:619–23. doi: 10.1039/c2nr32704j. [DOI] [PubMed] [Google Scholar]
- 93.Jin L, Yang K, Yao K, Zhang S, Tao H, Lee ST, et al. Functionalized graphene oxide in enzyme engineering: a selective modulator for enzyme activity and thermostability. ACS Nano. 2012;6:4864–75. doi: 10.1021/nn300217z. [DOI] [PubMed] [Google Scholar]
- 94.Moghimi SM. Cancer nanomedicine and the complement system activation paradigm: anaphylaxis and tumour growth. J Control Release. 2014;190:556–62. doi: 10.1016/j.jconrel.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 95.Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green ML, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 96.Hamad I, Christy Hunter A, Rutt KJ, Liu Z, Dai H, Moghimi SM. Complement activation by PEGylated single-walled carbon nanotubes is independent of C1q and alternative pathway turnover. Mol Immunol. 2008;45:3797–803. doi: 10.1016/j.molimm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tan X, Feng L, Zhang J, Yang K, Zhang S, Liu Z, et al. Functionalization of graphene oxide generates a unique interface for selective serum protein interactions. ACS Appl Mater Interfaces. 2013;5:1370–7. doi: 10.1021/am302706g. [DOI] [PubMed] [Google Scholar]
- 98.Moghimi SM, Wibroe PP, Wu L, Farhangrazi ZS. Insidious pathogen-mimicking properties of nanoparticles in triggering the lectin pathway of the complement system. Eur J Nanomed. 2015;7:263–8. [Google Scholar]
- 99.Bottini M, Rosato N, Bottini N. PEG-modified carbon nanotubes in biomedicine: current status and challenges ahead. Biomacromolecules. 2011;12:3381–93. doi: 10.1021/bm201020h. [DOI] [PubMed] [Google Scholar]
- 100.Pombo García K, Zarschler K, Barbaro L, Barreto JA, O’Malley W, Spiccia L, et al. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small. 2014;10:2516–29. doi: 10.1002/smll.201303540. [DOI] [PubMed] [Google Scholar]
- 101.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release. 2014;190:485–99. doi: 10.1016/j.jconrel.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krais A, Wortmann L, Hermanns L, Feliu N, Vahter M, Stucky S, et al. Targeted uptake of folic acid-functionalized iron oxide nanoparticles by ovarian cancer cells in the presence but not in the absence of serum. Nanomedicine. 2014;10:1421–31. doi: 10.1016/j.nano.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 104.Dai Q, Walkey C, Chan WC. Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angew Chem Int Ed Engl. 2014;53:5093–6. doi: 10.1002/anie.201309464. [DOI] [PubMed] [Google Scholar]
- 105.Dai Q, Yan Y, Ang CS, Kempe K, Kamphuis MM, Dodds SJ, et al. Monoclonal antibody-functionalized multilayered particles: targeting cancer cells in the presence of protein coronas. ACS Nano. 2015;9:2876–85. doi: 10.1021/nn506929e. [DOI] [PubMed] [Google Scholar]
- 106.Hadjidemetriou M, Al-Ahmady Z, Mazza M, Collins RF, Dawson K, Kostarelos K. In vivo biomolecule corona around blood-circulating, clinically used and antibody-targeted lipid bilayer nanoscale vesicles. ACS Nano. 2015;9:8142–56. doi: 10.1021/acsnano.5b03300. [DOI] [PubMed] [Google Scholar]
- 107.Sacchetti C, Rapini N, Magrini A, Cirelli E, Bellucci S, Mattei M, et al. In vivo targeting of intratumor regulatory T cells using PEG-modified single-walled carbon nanotubes. Bioconjug Chem. 2013;24:852–8. doi: 10.1021/bc400070q. [DOI] [PubMed] [Google Scholar]
- 108.Hong H, Yang K, Zhang Y, Engle JW, Feng L, Yang Y, et al. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano. 2012;6:2361–70. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hajipour MJ, Raheb J, Akhavan O, Arjmand S, Mashinchian O, Rahman M, et al. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale. 2015;7:8978–94. doi: 10.1039/c5nr00520e. [DOI] [PubMed] [Google Scholar]
