Abstract
Mating in Anopheles gambiae has been observed only in outdoor swarms. Here we evaluate if mating also occurs indoors. Mark release recapture of virgin males and females in natural houses showed that mating occurred over a single day even when mosquitoes can leave the house through exit traps and without adaptation to laboratory conditions. In these experiments, insemination rate in the M molecular form of An. gambiae (and An. arabiensis) was higher than that of the S form (15% vs. 6%). Under these conditions, smaller females of the M form mated more frequently than larger females of that form. Sampling mosquitoes throughout the day showed that both sexes enter houses around sunrise and leave around sunset, staying indoors together from dawn to dusk. In an area dominated by the M form, the daily rate of insemination in samples from exit traps was approximately 5% higher than in those from entry traps, implying that mating occurred indoors. Importantly, frequency of cross mating between the molecular forms was as high as that between members of the same form, indicating that indoors - assortative mating breaks down. Altogether, these results suggest that indoor mating is an alternative mating strategy of the M molecular form of An. gambiae. Because naturally occurring mating couples have not yet been observed indoors, this conclusion awaits validation.
Keywords: Anopheles gambiae, Alternative mating strategies, molecular forms
Introduction
Mosquito mating behavior is a poorly understood facet of their life, partly because direct observations are hampered by their small size, speed, and crepuscular or nocturnal activity. Hence, only a few studies address mating behavior of the principal African malaria vector, Anopheles gambiae under natural conditions (Charlwood and Jones 1980, Marchand 1984, Yuval et al. 1992, Charlwood et al. 2002b, Diabate et al. 2003, Diabate et al. 2006). The Anopheles gambiae complex consists of seven sibling species (Coluzzi et al. 1979, Coluzzi et al. 1985, Hunt et al. 1998, Toure et al. 1998). The M and S molecular forms of An. gambiae s.s. represent incipient species (e.g., (Coluzzi et al. 1985, Toure et al. 1998, della Torre et al. 2001, Favia et al. 2001, Gentile et al. 2001, Mukabayire et al. 2001, Lehmann et al. 2003, Turner et al. 2005), but see (Lanzaro et al. 1998, Yawson et al. 2007).
Many mosquito species initiate mating in swarms that typically consist of a group of males flying in zigzag pattern 1–3 m above ground (often over a distinct marker) and forming a distinct “cloud”, into which females fly and depart paired with a male. However, swarms are not involved in mating of many culicine and anopheline species (Nielsen and Haeger 1960, McIver et al. 1980, Lounibos et al. 1998) where mating occurs near the vertebrate host or near larval sites (Yuval 2006). As noted by (Downes 1969), swarming in different “arenas” can facilitate pre-mating isolation. The mating behavior of An. gambiae is key to understand the mechanisms of reproductive isolation of the partly-sympatric molecular forms and sibling species. Observing only five couples in 34 swarms (Marchand 1984) casts doubt if mating occurs only in swarms. Although more couples were observed in An. gambiae swarms elsewhere (Charlwood et al. 2002, Diabate et al. 2003, 2006), this issue remains relevant because mating can be initiated in different ways by different individuals within species (Emlen and Oring 1977, Thornhill and Alcock 1983, Yuval 2006). Alternative mating strategies are often used by males that have low prospects of mating under “standard” conditions because of competitive disadvantage, such as smaller body size (Thornhill and Alcock 1983, Yuval et al. 1993, Charlwood et al. 2002a, Okanda et al. 2002). For example, An. labranchiae atroparvus swarms outdoors and indoors, yet most females are mated in sheltered “resting” sites (Cambounac and Hill 1940).
Although mating in An. gambiae is initiated in outdoor swarms, the possibility that mating also occurs independent of swarms has not been addressed. To evaluate if An. gambiae mates indoors, we (i) conducted experiments using progeny of wild females in natural settings; (ii) determined if males and females naturally co-occur in houses, (iii) assessed if insemination rate is higher in females sampled from exit vs. entry traps as expected if mating indoors occurred naturally; and (iv) evaluated the specificity in indoor mating between the molecular forms.
Materials and methods
Study sites
Indoor mark, release, recapture mating experiments and assessment of the presence of males with virgin females indoors were conducted in the villages of Banambani (West 8° 3′ longitude and North 12° 48′), and Donéguébougou (West 7° 59′5″ longitude and North 12° 48′38″), located 3–4 km apart and approximately 15 km north of Bamako, Mali. These farming communities are situated among gently rolling hills covered with vegetation characteristics of wet savanna. Most houses have a single room, built of mud, shaped like a cylinder, and covered by a conic thatch roof.
Experiments comparing natural insemination rates in mosquitoes entering and exiting houses were carried out in the village Sokourani near Niono (14°17′ N, 8°5′W). It is a rice cultivation area located 350 km northeast of Bamako in a habitat of dry savanna where, away from the river and the irrigation canals, vegetation is sparse. The inhabitants live in mud-walled houses, typically designed as a rectangular prism (box), with one door and two windows. Unlike in villages near Bamako, in Niono, many inhabitants use bednets regularly, but most bednets are not impregnated with insecticides.
Mosquitoes used in release experiments
Females of An. gambiae s.l. were collected from houses in Banambani and Donéguébougou using aspirators during October-November 2004, July-August 2005, and July-August 2007. They were transported to the insectary at the Malaria Research and Training Center in the University of Bamako, and maintained at 27°C and relative humidity of 75% – 85%. Blood fed, half gravid and gravid females were placed in 1-gallon cages and kept for 2 days before they were placed individually in 50 ml Falcon tube containing 15 ml deionized water for oviposition. A strip of filter paper (2 cm wide) surrounded the water edge, providing a wet surface to collect the eggs. Females who laid eggs were identified to species and molecular form using molecular assays (Fanello et al. 2002). First instar larvae were pooled by species and molecular form in groups of 200 per pan. Pans (25 cm W x 30 cm l x 6 cm d) were filled with 400 ml of deionized water and 0.1 g ground fish food (Tetramin) was provided daily. When most larvae reached the 3rd instar, 400 ml of water were added. Pupae were collected daily and transferred into emergence cages. Emerging males and females were separated within 24 hours to prevent mating. Virginity of females was confirmed by dissection and examination of spermatheca (below) from 8–20 females from each cage. All verifications (n=339) were negative.
