Abstract
People living with human immunodeficiency virus (HIV) are living longer since the advent of effective combined antiretroviral therapy (cART). While cART substantially decreases the risk of developing some cancers, HIV-infected individuals remain at high risk for Kaposi sarcoma, lymphoma and several solid tumors. Currently HIV-infected patients represent an aging group, and malignancies have become a leading cause of morbidity and mortality. Tailored cancer-prevention strategies are needed for this population. In this review we describe the etiologic agents and pathogenesis of common malignancies in the setting of HIV, as well as current evidence for cancer prevention strategies and screening programs.
Introduction
There are approximately 1.2 million people living with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) in the United States (US) and more than 35 million HIV-infected worldwide1. HIV and associated immune suppression have been strongly associated with several mature B-cell lymphomas and Kaposi Sarcoma (KS) since the beginning of the AIDS epidemic. In women with HIV/AIDS, premalignant cervical lesions were also noted to be common. KS, certain non-Hodgkin lymphomas (NHL) and cervical cancer confer the diagnosis of AIDS in an HIV positive patient, and as such are referred to as AIDS defining malignancies (ADM). Over the years it has been recognized that several additional cancers occur more frequently in HIV-infected patients, such as lung cancer, hepatocellular carcinoma (HCC), anal cancer, oropharyngeal cancer, classical Hodgkin lymphoma and non-melanomatous skin cancer2,3. These neoplasms in HIV patients are referred to as non-AIDS defining malignancies (NADM). As the population of HIV-infected patients ages, incidental cancers not associated with HIV, such as breast, prostate and colon cancer are also increasingly seen.
Access to combined antiretroviral therapy (cART) has markedly decreased mortality among HIV-infected patients. This is largely due to a decrease in the incidence and mortality rate of opportunistic infections (OI)4–6. With decreases in infectious deaths, the population with HIV is living longer, and cancer has become a leading cause of morbidity and mortality in this patient population1,4,7,8. Additionally, at the beginning of the HIV epidemic, the majority of malignancies in HIV-infected patients were ADM. However, over the last two decades, the epidemiology of cancer in HIV-infected patients has markedly changed8. The incidences of cancers that occur most frequently in advanced immunosuppression have decreased in the setting of widespread use of cART9. While KS and mature B-cell lymphomas remain the commonest individual cancers in this population, collectively NADM and incidental cancers1 now comprise the majority of the cancer burden in HIV-infected populations in the US and represent a growing public health concern both in the US and globally.
Cancer is responsible for approximately one third of all deaths in people with HIV4. A recent study from France reported that respectively, ADM and NADM were the cause of death in 10% and 26% of HIV patients from 2000–20108. Both ADM and NADM are associated with decreased 10-year overall survival in HIV-infected patients, despite the use of cART. Health care disparities compromise outcomes for patients with HIV who develop cancer10,11. For these reasons, prevention and early detection of malignancies in HIV-infected patients are increasingly important in the US and globally.
The majority of cancers associated with HIV are linked to co-infection with oncogenic viruses, immunologic and inflammatory factors, and environmental conditions. The commonest oncogenic viruses in this patient population include Epstein-Barr virus (EBV), Kaposi sarcoma herpes-virus (KSHV) - also called human herpes-virus 8 (HHV-8), and human papilloma virus (HPV). Co-infection with hepatitis C virus (HCV) and hepatitis B virus (HBV) increases the risk of HCC in this population. A rare viral cause of cancer is Merkel cell polyomavirus12, which is the etiologic agent of Merkel cell carcinoma. Immune and inflammatory risk factors are closely related to HIV viremia and associated immunosuppression, and these directly and indirectly contribute to oncogenesis in several HIV-associated cancers. Further important and modifiable risk factors include cigarette smoking and sun exposure13,14.
In this review of cancer prevention in people with HIV, we will focus on the pathogenesis of ADM and NADM most strongly associated with HIV/AIDS (Table 1). We discuss the etiologic agents and pathogenesis of the commonest malignancies in HIV-infected patients. We review the mechanism of action of individual antiretroviral agents and the evidence for prevention of several cancers through the use of cART, control of viral co-infections and associated diseases, cancer screening, vaccination use, and behavioral modification.
Table 1.