- 110.Moghimi SM, Hunter AC, Andresen TL. Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective. Annu Rev Pharmacol Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 111.Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5:143–59. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3:151–7. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 114.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–23. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 115.Ali-Boucetta H, Kostarelos K. Pharmacology of carbon nanotubes: toxicokinetics, excretion and tissue accumulation. Adv Drug Deliv Rev. 2013;65:2111–9. doi: 10.1016/j.addr.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 116.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA. 2006;103:3357–62. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruggiero A, Villa CH, Bander E, Rey DA, Bergkvist M, Batt CA, et al. Paradoxical glomerular filtration of carbon nanotubes. Proc Natl Acad Sci USA. 2010;107:12369–74. doi: 10.1073/pnas.0913667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Jamal KT, Nunes A, Methven L, Ali-Boucetta H, Li S, Toma FM, et al. Degree of chemical functionalization of carbon nanotubes determines tissue distribution and excretion profile. Angew Chem Int Ed Engl. 2012;51:6389–93. doi: 10.1002/anie.201201991. [DOI] [PubMed] [Google Scholar]
- 119.Wang JT, Fabbro C, Venturelli E, Menard-Moyon C, Chaloin O, Da Ros T, et al. The relationship between the diameter of chemically-functionalized multi-walled carbon nanotubes and their organ biodistribution profiles in vivo. Biomaterials. 2014;35:9517–28. doi: 10.1016/j.biomaterials.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 120.Yang K, Wan J, Zhang S, Zhang Y, Lee ST, Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano. 2011;5:516–22. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 121.Jasim DA, Menard-Moyon C, Begin D, Bianco A, Kostarelos K. Tissue distribution and urinary excretion of intravenously administered chemically functionalized graphene oxide sheets. Chem Sci. 2015;6:3952–64. doi: 10.1039/c5sc00114e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wick P, Louw-Gaume AE, Kucki M, Krug HF, Kostarelos K, Fadeel B, et al. Classification framework for graphene-based materials. Angew Chem Int Ed Engl. 2014;53:7714–8. doi: 10.1002/anie.201403335. [DOI] [PubMed] [Google Scholar]
- 123.Cherukuri P, Gannon CJ, Leeuw TK, Schmidt HK, Smalley RE, Curley SA, et al. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc Natl Acad Sci USA. 2006;103:18882–6. doi: 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang ST, Guo W, Lin Y, Deng XY, Wang HF, Sun HF, et al. Biodistribution of pristine single-walled carbon nanotubes in vivo. J Phys Chem C. 2007;111:61–17764. [Google Scholar]
- 125.Chen S, Xiong C, Liu H, Wan Q, Hou J, He Q, et al. Mass spectrometry imaging reveals the sub-organ distribution of carbon nanomaterials. Nat Nanotechnol. 2015;10:176–82. doi: 10.1038/nnano.2014.282. [DOI] [PubMed] [Google Scholar]
- 126.Huang X, Zhang F, Zhu L, Choi KY, Guo N, Guo J, et al. Effect of injection routes on the biodistribution, clearance, and tumor uptake of carbon dots. ACS Nano. 2013;7:5684–93. doi: 10.1021/nn401911k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vachet RW. Molecular histology: More than a picture. Nat Nanotechnol. 2015;10:103–4. doi: 10.1038/nnano.2015.4. [DOI] [PubMed] [Google Scholar]
- 128.Girish CM, Sasidharan A, Gowd GS, Nair S, Koyakutty M. Confocal Raman imaging study showing macrophage mediated biodegradation of graphene in vivo. Adv Healthc Mater. 2013;2:1489–500. doi: 10.1002/adhm.201200489. [DOI] [PubMed] [Google Scholar]
- 129.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 130.Sacchetti C, Motamedchaboki K, Magrini A, Palmieri G, Mattei M, Bernardini S, et al. Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol-modified single-walled carbon nanotubes: potential implications on biological performance. ACS Nano. 2013;7:1974–89. doi: 10.1021/nn400409h. [DOI] [PubMed] [Google Scholar]
- 131.Yuan YY, Mao CQ, Du XJ, Du JZ, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv Mater. 2012;24:5476–80. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- 132.Smith BR, Ghosn EE, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat Nanotechnol. 2014;9:481–7. doi: 10.1038/nnano.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435–45. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kotchey GP, Zhao Y, Kagan VE, Star A. Peroxidase-mediated biodegradation of carbon nanotubes in vitro and in vivo. Adv Drug Deliv Rev. 2013;65:1921–32. doi: 10.1016/j.addr.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mukherjee SP, Bhattacharya K, Fadeel B. Carbon-Based Nanomaterials: From Biodegradation to Biomedical Applications. In: Bawa R, Szebeni J, editors. IMMUNE EFFECTS OF BIOPHARMACEUTICALS AND NANOMEDICINES. Pan Stanford Publishing; Singapore: 2015. [Google Scholar]