Indoor release experiments
Several one room houses in Banambani and Donéguébougou were selected for the experiments. Before release, all openings of the house in the village were sealed by nets and exit traps were mounted in the screen that covered the door opening (Fig. 1A). Five traps (30 cm x 30 cm x 30 cm) were mounted on the door, spaced more or less evenly from bottom to top. One trap (60 cm x 60 cm x 60 cm) was mounted on the window. To increase survival and recapture rate, spiders and cobwebs were removed, 3–5 wet towels were hung to increase air humidity, and three pads of cottons soaked in 5% sucrose solution were suspended by strings from the ceiling. A few hours before release, virgin females (2–5 d old) were dusted with a fluorescent dye (DayGloR, DayGlo Color Corporation). Each species and molecular form was marked with a different color. Males were not marked. Males and females were released into the house together, 4–1 hours before sunset. Mosquitoes were removed from exit traps continuously throughout the experiment to prevent mating in the exit traps and males and females were placed in separate cages. The next morning, mosquitoes were collected indoors using a different aspirator and a different cage for each mosquito color. Following the live collection, pyrethrum spray collection (PSC) was performed to collect all remaining mosquitoes.
Figure 1.
Mounting exit traps in MRR experiments in Doneguebougou (A) and in experiments to assess natural insemination in Niono using entry and exit traps (see text for details).
Insemination status was determined by examination (100X magnification or higher) of dissected spermatheca – pressed under a microscope cover slip, for the presence of sperm. All recaptured mosquitoes were preserved in 85% ethanol and their species and molecular form determined as described above. To determine whether smaller body size is a determinant of indoor mating, one wing of randomly selected females (n=190) was removed, mounted on a slide and measured to the nearest 0.1 mm using a dissecting scope equipped with micrometer ruler under 10X magnification.
Abundance of males and females in houses
Male and female mosquitoes were collected from five houses in Donéguébougou or Banambani by PSC at each of the following times: 06:00, 07:00, 17:00, 18:00, 18:30, 19:00, 20:00, 22:00, and 24:00. Weekly collections were performed from July to September 2005 alternately in the each village, allowing two weeks before repeated sampling of the same house.
Natural indoor mating assessment: using entry and exit traps
To ascertain if indoor mating occurs naturally, experiments were undertaken to compare female insemination rates in entry and exit traps mounted on houses during April-May 2006 in Sokourani (Niono), when mosquito density was high. If indoor mating occurs naturally, then insemination of females from exit traps was expected to be higher than in females from entry traps as long as mosquitoes can also enter and exit the house through another window or door which is not fitted with traps. We assume that indoor mortality is not higher in virgin females than in mated females. In the first experiment, funnel based entry and exit traps were used. Each trap measured 30 cm x 30 cm x 30 cm. Two exit and two entry traps were mounted on three different houses every other night for four nights. The traps were mounted in pairs on the door and on one window similar to those depicted in Figure 1A. Mosquitoes freely entered and exited the house via the other window which was left open. In the second experiment, larger traps (50 cm x 50 cm wide and 200 cm high) were used instead. One entry trap was mounted inside the house and one exit trap was mounted outside (Fig. 1B). Funnels were not used in these traps and mosquitoes flew into them in greater numbers. Mosquitoes entered and exited the house through the door opening, which was loosely covered by a cloth curtain, leaving space for flying mosquitoes. Such curtains were seen on many houses in that area (Fig. 1B). As customary in this region, three-four people slept in each house and often a few people slept outside. The experiment did not change any of these conditions, but we confirmed that no insecticide impregnated bednets have been used in experimental houses. Traps were installed before 14:00 for 24 hours (first series) or three-four consecutive days (second series). To prevent mating inside traps mosquitoes were continuously collected and males and females were immediately separated in different cups and pooled based on the following time intervals: 15:00 –17:00; 17:00 – 19:00; 19:00 – 20:00; 20:00 – 24:00; 24:00 – 03:00; 03:00 – 06:00; 06:00 – 09:00; 09:00 – 12:00; 12:00 –15:00. During the experiments, sunset and sunrise times were close to 19:00 and 06:00, respectively. The gonotrophic stage of females was recorded and their insemination status determined.
Specificity of indoor mating between the molecular forms
The mating specificity of the molecular forms was assessed using indoor MRR experiments in Banambani and Donéguébougou from July to August 2007. The same procedures described above were used to produce virgin offspring of wild females, conduct the MRR experiments, and process the adults, but no exit traps were used. Two experimental designs were used, both with males of one molecular form. In the single female design, the females were all of a single form, either the same- or the other- form of the males (i.e., ♂M♀M, ♂M♀S, ♂S♀S, and ♂S♀M). In mix female design, females from the two molecular forms were released together in equal numbers (i.e., ♂M♀M&S, ♂S♀M&S). Every combination in the single female design was replicated three times and those of the mix female design were replicated four times. The first two replications were carried out with 200 mosquitoes of each form and sex. In subsequent experiments, 150 males and a total of 150 females were used in both experimental designs (i.e., 75:75 M:S females in the mix female design). The fluorescent dyes were used alternately so, females of each form were marked with the same color combinations across replicates and color was not confounded in molecular form.
