Abstract
Background
The ability to exert self-control over temptation is a fundamental component of smoking behavior change. Transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC) has been shown to modulate cognitive control circuits. Although prior studies show that stimulation reduces cigarette craving and self-reported smoking, effects on ability to resist smoking have not been investigated directly.
Objectives
We assessed effects of a single 20-minute session of 1.0 mA anodal stimulation over the left DLPFC with cathodal stimulation over the right supra-orbital area (vs. sham stimulation) on ability to resist smoking in a validated smoking lapse paradigm.
Methods
Twenty-five participants completed two tDCS sessions (active and sham stimulation) in a within-subject, double-blind, randomized and counterbalanced order with a two-week washout period. Following overnight abstinence, participants received tDCS in the presence of smoking related cues; they had the option to smoke at any time or receive $1 for every 5 minutes they abstained. After 50 minutes, they participated in a one hour ad libitum smoking session. Primary and secondary outcomes were time to first cigarette and cigarette consumption, respectively.
Results
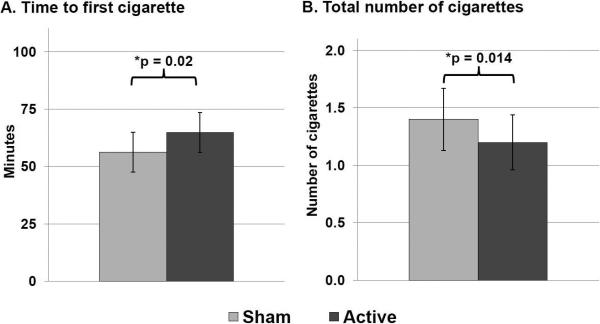
In multiple regression models, active tDCS (compared to sham) significantly increased latency to smoke (p = 0.02) and decreased the total number of cigarettes smoked (p = 0.014) during the session.
Conclusion
These findings suggest that acute anodal stimulation over the left DLPFC (with cathodal stimulation over the right supra-orbital area) can improve ability to resist smoking, supporting the therapeutic potential of tDCS for smoking cessation treatment.
Keywords: tDCS, nicotine addiction, smoking relapse
Introduction
Tobacco use accounts for over six million deaths each year worldwide [1] and takes a significant economic toll [1, 2]. Even with the best treatments available, most people revert to their former smoking practices [3]. While currently available treatments may address symptoms of nicotine withdrawal or alter the reinforcing effects of nicotine, they do not address the disruptive brain processes that may undermine attempts to quit smoking [4].
Abstinence from smoking produces cognitive impairments and altered brain functions that can make it more difficult to resist temptations to smoke (e.g., smoking cessation) [5]. For example, working memory performance and associated neural activity in the dorsolateral prefrontal cortices (DLPFC) are downregulated during nicotine withdrawal, compared to smoking satiety [6-9]. Reductions in working memory-related DLPFC activity predict smoking relapse above and beyond clinical and performance measures, with 81% accuracy [10]. The DLPFC is at the core of the brain's cognitive control network which supports behavioral self-control [11-14], suggesting that targeting DLPFC functions may be a therapeutic strategy to support smoking behavior change.
Emerging evidence shows that activity in the DLPFC and cognitive control circuits can be modulated using a noninvasive and safe intervention: direct current transcranial stimulation (tDCS) [15-17]. Active anodal tDCS administered to the DLPFC has been shown to increase task-related activity in the DLPFC during a risk sensitivity task, and to increase resting state functional connectivity in the attention network [18, 19]. In smokers, tDCS has been shown to reduce smoking cue-induced craving and self-reported cigarette intake [20-22]. However, these studies examined minimally-abstinent (1.5 hrs or less) smokers. Another study found that active tDCS versus sham reduced negative affect but not cigarette craving in smokers after overnight abstinence [23], but this study did not examine subsequent smoking behavior.