- 136.Ye R, Xiang C, Lin J, Peng Z, Huang K, Yan Z, et al. Coal as an abundant source of graphene quantum dots. Nat Commun. 2013;4:2943. doi: 10.1038/ncomms3943. [DOI] [PubMed] [Google Scholar]
- 137.Dong Y, Wan L, Cai J, Fang Q, Chi Y, Chen G. Natural carbon-based dots from humic substances. Sci Rep. 2015;5:10037. doi: 10.1038/srep10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Su Z, Zhou W, Zhang Y. New insight into the soot nanoparticles in a candle flame. Chem Commun. 2011;47:4700–02. doi: 10.1039/c0cc05785a. [DOI] [PubMed] [Google Scholar]
- 139.Li D, Mueller MB, Gilje S, Kaner RB, Wallace GG. Processable aqueous dispersions of graphene nanosheets. Nat Nanotech. 2008;3:101–105. doi: 10.1038/nnano.2007.451. [DOI] [PubMed] [Google Scholar]
- 140.Liu L, Ryu S, Tomasik MR, Stolyarova E, Jung N, Hybertsen MS, et al. Graphene oxidation: thickness-dependent etching and strong chemical doping. Nano Lett. 2008;8:1965–70. doi: 10.1021/nl0808684. [DOI] [PubMed] [Google Scholar]
- 141.Allen BL, Kichambre PD, Gou P, Vlasova II, Kapralov AA, Konduru N, et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8:3899–903. doi: 10.1021/nl802315h. [DOI] [PubMed] [Google Scholar]
- 142.Allen BL, Kotchey GP, Chen Y, Yanamala NVK, Klein-Seetharaman J, Kagan VE, et al. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J Am Chem Soc. 2009;131:17194–205. doi: 10.1021/ja9083623. [DOI] [PubMed] [Google Scholar]
- 143.Zhao Y, Allen BL, Star A. Enzymatic degradation of multiwalled carbon nanotubes. J Phys Chem A. 2011;115:9536–44. doi: 10.1021/jp112324d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Russier J, Menard-Moyon C, Venturelli E, Gravel E, Marcolongo G, Meneghetti M, et al. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale. 2011:3. doi: 10.1039/c0nr00779j. [DOI] [PubMed] [Google Scholar]
- 145.Flores-Cervantes DX, Maes HM, Schaffer A, Hollender J, Kohler H-PE. Slow biotransformation of carbon nanotubes by horseradish peroxidase. Environ Sci Technol. 2014;48:4826–4834. doi: 10.1021/es4053279. [DOI] [PubMed] [Google Scholar]
- 146.Kotchey GP, Allen BL, Vedala H, Yanamala N, Kapralov AA, Tyurina YY, et al. The enzymatic oxidation of graphene oxide. ACS Nano. 2011;5:2098–108. doi: 10.1021/nn103265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schreiner KM, Filley TR, Blanchette RA, Bowen BB, Bolskar RD, Hockaday WC, et al. White-rot basidiomycete-mediated decomposition of C60 fullerol. Environ Sci Technol. 2009;43:3162–8. doi: 10.1021/es801873q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berry TD, Filley TR, Blanchette RA. Oxidative enzymatic response of white-rot fungi to single-walled carbon nanotubes. Environ Pollut. 2014;193:197–204. doi: 10.1016/j.envpol.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 149.Lalwani G, Xing W, Sitharaman B. Enzymatic degradation of oxidized and reduced graphene nanoribbons by lignin peroxidase. J Mater Chem B Mater Biol Med. 2014;2:6354–62. doi: 10.1039/C4TB00976B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang L, Petersen EJ, Habteselassie MY, Mao L, Huang Q. Degradation of multiwall carbon nanotubes by bacteria. Environ Pollut. 2013;181:335–9. doi: 10.1016/j.envpol.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 151.Liu L, Zhu C, Fan M, Chen C, Huang Y, Hao Q, et al. Oxidation and degradation of graphitic materials by naphthalene-degrading bacteria. Nanoscale. 2015;7:13619–28. doi: 10.1039/c5nr02502h. [DOI] [PubMed] [Google Scholar]
- 152.Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotech. 2010;5:354–59. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Andón FT, Kapralov AA, Yanamala N, Feng W, Baygan A, Chambers BJ, et al. Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small. 2013;9:2721–9. doi: 10.1002/smll.201202508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bhattacharya K, El-Sayed R, Andon FT, Mukherjee SP, Gregory J, Li H, et al. Lactoperoxidase-mediated degradation of single-walled carbon nanotubes in the presence of pulmonary surfactant. Carbon. 2015;91:506–17. [Google Scholar]