In addition to analysis of female insemination status using logistic regression, we calculated a specificity index for each form in each experimental design in every replication as the difference between insemination rates of the pure vs. cross form standardized by the average insemination rate across forms: [Ip - Ic] / [(Ip + Ic)/2], where Ip and Ic represent the insemination rate of the females of the same and the other form as that of the males, respectively. The index varies from 2 (highest specificity) to −2 (complete preference of the other form), whereas 0 indicates no specificity.
Results
Insemination Rate in Indoor Mark Release and Recaptured Mosquitoes
To test whether An. gambiae might mate in natural houses, virgin fluorescently-marked females and unmarked males (2–5 d old, F1 offspring of wild caught females) were released into houses and insemination status was determined the next morning. Exit traps (Fig. 1A) allowed mosquitoes to leave the house. Overall across species and molecular forms, insemination rate was 13%, range: 1 to 27% (Table 1). The fraction of females and males collected in exit traps varied considerably between experiments (7–83% for females and 31–99% for males), possibly because of the relative humidity indoor or outdoor, moonlight intensity, etc. Overall, 62% of the females and 80% of the males were collected from exit traps. Insemination rates in females collected in houses (14%) and in the exit traps (13%) did not differ (P>0.28 G test; df =1). Mosquitoes could not mate in exit traps because they were removed as soon as they entered.
Table 1.
Assessment of indoor mating using mark, release, recapture experiments.
| Release Date | Female Recaptured/Released | Insemination rate (%) | |||||
|---|---|---|---|---|---|---|---|
| M | S | Ar | M | S | Ar | Mean | |
| 19-Nov-04 | 32/60 | 44/100 | 32/100 | 3.1 | 0 | 0 | 1.0 |
| 13-Jul-05 | 28/100 | 0/0 | 32/100 | 3.6 | - | 12.5 | 8.1 |
| 29-Jul-05 | 60/100 | 59/100 | 62/100 | 8.3 | 5.1 | 4.8 | 6.1 |
| 21-Aug-05 | 67/100 | 70/100 | 62/100 | 35.8 | 8.6 | 38.7 | 27.7 |
| 31-Aug-05 | 43/100 | 32/100 | 25/100 | 16.3 | 3.1 | 24 | 14.5 |
| Overall | 230/460 | 206/400 | 213/500 | 16.5 | 4.9 | 17.4 | 12.9 |
M, S and Ar represent M form, S form and An. arabiensis respectively. The number of females and males released per species/forms was equal. The insemination rates are based on dissection of all recaptured females
To compare the insemination rate of the species and forms we used logistic regression accommodating variation among experiments (excluding the experiment without the S form, Table 1). Differences between species and molecular forms were found (P<0.0001, Table 2) although insemination rates varied among experiments (P<0.0001, Table 2). The interaction between experiment and species/form was not significant (P>0.56, Table 2) and was removed. Insemination rate of the S form (6%) was lower (P<0.001) than that of the M form (15%) and An. arabiensis (18%), whereas the difference between the M form and An. arabiensis were not significant (P>0.8, Table 2). These results demonstrate that both species and molecular forms can mate indoors without adaptation to laboratory conditions even if they can leave the house via exit traps. The results suggest that indoor conditions are more suitable for An. arabiensis and the M form of An. gambiae than for the S form. Notably, nearly 90% of the females remained virgins under these conditions despite access to males and the advantage of early reproduction, suggesting that they preferred mating in outdoor swarms. Nevertheless, these experimental results do not demonstrate that this occurs naturally.
Table 2.
Body size (wing length, mm) difference between inseminated and uninseminated females
| ANOVA results | Means (mm) and significancea of difference | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Source | Df | F/MS | P | SpForm | Inseminated | Unnseminated | P |
| Model | 8 | 15.0/0.293 | 0.0001 | ||||
| Error | 219 | ND/0.020 | ND | Ar | 3.20 | 3.18 | 0.6 |
| Exp | 3 | 15.9/0.311 | 0.0001 | M | 2.97 | 3.08 | 0.0012 |
| Species/Form | 2 | 29.6/0.581 | 0.0009 | S | 3.15 | 3.20 | 0.3 |
| Insemination | 1 | 3.3/0.064 | 0.073 | ||||
| SpFo*Insemb | 2 | 4.1/0.080 | 0.018 | All | 3.11 | 3.16 | 0.073 |
Statistical significance of the contrast between inseminated vs. uninseminated females of An. arabiensis (Ar) and the M and S molecular forms of An. gambiae.
Interaction between Species/Form and Insemination.
In experimental houses, flight activity peaked approximately five-ten minutes after sunset and remained so for up to an hour. During this time, a humming sound, produced by flying mosquitoes was heard. Swarms, defined as groups of males flying together and forming a stationary “cloud”, were not observed indoors. Instead, many males and females concentrated on the bottom half of the screen covering the door and on the adjacent walls and engaged in short (ca 20 cm) flights. Small clusters of mosquitoes (6–20) were also observed near the eaves in houses with thatch roofs. Couples mating were observed flying and falling to ground near the better illuminated door area by twilight (this was especially noticeable if white sheets were spread on the floor). However, the events leading to couple formation were not observed.
To assess if female body size affected her probability of mating indoors, we compared wing length of all inseminated females and that of a random sample of uninseminated females from each replicate experiment. Overall, inseminated females tend to be smaller than uniseminated females (P=0.073), but only in the M form this difference was significant (P<0.0012, Table 2).