Common Malignancies in People with HIV in the cART Era
| Malignancy | Standard Incidence Ratio* (HIV only / AIDS) | Incidence in HIV (per 100,000 person-years) | Estimated % of all cancers† 2004–2007 in HIV/AIDS in US | Viral Associations | Smoking Association |
|---|---|---|---|---|---|
| AIDS-Defining Malignancies | |||||
| Non-Hodgkin lymphoma | |||||
| Systemic | 10–15 / 30–60 | >153¥ | 25.9% | EBV, KSHV | − |
| Primary CNS lymphoma | 250 / 1,020 | 27 | 3% | EBV | − |
| Kaposi sarcoma | 1,300 / 3,640 | 110 | 18.5% | KSHV | Inverse relation |
| Cervical cancer | 2.9 / 5.3 | 47 | 2.4% | HPV | + |
| Non-AIDS Defining Malignancies | |||||
| Lung cancer | 2.6 / 2.6 | 78 | 10% | − | + |
| Anal cancer | 9.2 / 20 | 59 | 5.7% | HPV | + |
| Classic Hodgkin lymphoma | 5.6 / 14 | 33 | 4.4% | EBV | − |
| Oropharyngeal carcinoma | 1.7 / 2.1 | 22 | 2.5% | HPV | + |
| Hepatocellular carcinoma | 2.7 / 3.3 | 32 | 2.3% | HBV, HCV | + |
| Non-melanomatous skin cancer | |||||
| Basal cell | 1.8/2.5 | 1197 | − | − | |
| Squamous cell | 1.6/4.2 | 405 | − | ? HPV (not established) | + |
Standard incidence ratio of patients in cohorts with 1) HIV but not AIDS and 2)
AIDS compared to the general United States population
Excluding non-melanomatous skin cancers
153 Includes diffuse large B-cell lymphoma and Burkitt lymphoma, but not other rarer histologies
Etiologic Agents and Pathogenesis
Human Immunodeficiency Virus
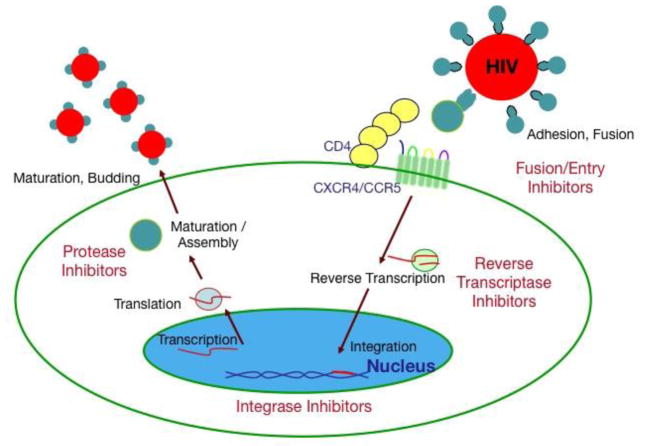
HIV, like other retroviruses, is enveloped and contains two strands of RNA. It is a lentivirus, a type of retrovirus that mainly infects CD4+ T-cells. The HIV lifecycle depends on several HIV encoded proteins (Figure 1). During HIV infection, viral envelope proteins gp120 and gp41 specifically bind to the CD4 receptor as well as the co-receptors CXCR415 or CCR516, which lead to fusion and entry into the cell. In the cytoplasm, HIV RNA undergoes reverse transcription by HIV-encoded reverse transcriptase (RT)17 to form a double stranded (DS) HIV DNA, which is further processed and chaperoned to the nucleus where it is integrated into the host DNA genome by an HIV integrase18. HIV can then be transcribed from this pro-viral DNA, which is followed by translation of HIV polyproteins. These polyproteins undergo secondary processing by an HIV aspartic protease19 to form mature protein components of an infectious virion. These virions are assembled and released by dying CD4+ T-cells. Untreated, HIV replicates rapidly, leading to rapid turnover of CD4+ T-cells20. It can also kill CD4+ T-cells by indirect mechanisms. Several steps in the lifecycle, including fusion, reverse transcription, integrase strand transfer and protease function can be effectively targeted by a variety of agents (Figure 1, Table 2). HIV mutations are common in the setting of uncontrolled viremia, and under the pressure of a single agent HIV regimen or suboptimal antiretroviral drug concentrations, this can quickly lead to drug resistance. However, cART, generally consisting of three drugs from at least two classes leads to effective control of HIV viremia and thwarts development of resistance. HIV can also infect macrophages and related cells such as microglia, which are important additional cellular reservoirs.
Figure 1. HIV lifecycle and steps targeted by antiretroviral therapy.
Current agents target human immunodeficiency virus (HIV) cell surface interactions that inhibit HIV – entry, or target HIV-encoded enzymes that are required for reverse transcription, integration, or protease activity. CD4- cluster of differentiation 4; CXCR4- chemokine receptor type 4; chemokine receptor type 5
Table 2.
United States Food and Drug Administration approved targeted antiretroviral agents for the control of HIV
| Class | Mechanism of action | Mechanism of resistance* | Specific agents |
|---|---|---|---|
| Fusion/Entry Inhibitors | Synthetic peptide corresponding to a region of the HIV envelope protein, gp41. Inhibits gp41-mediated virus entry. | HIV mutations leading to amino acid substitutions in positions 36–38 in HIV gp41. | Enfuvirtide (T20) |
| Imidazopyridine CCR5 ligand that alters conformation of extracellular loops (host targeted antiviral). Inhibits HIV binding and fusion to CCR5; active against CCR5-tropic HIV-1. | HIV cell entry through CXCR4 co-receptors. HIV gp120 V3 loop mutations leading to HIV binding to CCR5. | Maraviroc | |
| Nucloside Reverse Transcriptase inhibitors (NRTIs) | Deoxynucleoside analogue, active metabolites are competitive substrate inhibitors, incorporation by HIV reverse transcriptase leads to chain termination. | HIV reverse transcriptase (RT) inhibitors’ mutations that lead to discrimination against select triphosphate derivatives of NRTIs (i.e. M184V interferes with binding to lamivudine or emtricitibine triphosphate). Acquisition of thymidine analogue resistance mutations in RT that allow for excision of 3′ chain terminators (mechanism for zidovudine, stavudine, and tenofovir resistance). |
dTTP competitors:
|
| Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | Direct inhibitors of HIV reverse transcriptase through interaction with p66 subunit of HIV-1 reverse transcriptase. | Mutations in the NNRTI binding pocket (i.e. K103N) of HIV-1 reverse transcriptase that inhibit or modify interactions with NNRTIs. |
|
| Integrase Strand Transfer Inhibitors (INSTIs) | Interfaces with HIV- Integrase catalytic core domain as well as divalent cationic co- factors (Mg2+ or Mn2+), inhibiting the catalytic activity required for transfer of HIV cDNA. | Mutations in HIV-1 Integrase catalytic core domain (especially Q148H and N155H) that affect INSTI interactions. Q148H plus secondary HIV integrase mutations increase INSTI resistance. |
|
| Protease Inhibitors (PI) | Bind to Asp residues in the protease binding site, inhibiting the proteolytic cleavage of HIV polypeptides required for viral maturation and assembly. Darunavir also inhibits protease dimerization. | Mutations in the HIV protease that lead to a conformational change and lower affinity for PI in relation to other HIV polyprotein substrates. Most major mutations occur at the HIV protease catalytic site (i.e. V82F/I84V double mutation), although distal site mutations may also lead to resistance, notably L90M occurs at the PI dimerization interface. | Peptomimetics:
|
Specific mutations do not necessarily lead to cross-resistance for an entire class of antiretroviral agents, and are sometimes agent specific. Some anti-retroviral agents have been designed to address common mechanisms of resistance noted in the same class. Selection of effective antiviral therapy in patients with resistant HIV generally requires expert input from infectious disease physicians and pharmacologists.