Given the relevance of DLPFC activity for smoking relapse, we tested the hypothesis that anodal tDCS over the left DLPFC (with cathodal stimulation over the right supra-orbital area) after overnight abstinence will increase ability to resist temptations to smoke. This preliminary study employed a within-subject crossover design to compare active versus sham stimulation, such that each participant served as his/her own control, and utilized a validated smoking lapse paradigm that is sensitive to the effects of efficacious smoking treatments [24, 25]. We predicted that active stimulation (versus sham) would increase latency to first cigarette and decrease the total number of cigarettes smoked during the session.
Materials and Methods
Participants
All procedures were approved by the University of Pennsylvania Institutional Review Board and carried out in accordance with the Declaration of Helsinki. Healthy adults between the ages of 18 and 60 who reported smoking at least 10 cigarettes per day (CPD) for the past year and were not planning to quit smoking in the next 3 months were recruited through mass media. All participants provided written informed consent and completed an in-person eligibility screen including a urine drug screen, a breath alcohol test, and an expired breath carbon monoxide (CO) reading to confirm smoking status; women completed a urine pregnancy test. Individuals who self-reported a history of DSM-IV Axis I psychiatric or substance disorders (except nicotine dependence) and those taking psychotropic medications were excluded. Additional exclusion criteria included: current use of chewing tobacco, snuff, or smoking cessation products; pregnancy, planned pregnancy or breastfeeding; history of brain injury or seizures; presence of metal (other than dental apparatus) in the face or head; low or borderline intelligence (estimated IQ <90 on Shipley Institute of Living Scale [26]); and any impairment that would prevent task performance.
Procedures
Participants in this within-subject cross-over investigation completed two identical laboratory sessions (one session involving active stimulation and the other involving sham stimulation) scheduled at least two weeks apart to minimize carryover effects. Session order was double-blind, randomized and counterbalanced. Sessions started at approximately 9am (+/− 1 hr) after overnight abstinence (~12 hours). Participants provided an expired CO reading at the start of the session which was required to be <10ppm (or at least a 50% reduction from the reading obtained at the eligibility screen) in order to confirm compliance with the abstinence requirement. Following confirmation of eligibility, participants completed the Questionnaire on Smoking Urges – brief version (QSU-B; [27]) and then received 20 minutes of active or sham stimulation during a laboratory smoking lapse paradigm.
Smoking lapse paradigm
The smoking lapse paradigm was based on that designed by McKee & colleagues [24] and adapted for co-administration of tDCS. Participants were escorted to a 10 × 10 room equipped with industrial grade exhaust fans approved for exhaust of cigarette smoke, a comfortable couch, a coffee table, and a portable smoking topography unit (Clinical Research Smoking Systems, Plowshare Technologies/Borgwaldt, Baltimore, MD). A pack of cigarettes of the participant's preferred brand was placed in view on the table along with a lighter and ashtray. Prior to initiating tDCS, participants were instructed that over the next 50 minutes they should try not to smoke. Participants could choose to start smoking at any time, but for each 5 minute period that they were able to delay, they would earn $1.00 (up to a maximum of $10; [24]). Once they decided to smoke, they could smoke as little or as much as they wished through the smoking topography device. Participants were permitted to read books or magazines but were asked to refrain from using smartphones or other electronic devices. Once instructions were delivered, tDCS commenced. The tDCS equipment remained in place until the end of the 50 minute resist period to avoid influencing the participant's smoking behavior; however, stimulation was delivered only for the first 20 minutes. Participants remained in the smoking room for the full 50 minutes, regardless of when they decided to smoke, so that all participants would begin the subsequent ad libitum period at the same time relative to tDCS administration (30 minutes post-stimulation). Time to first cigarette (latency to smoke) and total number of cigarettes smoked were recorded electronically via the smoking topography device.