Abundance of males and females in houses
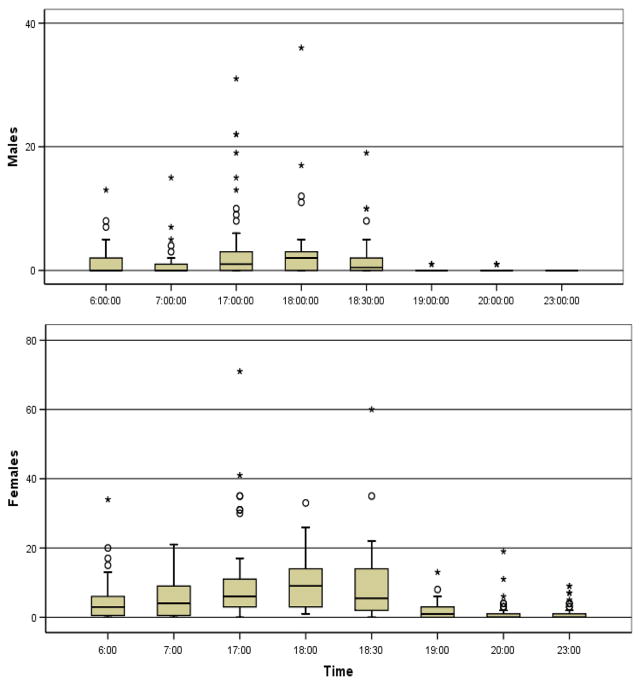
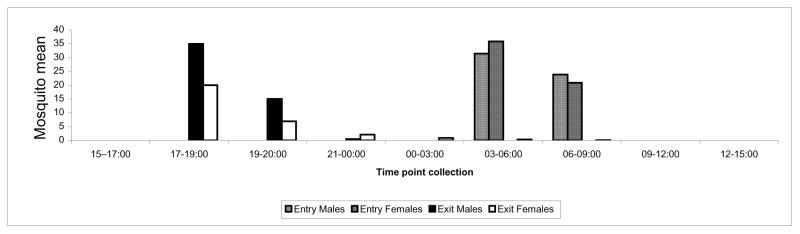
Collections of males and females in houses were conducted throughout the day in Banambani and Donéguébougou by PSC. Male density indoors increased rapidly at sunrise (06:15) and remained stable (average = 4 males per house, max = 36) until sunset (18:45, sunset time varied between 18:30 and 19:00 during the experiment). Male density declined sharply to near zero by 19:00 (Fig. 2), precluding indoor mating during the rest of the night when male density was effectively zero. Female density indoors followed this pattern but female density remained above zero throughout the night (Fig. 2). Notably, the number of males (and females) was considerably higher in certain houses. Males and females represented both molecular forms (M: 38%, S: 42%) and An. arabiensis (20%). Indoors, 30% of females were virgin. Although they were present in houses throughout the day, relative abundance of virgin females varied among different times (P<0.0074, Chisq=17.6, df=6), reaching peak abundance at 18:30 (37%) and dropping to 17% at 19:00 (not shown). Departure of virgin females from houses, mating indoors, and entry of inseminated females indoors, could explain the rapid decline in virgin females over this short period. Co-habitation satisfies a fundamental requirement for indoor mating and increased presence of virgin females indoors before and near sunset could provide an opportunity for mating. Samples from exit and entry traps mounted on houses in Niono (see Materials & Methods), similarly showed that males and females enter houses around sunrise and exit during and after sunset (Fig. 3). Altogether, these results suggest that both sexes rest indoors during the day, exit at dusk to swarm and sugar feed, and enter houses mostly during dawn. Accordingly, the main window of time available for indoor mating is during the day and around sunset.
Figure 2.
Mosquito density in houses estimated using pyrethrum spray catch in Doneguebougou and Banambani (pooled) over time showing variation among houses. In box-whisker plot, the box extends between the 25th and the 75th percentile, i.e., across one inter quartile range (IQR), and the whiskers extend up to the most extreme value, but not beyond 1.5 times the IQR. Outlier values located 1.5–3 IQR from the median are shown as “○” and extreme outliers located over 3 IQR are shown as ‘*’
Figure 3.
Mean number of males and females collected in entry and exit traps mounted on houses in Niono over time.
Natural indoor mating
To determine if indoor mating in An. gambiae occurs naturally, we compared insemination rates in samples of females from entry and exit traps mounted on a house for 24 hours (or longer) of continuous collection. To allow free entry and exit of mosquitoes, traps were mounted on one window and the door, leaving another window open (first experiment) or on both windows, leaving the door open (second experiment, see Materials & Methods). If mating does not occur indoors, insemination rate of females exiting and entering the house over 24 hr, is expected to be equal, but if mating occurs indoors, the insemination rate in females exiting the house is expected to be higher than that in females entering the house. Experiments were conducted in the rice cultivation area near Niono, where the M form of An. gambiae exclusively abounds (Dolo et al. 2004). During our experiments, the M form was the only member of An. gambiae identified (Exp 1: 309 M form and 10 unidentified; Exp 2: 260 M form and 25 unidentified).