- 155.Bai H, Kotchey GP, Saidi WA, Bythell BJ, Jarvis JM, Marshall AG, et al. Insight into the mechanism of graphene oxide degradation via the photo-fenton reaction. J Phys Chem C. 2014;118:10519–29. doi: 10.1021/jp503413s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pan S, Sardesai NP, Liu H, Rusling JF. Assessing DNA damage from enzyme-oxidized single-walled carbon nanotubes. Tox Res. 2013;2:375–8. doi: 10.1039/C3TX50022E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu N, Li J, Tian R, Peng YY. Binding of human serum albumin to single-walled carbon nanotubes activated neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Chem Res Toxicol. 2014;27:1070–7. doi: 10.1021/tx5001317. [DOI] [PubMed] [Google Scholar]
- 158.Shvedova AA, Kapralov AA, Feng WH, Kisin ER, Murray AR, Mercer RR, et al. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS One. 2012;7:e30923. doi: 10.1371/journal.pone.0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farrera C, Bhattacharya K, Lazzaretto B, Andón FT, Hultenby K, Kotchey GP, et al. Extracellular entrapment and degradation of single-walled carbon nanotubes. Nanoscale. 2014;6:6974–83. doi: 10.1039/c3nr06047k. [DOI] [PubMed] [Google Scholar]
- 160.Kagan VE, Kapralov AA, StCroix CM, Watkins SC, Kisin ER, Kotchey GP, et al. Lung macrophages “digest” carbon nantubes using a superoxide/peroxynitrite oxidative pathway. ACS Nano. 2014;8:5610–21. doi: 10.1021/nn406484b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang M, Yang M, Bussy C, Iijima S, Kostarelos K, Yudasaka M. Biodegradation of carbon nanohorns in macrophage cells. Nanoscale. 2015;7:2834–40. doi: 10.1039/c4nr06175f. [DOI] [PubMed] [Google Scholar]
- 162.Elgrabli D, Dachraoui W, Ménard-Moyon C, Liu XJ, Bégin D, Bégin-Colin S, et al. Carbon nanotube degradation in macrophages: live nanoscale monitoring and understanding of biological pathway. ACS Nano. 2015 Sep 10; doi: 10.1021/acsnano.5b03708. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 163.Nunes A, Bussy C, Gherardini L, Meneghetti M, Herrero MA, Bianco A, et al. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine (Lond) 2012;7:1485–94. doi: 10.2217/nnm.12.33. [DOI] [PubMed] [Google Scholar]
- 164.Bhattacharya K, Sacchetti C, El-Sayed R, Fornara A, Kotchey GP, Gaugler JA, et al. Enzymatic ‘stripping’ and degradation of PEGylated carbon nanotubes. Nanoscale. 2014;6:14686. doi: 10.1039/c4nr03604b. [DOI] [PubMed] [Google Scholar]
- 165.Yang ST, Wang H, Meziani MJ, Liu Y, Wang X, Sun YP. Biodefunctionalization of functionalized single-walled carbon nanotubes in mice. Biomacromolecules. 2009;10:2009–12. doi: 10.1021/bm900263z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Allen BL, Kichambare PD, Star A. Synthesis, characterization, and manipulation of nitrogen-doped carbon nanotube cups. ACS Nano. 2008;2:1914–20. doi: 10.1021/nn800355v. [DOI] [PubMed] [Google Scholar]
- 167.Zhao Y, Burkert SC, Tang Y, Sorescu DC, Kapralov AA, Shurin GV, et al. Nano-gold corking and enzymatic uncorking of carbon nanotube cups. J Am Chem Soc. 2015;137:675–84. doi: 10.1021/ja511843w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Seo W, Kapralov AA, Shurin GV, Shurin MR, Kagan VE, Star A. Payload drug vs. nanocarrier biodegradation by myeloperoxidase- and peroxynitrite-mediated oxidations: pharmacokinetic implications. Nanoscale. 2015;7:8689–94. doi: 10.1039/c5nr00251f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kotchey GP, Allen BL, Vedala H, Yanamala N, Kapralov AA, Tyurina YY, et al. The enzymatic oxidation of graphene oxide. ACS Nano. 2011;5:2098–108. doi: 10.1021/nn103265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kurapati R, Russier J, Squillaci MA, Trossi E, Menard-Moyon C, Del Rio-Castillo AE, et al. Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase. Small. 2015;11:3985–94. doi: 10.1002/smll.201500038. [DOI] [PubMed] [Google Scholar]
- 171.Li Y, Feng L, Shi X, Wang X, Yang Y, Yang K, et al. Surface coating-dependent cytotoxicity and degradation of graphene derivatives: towards the design of non-toxic, degradable nano-graphene. Small. 2014;10:1544–54. doi: 10.1002/smll.201303234. [DOI] [PubMed] [Google Scholar]
- 172.Andón FT, Fadeel B. Nanotoxicology: Towards Safety-by-Design. In: Alonso MJ, Garcia-Fuentes M, editors. NANO-ONCOLOGICALS: NEW TARGETING AND DELIVERY APPROACHES. Springer; Heidelberg: 2014. [Google Scholar]