After the 50 minute resist period ended, the tDCS equipment was removed and participants were given new instructions for a 60 minute ad libitum smoking period. During this period, participants were given eight cigarettes of their preferred brand and informed that they had a $4.00 “tab” with the researchers. Participants were instructed to smoke as much or as little as they wished through the smoking topography device, but that for each cigarette lit, they would “lose $0.50 from their $4.00 tab.” Participants received any money left in the tab at the end of the session. Self-reported smoking behavior (number of cigarettes smoked) for the 24 hours following the tDCS session was assessed using a timeline follow-back method during a follow-up call the next day.
tDCS procedures
A Magstim Eldith 1 Channel DC Stimulator Plus was used to apply a constant direct current via two 5cm × 5cm electrodes covered in saline-soaked sponges. Electrode placement was determined using the international 10-20 system developed for EEG [28]; the anodal electrode was placed over the F3 area for stimulation over the left DLPFC and the cathode was placed over the right supra-orbital area. During the active condition, current was ramped up to 1.0 mA over 30s, maintained for 19 minutes and ramped down over 30s (total stimulation period 20 min). During the sham condition, the current was ramped up to 1.0 mA over 30s and immediately ramped down at the beginning and end of the 20 minute period. This process mimics the skin sensations experienced during active stimulation; most participants cannot distinguish between real and sham tDCS using this procedure [29].
Measures
Outcomes
The primary outcome was latency to smoke (in minutes) and the secondary outcome was total number of cigarettes smoked during the resist and ad libitum periods. Latency to smoke was set to 110 minutes if a participant did not smoke during either period (i.e. the combined duration of the abstinence period plus the ad libitum smoking period). As exploratory measures, we examined smoking topography (total puff volume) and self-reported smoking behavior for the 24 hours following the tDCS session. Total puff volume was set to zero if a participant did not smoke during either period.
Baseline measures and covariates
Nicotine dependence was assessed at the eligibility session using the Fagerström Test for Nicotine Dependence (FTND, [30]). Pre-stimulation breath CO was obtained at each tDCS session to confirm compliance with the overnight abstinence requirement. Smoking urges (QSU-B) were assessed prior to stimulation [27], but were not assessed as an outcome measure due to potential confounding based on whether or not the participant smoked during the session.
Treatment-related measures
Side effects of tDCS and blinding success were assessed using a questionnaire [31]. Participants were asked to rate to what extent they experienced 10 potential side effects of tDCS during and after stimulation (headache, difficulty concentrating, acute mood changes, changes in visual perception, tingling, itching, burning, pain, fatigue, and nervousness), plus visual sensation at the start or end of stimulation, on a scale from 0 (not at all) to 10 (to a high degree). Participants were also asked to guess at which session they had received active stimulation and which session involved sham stimulation. Because we included this question, the questionnaire was administered only at the end of the second session to avoid drawing attention to the difference and influencing future sessions.
Analysis
Descriptive statistics were obtained for all variables. Effects of stimulation on the smoking behavior outcomes were modeled using regression with subject-level random effects, and estimated using maximum likelihood techniques (Stata xt-reg; Stata Corporation, College Station, TX, USA) with linear mixed effects models. Pre-session craving for negative reinforcement (QSU-B Factor 2) and FTND score were included as covariates in the multiple regression models based on prior associations with latency to smoke and number of cigarettes smoked in the smoking lapse paradigm [24, 32]; session order (active first vs. sham first) was also included as a covariate. Session order by stimulation interaction effects, age, and sex were tested but allowed to drop from the model if non-significant. We used an adjusted alpha of p<0.025 to correct for testing the two primary outcome measures.
Results
Descriptive Data
Twenty-eight subjects completed session 1. One subject withdrew from the study prior to session 2 and two were ineligible at session 2 due to non-compliance with the abstinence requirement, leaving 25 subjects included in the analysis. The sample was predominantly male (n = 15, 60%) and African-American (n = 16, 64%); most had completed at least some college or beyond (n = 14, 56%). The mean age was 42.1 years (SD 11.2), the mean cigarettes per day was 15.2 (SD 4.4), mean expired CO at the eligibility screen was 19.1ppm (SD 5.9), and the mean FTND score was 5.4 (SD 1.9).
Expired CO readings at the start of the tDCS sessions were significantly reduced compared to the eligibility screen (p < 0.0001) indicating compliance with the abstinence requirement. Participants reported abstaining from cigarettes for a mean of 12.6 hrs (range: 11-16 hrs). Expired CO and pre-stimulation smoking urges did not differ between active and sham sessions (p > 0.1), which is expected because the same abstinence requirements were used for both sessions.
Outcome Data
In the multiple regression models, active stimulation significantly increased latency to smoke [mean time to first cigarette: 56.2 min (SD 43.1) during the sham session and 64.8 min (SD 43.5) during the active session; β = 9.6, 95% Confidence Interval (CI) 1.5 to 17.7, p = 0.02]. Active stimulation also significantly decreased the total number of cigarettes smoked during the session [mean total cigarettes smoked: 1.44 (SD 1.3) during the sham session and 1.20 (SD 1.2) during active session; β = −0.27, 95% CI −0.49 to −0.06, p = 0.014; Figure 1]. Nicotine dependence (FTND score) was negatively associated with latency to smoke (β = −10.9, p = 0.003) and positively associated with total number of cigarettes smoked (β = 0.26, p = 0.02). Urge to smoke for negative reinforcement (QSU-B Factor 2) was positively associated with total number of cigarettes smoked (β = 0.05, p = 0.03) but not with latency to smoke (p = 0.06). There was also a trend toward decreased total puff volume during the active session compared to sham which did not reach significance (p = 0.07). There was no effect of stimulation on self-reported number of cigarettes smoked in the 24 hours following the tDCS session (p > 0.8). Age, sex, and session order were not significantly associated with any of the outcomes (ps > 0.05) and there were no stimulation by session order interaction effects (ps > 0.4).
Figure 1. Effects of tDCS on smoking behavior.
Latency to smoke and total number of cigarettes smoked in each session (sham vs. active stimulation); error bars are standard error of the mean. In the multiple regression models, active stimulation significantly increased latency to smoke (β = 9.6, 95% Confidence Interval (CI) 1.5 to 17.7, p = 0.02) and decreased the number of cigarettes consumed during the session (β = −0.27, 95% CI −0.49 to −0.06, p = 0.014).
tDCS Side Effects and Blinding
The most commonly reported side effect of stimulation was tingling at the site of the electrode (reported by 92% of participants), followed by itching (80%), burning sensation (64%), fatigue (56%), nervousness (48%), difficulty concentrating (48%), mood change (44%), pain (36%), headache (36%), and visual sensation at the start or end of stimulation (24%). Side effects were generally mild (mean rating <5 out of 10) except for tingling (mean rating 6.0, SD 2.9) and itching (mean rating 5.0, SD 3.4). Because we recorded side effects only at the end of the second session, we could not assess whether side effects differed for active compared to sham stimulation. Participants were not able to correctly identify active stimulation versus sham at a rate significantly greater than chance (64% correct, one sample binomial test p > 0.1), indicating successful blinding to stimulation type.
Discussion
This study is the first to demonstrate a significant effect of active anodal tDCS over the left DLPFC (with cathodal stimulation over the right supra-orbital area) on ability to resist smoking in the presence of in vivo smoking cues. Smokers in this within-subject cross-over study were able to delay smoking for an average of ~9 minutes longer when they received active stimulation compared to when they received sham stimulation. Active stimulation also reduced the total amount of cigarettes smoked by about a quarter of a cigarette, an approximately 17% reduction in cigarette intake during the two-hour laboratory session.
Although increasing latency to smoke by ~9 minutes may appear to be a small improvement, the ability to delay smoking for a short time might allow smokers to wait out acute urges which are typically of limited duration [33-35]. Although further research is necessary to examine whether the effects of acute tDCS on smoking behavior are persistent, a reduction of one-fourth of a cigarette every 2 hours could potentially translate into a reduction in daily smoking of 2 cigarettes over a 16 hour waking period. In a clinical trial examining the effects of nicotine replacement therapy and motivational advice in smokers unmotivated to quit, Carpenter and colleagues [36] found that every 1% reduction in daily cigarettes smoked over six weeks increased the odds of making a quit attempt by 2% and of achieving abstinence at 24 weeks by 3%, which suggests that even a small reduction in intake may be clinically relevant. Although we did not see an effect of stimulation on self-reported smoking quantity in the 24 hours following the session, smokers in this study were not currently interested in quitting and were not instructed to attempt to reduce their smoking after the session. The effects of stimulation on smoking behavior during the laboratory session, when smokers were actively attempting to resist smoking, are promising.
The smoking paradigm utilized in this study was adapted from a validated smoking lapse paradigm which has been shown to be sensitive to identifying treatments known to be efficacious for smoking cessation [24]. However, the effect sizes in the current study are smaller than the effects previously noted for the smoking cessation medications varenicline and bupropion in this paradigm. Following administration to steady state, these efficacious smoking cessation medications were shown to increase latency to smoke by approximately 20 minutes and number of cigarettes smoked by about half, but only among highly dependent smokers (those who report smoking within five minutes of waking; [24]). The number of participants in our sample who reported smoking within five minutes of waking was too small (n = 4) to test for an interaction effect; however, the effects of a single acute dose of active tDCS were observed in the full sample and were not restricted to highly dependent smokers.
Our findings add to literature reports of overall reductions in daily cigarette intake during multi-day treatment periods involving daily sessions of active anodal stimulation over the left or right DLPFC or other frontal regions [20, 22, 37]. These studies reported that daily cigarette consumption was approximately 30% lower during the active stimulation period compared to the sham period, which is slightly greater than the reduction in intake noted during our laboratory study. This difference may be due to the extended treatment period (five daily sessions versus a single acute session), differences in tDCS dosage (2.0 mA versus 1.0 mA in the current study), or placement of the cathodal electrode (over the right DLPFC compared to over the supra-orbital area in the current study). Some studies have also demonstrated an acute effect of active anodal stimulation over the left or right DLPFC on cue-induced craving for cigarettes [20-22], although another study reported no effect of anodal stimulation of the left DLPFC on cue-induced craving following overnight abstinence [23]. Although our participants were exposed to cigarette cues during the smoking lapse paradigm, we did not include craving as an outcome measure due to the fact that post-session craving levels would be confounded by the participant's decision to smoke or not smoke during the lapse paradigm.
An interesting feature of the smoking lapse paradigm is its manipulation of reward: first, the paradigm offers money as an alternative reward for smokers who successfully resist smoking; and second, the two periods (resist and ad libitum) utilize different approaches to reward. During the resist period, the monetary reward is presented as a positive reinforcer; smokers can earn $1 for every five minutes they resist smoking. During the ad libitum period, the monetary reward is a negative reinforcer; smokers are told that they have potential earnings of $4, but that they will lose $0.50 from this total for every cigarette they smoke [24]. Prior work has demonstrated that tDCS effects may be reward-dependent; for example, Fecteau and colleagues found that active stimulation over the DLPFC increased the likelihood that smokers would reject offers of cigarettes during an Ultimatum game, but had no effect on the frequency with which they rejected offers of money [20]. Additional research employing variations of the smoking lapse paradigm may be useful to evaluate whether the effects of tDCS on smoking behavior are sensitive to the nature or presentation of alternative rewards.
Non-invasive brain stimulation with tDCS has been investigated as a potential treatment for addiction and obesity, two conditions which share similar features such as craving, compulsive behavior, and impaired decision-making capability [38, 39]. A recent meta-analysis of studies utilizing tDCS or repetitive transcranial magnetic stimulation (rTMS) found that stimulation of the DLPFC reduced craving for nicotine, alcohol, and marijuana in addicted individuals, and reduced craving for food in subjects who normally experienced strong food cravings [40]. It has been suggested that stimulation of the DLPFC may alter smoking behavior and substance use by modulating cognitive control circuits involved in decision-making behavior and regulation of craving [20-22, 41]. Another study found that tDCS targeting other fronto-parietal regions involved in cognitive control also reduced cigarette consumption [37]. Modulation of these circuits may help to suppress craving or reduce risk-seeking behavior by enhancing executive function, which contributes to improved control over impulsive, reward-motivated behaviors [20, 38-40, 42].
Studies of tDCS effects on cognitive function have proposed that achieving optimal benefits may require co-administration of a training task designed to engage the desired function (i.e., working memory is enhanced when tDCS is co-administered with a working memory task such as the n-back; [43, 44]). In the present study, we considered the instruction to “try to resist smoking” to be a task engaging the cognitive control circuit. Supporting this decision are neuroimaging studies which have shown that resisting cigarette cue-induced craving is associated with greater activation in parts of the attention and decision-making networks including the prefrontal cortex [45, 46]. Some participants did actively engage in this task, as demonstrated by comments recorded during a post-session thought-listing task (i.e., “I was thinking about smoking cigarettes but didn't want to give in to the temptation”; “I was determined not to smoke”; etc.). However, some participants chose to “give in” and smoke during the 20-minute tDCS administration period. Therefore, it could be argued that the training task was not standardized across all participants; some participants may in fact have engaged in “training” the very behavior they were instructed to avoid (i.e., smoking). Utilization of a standardized training task such as a response inhibition task to engage the cognitive control circuit during tDCS might strengthen the results. Furthermore, nicotine withdrawal has been shown to reduce tDCS-induced neural plasticity, and acute administration of nicotine reverses this effect [47]; however, it is not known whether re-introduction of nicotine during a withdrawal state (as seen in participants who smoked during stimulation) might influence the effects of stimulation on the ability to resist subsequent smoking. Although this would not have affected our primary outcome of latency to smoke, future studies could explore the effects of nicotine intake during tDCS on subsequent smoking behavior.
Strengths of our study include the use of a validated laboratory model of smoking lapse with direct observation of smoking behavior and the within-subject crossover design which allowed each participant to serve as his/her own control. Limitations include the use of a single acute dose of tDCS and the short observation period. Further research is necessary to investigate whether the effects observed here are persistent over time, may be enhanced with multiple tDCS sessions, or may benefit from a standardized task to engage cognitive control circuits during stimulation. Furthermore, future studies may evaluate treatment-seeking smokers since they may respond differently than non-treatment-seekers in medication and cessation paradigms [48]. However, our findings that active anodal tDCS over the left DLPFC with cathodal tDCS over the right supra-orbital area increased latency to smoke and decreased cigarette consumption in a validated model of smoking lapse suggests that tDCS may offer promise as a novel treatment for smoking cessation.
Highlights.
We examine effects of acute brain stimulation in a validated smoking lapse paradigm.
Active anodal tDCS over left DLPFC vs. sham stimulation increased latency to smoke.
Active tDCS (vs. sham) decreased the total number of cigarettes smoked.
Acute tDCS over the left DLPFC shows promise for smoking cessation treatment.
Acknowledgements
This research was supported by a grant from the National Institutes of Health (1-R35-CA197461 to C.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could aect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization . WHO report on the global tobacco epidemic, 2013. Geneva, Switzerland: 2013. [Google Scholar]
- 2.Centers for Disease Control and Prevention Smoking attributable mortality, years of potential life lost, and productivity losses. MMWR. 2008;57:1226–28. [PubMed] [Google Scholar]
- 3.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol. 2013;48:1–19. doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014;76 Pt B:581–91. doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falcone M, Wileyto EP, Ruparel K, Gerraty RT, LaPrate L, Detre JA, et al. Age-related differences in working memory deficits during nicotine withdrawal. Addict Biol. 2014;19:907–17. doi: 10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loughead J, Ray R, Wileyto EP, Ruparel K, O'Donnell GP, Senecal N, et al. Brain activity and emotional processing in smokers treated with varenicline. Addict Biol. 2013;18:732–8. doi: 10.1111/j.1369-1600.2011.00324.x. [DOI] [PubMed] [Google Scholar]
- 8.Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, et al. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–21. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–9. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, et al. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40:1311–20. doi: 10.1038/npp.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 12.Kool W, McGuire JT, Wang GJ, Botvinick MM. Neural and behavioral evidence for an intrinsic cost of self-control. PLoS One. 2013;8:e72626. doi: 10.1371/journal.pone.0072626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botvinick M, Braver T. Motivation and Cognitive Control: From Behavior to Neural Mechanism. Annu Rev Psychol. 2014;66:83–113. doi: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- 14.Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, et al. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2004;20:132–43. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Coffman BA, Clark VP, Parasuraman R. Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage. 2014;85 Pt 3:895–908. doi: 10.1016/j.neuroimage.2013.07.083. [DOI] [PubMed] [Google Scholar]
- 16.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–78. doi: 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snowball A, Tachtsidis I, Popescu T, Thompson J, Delazer M, Zamarian L, et al. Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Curr Biol. 2013;23:987–92. doi: 10.1016/j.cub.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: a tDCS-fMRI study. Hum Brain Mapp. 2014;35:3673–86. doi: 10.1002/hbm.22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pena-Gomez C, Sala-Lonch R, Junque C, Clemente IC, Vidal D, Bargallo N, et al. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimul. 2012;5:252–63. doi: 10.1016/j.brs.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D, et al. Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend. 2014;140:78–84. doi: 10.1016/j.drugalcdep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry. 2008;69:32–40. doi: 10.4088/jcp.v69n0105. [DOI] [PubMed] [Google Scholar]
- 22.Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett. 2009;463:82–6. doi: 10.1016/j.neulet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Fregni F, Brody AL, Rahman AS. Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front Psychiatry. 2013;4:112. doi: 10.3389/fpsyt.2013.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14:1362–71. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, et al. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol. 2015;29:300–11. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zachary RA. Shipley Institute of Living Scale: Revised Manual. Western Psychological Services; Los Angeles: 1986. [Google Scholar]
- 27.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 28.Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:370–5. [PubMed] [Google Scholar]
- 29.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5:155–62. doi: 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roche DJ, Bujarski S, Moallem NR, Guzman I, Shapiro JR, Ray LA. Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology (Berl) 2014;231:2889–97. doi: 10.1007/s00213-014-3465-x. [DOI] [PubMed] [Google Scholar]
- 33.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 34.Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt's cognitive-behavioral model. Alcohol Res Health. 1999;23:151–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Sayette MA, Loewenstein G, Kirchner TR, Travis T. Effects of smoking urge on temporal cognition. Psychol Addict Behav. 2005;19:88–93. doi: 10.1037/0893-164X.19.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72:371–81. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- 37.Meng Z, Liu C, Yu C, Ma Y. Transcranial direct current stimulation of the frontal-parietal- temporal area attenuates smoking behavior. J Psychiatr Res. 2014;54:19–25. doi: 10.1016/j.jpsychires.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev. 2010;34:559–74. doi: 10.1016/j.neubiorev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Fecteau S, Fregni F, Boggio PS, Camprodon JA, Pascual-Leone A. Neuromodulation of decision-making in the addictive brain. Subst Use Misuse. 2010;45:1766–86. doi: 10.3109/10826084.2010.482434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev. 2013;37:2472–80. doi: 10.1016/j.neubiorev.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013;6:221–30. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Theoret H, Boggio PS, et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J Neurosci. 2007;27:6212–8. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill J, Shah-Basak PP, Hamilton R. It's the thought that counts: examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain Stimul. 2015;8:253–9. doi: 10.1016/j.brs.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011;4:84–9. doi: 10.1016/j.brs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Hartwell KJ, Johnson KA, Li X, Myrick H, LeMatty T, George MS, et al. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16:654–66. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartwell KJ, Lematty T, McRae-Clark AL, Gray KM, George MS, Brady KT. Resisting the urge to smoke and craving during a smoking quit attempt on varenicline: results from a pilot fMRI study. Am J Drug Alcohol Abuse. 2013;39:92–8. doi: 10.3109/00952990.2012.750665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grundey J, Thirugnanasambandam N, Kaminsky K, Drees A, Skwirba AC, Lang N, et al. Neuroplasticity in cigarette smokers is altered under withdrawal and partially restituted by nicotine exposition. J Neurosci. 2012;32:4156–62. doi: 10.1523/JNEUROSCI.3660-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkins KA, Lerman C, Stitzer M, Fonte CA, Briski JL, Scott JA, et al. Development of procedures for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2008;84:216–21. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]