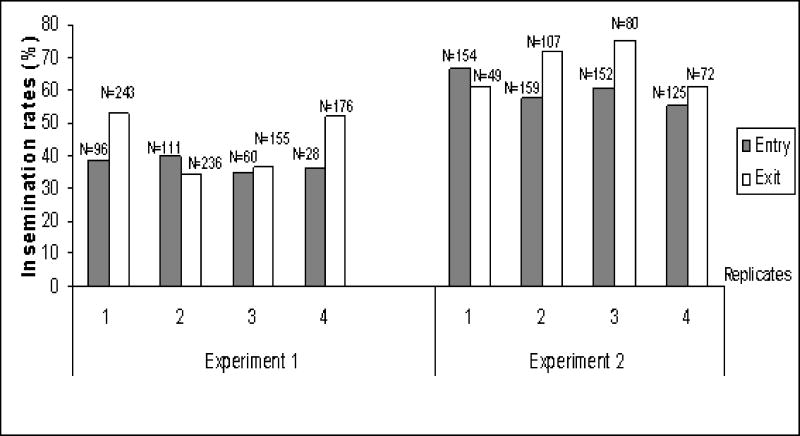
In the first experiment, two exit- and two entry traps (see Materials & Methods) were mounted on three different houses every other day for four days (Fig. 4). Overall insemination rate in exit traps (44.0%) was greater than in entry traps (37.8%; N = 1166; P = 0.036; One side Fisher Exact Test). The number of exiting females (870) was larger than those entering (296), indicating that mosquitoes avoid the entry traps. Although this did not necessarily bias the results, the traps were modified (see Materials & Methods) in the second experiment. The modified entry traps collected a total of 590 females vs. 308 captured in the modified exit traps. The traps were used for 3–4 days consecutively on each house. As in the previous experiment, overall insemination rate in exit traps (68.5%) was greater than in entry traps (60.2%; N = 898; P<0.008; One side Fisher Exact Test). When tested separately, significantly higher rate of insemination in exiting vs. entering females was detected only in certain days (Fig. 4) and houses (not shown), probably reflecting differences in power due to sample size as well as difference in conditions suitable for indoor mating, but no heterogeneity was detected among days (across houses) and among houses (Fig. 4; P>0.08, Breslow-Day Test for Homogeneity) in both series of experiments. Corresponding to the movement pattern into and from houses (Fig. 3), higher fraction of virgin females entered the house at dawn, whilst higher fraction of inseminated females exited at dusk and throughout the night (not shown).
Figure 4.
Insemination rates in entry and exit traps over consecutive days. Bars represent a mean across houses pooled by day. Numbers above bars denote the number of total females sampled.
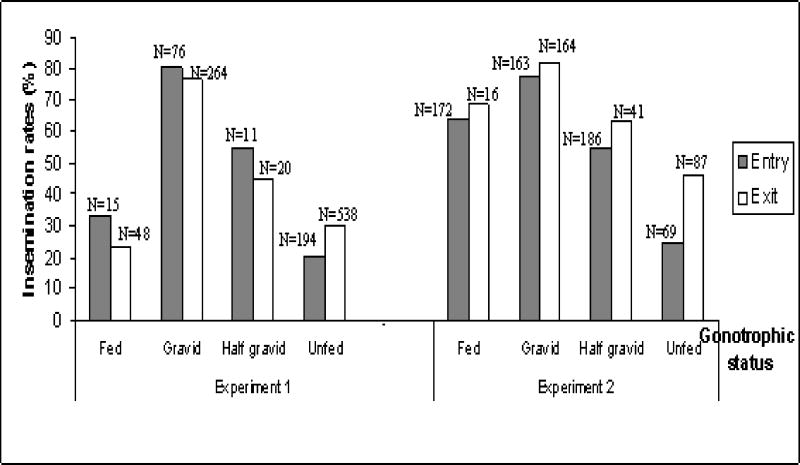
Insemination rate varied with the gonotrophic state, being highest in gravid females and lowest in unfed females (Fig. 5; P<0.001, Likelihood Ratio Chi-Square>90, df=3). Higher insemination rates in exit vs. entry traps were detected in unfed females in both experiments (Fig. 5; P<0.013, Likelihood Ratio Chi-Square>6, df=1) and was not significant in the other gonotrophic states (Fig. 5; P>0.4, Likelihood Ratio test, df=1), suggesting that some unfed virgin females entered to the house to mate. Importantly, higher insemination rate in females from exit traps was observed in the same physiological state (unfed females), indicating that the difference between exit and entry traps was not confounded by higher frequency of gravid and half-gravid females. These results suggest that the M molecular form of An. gambiae in Niono mates indoors naturally. Indoor mating may occur in certain houses and days in different intensity. Because conventional outdoor swarms were observed nearby and insemination rate in exit traps was near 5% higher than that in entry traps, we infer that only a fraction of mosquitoes mate indoors.
Figure 5.
Insemination rates of females in different gonotrophic states in entry and exit traps. Stars indicate significant difference between adjacent bars entry and exit traps (using one side Fisher Exact Test). Numbers above bars denote the number of total females sampled.
Specificity of Indoor Mating
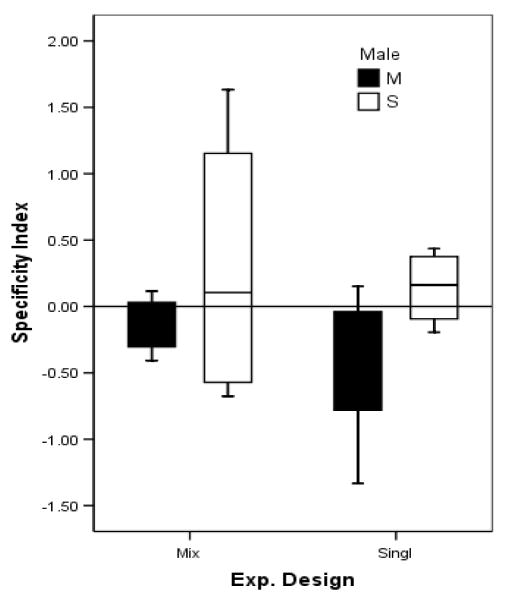
The specificity of mating indoors was evaluated using MRR experiments in which virgin males of one molecular form were released into experimental houses with virgin females of both forms (mix female design) or of one form (single female design). Logistic regression of female insemination status showed strong male form effect (P<0.001, Table 3) ascribed to higher insemination of females of both forms by males of the M form (33%) compared with males of the S (9%), whereas insemination rate in the females of the S molecular form (19.5%) was slightly higher than that of the M form females (15.1%, P<0.007, Table 3). Importantly, crossmating (16.1%) was as common as mating between members of the same form (18.9%) providing no evidence for indoor mating specificity as reflected by the insignificant interaction between male and female forms (P<0.34). Likewise, specificity index, calculated in each experiment as the difference in insemination rate between pure- and cross- form, standardized by the average mating rate (see M&M) depicted slightly higher specificity (positive values) involving S males (Fig. 6), but the difference between forms was not significant (P>0.06) and even specificity of the S form was minimal. Because the molecular forms mate assortatively (Tripet et al. 2001) and M/S hybrids are rare (della Torre et al. 2005), indoor mating cannot occur frequently, unless it involves a single form.
Table 3.
Mating specificity indoors measured as frequency of insemination in experiments with different form combinations of males and females using a logistic regression. In single female design, females were of the same or other form whereas in mixed female design, females of both forms were present. In both designs males were exclusively of one form.
| Source | Estimate | DF | Chi-Square | Pr > ChiSq |
|---|---|---|---|---|
| Intercept | 1.50 | 1 | 656.30 | <.0001 |
| ExpDesign (Single/Mix) | −0.13 | 1 | 6.22 | 0.0126 |
| MaleForm | −0.77 | 1 | 175.51 | <.0001 |
| FemaleForm | 0.16 | 1 | 7.33 | 0.0068 |
| MaleForm*FemaleForm | 0.06 | 1 | 0.88 | 0.3482 |
| Likelihood Ratio | -- | 3 | 5.31 | 0.1504 |
Figure 6.
Mating specificity between the molecular forms of An. gambiae in experiments with males of one form in the single female design (females of the same or other form than the male) or mixed female design (females of both forms with males of one form). Specificity index is calculated as the difference in insemination rate between pure and cross –form standardized by the average insemination rate in each experiment replicate. The index ranges from 2 (highest specificity) to −2 (complete preference of the other form), 0 indicates no specificity.
Discussion
As in many dipterans, mating in An. gambiae is initiated in swarms (Charlwood et al. 1980, 2002a, 2002b, Marchand 1984, Diabate et al. 2005, 2006). However, in many species, swarms represent one of two alternative mating strategies (e.g., Thornhill and Alcock 1983). The ceratopoginid Culicoides nubeculosus usually swarms, but males also perch on the host (cows) and initiate mating with females who land to blood feed (Downes 1955). Unlike large chironomid midge males that swarm, smaller conspecific males mate with females aggregated in vegetation near swarms (McLachlan and Neems 1989). Aedes aegypti males form various types of swarms but also approach females near the host (e.g., (Hartberg 1971). An. labranchiae atroparvus swarms outdoors and indoors, yet most mating events occur in sheltered resting sites (Cambournac and Hill, 1940).
Whether indoor mating represents an alternative mating tactic in An. gambiae is the focus of our study. The evidence supporting this hypothesis consists of (i) observing mating of 2–6 d old virgin F1 females, released into natural houses over a single day when they can leave the house through exit traps and without prior adaptation to laboratory conditions; (ii) males and virgin females are naturally found in houses from sunrise and until an hour after sunset (throughout the day in Niono); and (iii) the rate of insemination in samples of the M form from exit traps was higher than in those from entry traps, implicating that some mating occurred indoors. However, assortative mating of the molecular forms was not observed indoors. Because assortative mating is a key characteristic of the molecular forms (Tripet et al. 2001) and because hybrids are rare in Mali (Tripet et al. 2001, della Torre et al. 2005), indoor mating may occur in one but not in both forms. Notably, the evidence showing that indoor mating is an alternative mating strategy of An. gambiae pertains primarily to the M molecular form. Indoor MRR experiments showed substantially higher insemination rate in the M molecular form and An. arabiensis compared with the S form (15 vs. 6%). In these experiments, mated females were smaller than virgin females; but this difference was only found in the M form. The higher insemination rate in females from exit vs. entry traps was also found in the M form. This evidence remains short of direct observation of naturally occurring mating couples indoors and therefore is not definite. However, observing mosquito couples under dark settings on the background of mud walls and thatch roof is not a trivial task.
The relationships between indoor mating and density, sex ratio, form composition indoors, conditions indoors and outdoors are not known. The insemination rate in the indoor MRR experiments (~10%/day) represent a unique subset of these parameters and therefore may not provide a “general” rate of indoor mating. The difference between exit and entry traps in Niono (~5%/day) is a more meaningful estimate for natural indoor mating under these conditions. As virgin females constituted about 40% of the total females in Niono, then indoor mating constitutes approximately 12% (5/40) of total mating whilst 88% occurs in outdoor swarms.
Mating systems are shaped by the species phylogenetic history, evolutionary constraints, as well as by its unique ecological factors (Emlen and Oring 1977, Thornhill and Alcock 1983, Yuval and Fritz 1994, Yuval 2006). Alternative mating strategies are commonly used by males that have low prospects to mate under “standard” conditions because of competitive disadvantage, such as smaller body size (Thornhill and Alcock 1983, Yuval, 1993). Accordingly, indoor mating may have evolved to circumvent low prospects of certain males to mate in swarms. That smaller M form females mated indoor more frequently than larger females provides some support to this explanation, if smaller males mate more successfully with smaller females. Possibly, indoor mating may have evolved as it enhances prospects of mating under conditions that are not favorable for swarms, such as dry, hot, and windy weather (e.g., during the period of Harmattan). Moreover, such conditions prevail when population density decreases, so swarms are probably rare and localizing them would require higher energetic expanse, and increased risk of predation for both sexes. Under such conditions, meeting at the resting sites, which are also the females “feeding grounds”, is expected to reduce the above costs. Consistent with that explanation, the arid-adapted An. arabiensis and the M form of An. gambiae mated indoors in MRR experiments more frequently than the humid-adapted S form. On the other hand, mating in swarms probably provides females with better mate choice. To better understand how both mating strategies are maintained, more information is needed on the relative contribution of indoor mating during different seasons and population densities as well as on the differences between the males and females that participate in indoor vs. outdoor mating activities during varied conditions.
Indoor mating provides insights on important aspects of An. gambiae mating behaviors. The low specificity of indoor mating indicates that chemical cues such as pheromones and cuticular hydrocarbons do not play a major role in form recognition, unless such cues are not released or perceived away from a swarm. Likewise, flight tone is not a primary form recognition cue (Tripet et al. 2004), unless flying is not involved in couple formation indoors. Because we used laboratory reared F1s in our experiments, we cannot rule out that larvae growing in natural habitats acquire cues or capacity to respond to differences between adults that promote assortative mating. However, larvae of both forms naturally co-habit many larval sites (Edillo et al. 2002), and the F1 larvae were raised in separate pans according to molecular form. Alternatively, these results suggest that form specificity relies on spatial segregation of mating arenas; because once sexually active males and females are brought together into close range their mating is not assortative. This is supported by the absence of specificity in experiments without exit traps (specificity experiments) compared with the two and a half fold higher indoor mating rate of the M vs. S forms in houses with exit traps (MRR experiments). Supposedly virgin females of the S form depart the house prior to indoor mating or are less receptive to mating indoors. Female receptivity in Aedes ageypti determined if insemination will occur after coupling (Gwadz et al. 1971).
Spatial segregation between swarms of the molecular forms was described previously (Diabate et al. 2006) and recent results in Donéguébougou reveal striking spatial segregation between swarms of the molecular forms (Diabate et al. unpublished). Indoor mating might reflect the plasticity of mating behavior which probably has implications for the evolution of mating barriers and for introgression events between sibling species and molecular forms. Rare situations where males and females of different molecular forms (and sibling species) are confined in houses may result in introgression events even if specificity in outdoor swarms is absolute. Rare introgression events could have important ramifications for evolution of these species. We believe that these results will encourage further work on mating behavior of An. gambiae and focus attention on indoor mating as a possible alternative mating strategy in this complex of mosquito species. Evaluating the relative importance of these mating strategies in different conditions and understanding the reproductive isolation mechanisms between siblings and incipient species and the conditions, where cross-mating occurs – are key aspects for future research.
Acknowledgments
We thank the villagers of Donéguébougou, Banambani and Sokourani for their kindness and generosity by accepting the mosquito collection in their houses. We thank Drs. Jose’ Ribeiro, Nicholas Manoukis, Richard Sakai, Robert Gwadz, and especially Drs. Abdoulaye Diabate and Boaz Yuval for helpful comments and discussions about this study. The authors thank also the Malian team at MRTC for their support. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
References
- Cambounac FJC, Hill RB. Observation on the Swarming of Anopheles Maculipennis, Var. Atroparvus. Am J Trop Med. 1940:133–140. [Google Scholar]
- Charlwood JD, Jones MDR. Mating in the mosquito, Anopheles gambiae. s.l. II. Swarming behaviour. Physiological Entomology. 1980;5:315–320. [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Ferreira C, Do Rosario VE. Male size does not affect mating success (of Anopheles gambiae in Sao Tome) Med Vet Entomol. 2002a;16:109–11. doi: 10.1046/j.0269-283x.2002.00342.x. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Madsen H, Ferreira C, do Rosario VE. The swarming and mating behaviour of Anopheles gambiae s.s. (Diptera: Culicidae) from Sao Tome Island. J Vector Ecol. 2002b;27:178–83. [PubMed] [Google Scholar]
- Coluzzi M, Petrarca V, Di Deco MA. Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Bollettino di Zoologia. 1985;52:45–63. [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- della Torre A, Tu Z, Petrarca V. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochem Mol Biol. 2005;35:755–69. doi: 10.1016/j.ibmb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- della Torre A, Fanello C, Akogbeto M, Dossou-yovo J, Favia G, Petrarca V, Coluzzi M. Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol Biol. 2001;10:9–18. doi: 10.1046/j.1365-2583.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- Diabate A, Dabire RK, Kengne P, Brengues C, Baldet T, Ouari A, Simard F, Lehmann T. Mixed swarms of the molecular M and S forms of Anopheles gambiae (Diptera: Culicidae) in sympatric area from Burkina Faso. J Med Entomol. 2006;43:480–3. doi: 10.1603/0022-2585(2006)43[480:msotmm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Diabate A, Baldet T, Brengues C, Kengne P, Dabire KR, Simard F, Chandre F, Hougard JM, Hemingway J, Ouedraogo JB, Fontenille D. Natural swarming behaviour of the molecular M form of Anopheles gambiae. Trans R Soc Trop Med Hyg. 2003;97:713–6. doi: 10.1016/s0035-9203(03)80110-4. [DOI] [PubMed] [Google Scholar]
- Dolo G, Briet OJT, Dao A, Traore S, Bouare M, Sogoba N, Niare O, Bagayogo M, Sangare D, Teuscher T, Toure YT. Malaria transmission in relation to the rice cultivation in the irrigated Sahel of Mali, West Africa. Acta Tropica. 2004;89:147–159. doi: 10.1016/j.actatropica.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Downes JA. Observations on the swarming flight and mating of Culicoides (Diptera : Ceratopogonidae) Transactions of the Royal Entomological Society of London. 1955;106:213–236. [Google Scholar]
- Downes JA. The swarming and mating flight of Diptera. Annu Rev Entomol. 1969;14:271–298. [Google Scholar]
- Edillo FE, Toure YT, Lanzaro GC, Dolo G, Taylor CE. Spatial and habitat distribution of Anopheles gambiae and Anopheles arabiensis (Diptera: Culicidae) in Banambani village, Mali. J Med Entomol. 2002;39:70–7. doi: 10.1603/0022-2585-39.1.70. [DOI] [PubMed] [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–23. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Fanello C, Santolamazza F, della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–4. doi: 10.1046/j.1365-2915.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- Favia G, Lanfrancotti A, Spanos L, Siden-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- Gentile G, Slotman M, Ketmaier V, Powell JR, Caccone A. Attempts to molecularly distinguish cryptic taxa in Anopheles gambiae s.s. Insect Mol Biol. 2001;10:25–32. doi: 10.1046/j.1365-2583.2001.00237.x. [DOI] [PubMed] [Google Scholar]
- Gwadz R, Craig GB, Jr, Hickey WA. Female sexual behavior as the mechanism rendering Aedes aegypti refractory to insemination. J Am Mosq Contr Assoc. 1971;14:210–213. doi: 10.2307/1540069. [DOI] [PubMed] [Google Scholar]
- Hartberg WK. Observations on the mating behaviour of Aedes aegypti in nature. Bull World Health Organ. 1971;45:847–50. [PMC free article] [PubMed] [Google Scholar]
- Hunt RH, Coetzee M, Fettene M. The Anopheles gambiae complex: a new species from Ethiopia. Trans R Soc Trop Med Hyg. 1998;92:231–235. doi: 10.1016/s0035-9203(98)90761-1. [DOI] [PubMed] [Google Scholar]
- Lanzaro GC, Toure YT, Carnahan J, Zheng L, Dolo G, Traore S, Petrarca V, Vernick KD, Taylor CE. Complexities in the genetic structure of Anopheles gambiae populations in west Africa as revealed by microsatellite DNA analysis. Proc Natl Acad Sci USA. 1998;95:14260–14265. doi: 10.1073/pnas.95.24.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, Licht M, Elissa N, Maega BT, Chimumbwa JM, Watsenga FT, Wondji CS, Simard F, Hawley WA. Population Structure of Anopheles gambiae in Africa. J Hered. 2003;94:133–47. doi: 10.1093/jhered/esg024. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Lima DC, Lourenco-de-Oliveira R. Prompt mating of released Anopheles darlingi in western Amazonian Brazil. J Am Mosq Control Assoc. 1998;14:210–3. [PubMed] [Google Scholar]
- Marchand RP. Field observations on swarming and mating in Anopheles gambiae mosquitoes in Tanzania. Neth J Zool. 1984;34:367–387. [Google Scholar]
- McIver SB, Wilkes TJ, Gillies MT. Attraction to mammals of male Mansonia (Mansonioides) (Diptera: Culicidae) Bull Entomol Res. 1980;70:11–16. [Google Scholar]
- McLachlan A, Neems R. An alternative mating system in small male insects. Ecological Entomology. 1989;14:85–91. [Google Scholar]
- Mukabayire O, Caridi J, Wang X, Toure YT, Coluzzi M, Besansky NJ. Patterns of DNA sequence variation in chromosomally recognized taxa of Anopheles gambiae: evidence from rDNA and single-copy loci. Insect Mol Biol. 2001;10:33–46. doi: 10.1046/j.1365-2583.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- Nielsen ET, Haeger JS. Swarming and mating in mosquitoes. Misc Publ Entomol. 1960;1:79–95. [Google Scholar]
- Okanda F, Dao A, Njiru B, Arija J, Akelo H, Toure Y, Odulaja A, Beier J, Githure J, Yan G, Gouagna L, Knols B, Killeen G. Behavioural determinants of gene flow in malaria vector populations: Anopheles gambiae males select large females as mates. Malar J. 2002;1:10. doi: 10.1186/1475-2875-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill R, Alcock J. The Evolution of Insect Mating Systems. Harvard University Press; Cambridge, MA: 1983. [Google Scholar]
- Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- Tripet F, Dolo G, Traore S, Lanzaro GC. The “wingbeat hypothesis” of reproductive isolation between members of the Anopheles gambiae complex (Diptera: Culicidae) does not fly. Journal of Medical Entomology. 2004 May;41(3) doi: 10.1603/0022-2585-41.3.375. [DOI] [PubMed] [Google Scholar]
- Tripet F, Toure YT, Taylor CE, Norris DE, Dolo G, Lanzaro GC. DNA analysis of transferred sperm reveals significant levels of gene flow between molecular forms of Anopheles gambiae. Mol Ecol. 2001;10:1725–1732. doi: 10.1046/j.0962-1083.2001.01301.x. [DOI] [PubMed] [Google Scholar]
- Turner TL, Hahn MW, Nuzhdin SV. Genomic islands of speciation in Anopheles gambiae. PLoS Biol. 2005;3:e285. doi: 10.1371/journal.pbio.0030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawson AE, Weetman D, Wilson MD, Donnelly MJ. Ecological zones rather than molecular forms predict genetic differentiation in the malaria vector Anopheles gambiae s.s. in Ghana. Genetics. 2007;175:751–61. doi: 10.1534/genetics.106.065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval B. Mating systems of blood-feeding flies. Annu Rev Entomol. 2006;51:413–40. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
- Yuval B, Fritz GN. Multiple mating in female mosquitoes –evidence from a field population of Anopheles freeborni (Diptera: Culicidae) Bull Entomol Res. 1994;84:137–140. [Google Scholar]
- Yuval B, Wekesa JW, Washino RK. Swarming and mating in Anopheles freeborni: preliminary observations and methods for field studies. Proceeding of the Californian mosquito Vector Control Association. 1992;60:76–81. [Google Scholar]
- Yuval B, Wekesa JW, Washino RK. Effects of body size on swarming behaviour and mating success of male Anopheles freeborni (Diptera: Culicidae) J Insect Behav. 1993;6:333–342. [Google Scholar]