Abstract
Dendrimers possess discrete highly compact nanostructures constituted of successive branched layers. Soon after the inception of dendrimers, recognition of their tunable structures and biologically favorable properties provoked a great enthusiasm in delving deeply into the utility of dendrimers for biomedical and pharmaceutical applications. One of the most important nanotechnology applications is the development of nanomedicines for targeted cancer therapies. Tremendous success in targeted therapies has been achieved with the use of dendrimer-based nanomedicines. This article provides a concise review on latest advances in the utility of dendrimers in immunotherapies and hormone therapies.
Keywords: dendrimer, critical nanoscale design parameters, immunotherapy, hormone therapy
Introduction
Dendrimers possess discrete highly compact nanostructures constituted of successive branched layers, which are commonly referred to as generations.1, 2 Accompanying dendrimer generation increase, the number of branches and surface groups increases exponentially along with a stepwise increase in size. Soon after the inception of dendrimers, recognition of their tunable structures and biologically favorable properties provoked a great enthusiasm in delving deeply into the utility of dendrimers for biomedical and pharmaceutical applications.3, 4 Dendrimers offer a high degree of flexibility in drug loading. Drugs can be either covalently conjugated to the dendrimer surface5–10 or physically encapsulated into the inner core.11–13 Gene plasmids and nucleic acids can be complexed with dendrimers through electrostatic interaction.14–21 Burgeoning evidence shows that dendrimers empower development of effective theranostic platforms as supported by numerous in vitro and pre-clinical studies. Intense efforts have been invested in developing dendrimer-based nanomedicines and translating them into clinical applications.2 A few dendrimer-based nanomedicine products such as VivaGel®–a new antimicrobial agent– have been successfully commercialized. Those with encouraging preclinical results, for example, Starpharma's dendrimer-enhanced docetaxel (commonly known as DEP™ docetaxel), have moved to clinical trials or are in the pipeline for FDA regulatory approval reviews.
There is a widespread interest in diverse topics ranging from synthesis and functionalization of dendrimers, design principles and considerations for the utility of dendrimer features, to dendrimer-based drug delivery systems for treatment of various diseases.22–24 One of the most important nanotechnology applications is the development of nanomedicines for targeted cancer therapies. Different from conventional chemotherapy acting on rapidly dividing normal and cancerous cells, targeted therapies interfere with specific molecular targets that are involved in cancer growth, progression, and spread. Tremendous success in targeted therapies has been achieved with the use of dendrimer-based nanomedicines. Targeted therapies have been grouped to different categories including hormone therapies, signal transduction inhibitors, gene therapy, apoptosis inducer, angiogenesis inhibitor, immunotherapies, and toxin delivery molecules. This article provides a concise review on latest advances in the utility of dendrimers in immunotherapies and hormone therapies.
Dendrimer nanomedicine design considerations: critical nanoscale design parameters (CNDPs)
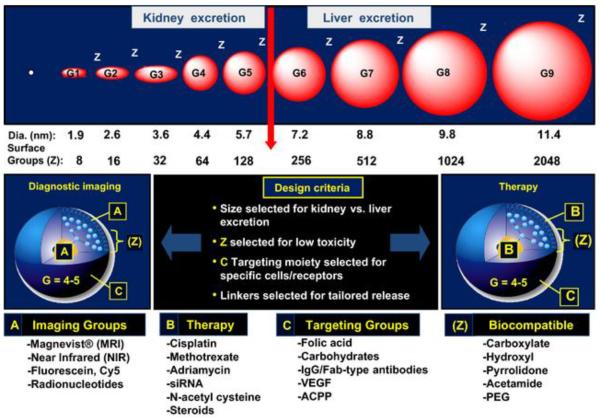
Critical nanoscale design parameters (CNDPs), namely size, shape, surface chemistry, flexibility/rigidity, elemental composition, and architecture, have been applied to establish a nanoperiodic framework to understand nanoparticle structure control and atom-like combining properties of dendrimers.2, 25 It is gaining increasing recognition that pharmacokinetics, pharmacodynamics, and toxicokinetics of nanomedicines in preclinical and clinical are also influenced by the CNDPs mentioned above. Uniquely, dendrimers offer defined nanostructures that can be engineered through any one of the six CNDPs (Figure 1). Therefore, CNDP-directed design strategies can be implemented to develop dendrimer-based nanomedicines and offer a powerful tool for elucidation of structure-property-function relationships and optimization of the nanomedicines in the context of intended clinical applications (Figure 1).2
Figure 1.