The incidence of ADM is substantially increased in HIV-infected patients. This is particularly true for certain NHLs and KS, for which both HIV viremia as well as HIV-induced immunosuppression as measured by CD4+ T-cell counts, are strongly correlated with incidence21. Several overlapping mechanisms of oncogenesis have been proposed, including decreased immune surveillance, increased activation of both the innate and acquired immune system, and possibly direct HIV effects on proliferation of certain cell populations, and possibly other mechanisms.
Untreated HIV-infection leads to decreased CD4+ T-cell count. Lack of virus-specific or tumor-specific CD4+ T-cells is an important mechanism for development of some tumors, especially those occurring at the lowest CD4+ T-cell counts. This is best studied for EBV, KSHV and HPV specific T-cells. CD4+ T-cells targeting the latency associated EBV nuclear antigen (EBNA) 1 are depleted in both systemic AIDS-related NHL22 and AIDS-related primary central nervous system lymphoma (PCNSL)15. Both absolute decreases in CD4+ T-cell counts and lack of KSHV-specific T-cell immunity are associated with incident KS23. Functional deficits such as decreased IFN-gamma production have been observed in patients with KS23, and an immunosenescent CD4+ T–cell phenotype, marked by increased populations of CD4+ CD57+ T-cells, is associated with KS tumorigenesis. CD4+ T-cell depletion also diminishes control of carcinogenic high-risk HPV infection and is associated with cervical and anal intraepithelial neoplasia. HIV-infected patients with HPV-16 positive genital lesions can lack detectable CD4+ T-cell immunity against HPV viral proteins24.
HIV Viremia Promotes Immune Activation
In addition to defects in T-cell immunity, HIV infection is associated with chronic inflammation through different mechanisms. Immune activation in the setting of HIV appears particularly important in the pathogenesis of AIDS-related NHLs and some KSHV-associated malignancies (discussed below). AIDS-related lymphomas are aggressive B-cell lymphomas that largely arise from germinal center or post-germinal center B-cells. Several circulating biomarkers have been correlated with B-cell activation and expansion, such as interleukin (IL)-6, IL-10, CD30, CD27 and CD23, neopterin, interferon gamma-induced protein 10 (IP-10), tumor necrosis factor alpha (TNF-alpha), and microRNA (miR2-121)25–27. The epidemiologic observation that elevated levels of serum free light chains are a risk factor for lymphoma in patients with HIV further supports the role of B-cell lymphoproliferation in pathogenesis28. Interestingly, elevated plasma levels of CXCL13, a B-cell chemokine strongly associated with NHL risk, is elevated in the setting of uncontrolled HIV viremia but decreases with cART29, supporting one potential mechanistic role for cART in NHL prevention.
Early in infection, HIV depletes gut associated CD4+ T-cells. At the same time, HIV is associated with CD8+ T-cell30, B-cell31 and monocyte activation, which promotes an inflammatory milieu32 and resultant decreased mucosal integrity33. Preferential loss of gut CD4+ TH17 cells has been posited to play an important mechanistic role in this process34. Interestingly, recent in vivo studies showed that a model retrovirus, simian immunodeficiency virus (SIV), disrupts overall microRNA (miRNA) expression and diversity of commonly expressed miRNAs in the small intestinal mucosa, perhaps contributing to epithelial barrier dysfunction in this setting35. Associated microbial translocation is then associated with systemic immune activation. Lipopolysaccharide (LPS), the major component of the bacterial wall of gram-negative microorganisms, is recognized by toll like receptors 2 and 4 (TLR2, TLR4) and stimulates several immune cell populations. Systemic LPS levels are increased in HIV-infected patients, especially in the setting of HIV viremia. This increase in circulating LPS occurs in conjunction with chronic stimulation of monocytes and an increase in circulating CD8+ T-cells with an activated CD38+ HLA-DR+ phenotype (polyclonal T-cell activation) and an increase in measures of immune activation such as soluble CD14 (sCD14). CD4+ T-cell depletion and immune activation are reversible with cART36, especially when started soon after HIV infection37. However, gut associated lymphoid tissue abnormalities appear to improve slowly, and can persist even after years of effective cART30. Inflammatory biomarkers have been shown not only to correlate with HIV disease progression and poor response to cART38, but also development of AIDS-related lymphoma39. For example, elevated levels of sCD14 and LPS were significantly associated respectively with a 2.7- and 3.2-fold risk for NHL, implicating gut dysfunction in lymphomagenesis. More studies are required to evaluate this as a risk factor for the development of other malignancies in HIV infected patients.
HIV is associated with chronic B-cell activation and B-cell exhaustion has been described, characterized by increased frequencies of circulating tissue-like memory B-cells40. Chronic stimulation of B cells from HIV viremia is partially reversed with cART; however markers of exhaustion are not completely reversed to compared to HIV-negative individuals. B-cell lymphomagenesis in the setting of HIV-associated inflammation involves chronic antigen stimulation and associated B-cell immune hyperactivation. B-cell activation may occur through LPS or other infectious antigens binding to TLR. HIV itself appears to be able to stimulate B-cells though both CD40 and the B-cell receptor. CD40 is a membrane protein in the tumor necrosis factor (TNF) receptor family that is found on the surface of B cells, macrophages, dendritic and endothelial cells. CD40 ligand (CD40L) is found on CD4+ T-cells, and the incorporation of CD40L into HIV virions may lead to B-cell activation. Additionally, HIV upregulates the Syk and Jnk pathways through the B-cell receptor. A consequence of B-cell activation in lymphatic germinal centers is upregulation of activation induced cytidine deaminase (AICD), an enzyme required for immunoglobulin (Ig) class switch recombination and somatic hypermutation42. However, AICD is also responsible for translocations that potentiate lymphoma development (i.e. c-Myc/IgH) as well as other recurrent mutations (i.e. in the promoter region of BCL6) that contribute to lymphomagenesis. In the setting of HIV, c-Myc/IgH contributes to the pathogenesis of Burkitt lymphoma43, a large number of diffuse large B-cell lymphomas, and plasmablastic lymphoma.
Kaposi Sarcoma Herpesvirus (KSHV)
KSHV is a gamma herpesvirus that is a necessary but not sufficient etiologic agent of KS, a plasmablastic form of multicentric Castleman disease (KSHV-MCD) and primary effusion lymphoma (PEL)44,45. Early in the HIV/AIDS epidemic, as many as 20–30% of men who have sex with men (MSM) AIDS patients developed KS. In the cART era, the incidence of KS has decreased 84% in HIV-infected patients, which appears largely to be due to control of HIV viremia and resultant improvement in T-cell mediated immunity46.
KSHV seroprevalence varies substantially between populations; in the general US population it is less than 5%. In contrast, it ranges from 10% to 25% in the Mediterranean area47 and from 20% to 60% in certain regions of Africa and among MSM48. KSHV DNA has been detected in saliva, and saliva exchange is considered the major transmission route49. KSHV seroprevalence is associated with sanitation, number of sexual partners, and saliva exchange behaviors during sex or in some cultures, pre-mastication of food49,50. KSHV infection is also associated with other infections such as malaria and HIV51, which may be due in part to the effect of certain infections on increasing KSHV salivary shedding52.
In addition to its effect on KSHV transmission, an HIV encoded protein, trans-activator of transcription (tat), has also been directly implicated in KS pathogenesis. Intriguingly, in vitro, HIV tat appears permissive for infectivity of KSHV in cultured endothelial cells53. In transgenic mice models, tat induces dermal lesions similar to KS54, and in cell culture, tat alone and synergistically with human basic fibroblast growth factor (bFGF) promotes spindle cell proliferations derived from KS lesions55. More recently, HIV tat was found to promote KSHV-encoded viral IL-6 (vIL-6, discussed below) induced angiogenesis and tumorigenesis in vitro through activation of the PI3K/PTEN/AKT/GSK-3beta signaling pathway56. Thus, treatment of HIV may have an additional protective effect against KSHV-associated malignancies through decreasing HIV tat.
KSHV can infect a variety of cell types, including endothelial cells, B-cells and monocytes. KSHV has a large double stranded DNA genome that encodes a number of mimics of human genes, many of which have immunologic or angiogenic properties57. Viral oncogenesis is complex; KSHV-associated tumors do not appear to be driven by a single viral oncogene. Some of the better-studied KSHV-encoded oncogenes include those that encode a constitutively active transmembrane viral G-protein coupled receptor (vGPCR) that is a homologue of the IL-8 receptor, CXCR158, viral interleukin-6 (vIL-6) and a viral FLICE-inhibitory protein (vFLIP) that activates nuclear factor-κB (NF-κB) signaling by binding to the inhibitor of IκB kinase-γ59. More recently, miRNAs were shown to be derived from 12 precursor miRNAs (pre-miRNAs) encoded by sequences in the latency locus of the KSHV genome60. These miRNAs can contribute to oncogenesis by modulating cell differentiation60, cytokine production, and immune receptor signaling61.
Epstein-Barr-Virus (EBV)
Like KSHV, EBV is a DNA gamma herpesvirus. EBV infection is almost universal by adulthood. Acute infection can cause infectious mononucleosis, but in immune-competent hosts, the virus then generally forms an asymptomatic latent chronic infection in which the virus remains in a latent state, primarily in B cells. However, EBV is associated with several lymphomas in the setting of HIV. The malignant cells are EBV-infected in a majority of cases of AIDS-related PCNSL, immunoblastic forms of systemic diffuse large B-cell lymphoma (DLBCL) occurring at low CD4+ T-cell counts, plasmablastic lymphoma, and classical Hodgkin lymphoma, as well as a proportion of Burkitt lymphoma and primary effusion lymphoma cases. EBV is also associated with rare cases of leiomyosarcoma in children with HIV.
EBV encodes several latency-associated genes that are variably expressed during primary and chronic infection, and which may contribute to lymphomagenesis. One important gene is EBNA1, which is expressed in all latently infected cells and can induce oxidative stress as well as promote telomere dysfunction62. LMP2 is expressed in a more limited fashion but is sufficient for B-cell transformation in vitro. More recently, it has been recognized that EBV encodes viral miRNAs that play an important role in modifying the cellular environment through a variety of mechanisms such as inducing resistance to apoptosis, modulating the latency to lytic transition, regulating angiogenesis63 and regulating cellular oncogenes64.
EBV-associated lymphomas can be classified into 3 different categories based on the variable expression of EBV viral proteins and RNA, and these latency patterns provide insight into disease pathogenesis. Latency 1 tumors generally occur at relatively preserved CD4+ T-cell counts. The tumor cells express EBV nuclear antigens (EBNA) 1, EBV-encoded RNA (EBER) and several microRNAs, and include monomorphic cases of DLBCL, Burkitt lymphoma, and plasmablastic lymphoma. There is overlap between Latency 1 EBV+ lymphomas and translocations involving c-Myc and immunoglobulin genes, mainly t(8;14) (c-Myc/IgH). C-Myc translocations are noted in the majority of plasmablastic lymphomas, as well as Burkitt lymphoma and 25% of DLBCL, regardless of EBV status. When present, c-Myc translocations likely drive the high proliferative rate in these tumors. Interestingly, in EBV+ cases of Burkitt lymphoma, EBNA1 may inhibit c-Myc induced apoptosis, and thereby contribute to oncogenesis65.
Classical Hodgkin lymphoma has latency 2 pattern. The EBV-infected Reed-Sternberg cells express EBER, EBNA1, miRNA and latent membrane protein (LMP) 2 with varying LMP1 levels. EBV-encoded genes may play a more important role in these tumors through proliferative signaling and modulation of the immune response. LMP166,67, a constitutively active homologue of CD40 that upregulates NF-κB signaling and IRF4 expression68, and LMP2A that mimics B-cell receptor signaling both contribute to proliferation69. EBNA1 appears to influence cytokine networks by stimulating production of chemokines such as CXCL10 and CCL2070 that attract T-regulatory cells and modulate an immunosuppressive microenvironment.
EBV type 3 latency pattern is the most immunogenic, and is characterized by the expression of all six EBV-nuclear antigens (EBNA) and all 3 LMPs. Tumors in which EBV usually expresses a type 3 latency pattern include AIDS-related PCNSL and immunoblastic DLBCL occurring at low CD4+ T-cell counts. EBV-encoded gene products play an important role in the pathogenesis of these tumors. Latency 3 tumors occur at the lowest CD4+ T-cell counts, and CD4+ T-cell immune reconstitution with cART is most important in prevention and treatment of this category of EBV-associated lymphomas. More recently, strong upregulation of the immune modulatory molecule, PD-L1, has been noted in EBV-associated DLBCL and classic Hodgkin lymphoma but not EBV-associated Burkitt lymphoma, supporting an association between EBV and tumor immune evasion in latency 2 and latency 3 tumors 71.
Human Papilloma Virus
Human papilloma virus (HPV) is the etiologic agent of the majority of cases of cervical cancer and anal cancer, as well as a proportion of head and neck squamous cell cancers (HNSCC) in the general population and in people with HIV. Most HPV-associated HNSCC involve the oropharynx, and a recent large case series of head and neck cancers in HIV infected individuals found that 64% of HIV oropharyngeal cases were HPV positive72. Rare HPV-associated malignancies include cancer of the vulva and penis. HIV infection and associated immunosuppression are associated with an increased risk for HPV associated invasive cancers in these sites73. High-grade squamous intraepithelial lesions (HSIL) precede the development of cervical or anal cancer, and HIV/AIDS is associated with an increased risk of HSIL74. Given the high prevalence of HSIL, screening for cervical cancer and possibly anal cancer is important in people with HIV.
The HPV virion contains a double-stranded, circular DNA genome covered by a capsid. HPV can infect cells of the basal layer of squamous epithelium that have been exposed due to microabrasions. The main viral capsid proteins are L1 and L2. The genome is divided into three regions: early, late and long control (LCR) or non-coding (NC). The early region contains the regulatory proteins (E1, E2, E4, E5, E6 and E7). The latter two are viral oncogenes that promote cellular proliferation and are responsible for the carcinogenesis of high-risk HPV types. Multiple HPV genotypes have been associated with HSIL and cervical cancer; these are called “high risk” genotypes. HPV-16 accounts for about 50% of cases of HSIL and cervical cancer. Other high-risk types include 18, 31, 35, 45, 51, 52 and 5875,76. High-risk HPV are more prevalent among HIV-infected individuals, which can also contribute to a higher prevalence of HPV-associated malignancies in this population77.
Several factors have been proposed to explain the increased risk of HPV-related cancers in the HIV population. HIV-encoded proteins tat and gp120 disrupt the integrity of mucosal epithelium and may facilitate the penetration of HPV78. Persistent HPV infection and associated anal and cervical HSIL in the setting of HIV are also highly dependent on the immune status of the host and have been associated with decreases in CD4+ T-cell counts and increases in HIV viral load79. HPV-specific immune defects, including defective CD4+ T-cell response and increases in CD4+ T regulatory cells are also demonstrable in this setting24,80. Quantitative defects in perforin granule release by CD8+ T cells in HPV/HIV co-infected patients may further contribute to pathogenesis81. Polymorphisms in human genes that code for proteins targeted by E6 and E7 or those associated with IL-2 and IL-7 signaling are host factors that may further modulate HPV clearance82,83.
It has been hypothesized that HIV-induced immune suppression enhances the risk of HPV-associated tumors by being permissive for chronic HPV infection, thus allowing premalignant lesions to accumulate genetic damage and progress through increasing dysplasia to cancer. Two HPV-encoded genes have been strongly implicated in this oncogenic process. E6 from high-risk HPV strains interacts with human E6 associated protein (E6AP), forming an E3 ubiquitin complex that targets specific proteins such as p53 for proteosomal degradation. High-risk E7 also modulates ubiquitin ligase activity, leading to degradation of the retinoblastoma tumor suppressor (pRb). Besides E6AP, E6 also interacts with and modulates the function of several other regulatory proteins, leading to suppression of apoptosis, disruption of cell adhesion and epithelial differentiation, activation of telomerase reverse transcriptase (TERT), and reduction of immune recognition through modulation of interferon responses84,85.
Hepatitis B Virus
Hepatitis B virus (HBV) is a member of the Hepadnaviridae family, which infects hepatocytes and increases the risk of HCC by 5–15 fold. Co-infection with HBV is frequent in people living with HIV (PLWH) due to the common route of transmission (sexual, parenteral and perinatal). It is estimated that worldwide the prevalence of co-infection, assessed by persistent hepatitis B surface antigen (HBsAg), in HIV-infected patients is 5–25%, with higher prevalence in regions of Asia and Africa 86. Patients with detectable HBsAg in serum for longer than 6 months are considered as having chronic hepatitis B, a condition that can lead to cirrhosis, liver failure and hepatocellular carcinoma (HCC) with significant associated morbidity and mortality. HBV-induced inflammation is believed to play a major role in the development of HCC. Uncontrolled HIV is associated with increased HBV viral load87 and increased liver-associated mortality, especially in severely immunosuppressed patients in the pre-cART era88. Patients co-infected with HIV and HBV may progress more rapidly to liver disease and have a higher risk of developing HCC89. The risk of developing HCC is 3–8% per year in HBV-infected patients with established cirrhosis90. Alcohol use may further promote carcinogenesis.
The hepatitis B encoded protein X antigen (HBx) is a transactivating viral-encoded protein that is implicated in hepatocyte proliferation through its effect on the expression of a range of proto-oncogenes and microRNAs91, such as TGF-beta, Wnt/beta-catenin, JAK/STAT, PI3K, Ras-raf-MAPK along with inhibition of p53 and Fas-mediated apoptosis92. Additionally, HIV proteins (tat, gp120) may act on hepatic stellate cells (HSC) to induce inflammation and contribute to fibrosis93. Genomic integration of HBV is present in the majority of cases in which HBV-infection leads to HCC94, and appears to be facilitated by DNA damage in the setting of chronic inflammation and oxidative stress. HBV integration is not sufficient for patients to develop HCC, and oncogenesis likely depends on either integration site mutagenesis (i.e. insertion near MLL4, ANGPT1, PDGFRB and hTERT genes have been reported in some HCC patients) or more generalized HBV-associated genomic instability. Secondary mutations in human genes are required in HCC, the most common being mutations in TP53 (>30% of cases) and CTTNB1 (20%)95.
Hepatitis C Virus (HCV)
HCV is a single stranded RNA virus belonging to the Flaviviridae family. HCV co-infection rates among HIV-infected persons are estimated at 25–30%96. Individuals with HIV and HCV who develop HCC are often diagnosed with more advanced disease than those with HCV alone97. Unlike HBV, HCV is not able to integrate into the human genome. Chronic HCV infection promotes host responses that lead to inflammation, oxidative stress and associated DNA damage, steatosis and cirrhosis. These processes then contribute to the pathogenesis of HCC. The annual risk of developing HCC in HCV cirrhotic patients is around 1–7%98. Additionally, several HCV encoded proteins have a potential role in oncogenesis. The best studied is the HCV core protein, which is associated with induction of oxidative stress, modulation of p53, upregulation of TGF-beta, and activation of the Raf/MAPK pathway; these are but a few of the proposed mechanisms92. Additionally, an HCV encoded nonstructural protein; NS5A promotes cell survival through activation of PI3-kinase/akt 99 and stabilization of beta-catenin100. As with HBV-associated HCC, secondary mutations are required95. HCV infection is also associated with several forms of NHL, including marginal zone lymphoma, diffuse large B-cell lymphoma, and lymphoplasmacytic lymphoma101.
Smoking
Tobacco smoke contains more than 60 known carcinogens, including polycystic hydrocarbons (PAHs) and nitrosamines102, which cause DNA adducts. These in turn lead to mutations in TP53, RAS and other genes in lung cancer103. Tobacco-induced promoter hypermethylation is an early epigenetic event that is implicated in lung carcinogenesis104. In addition to being a carcinogen, tobacco smoke may have tumor promoter effects by triggering and maintaining chronic inflammation through modulation of inflammatory signaling105,106. For example, the nicotine-derived nitrosamine ketone (NKK) is implicated in IL-6 upregulation and lung cancer tumorigenesis through gp130 pathways107. Smoking is a modifiable risk factor for cancer. Patients at risk for HIV have relatively high rates of smoking compared to the general population, and over 70–85% of some HIV-infected populations smoke108. Smoking increases the risk of several important cancers affecting people with HIV, including lung, head and neck, esophageal, stomach, pancreatic, liver, anal and cervical cancers.
Lung cancer risk is increased by approximately 5-fold in people with HIV, and in most large studies, HIV remains an independent risk factor for lung cancer even after correcting for smoking109. Lung cancer is the most common NADM in the cART era, representing a leading cause of morbidity and mortality110. Aging of the HIV-infected population contributes to the increasing burden of lung cancer. However, the median age of diagnosis of lung cancer in HIV-infected people is 10–15 years younger than that in HIV uninfected patients111. Even when adjusted for the different age distributions of the underlying populations, age at diagnosis is 4 years younger in HIV-infected versus uninfected individuals112. Increasing evidence suggests chronic inflammation as well as immunosuppression play important roles in lung carcinogenesis, and these factors may be of particular mechanistic importance in people with HIV106,113,114. A large study in non-HIV patients showed that elevated serum levels of IL-8 and C-reactive protein (CRP) were associated with lung cancer, after adjustment for cigarette smoking115. The evidence regarding the degree of immunosuppression (based on CD4+ T-cell counts) as a risk factor for lung cancer is controversial. Several studies showed that the degree and duration of immunodeficiency and low CD4+ T-cell counts (< 200–500/mm3) is associated with lung cancer risk116,117, whereas others failed to come to the same conclusion when adjusted for smoking and age108,118,119.
HIV-infected patients are at an approximately 2–4 fold increased risk of developing both HPV-associated and HPV-unassociated head and neck squamous cell carcinomas (HNSCC)120 compared to the general population. Collectively, head and neck cancers are the fourth commonest NADMs. Like lung cancer, laryngeal cancer occurs at younger ages in people with HIV112. In addition to smoking, risk factors for the development of (HNSCC) include alcohol, older age, and in some cases HPV72. The role of HIV in the pathogenesis of HNSCC is not completely clear; however reduced CD4+ T-cell count is an important risk factor suggesting that HIV-associated immunosuppression is a contributory factor, especially for those cases associated with HPV9,72,108,121.
A modest but significant increased risk of esophageal (both adenocarcinoma and squamous) and stomach cancers has been noted in US patients with HIV, with the highest risk seen in upper esophageal squamous cell carcinoma9,122. Age is an important risk factor in this patient population. Additional studies are required to determine whether this elevated risk is independent of tobacco or alcohol or is related to any specific infectious agents such as H. pylori. Pancreatic cancer risk is also increased in the setting of HIV9, and mortality appears substantially increased, even in the setting of HIV control and relatively preserved CD4+ T-cell counts123. In addition to cigarette smoking, a well-established risk factor for pancreatic cancer in the general population is chronic inflammation (pancreatitis)124. Interestingly, HIV/AIDS has been associated with increased risk of pancreatitis. Major risk factors in this population include low CD4+ T-cell count, opportunistic infections, specific medications, and female gender125. While some older antiretroviral nucleoside reverse transcriptase inhibitors, as well as pentamidine used for prophylaxis and treatment of Pneumocystis pneumonia, are also associated with pancreatitis, the risk of pancreatitis is substantially lower with current antiretroviral drugs125,126. A potential role of inflammation in pancreatic cancer in people with HIV requires further study.
Sun exposure
HIV-infected patients have an increased risk of developing non-melanoma skin cancer (NMSC) (approximately 2-fold higher than the general population; cumulative risk 6%), most commonly squamous cell and basal cell carcinomas3. Interestingly, risk is lower than that of immunosuppressed transplant patients (cumulative risk 30–70%)127–129, and HIV-associated NMSC is not strongly associated with CD4+ T-cell counts 129,130. Major risk factors for both basal cell and squamous cell carcinoma of the skin in HIV-infected patients appear to be more similar to those in the HIV-negative counterpart, and include fair skin, aging, a positive family history, and cumulative sun exposure129,131,132. In the US, NMSC are most common in white/non-Hispanics, and recurrent NMSC is common129,133. Ultraviolet-B (UVB) radiation induced DNA damage, from which mutations arise, likely plays a central role. In general, HPV does not appear to have an etiologic role in non-anogenital SCC of the skin in HIV-infected patients, although HPV-association has been noted in some cases132,134. HIV patients are also at a higher risk of developing Merkel cell carcinoma, which is a virally-associated skin cancer caused by Merkel cell polyomavirus135,136, and immunosuppression likely plays an etiologic role in this uncommon tumor137.
HIV-infected patients also have an increased risk of developing melanoma, even after adjusting for ethnicity and race14,127. While immunodeficiency, immunosenescence and increased inflammation or increased medical surveillance possibly increase risk, behavioral factors associated with UVB induced DNA damage, such as use of tanning beds and excessive sun exposure likely are major contributors 138,139.
Cancer prevention interventions
Antiretroviral therapy
The introduction of cART in 1996, and its broad availability led to decreased infectious mortality, as well as a decrease in incidence and mortality from Kaposi sarcoma, systemic NHL and AIDS-related PCNSL1,46,140. In the US, it is estimated that KS incidence decreased by 84%, and non-Hodgkin lymphoma incidence decreased by 57%. To a large extent, CD4+ T-cells are the best predictor of KS and NHL risk, especially for lymphoma histologies such as PCNSL, which are mostly closely associated with the degree of immunosuppression. Large cohort studies also show that cumulative HIV viremia is independently associated with increased risk and cART with a decreased risk for developing these ADMs116,141,142, while time on cART appears to be protective143.
Prevention of KS and NHL with cART has also been demonstrated in a randomized controlled trial. The Strategies for Management of Antiretroviral Therapy (SMART) study randomized patients with HIV and a CD4+ T-cell count greater than 350 cells/mL to continuous cART or a CD4+ T cell-guided ART, where cART was given when CD4+ T-cell count was less than 250 cells/mL and stopped once over 350 cells/mL144,145. The ADM rate was significantly higher in the CD4+ T-cell guided therapy (driven by lower CD4+ T-cell counts and higher viral load). Other outcomes were also improved in the continuous therapy arm, and provided Level 1 evidence for using higher CD4+ T-cell count thresholds for initiating cART in HIV-infected patients. A subset analysis from the same study found that elevated interleukin-6 (IL-6) levels and other inflammatory biomarkers on the continuous cART arm were associated with increased risk of cancer16.
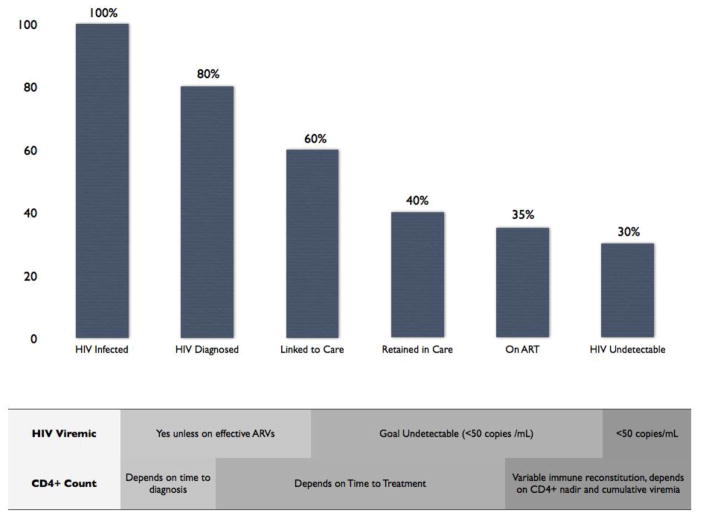
While decreases in KS and NHL have been substantial, the incidences of these malignancies remain markedly increased compared to the general population. Estimates of the percent of HIV-infected patients with controlled HIV viremia on cART is generally less than 50%. Many HIV-infected patients are still not receiving cART either because they are not aware they have HIV or because they are not engaged in appropriate medical care. It is estimated that 18% of HIV-infected patients in the US are unaware of their diagnosis146 Improved testing in high risk patient populations, as well as methods to increase adherence to prescribed cART, are required. Potential further prevention may be possible with implementation of programs designed to increase HIV diagnosis, improve linkage and retention in care, and increase adherence. (Figure 2) Furthermore, recent modification of treatment guidelines to include patients with higher CD4+ T-cell counts should lead to a larger proportion of the HIV-infected population on cART. Prevention of KS and lymphoma through increasing availability of cART is particularly important in sub-Saharan Africa, where KSHV seroprevalance is increased in both men and women, there is a large burden of these ADM, and chemotherapy treatment options are more limited.
Figure 2. Gaps in implementation of combination antiretroviral therapy.
Populations infected with HIV are heterogeneous in terms of time with uncontrolled HIV viremia, extent of immune depletion, and time on antiretroviral therapy (cART). Globally, percent of HIV-infected patients on cART varies between countries and is increasing over time. Presented data is based on 2011 United States estimates. Further cancer prevention through increased cART coverage is likely achievable. Considerations regarding effects of timing of initiation of cART on cancer risk factors are noted.
A preventive effect of cART was less readily apparent in the initial epidemiologic studies evaluating other types of cancers. Indeed in the cART era, the burden of many NADM increased9,46,110, in part due to demographic changes in the underlying population such as decreased infectious mortality, aging of the HIV-infected population, and in the case of classical Hodgkin lymphoma, a very high incidence noted during the first year on cART147. Nonetheless, several lines of emerging evidence suggest a potential protective effect of long-term cART against a broader range of pre-malignant lesions and malignancies.
Importantly, cART and associated immune reconstitution decrease the risk of pre-malignant cervical and anal HPV-associated HSIL among HIV-infected individuals148. Indeed, well-controlled HIV viremia is associated with half of the risk of developing cervical or anal HSIL after adjusting for CD4+ T-cell counts149. This effect may lead to decreased progression of some HSIL to invasive cancers.
With increased experience of large populations of patients on long-term cART, evidence supporting a role for cART in preventing cervical cancer and certain other NADM is growing. Importantly, large cohort studies consistently show an increased risk for both ADM and NADM in patients with low CD4+ T-cell counts (<350–500 cells/mm3)116,117,141,150. Therefore maintenance of T-cell immunity with cART could potentially play an important preventative role, especially when cART is initiated earlier in the natural history of infection. The effect of cART on cancer incidence is likely to be heterogeneous across cancer types. While some studies show no decreased risk of NADM as a whole for patients on cART139,141,143, a few large studies have identified HIV viremia as an independent risk factor for cervical cancer and anal cancer149,151,152, while others have found time on cART (or controlled HIV viremia) associated with decreased risk of certain non-virally associated NADM (largely lung cancer)153, as well as classical Hodgkin lymphoma147 and anal cancer152. Differences between studies may depend in part on the definition of HIV viremia, with percent time with undetectable HIV a more sensitive measure of cART effect than single measurements of HIV viremia. Follow-up time also appears important, as decreasing incidence for certain cancers may become apparent only after 5 or more years110,147.
Screening for HPV-associated malignancies
Cervical Cancer Screening
The incidence of cervical cancer in the United States has been reported at 8 cases per 100,000 women per year in the general population, and as high as 26 per 100,000 HIV-infected women154. HIV-infected women have an increased incidence of pre-malignant lesions, including low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL) and cancers155. In addition to that, women with HIV have higher recurrence rates of cervical HSIL following treatment156. Current guidelines note that HIV-infected women require more intense surveillance for cervical cancer than the general population.
Currently, the U.S. