Abstract
The endocannabinoid system (ECS) is a widespread neuromodulatory system that plays important roles in central nervous system (CNS) development, synaptic plasticity, and the response to endogenous and environmental insults. The ECS is comprised of cannabinoid receptors, endogenous cannabinoids (endocannabinoids), and the enzymes responsible for the synthesis and degradation of the endocannabinoids. The most abundant cannabinoid receptor is the CB1 cannabinoid receptors, however CB2 cannabinoid receptors, transient receptor potential (TRP) channels, and peroxisome proliferator activated receptors (PPAR’s) are also engaged by some cannabinoids. Exogenous cannabinoids, such as tetrahydrocannabinol, produce their biological effects through their interactions with cannabinoid receptors. 2-arachidonoyl glycerol (2-AG) and arachidonoyl ethanolamide (anandamide) are the best-studied endogenous cannabinoids. Despite similarities in chemical structure, 2-AG and anandamide are synthesized and degraded by distinct enzymatic pathways, which impart fundamentally different physiological and pathophysiological roles to these two endocannabinoids. Because of the pervasive social use of cannabis and the involvement of endocannabinoids in a multitude of biological processes, much has been learned about the physiological and pathophysiological roles of the ECS. This review will provide an introduction to the ECS with an emphasis on its role in synaptic plasticity and how the ECS is perturbed in schizophrenia.
Keywords: cannabinoid, cannabis, lipid signaling, retrograde messenger, schizophrenia, synaptic plasticity
Introduction
The endocannabinoid system (ECS) has emerged as an important neuromodulatory system over the last twenty-five years. Relevant to the topic of this special issue of Biological Psychiatry, perturbations of the ECS are involved in several psychiatric disorders, including schizophrenia. The ECS is comprised of endogenous cannabinoids (endocannabinoids), cannabinoid receptors, and the enzymes responsible for the synthesis and degradation of endocannabinoids (Fig. 1). Each of these components will be introduced in this chapter, with an emphasis on their potential involvement in psychosis.
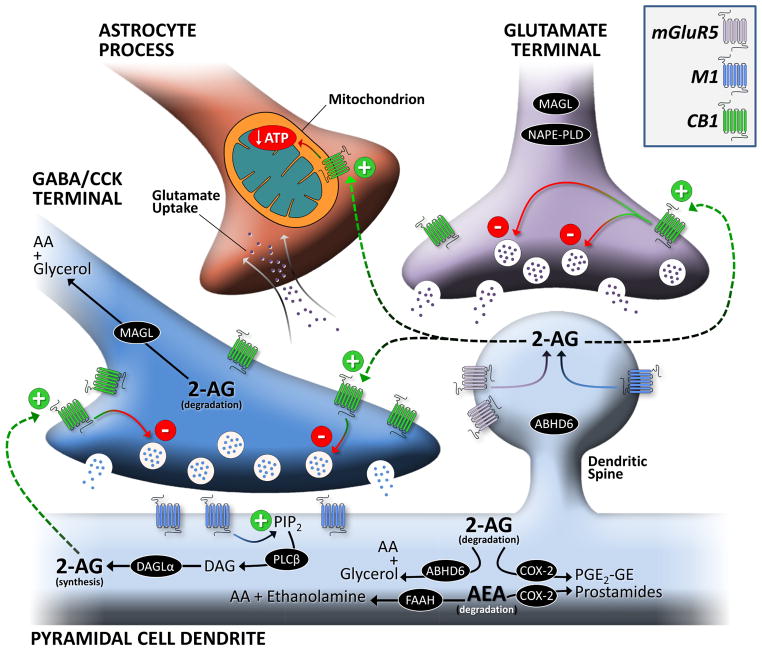
Fig. 1. Overview of the localization of endocannabinoid system components at the synapse.
Schematic of an inhibitory and excitatory terminal synapsing onto the dendritic shaft of a representative cortical principal neuron. Abbreviations: ABHD6, alpha/beta domain-containing hydrolase 6; CB1, CB1 cannabinoid receptor; CCK, cholecystokinin; COX-2, cyclooxygenase-2; DAGLα, diacylglycerol lipase α; M1, M1 muscarinic receptor; MAGL, monoacylglycerol lipase; mGluR5, metabotropic glutamate receptor 5; NAPE-PLD, N-arachidonoyl phosphatidyl ethanolamine-preferring phospholipase D; PLCβ, phospholipase C β. The increased number of CB1 receptors on the CCK/GABA terminal represents the higher density of CB1 receptors found on these axon terminals.
Endogenous cannabinoids are endogenous lipids that engage cannabinoid receptors (see below), affecting behavior in a fashion that at least partially recapitulates the effects produced by the psychoactive components of cannabis, most notably Δ-9-THC ((–)-trans-Δ9-tetrahydrocannabinol; THC). The first discovered and best-characterized endocannabinoids are anandamide (arachidonoyl ethanolamide) and 2-arachidonoyl glycerol (2-AG). An important feature of these endocannabinoids is that their precursors are present in lipid membranes. Upon demand (typically by activation of certain G protein-coupled receptors or by depolarization), endocannabinoids are liberated in one or two rapid enzymatic steps and released into the extracellular space. This contrasts with classical neurotransmitters that are synthesized ahead of time and stored in synaptic vesicles. The intrinsic efficacy of the endogenous cannabinoids varies—2-AG is a high efficacy agonist for both CB1 and CB2 receptors, however anandamide is a low efficacy agonist at CB1 receptors and a very low efficacy agonist at CB2 receptors (1, 2). Thus, in systems with low receptor expression or when receptors couple weakly to signaling pathways anandamide can antagonize the effects of more efficacious agonists (3). Additional endogenous substances (e.g., virodhamine and 2-arachidonoyl glycerol ether (4)) may expand the repertoire of endocannabinoids, however the biology of these compounds are not as well developed as the biology of anandamide and 2-AG, so they will not be considered further in this review. This chapter will introduce the components of the endocannabinoid system and discuss their role in modulating synaptic transmission. Chapters 6 and 8 will consider the extensive functions of cannabinoids in neurodevelopment and how perturbation of these functions may increase an individual’s risk to develop a psychiatric disorder.
Cannabinoid receptors
The effects of endocannabinoids are primarily mediated by CB1 and CB2 cannabinoid receptors (4), with other receptors (such as PPAR’s and Transient Receptor Potential (TRP)) channels (see below)) also mediating some endocannabinoid actions, particularly of the acylethanolamides. As discussed in more detail in Chapter 12, polymorphisms of cannabinoid receptor and endocannabinoid system genes are variably associated with schizophrenia (5–8) and possibly with response to atypical antipsychotics (9). Both CB1 and CB2 cannabinoid receptors are G protein-coupled receptors (GPCR’s), which primarily couple to G proteins of the Gi and Go classes (4). As such, their activation inhibits adenylyl cyclases and certain voltage dependent calcium channels and activates several MAP kinases and inwardly rectifying potassium channels, with some variation depending on the particular type of cell (4). Thus, activation of CB1 or CB2 receptors exerts diverse consequences on cellular physiology, including synaptic function, gene transcription, cell motility, etc. (4).
CB1 receptors are abundant in the central nervous system (CNS), particularly in cortex, basal ganglia, hippocampus, and cerebellum (10). The majority of CB1 receptors are present on axon terminals and pre-terminal axon segments, while sparing the active zone (11) (Fig. 1). Cortical and hippocampal CB1 receptors are particularly enriched on cholecystokinin (CCK) positive interneurons (low threshold spiking interneurons) (12–14), and are widely expressed at lower (but still functionally important) levels in glutamatergic neurons (15). CB1 receptors are highly abundant in medium spiny neurons in both the dorsal and ventral striatum (16–18). Expression is particularly high on the direct pathway axons as they enter the globus pallidus heading towards the substantia nigra (19). Cerebellar CB1 receptors are found in parallel and climbing fibers, as well as in basket cells (20, 21). While CB1 has been detected on many neurons, functionally relevant expression of CB1 in glial elements has also been reported by a number of independent groups (22–24).
CB2 receptors are expressed at much lower levels in the CNS compared to CB1. This receptor is primarily present in microglia and vascular elements (25, 26). However, CB2 does appear to be expressed by some neurons, particularly under certain pathological conditions (e.g., nerve injury) (27, 28), and see (29) for a discussion of the caveats on examining CB2 in the brain. Accumulating genetic and animal model evidence suggests a link between CB2 receptors and an increased risk for schizophrenia (5, 30–32), however if this due to neuronal CB2, microglial CB2, or a neurodevelopmental role of CB2 remains an unknown, but important question. A particularly interesting feature of CB2 receptors is that they appear to be highly inducible, with expression in CB2 increasing up to 100 fold following tissue injury or during inflammation (33). It remains to be determined whether observed increases in CNS CB2 is due to increased expression of CB2 on cells intrinsic to the CNS, or is a result of the migration (e.g. CB2-expressing monocytes) of peripheral immune cells into the CNS.
TRP channels, especially TRPV1, are activated by anandamide under certain conditions (34). The relative roles of cannabinoid receptors and TRP channels in anandamide’s actions appear variable. Anandamide also activates PPARalpha and gamma, with significant effects on gene transcription (35, 36). It is important to keep in mind that increasing anandamide by decreasing its degradation by inhibition of fatty acid aminohydrolase (FAAH) also increases levels of other N-acylamides, which can modulate PPARα (37, 38).
Endocannabinoid synthesis
Despite anandamide and 2-AG both containing arachidonic acid, their routes of synthesis and degradation in vivo are almost completely distinct and are mediated by different enzymes (39). Most anandamide appears to be produced from N-arachidonoyl phosphatidyl ethanol (NAPE), while 2-AG is produced from 2-arachidonoyl-containing phospholipids, primarily arachidonoyl-containing phosphatidyl inositol bis-phosphate (PIP2) (Fig. 2). An important consideration in 2-AG biology is that, in addition to serving as an endogenous ligand for cannabinoid receptors, 2-AG is an important metabolic intermediate in lipid synthesis and also serves as a major source of arachidonic acid in prostaglandin synthesis (40). Thus, manipulation of 2-AG production and degradation can have wide-ranging effects that are quite independent of ECS. For example, 2-AG in the brain, liver, and lung, but not in the gut, heart, kidney, or spleen, is the major source of arachidonic acid used for prostaglandin synthesis (40). A second implication is that the measurement of bulk tissue levels of 2-AG is an indirect measure of “synaptically-active” or “interstitial” 2-AG, which is most relevant for cannabinoid receptor signaling and might be more accurately measured by microdialysis (41).
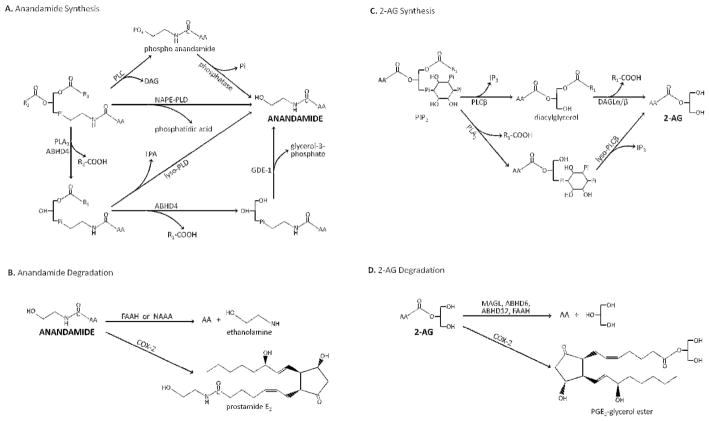
Fig. 2. Potential synthetic and degradative pathways for anandamide and 2-AG.
A. Primary synthetic pathways for anandamide. B. Primary degradative pathways for anandamide. C. Primary synthetic pathways for 2-AG. D. Primary degradative pathways for 2-AG. Only major pathways are shown. More comprehensive details can be found in recent reviews (131–133). Abbreviations: AA, arachidonic acid; ABHD4, alpha/beta domain-containing hydrolase 4; ABHD6, alpha/beta domain-containing hydrolase 6; ABHD12, alpha/beta domain-containing hydrolase 12; COX-2, cyclooxygenase-2; DAG, diacylglycerol; DAGL, diacylglycerol lipase; FAAH, fatty acid aminohydrolase; GDE-1, glycerophosphodiesterase GDE-1; IP3, inositol tris-phosphate; LPA, lyso-phosphatidic acid; lyso-PLC, lyso-phospholipid-preferring phospholipase C; MAGL, monoacyl glycerol lipase; NAAA, N-acyl ethanolamine amino hydrolase; NAPE-PLD, N-arachidonoyl phosphatidyl ethanol-preferring phospholipase D; PIP2, phosphatidyl inositol bis-phosphate; Pi, PO4; PLA2, phospholipase A2; PLC, phospholipase C;
Synthesis of anandamide has been proposed to occur by multiple pathways (Fig. 2A), presumably this varies among brain regions and different pathways may be favored for distinct physiological and pathophysiological processes. Several groups have found alterations in levels of anandamide and related acylamides in schizophrenic individuals (42–44) and this is discussed in more detail in Chapter 14. Interestingly, D2-like dopamine receptor stimulation increases anandamide levels in the striatum and CB1 receptor antagonists suppress the increased locomotion seen with a D2-like receptor agonist (45). Thus, elucidating anandamide synthetic pathways may be important for understanding the etiology of schizophrenia. Four routes for anandamide synthesis have been proposed: NAPE-PLD (46), NAPE-phospholipase C (PLC) followed by phosphatase (47), dual hydrolysis of the acyl groups by the phospholipase B, ABHD4, followed by hydrolysis by GDE1 (48), and hydrolysis of one acyl group, followed by the liberation of anandamide by the action of a lyso-NAPE-PLD (49) (Fig. 2A). Hydrolysis of NAPE by a NAPE-specific phospholipase D was the first route identified for synthesis of anandamide from cells (50). A mammalian calcium-stimulated NAPE-PLD has been cloned and characterized (51). Genetic deletion of NAPE-PLD has variable effects on anandamide levels (47, 52, 53) and NAPE-PLD distribution only partially overlaps with CB1 receptor distribution. The next best understood route of anandamide synthesis is cleavage of the NAPE phosphodiester bond by a NAPE-selective phospholipase C (PLC) followed by dephosphorylation of the resulting phospho-anandamide (47) to liberate anandamide. This pathway has been most thoroughly studied in immune cells; however it may also be present in brain (47). The remaining two synthetic pathways have been delineated in expression systems, but their roles in producing CNS anandamide remain to be elucidated (49).
The synthetic pathways for 2-AG are simpler than those for anandamide (Fig. 2C). Most 2-AG appears to be made by the sequential hydrolysis of an arachidonoyl-containing PIP2 (often 1-stearoyl-2-arachidonoyl-sn-glycerol (54, 55)) by a PLCβ followed by hydrolysis of the resulting diacylglycerol by diacylglycerol lipase (DAGL) (56). The first pathway will be engaged following the stimulation of receptors that activate PLC (e.g., receptors such group I metabotropic glutamate, M1 or M3 muscarinic, orexin A, etc.) will often lead to production of 2-AG. Two isoforms of DAGL have been found, DAGLα and DAGLβ (57). Based on knockout mice data, DAGLα appears to be the isoform responsible for most 2-AG production that is contributes to synaptic plasticity in the adult CNS (58, 59). Anatomical studies place receptors such as mGluR5 and DAGLα in close proximity to one another in dendritic spines, apposed to presynaptic CB1 receptors (60–62). While DAGLα seems the dominant 2-AG producing lipase in adult CNS, DAGLβ may contribute to synaptic 2-AG under certain conditions (63) and plays an important role in the generation of 2-AG during immune responses (64). A secondary pathway for 2-AG synthesis could be cleavage of the phosphatidyl inositol precursor by a phospholipase A, followed by hydrolysis of the phosphate ester bond by a lyso-phospholipase C, however the importance of this pathway in brain remains to be established.
Endocannabinoid degradation
Anandamide degradation in the CNS is primarily by the enzyme fatty acid amino hydrolase (FAAH) (Fig. 2B) (65). As its name suggests, FAAH degrades multiple fatty acid amides, including palmitoyl and oleoyl ethanolamide. This has important experimental and therapeutic implications as inhibition of FAAH increases levels of these ethanolamides, which have widespread actions independent of cannabinoid receptors for example, (34, 38). A second pathway for anandamide degradation is via oxidation by cyclooxygenase-2 (COX-2), to create prostamides (Fig. 2B) (66). These compounds have distinct biological actions that are independent of cannabinoid receptors, have their own unique pharmacology and have a significant role as a therapy for intraocular hypertension (66, 67). The differences in structure between arachidonic acid and anandamide are sufficient to allow the development of COX-2 inhibitors that inhibit anandamide oxidation without affecting prostaglandin formation (68). Furthermore, COX-2 is reasonably selective for anandamide over other acyl ethanolamides, so its inhibition offers a more selective way to increase anandamide when compared to inhibition of FAAH (69). A third potential route of anandamide degradation is via N-acylethanolamine-hydrolyzing acid amidase (NAAA) (Fig. 2B) (70). Inhibition of FAAH may shunt anandamide metabolism to one of these alternative pathways, altering cell functions that may be independent of cannabinoid receptor engagement.
2-AG degradation is primarily due to three hydrolytic enzymes, monoacylglycerol lipase (MGL) and alpha/beta domain hydrolases 6 and 12 (ABHD6 and 12) (Fig. 2D)(71). Additionally, 2-AG can be oxidized by COX-2 (69), and hydrolyzed under some conditions by FAAH. The first three enzymes have different subcellular localizations, which likely define degradation of 2-AG in different cellular compartments. MGL is widespread and in the adult nervous system is primarily localized in synaptic terminals (72). It appears to account for the majority of 2-AG hydrolysis in a broad survey of brain 2-AG hydrolytic activity (71). One consequence of MGL inhibition is increased 2-AG signaling at CNS CB1 receptors e.g. (73–76), however, it also reduces available levels of arachidonic acid, which is required for prostaglandin synthesis. Subsequently, prostaglandin-mediated inflammatory processes are lessened by MGL inhibition (40). In contrast to the presynaptic localization of MGL (17, 18), ABHD6 is primarily localized to dendrites and dendritic spines of excitatory neurons in cortex (77). Inhibition of ABHD6 also increases 2-AG signaling through CB1 receptors in the CNS (77, 78). ABHD6 also has significant functions outside of the CNS e.g., (79, 80), which will complicate the application of ABHD6-based therapies to CNS disorders. ABHD12 is the other major hydrolytic enzyme suggested to be involved in the hydrolysis of 2-AG in brain. While its role in 2-AG metabolism in vivo is not firmly established, it plays a significant role in the degradation of long chain lyso-phosphatidylserines (81). Intriguingly, in humans ABHD12 loss of function mutations are associated with the neurodegenerative PHARC syndrome (82) and increased levels of long chain lysophosphatidyl serine (83). COX-2 metabolism of 2-AG (Fig. 2D) in the CNS is considerable, as evidenced by increased 2-AG signaling through CB1 receptors followed by inhibition of COX-2 (84, 85). Interestingly, a major oxidative metabolite of 2-AG, PGE2-glycerol ester (PGE2-GE), potentiates synaptic transmission, enhances synaptic plasticity, and produces hyperalgesia (86–88). As 2-AG acting via CB1 receptors generally suppresses synaptic transmission and neuronal excitability (see below) and PGE2-GE is excitatory, changes in COX-2 levels or activity may have profound effects on CNS network activity.
Endocannabinoids as retrograde synaptic messengers
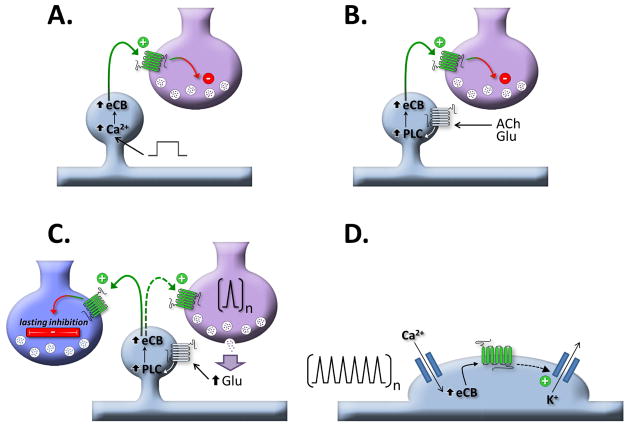
The presynaptic localization of CB1 receptors and their ability to inhibit synaptic transmission, coupled with the postsynaptic localization of some endocannabinoid synthesizing enzymes, and the observation that postsynaptic activity (specifically, increases in intracellular calcium and activation of Gq/11-linked G protein-coupled receptors) increase endocannabinoid production, suggest that endocannabinoids, particularly 2-AG, may be a retrograde messenger. This hypothesis is supported by considerable experimental evidence (reviewed by, (89)). Three basic forms on endocannabinoid-mediated synaptic plasticity involving endocannabinoids as retrograde messengers have been described (Fig. 3). These are: (1) depolarization-induced suppression of inhibition (DSI)/depolarization-induced suppression of excitation (DSE), (2) metabotropic-induced suppression of inhibition (MSI)/metabotropic-induced suppression of excitation (MSE) (also known as synaptically-evoked suppression of inhibition/excitation (SSE/SSI) (21) or endocannabinoid-mediated short term depression (eCB-STD) (89)), and (3) endocannabinoid-mediated long term depression (eCB-LTD).
Fig. 3. Forms of endocannabinoid-mediated synaptic plasticity and endocannabinoid mediated cell-autonomous regulation of excitability.
A. Depolarization-induced suppression of excitation/inhibition. B. Metabotropic-induced suppression of excitation/inhibition. C. Homosynaptic and heterosynaptic long-term depression. D. Slow self inhibition. See text for details.
DSI/DSE
Depolarization-induced suppression of inhibition (or excitation) (Fig. 3A) is found in many neurons. DSI is the transient suppression of inhibitory input onto a neuron following the strong activation (repeated action potential or a step depolarization) that last for a few tens of seconds (90, 91). DSE is precisely the same phenomenon, except excitatory inputs are affected (92). In 2001 three groups published the finding that endocannabinoids are likely the retrograde messenger for DSI and DSE in hippocampus and cerebellum (92–94). A general finding is that inhibitory synapses are more sensitive to depolarization-induced suppression of synaptic transmission than excitatory synapses (95). A great deal of work in subsequent years has investigated mechanisms and extended these results to many other neurons and brain regions, suggesting endocannabinoids (likely, 2-AG) are a major contributor to short term synaptic plasticity reviewed by (89, 96, 97).
Interestingly, tonic activation of CB1 receptors by endocannabinoids is evident at several inhibitory synapses (98, 99), which may have important functional consequences when these synapses are exposed to THC. While DSE/DSI are primarily discussed in terms of inhibition of glutamate or GABA release, it is important to keep in mind that activation of CB1 receptors can also inhibit release of peptides, such as CCK often found in CB1 receptor positive terminals (100).
MSI/MSE
Metabotropic induced suppression of inhibition (or excitation) (Fig. 3B) is a similarly ubiquitous form of endocannabinoid-mediated short-term synaptic plasticity. It occurs following the engagement of a post-synaptic Gq/11-linked GPCR and the activation of a phospholipase Cβ. The diacylglycerol produced by the phospholipase C is then deacylated by diacyl glycerol lipase to yield 2-AG, which diffuses presynaptically to activate CB1 receptors and suppress synaptic transmission. MSI and MSE are elicited by a wide number of Gq/11 coupled GPCR’s, including mGluR1, mGluR5, M1, M3, orexinA, CCKA, and α1 adrenergic receptors, among others (89). The calcium sensitivity of PLCβ1 (101) results in a synergistic interaction between depolarization- and metabotropic-induced suppressions of inhibition/excitation. Thus, these two forms of retrograde synaptic plasticity can serve as a coincidence detector of Gq/11-linked signaling and post-synaptic depolarization or calcium influx (102, 103).
LTD
Long-term depression (LTD) is a ubiquitous form of a long-lasting inhibition of synaptic strength and is elicited by multiple mechanisms. Endocannabinoids can induce both homosynaptic and heterosynaptic LTD (eLTD) (Fig. 3C). Homosynaptic eLTD is LTD at the synapse being stimulated. It is typically evoked by persistent low frequency stimulation and is prominent at glutamatergic synapse in both dorsal and ventral striatum (104, 105). Heterosynaptic eLTD occurs at synapses adjacent to the stimulated synapses. For example, stimulation of Schaffer collaterals in hippocampal CA1 leads to a persistent decrease in GABAergic inhibition of CA1 pyramidal neurons (106). The mechanism of eLTD at hippocampal inhibitory synapses appears to require inhibition of adenylyl cyclase and the involvement of the presynaptic proteins, RIM1α and RAB3B (107, 108). This is a very interesting form of metaplasticity as by removing inhibition, eLTD of inhibitory synapses increases dendritic excitability, which will potentiate excitatory transmission over a narrow spatial domain (109). eLTD appears to be involved in the maturation of cortical circuits (110), so it is tempting to speculate that its disruption by THC and related cannabinoids in cannabis might lead to subtle developmental abnormalities, which when accompanied by other environmental or genetic insults may predispose an individual to psychiatric disease. Mechanistically diverse forms of endocannabinoid-induced LTD have also been described, which either involve (111), or don’t involve (112) CB1 receptors.
SSI
In addition to inducing several forms of synaptic plasticity, endocannabinoids, particularly 2-AG, also can directly suppress neuronal excitability through a process termed slow-self inhibition (SSI) (113) (Fig. 3D). SSI is most prominent in low threshold-spiking cortical interneurons (113) and cerebellar basket cells (114), but also appears to be present in some cortical principal cells (115). The mechanism of SSI appears to involve synthesis of 2-AG during intense stimulation of the neuron, activation of somatic CB1 receptors, and activation of a somatic potassium conductance, likely an inwardly rectifying potassium channel (116).
Interactions between THC and endocannabinoids
The predicted and observed interactions between THC and the endocannabinoids with CB1 receptors are potentially complex and deserve additional consideration. Both THC and anandamide are low efficacy agonists e.g., (2). Under conditions of either low receptor density or limiting post-receptor effectors (117), they may antagonize CB1 receptor signaling elicited by 2-AG. Indeed, this has been observed in several systems (111, 118, 119). However, in other systems THC (and anandamide) acts as an efficacious CB1 receptor agonist (120, 121). So, what is going on in the brain of a person imbibing in cannabis? Evidence that acute responses to cannabis involves both agonism and antagonism of CB1 receptor signaling is the observation that even repeated, very high doses of the CB1 receptor antagonist, rimonabant, modestly attenuated the subjective measures of “high,” while substantially suppressing the tachycardia induced by cannabis (122). This contrasts to the rapid reversal of the subjective effects of morphine following the administration of naloxone (123). Similarly, oral rimonabant did not elicit a precipitated withdrawal syndrome in humans taking moderate doses of THC in a supervised environment (124). However, following chronic high dose THC in rodents, rimonabant elicits a robust withdrawal syndrome (125). These two observations may be reconciled by noting that the THC’s low efficacy, coupled with the sparse receptor occupancy likely attained in casual human cannabis use, compared to what can be achieved in experimental models, in the clinical setting (126, 127), or with cannabis strains of high THC content (128, 129), may result in milder acute effects in population studies. Finally, the use of highly potent, highly efficacious cannabinoid receptors agonists typically present in synthetic marijuana preparations (“spice”) results in a greater incidence of adverse psychiatric effects, that may be attributable to their higher intrinsic efficacy (130). In summary, the interactions of THC, CB1, and the endocannabinoids are more complex than THC simply “hijacking” CB1 receptors as another agonist and need to be carefully considered.
Summary
An involvement of the endocannabinoid system with schizophrenia is supported both by the epidemiological observation that increased cannabis use is associated with a heightened risk for schizophrenia and that acute consumption of cannabis or synthetic cannabinoids can elicit psychotic symptoms in susceptible individuals. It is likely that the former observation has its basis in cannabis interfering with the neurodevelopmental roles of endocannabinoids, while the latter observation is due to interactions between THC in cannabis with ongoing endocannabinoid-mediated synaptic plasticity. Focusing on the latter, several challenges remain: 1. What is the role and mechanism by which cannabidiol attenuates the acute effects of THC? 2. Will the synthetic cannabinoids found in “spice” preparations produce more severe psychotic symptoms? 3. What are the roles of CB1 receptors on non-neuronal CNS cells (oligodendrocytes, astrocytes, and microglia) in mediating the acute effects of cannabis? 4. What is the role and mechanism underlying the relationship between CB2 and schizophrenia? 5. Is there a physiological basis for the observation that many schizophrenic patients regularly use cannabis? 6. Will manipulations of the endocannabinoid system by therapeutically beneficial in schizophrenia? The answers to these questions will greatly enhance our understanding of the relationship between cannabis, the endocannabinoid system and schizophrenia.
Acknowledgments
Supported by NS086794 (HCL), NS048884 (HCL), DA021696 (KM), and DA011322 (KM).
Footnotes
Conflict of interest
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57:1045–1050. [PubMed] [Google Scholar]
- 2.Luk T, Jin W, Zvonok A, Lu D, Lin XZ, Chavkin C, et al. Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist. Br J Pharmacol. 2004;142:495–500. doi: 10.1038/sj.bjp.0705792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- 4.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 5.Bae JS, Kim JY, Park BL, Kim JH, Kim B, Park CS, et al. Genetic association analysis of CNR1 and CNR2 polymorphisms with schizophrenia in a Korean population. Psychiatr Genet. 2014;24:225–229. doi: 10.1097/YPG.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 6.Seifert J, Ossege S, Emrich HM, Schneider U, Stuhrmann M. No association of CNR1 gene variations with susceptibility to schizophrenia. Neurosci Lett. 2007;426:29–33. doi: 10.1016/j.neulet.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Ujike H, Takaki M, Nakata K, Tanaka Y, Takeda T, Kodama M, et al. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol Psychiatry. 2002;7:515–518. doi: 10.1038/sj.mp.4001029. [DOI] [PubMed] [Google Scholar]
- 8.Norrod AG, Puffenbarger RA. Genetic polymorphisms of the endocannabinoid system. Chem Biodivers. 2007;4:1926–1932. doi: 10.1002/cbdv.200790160. [DOI] [PubMed] [Google Scholar]
- 9.Hamdani N, Tabeze JP, Ramoz N, Ades J, Hamon M, Sarfati Y, et al. The CNR1 gene as a pharmacogenetic factor for antipsychotics rather than a susceptibility gene for schizophrenia. Eur Neuropsychopharmacol. 2008;18:34–40. doi: 10.1016/j.euroneuro.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- 11.Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136:811–822. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 13.Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 16.Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matyas F, Watanabe M, Mackie K, Katona I, Freund TF. Molecular architecture of the cannabinoid signaling system in the core of the nucleus accumbens. Ideggyogy Sz. 2007;60:187–191. [PubMed] [Google Scholar]
- 19.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 20.Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, et al. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509:400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- 21.Safo PK, Cravatt BF, Regehr WG. Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum. 2006;5:134–145. doi: 10.1080/14734220600791477. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, et al. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 28.Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiguro H, Horiuchi Y, Ishikawa M, Koga M, Imai K, Suzuki Y, et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry. 2010;67:974–982. doi: 10.1016/j.biopsych.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Khella R, Short JL, Malone DT. CB2 receptor agonism reverses MK-801-induced disruptions of prepulse inhibition in mice. Psychopharmacology (Berl) 2014;231:3071–3087. doi: 10.1007/s00213-014-3481-x. [DOI] [PubMed] [Google Scholar]
- 32.Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36:1489–1504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- 34.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 35.Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 38.Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, et al. Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors. Addict Biol. 2010;15:277–288. doi: 10.1111/j.1369-1600.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- 43.Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- 44.Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry. 2009;194:371–372. doi: 10.1192/bjp.bp.108.053843. [DOI] [PubMed] [Google Scholar]
- 45.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 46.Schmid PC, Reddy PV, Natarajan V, Schmid HH. Metabolism of N-acylethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J Biol Chem. 1983;258:9302–9306. [PubMed] [Google Scholar]
- 47.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol Biosyst. 2010;6:1411–1418. doi: 10.1039/c000237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuboi K, Ikematsu N, Uyama T, Deutsch DG, Tokumura A, Ueda N. Biosynthetic pathways of bioactive N-acylethanolamines in brain. CNS Neurol Disord Drug Targets. 2013;12:7–16. doi: 10.2174/1871527311312010005. [DOI] [PubMed] [Google Scholar]
- 50.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 51.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 52.Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsuboi K, Okamoto Y, Ikematsu N, Inoue M, Shimizu Y, Uyama T, et al. Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Biochim Biophys Acta. 2011;1811:565–577. doi: 10.1016/j.bbalip.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Shonesy BC, Winder DG, Patel S, Colbran RJ. The initiation of synaptic 2-AG mobilization requires both an increased supply of diacylglycerol precursor and increased postsynaptic calcium. Neuropharmacology. 2015;91:57–62. doi: 10.1016/j.neuropharm.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–621. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- 56.Murataeva N, Straiker A, Mackie K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol. 2014;171:1379–1391. doi: 10.1111/bph.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 63.Jain T, Wager-Miller J, Mackie K, Straiker A. Diacylglycerol lipasealpha (DAGLalpha) and DAGLbeta cooperatively regulate the production of 2-arachidonoyl glycerol in autaptic hippocampal neurons. Mol Pharmacol. 2013;84:296–302. doi: 10.1124/mol.113.085217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu KL, Tsuboi K, Adibekian A, Pugh H, Masuda K, Cravatt BF. DAGLbeta inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol. 2012;8:999–1007. doi: 10.1038/nchembio.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 66.Woodward DF, Liang Y, Krauss AH. Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br J Pharmacol. 2008;153:410–419. doi: 10.1038/sj.bjp.0707434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urquhart P, Nicolaou A, Woodward DF. Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Biochim Biophys Acta. 2015;1851:366–376. doi: 10.1016/j.bbalip.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 68.Hermanson DJ, Hartley ND, Gamble-George J, Brown N, Shonesy BC, Kingsley PJ, et al. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat Neurosci. 2013;16:1291–1298. doi: 10.1038/nn.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci. 2014;35:358–367. doi: 10.1016/j.tips.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- 71.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ludanyi A, Hu SS, Yamazaki M, Tanimura A, Piomelli D, Watanabe M, et al. Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience. 2011;174:50–63. doi: 10.1016/j.neuroscience.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, et al. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014;171:1392–1407. doi: 10.1111/bph.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, et al. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 2013;345:492–501. doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2011;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naydenov AV, Horne EA, Cheah CS, Swinney K, Hsu KL, Cao JK, et al. ABHD6 blockade exerts antiepileptic activity in PTZ-induced seizures and in spontaneous seizures in R6/2 mice. Neuron. 2014;83:361–371. doi: 10.1016/j.neuron.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomas G, Betters JL, Lord CC, Brown AL, Marshall S, Ferguson D, et al. The serine hydrolase ABHD6 Is a critical regulator of the metabolic syndrome. Cell Rep. 2013;5:508–520. doi: 10.1016/j.celrep.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oparina NY, Delgado-Vega AM, Martinez-Bueno M, Magro-Checa C, Fernandez C, Castro RO, et al. PXK locus in systemic lupus erythematosus: fine mapping and functional analysis reveals novel susceptibility gene ABHD6. Ann Rheum Dis. 2015;74:e14. doi: 10.1136/annrheumdis-2013-204909. [DOI] [PubMed] [Google Scholar]
- 81.Blankman JL, Long JZ, Trauger SA, Siuzdak G, Cravatt BF. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc Natl Acad Sci U S A. 2013;110:1500–1505. doi: 10.1073/pnas.1217121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fiskerstrand T, H’Mida-Ben Brahim D, Johansson S, M’Zahem A, Haukanes BI, Drouot N, et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: An inborn error of endocannabinoid metabolism. Am J Hum Genet. 2010;87:410–417. doi: 10.1016/j.ajhg.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamat SS, Camara K, Parsons WH, Chen DH, Dix MM, Bird TD, et al. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat Chem Biol. 2015;11:164–171. doi: 10.1038/nchembio.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- 85.Straiker A, Wager-Miller J, Hu SS, Blankman JL, Cravatt BF, Mackie K. COX-2 and fatty acid amide hydrolase can regulate the time course of depolarization-induced suppression of excitation. Br J Pharmacol. 2011;164:1672–1683. doi: 10.1111/j.1476-5381.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sang N, Zhang J, Chen C. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. J Physiol. 2006;572:735–745. doi: 10.1113/jphysiol.2006.105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang H, Zhang J, Andreasson K, Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol Cell Neurosci. 2008;37:682–695. doi: 10.1016/j.mcn.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu SS, Bradshaw HB, Chen JS, Tan B, Walker JM. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. Br J Pharmacol. 2008;153:1538–1549. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 90.Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vincent P, Armstrong CM, Marty A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J Physiol. 1992;456:453–471. doi: 10.1113/jphysiol.1992.sp019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 93.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 94.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 95.Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alger BE. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001) what we still do not know. J Physiol. 2012;590:2203–2212. doi: 10.1113/jphysiol.2011.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beinfeld MC, Connolly K. Activation of CB1 cannabinoid receptors in rat hippocampal slices inhibits potassium-evoked cholecystokinin release, a possible mechanism contributing to the spatial memory defects produced by cannabinoids. Neurosci Lett. 2001;301:69–71. doi: 10.1016/s0304-3940(01)01591-9. [DOI] [PubMed] [Google Scholar]
- 101.Taylor SJ, Chae HZ, Rhee SG, Exton JH. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991;350:516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- 102.Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, et al. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 105.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 107.Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsetsenis T, Younts TJ, Chiu CQ, Kaeser PS, Castillo PE, Sudhof TC. Rab3B protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc Natl Acad Sci U S A. 2011;108:14300–14305. doi: 10.1073/pnas.1112237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 110.Jiang B, Huang S, de Pasquale R, Millman D, Song L, Lee HK, et al. The maturation of GABAergic transmission in visual cortex requires endocannabinoid-mediated LTD of inhibitory inputs during a critical period. Neuron. 2010;66:248–259. doi: 10.1016/j.neuron.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kellogg R, Mackie K, Straiker A. Cannabinoid CB1 receptor-dependent long-term depression in autaptic excitatory neurons. J Neurophysiol. 2009;102:1160–1171. doi: 10.1152/jn.00266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- 114.Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 115.Marinelli S, Pacioni S, Cannich A, Marsicano G, Bacci A. Selfmodulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12:1488–1490. doi: 10.1038/nn.2430. [DOI] [PubMed] [Google Scholar]
- 116.Marinelli S, Pacioni S, Bisogno T, Di Marzo V, Prince DA, Huguenard JR, et al. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J Neurosci. 2008;28:13532–13541. doi: 10.1523/JNEUROSCI.0847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kenakin T. Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov. 2002;1:103–110. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- 118.Kelley BG, Thayer SA. Delta 9-tetrahydrocannabinol antagonizes endocannabinoid modulation of synaptic transmission between hippocampal neurons in culture. Neuropharmacology. 2004;46:709–715. doi: 10.1016/j.neuropharm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 119.Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laaris N, Good CH, Lupica CR. Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology. 2010;59:121–127. doi: 10.1016/j.neuropharm.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Evans JM, Hogg MI, Lunn JN, Rosen M. Degree and duration of reversal by naloxone of effects of morphine in conscious subjects. Br Med J. 1974;2:589–591. doi: 10.1136/bmj.2.5919.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gorelick DA, Goodwin RS, Schwilke E, Schwope DM, Darwin WD, Kelly DL, et al. Antagonist-elicited cannabis withdrawal in humans. J Clin Psychopharmacol. 2011;31:603–612. doi: 10.1097/JCP.0b013e31822befc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsou K, Patrick SL, Walker JM. Physical withdrawal in rats tolerant to delta 9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur J Pharmacol. 1995;280:R13–15. doi: 10.1016/0014-2999(95)00360-w. [DOI] [PubMed] [Google Scholar]
- 126.Morrison PD, Zois V, McKeown DA, Lee TD, Holt DW, Powell JF, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–1616. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- 127.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 128.Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488–491. doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Amsterdam J, Brunt T, van den Brink W. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J Psychopharmacol. 2015 doi: 10.1177/0269881114565142. [DOI] [PubMed] [Google Scholar]
- 131.Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ueda N, Tsuboi K, Uyama T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA) Prog Lipid Res. 2010;49:299–315. doi: 10.1016/j.plipres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 133.Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]