A schematic illustration of dendrimer critical nanoscale design parameter control and engineering for optimizing prototypes suitable for various nanomedical applications. (i) Size control (approximately 1 nm per generation) with mathematically defined polyvalent surface functionality; (ii) polyvalent dendrimer surface chemistry can be chemically partitioned into imaging groups (A), therapy with cleavable linkers (B), targeting groups (C) and biocompatible or circulatory enhancement groups (Z). Cited from reference2 with permission.
It is worth noting that clearance pathway of PAMAM dendrimers shows clear size (or generation)- and charge-dependence.2, 26 PAMAM dendrimers of generation 5 or lower can be sufficiently eliminated via glomerular filtration in the pathway of renal excretion. However, when size of dendrimers is close to the filtration size threshold, charge plays a significant role. The highly negatively charged glomerular basement membrane makes it more difficult for anionic dendrimers to filtrate. As dendrimers become larger in size, their elimination relies on hepatic clearance. Dendrimer surface functionalization often leads to size increase and charge changes. Such design parameters will help predict in vivo biodistribution and clearance patterns of dendrimer nanomedicine and create more effective delivery systems.
Dendrimer-ligand conjugates for tumor cell targeting
Immunotherapies may harness the immune system to attack cancer cells with the aid of dendrimers. One strategy is to prepare immunodendrimers by surface functionalizing dendrimers with monoclonal antibodies (mAbs) that bind to the surface of cancer cells. The multivalency and hydrophobic inner core of dendrimers can then be utilized to deliver toxic molecules through covalent conjugation and encapsulation, respectively, to kill cancer cells precisely with guidance of the conjugated mAbs.
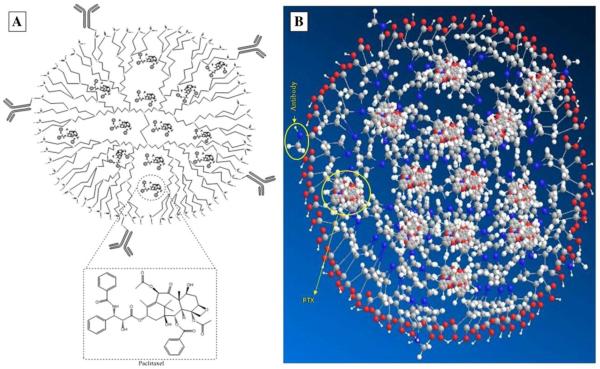
Over 70% of ovarian adenocarcinoma overly express mesothelin, a glycosyl-phosphatidyl inositol (GPI)-linked membrane protein, which can be recognized by monoclonal antibody K1 (mAbK1). Immunodendrimers were made towards ovarian cancer treatment by conjugating mAbK1 to half-generation poly (propylene imine) (PPI) dendrimers (Figure 2). Paclitaxel (PTX) was encapsulated into the hydrophobic nanodomains of PPI dendrimers. Nearly 30% of the drug was entrapped in dendrimers at a drug to dendrimer ratio of 4:1. The resulting mAbK1-PPI-PTX was tested in an ovarian cancer model, and it was found to reduce tumor volume and extend animal survival more significantly through enhanced drug uptake in the tumor.27
Figure 2.
(A) Pictorial presentation. (B) Molecular model of PTX loaded immunodendrimer (representation—red circle for oxygen; blue for nitrogen; white for hydrogen, and gray for carbon atom). Cited from reference27 with permission.
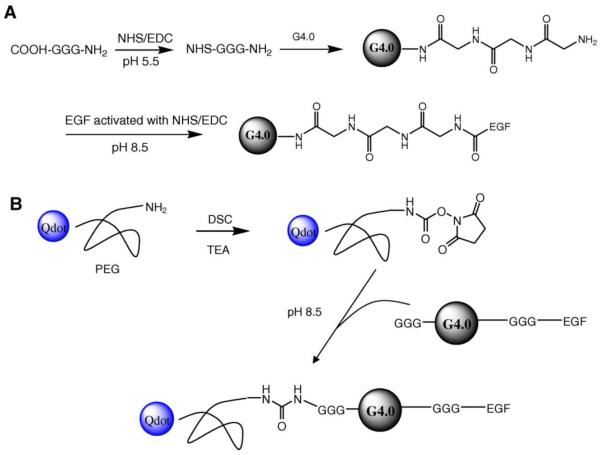
In most cases, cancer cells express much higher levels of receptors on the cell surface, such as folate receptor, EGFR. In addition to mAbs, ligands that bind to those receptors can be conjugated to the dendrimer to make targeted dendrimers. It has been widely accepted that multivalent conjugation of folic acid to the dendrimers makes the conjugates have high avidity to folate binding protein. In one of our latest publications, we reported functionalization of dendrimers with EGF peptide ligands for EGFR-mediated gene delivery.17 In this particular system, the dendrimer surface was uniformly coated with triglycine (GGG) spacer for improving cytocompatibility while maintaining positive charges for complexation with nucleic acids (Figure 3). Quantum dots were conjugated to the dendrimer via a polyethylene glycol (PEG) spacer for imaging purpose. Vimentin shRNA (shVIM) and yellow fluorescent protein (YFP) siRNA can be delivered to head and neck cancer cells HN12 cells more efficiently to exert knockdown effects. Selectivity of the EGF-conjugated dendrimers against tumor cells was also demonstrated.
Figure 3.
(A) EGF-triglycine (GGG)-dendrimer conjugates, (B) EGF-triglycine-dendrimer conjugates labeled with Qdots which were coated with amine-derivatized PEG. Cited from reference17 with permission.
Most recently, van Dongen et al. creatively synthesized a monovalent folic acid-dendrimer conjugate using click chemistry.28 They used the synthesized monovalent folic acid-dendrimer conjugate to study how the folic acid-polymer conjugate binds to a folate binding protein surface. Their seminal work showed that monovalent folic acid-dendrimer conjugate also has increased avidity, and they argued that the increased avidity results from a two-phase folate-keyed interaction: an initial, reversible, FA concentration dependent key–lock/van der Waals binding between the conjugate and protein, and then an irreversible interaction between the dendrimer and protein surfaces (Figure 4, model C). Their findings provide new insight into rational design of targeted dendrimers based upon the utility of multivalency of dendrimers.
Figure 4.
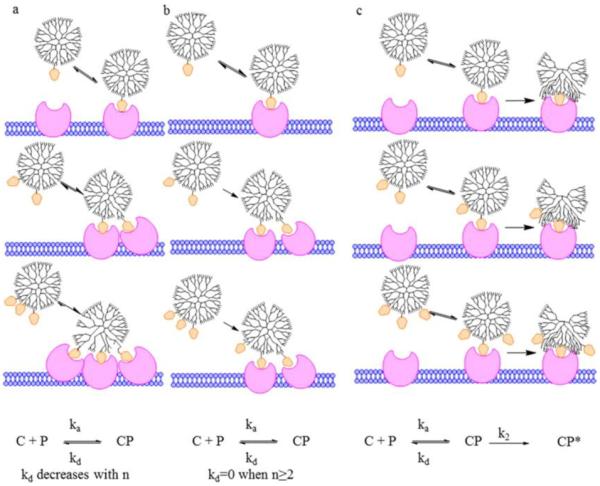
Proposed models for enhanced G5–FA binding to FBP. (a) Multivalent binding increases avidity with increasing valency. (b) Any multivalent binding (2 or more interactions) is irreversible, and monovalent binding is reversible. (c) FA “keys” the initial interaction between conjugate and FBP, which is followed by strong nonspecific interaction between the dendrimer and protein. C represents G5–FAn conjugate, P is FBP, CP a complex between a conjugate and n ≥ 1 FBP. CP* is a tight complex formed by a conformation change in the polymer and the resulting polymer–protein interaction. C represents G5–Fan conjugate, P is FBP, CP a complex between a conjugate and n ≥ 1 FBP. CP* is a tight complex formed by a conformation change in the polymer and the resulting polymer–protein interaction. Cited from reference28 with permission.
Dendrimer-antigen conjugates for immune response stimulation
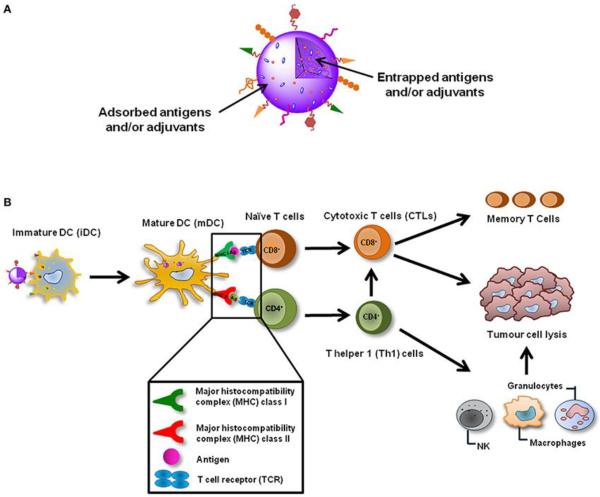
Dendritic cells (DCs) are antigen presenting cells of the immune system. DCs present antigens to T cells and initiate the immune response as illustrated in Figure 5.29 In recognition of the importance of DCs in immunotherapy, tumor-associated antigens (TAAs) and adjuvants can be simultaneously or respectively loaded onto nanoparticles and taken up by the same DC to initiate the immune response through coordinated activation. In particular, TAAs presented via major histocompatibilitycomplex (MHC) class I and class II molecules can be recognized by CD8+ and CD4+ naïve T cells through T-cell receptors (TCRs). Activated CD8+ T cells differentiate into memory T cells and cytotoxic T lymphocytes (CTLs) that are responsible for killing tumor cells. In the meantime, CD4+ T cells differentiate in type 1 T helper (Th1) cells, which help potentiate the action of CTLs and activate those cells of the innate immune system, such as natural killer (NK) cells, granulocytes and macrophages to strengthen tumor destruction process.
Figure 5.
Nanoparticle-based cancer vaccines can be targeted to DCs in vivo and after their internalization induce the maturation of these cells. Cited from reference29 with permission.
As discussed above, delivery of antigens to DCs is a critical step for successfully triggering immune response but antigen delivery efficiency is rather limited. Dendrimers were recently biofunctionalized to enhance efficiency of antigen delivery to dendritic cells.30 DC-SIGN (dendritic cell-specific ICAM-3-grabbing non-integrin) is a C-type lectin receptor present on the surface of DCs and can be targeted for antigen delivery to DCs. Lewisb (Leb) glycopeptides are natural ligands that bind to DC-SIGN. In this work, Leb was conjugated to the dendrimer surface (Figure 6). Interestingly, an increase in DC-SIGN ligand multivalency directly results in linearly enhanced binding.
Figure 6.
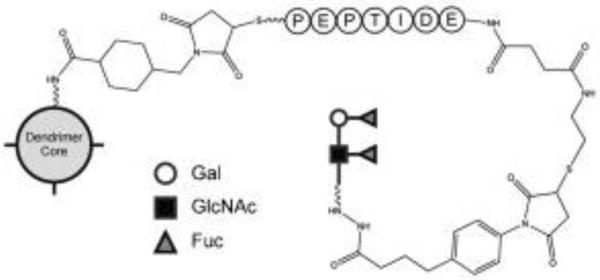
Multivalency enhances avidity of DC-SIGN for its ligands. Glycopeptide dendrimers are synthesized sequentially over PAMAM dendrimers functionalized with primary amines. A maleimide linker is used to attach the C-terminal Cys of the CKOTI/II peptide to the dendrimer. At the other end a glycan is attached to the N-terminus via reductive amination to the side chain of a Lys. Adapted from reference30 with permission.
Dendrimer-cell hybrids
The ability of anticancer drugs to precisely attack cancer cells influences cancer treatment effectiveness. Although nanostructured materials and cells have been actively researched as carriers for drug delivery, each type has its distinct advantages as well as apparent limitations in terms of anticancer drug delivery. Heterogeneities of vascular permeability and the complex microenvironment of cancer biology are barriers for effective delivery of drug-carrying nanoparticles including those decorated with tumor-specific ligand relies heavily on passive mechanisms such as the enhanced permeability and retention (EPR) effect. Only a small percentage of the injected drug can finally accumulate in the tumor with the aid of nanoparticles, in large part, due to rapid clearance by immune cells in the liver and spleen. Using cells to deliver drugs has also attracted considerable attention. Immune cells including T cells and macrophages have built-in cancer-targeting or-attacking abilities and seem suitable for anticancer drug delivery because they respond to tumor invasion, recurrence, and metastasis or tumor hypoxia and actively infiltrate in the tumor.31–38 Such cellular vehicles with have a great potential to deliver therapeutic compounds. Nonetheless, none of the approaches mentioned above is applicable to cell-based anticancer drug delivery. Anticancer drugs cannot be directly loaded into the cellular vehicle or loosely encapsulated on the cell surface because of the detrimental impact of their inherent toxicity on cell viability and functions. Resolution of this issue is critical to the realization of the potential of this therapeutic approach.
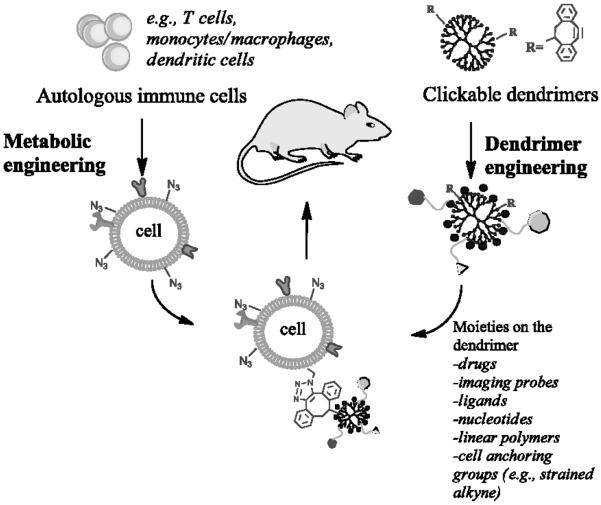
Yang's team developed a delivery platform by hybridizing immune cell and dendrimers via highly efficient highly selective bioorthogonal reaction on the cell surface (Figure 7).39 The resulting hybrids remained good viability and motility, indicating the mildness of the chemistry and noninvasiveness to the cellular vehicle. This hybrid system takes advantage of multivalency of PAMAM dendrimers for delivery of compounds of interest such as drugs and imaging agents. This approach provides a new way to utilize dendrimers and immune cells and make it possible to develop personalized immunotherapy.
Figure 7.
Click hybridization of immune cells and polyamidoamine dendrimers. Cited from reference39 with permission.
Dendrimer-based hormone therapy
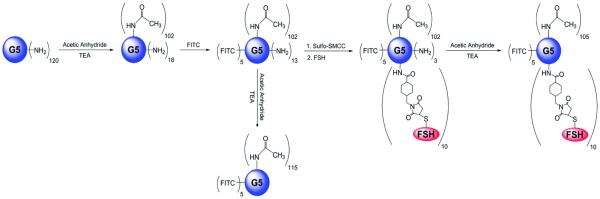
Hormone therapies exert therapeutic effectiveness by preventing production of hormones or impeding hormone activities.40 Similar to other cancer types, receptors that are overexpressed can be utilized to develop targeted therapy. For instance, elevated expression of follicle stimulating hormone receptor (FSHR) in ovarian cancers has been confirmed.41, 42 Thus, targeted ovarian cancer therapy can be achieved by designing drug delivery systems carrying FSH ligand. Recently, Modi et al. designed a dendrimer-based platform to target FSHR. The platform was constructed by coupling the binding peptide domain of FSH (FSH33) to PAMAM dendrimer G5.0 (Figure 8).43 The high selectivity of FSH33-dendrimer conjugates was confirmed. Furthermore, the conjugates displayed receptor antagonistic effect by downregulating surviving, an anti-apoptotic protein.
Figure 8.
FSH33-targeted G5 PAMAM dendrimer conjugates. Cited from reference43 with permission.
Conclusions and outlook
High-quality dendrimer products in high quantities have been made available for fundamental research and translational studies owing to commercialization of PAMAM dendrimers by several companies such as Dendritech (Midland, Michigan) and NanoSynthons (Mt. Pleasant, Michigan). The commercially available dendrimers serve as a reliable source of building blocks for development of dendrimer nanomedicine products on a large scale. Nonetheless, manufacturing of functionalized dendrimers with uniform loading of drugs, ligands, and other moieties on the dendrimer surface is challenging and remains to be solved for consistent therapeutic effects.44 Efficient and robust dendrimer surface chemistries such as click chemistry45 must be developed to tackle the heterogeneity of dendrimer surface functionalization and enable scaling up of dendrimer nanomedicine products. Factors
Cancer therapy strategies have diversified considerably. Mechanisms for cancer therapies also include blockage of signal transduction inhibitor activities, modulation of gene expression, induction of apoptosis, inhibition of angiogenesis and utility of cancer vaccines. Nanostructured carriers including dendrimers are expected to continue to play an important role in delivering payload to the right target at the tissue, cellular, or subcellular levels. Interesting and astonishing biological activities of dendrimers, particularly PAMAM dendrimers, have been revealed lately. In particular, PAMAM dendrimers show anti-inflammatory activities via different mechanisms determined by surface groups.46, 47 Neutral dendrimers have been found to target inflammatory cells within the brain. They can localize in activated microglia and astrocytes in the presence of neuroinflammation.48 Activated (i.e., SuperFect) and non-activated (i.e., Polyfect) PAMAM dendrimers can differentially modulate EGFR signaling pathway.49 Such intrinsic therapeutic functions endowed by dendrimers themselves may be integrated into drug delivery system design and offer additional therapeutic benefits for cancer therapy.
Acknowledgments
This work was supported, in part, by the National Science Foundation (CAREER award CBET0954957), National Institutes of Health (R01EY024072), and Virginia Commonwealth University Massey Cancer Center (multi-investigator award 2013-MIP-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. Dendritic macromolecules: synthesis of starburst dendrimers. Macromolecules. 1986;19:2466–8. [Google Scholar]
- 2.Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J Intern Med. 2014;276:579–617. doi: 10.1111/joim.12280. [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Frechet JMJ. Designing dendrimers for drug delivery. Pharmaceutical Science & Technology Today. 1999;2:393–401. doi: 10.1016/s1461-5347(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 4.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Lopina ST. Stealth dendrimers for antiarrhythmic quinidine delivery. J. Mater. Sci.: Mater. Med. 2007;18:2061–2065. doi: 10.1007/s10856-007-3144-0. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Lopina ST. In vitro enzymatic stability of dendritic peptides. J Biomed Mater Res, Part A. 2006;76A:398–407. doi: 10.1002/jbm.a.30529. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Lopina ST. Extended release of a novel antidepressant, venlafaxine, based on anionic polyamidoamine dendrimers and poly(ethylene glycol)-containing semi-interpenetrating networks. J Biomed Mater Res. 2005;72A:107–14. doi: 10.1002/jbm.a.30220. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Lopina ST. Penicillin V-conjugated PEG-PAMAM star polymers. J Biomater Sci Polym Ed. 2003;14:1043–56. doi: 10.1163/156856203769231556. [DOI] [PubMed] [Google Scholar]
- 9.She WC, Pan DY, Luo K, He B, Cheng G, Zhang CY, et al. PEGylated Dendrimer-Doxorubicin Cojugates as pH-Sensitive Drug Delivery Systems: Synthesis and In Vitro Characterization. Journal of Biomedical Nanotechnology. 2015;11:964–978. doi: 10.1166/jbn.2015.1865. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Jeong YI, Park HK, Kang DH, Oh JS, Lee SG, et al. Enzyme-responsive doxorubicin release from dendrimer nanoparticles for anticancer drug delivery. Int J Nanomed. 2015;10:5489–5503. doi: 10.2147/IJN.S87145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Morris JJ, Lopina ST. Polyethylene glycol-polyamidoamine dendritic micelle as solubility enhancer and the effect of the length of polyethylene glycol arms on the solubility of pyrene in water. J. Colloid Interface Sci. 2004;273:148–154. doi: 10.1016/j.jcis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar K, Yang H. Encapsulation and extended release of anti-cancer anastrozole by stealth nanoparticles. Drug Deliv. 2008;15:343–6. doi: 10.1080/10717540802035343. [DOI] [PubMed] [Google Scholar]
- 13.Zhu JY, Xiong ZJ, Shen MW, Shi XY. Encapsulation of doxorubicin within multifunctional gadolinium-loaded dendrimer nanocomplexes for targeted theranostics of cancer cells. Rsc Adv. 2015;5:30286–30296. [Google Scholar]
- 14.Bai CZ, Choi S, Nam K, An S, Park JS. Arginine modified PAMAM dendrimer for interferon beta gene delivery to malignant glioma. Int J Pharm. 2013;445:79–87. doi: 10.1016/j.ijpharm.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 15.Mishra MK, Gerard HC, Whittum-Hudson JA, Hudson AP, Kannan RM. Dendrimer-enabled modulation of gene expression in Chlamydia trachomatis. Mol Pharm. 2012;9:413–21. doi: 10.1021/mp200512f. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigo AC, Rivilla I, Perez-Martinez FC, Monteagudo S, Ocana V, Guerra J, et al. Efficient, non-toxic hybrid PPV-PAMAM dendrimer as a gene carrier for neuronal cells. Biomacromolecules. 2011;12:1205–13. doi: 10.1021/bm1014987. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Q, Lee E, Yeudall WA, Yang H. Dendrimer-triglycine-EGF nanoparticles for tumor imaging and targeted nucleic acid and drug delivery. Oral Oncol. 2010;46:698–704. doi: 10.1016/j.oraloncology.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Q, Yeudall WA, Yang H. PEGylated polyamidoamine dendrimers with bisaryl hydrazone linkages for enhanced gene delivery. Biomacromolecules. 2010;11:1940–7. doi: 10.1021/bm100589g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Q, Yeudall WA, Yang H. Thermoresponsive Dendritic Facial Amphiphiles for Gene Delivery. Nanomedicine and Nanobiology. 2014;1:64–69. [Google Scholar]
- 20.Guan LM, Huang SP, Chen Z, Li YC, Liu K, Liu Y, et al. Low cytotoxicity fluorescent PAMAM dendrimer as gene carriers for monitoring the delivery of siRNA. J Nanopart Res. 2015;17 [Google Scholar]
- 21.Xiao S, Castro R, Rodrigues J, Shi XY, Tomas H. PAMAM Dendrimer/pDNA Functionalized-Magnetic Iron Oxide Nanoparticles for Gene Delivery. Journal of Biomedical Nanotechnology. 2015;11:1370–1384. doi: 10.1166/jbn.2015.2101. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Kao WJ. Dendrimers for pharmaceutical and biomedical applications. J Biomater Sci Polym Ed. 2006;17:3–19. doi: 10.1163/156856206774879171. [DOI] [PubMed] [Google Scholar]
- 23.Yang H. Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res. 2010;27:1759–71. doi: 10.1007/s11095-010-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leyuan X, Yeudall WA, Hu Y. Dendrimer-Based RNA Interference Delivery for Cancer Therapy Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications. American Chemical Society. 2013:197–213. [Google Scholar]
- 25.Tomalia DA. Dendrons/dendrimers: quantized, nano-element like building blocks for soft-soft and soft-hard nano-compound synthesis. Soft Matter. 2010;6:456–474. [Google Scholar]
- 26.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 2008;3:703–17. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain NK, Tare MS, Mishra V, Tripathi PK. The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomedicine. 2015;11:207–18. doi: 10.1016/j.nano.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 28.van Dongen MA, Silpe JE, Dougherty CA, Kanduluru AK, Choi SK, Orr BG, et al. Avidity mechanism of dendrimer-folic acid conjugates. Mol Pharm. 2014;11:1696–706. doi: 10.1021/mp5000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S, et al. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front Chem. 2014;2:105. doi: 10.3389/fchem.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Vallejo JJ, Ambrosini M, Overbeek A, van Riel WE, Bloem K, Unger WW, et al. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol Immunol. 2013;53:387–97. doi: 10.1016/j.molimm.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 32.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4:437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths L, Binley K, Iqball S, Kan O, Maxwell P, Ratcliffe P, et al. The macrophage - a novel system to deliver gene therapy to pathological hypoxia. Gene Ther. 2000;7:255–62. doi: 10.1038/sj.gt.3301058. [DOI] [PubMed] [Google Scholar]
- 34.Paul S, Snary D, Hoebeke J, Allen D, Balloul JM, Bizouarne N, et al. Targeted macrophage cytotoxicity using a nonreplicative live vector expressing a tumor-specific single-chain variable region fragment. Hum Gene Ther. 2000;11:1417–28. doi: 10.1089/10430340050057495. [DOI] [PubMed] [Google Scholar]
- 35.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–65. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 38.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–41. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, Zolotarskaya OY, Yeudall WA, Yang H. Click hybridization of immune cells and polyamidoamine dendrimers. Adv Healthc Mater. 2014;3:1430–8. doi: 10.1002/adhm.201300515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 41.Bose CK. Follicle stimulating hormone receptor in ovarian surface epithelium and epithelial ovarian cancer. Oncol Res. 2008;17:231–8. doi: 10.3727/096504008786111383. [DOI] [PubMed] [Google Scholar]
- 42.Choi JH, Choi KC, Auersperg N, Leung PC. Overexpression of follicle-stimulating hormone receptor activates oncogenic pathways in preneoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2004;89:5508–16. doi: 10.1210/jc.2004-0044. [DOI] [PubMed] [Google Scholar]
- 43.Modi DA, Sunoqrot S, Bugno J, Lantvit DD, Hong S, Burdette JE. Targeting of follicle stimulating hormone peptide-conjugated dendrimers to ovarian cancer cells. Nanoscale. 2014;6:2812–20. doi: 10.1039/c3nr05042d. [DOI] [PubMed] [Google Scholar]
- 44.Shi XY, Majoros IJ, Patri AK, Bi XD, Islam MT, Desai A, et al. Molecular heterogeneity analysis of poly(amidoamine) dendrimer-based mono- and multifunctional nanodevices by capillary electrophoresis. Analyst. 2006;131:374–381. doi: 10.1039/b515624f. [DOI] [PubMed] [Google Scholar]
- 45.Zolotarskaya OY, Xu LY, Valerie K, Yang H. Click synthesis of a polyamidoamine dendrimer-based camptothecin prodrug. Rsc Adv. 2015;5:58600–58608. doi: 10.1039/C5RA07987J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Y, Han Y, Liu L, Shen W, Zhang H, Wang Y, et al. Protective effects and mechanisms of G5 PAMAM dendrimers against acute pancreatitis induced by caerulein in mice. Biomacromolecules. 2015;16:174–82. doi: 10.1021/bm501390d. [DOI] [PubMed] [Google Scholar]
- 47.Chauhan AS, Diwan PV, Jain NK, Tomalia DA. Unexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimers. Biomacromolecules. 2009;10:1195–202. doi: 10.1021/bm9000298. [DOI] [PubMed] [Google Scholar]
- 48.Dai H, Navath RS, Balakrishnan B, Guru BR, Mishra MK, Romero R, et al. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine (Lond) 2010;5:1317–29. doi: 10.2217/nnm.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akhtar S, Al-Zaid B, El-Hashim AZ, Chandrasekhar B, Attur S, Yousif MH, et al. Cationic Polyamidoamine Dendrimers as Modulators of EGFR Signaling In Vitro and In Vivo. PLoS One. 2015;10:e0132215. doi: 10.1371/journal.pone.0132215. [DOI] [PMC free article] [PubMed] [Google Scholar]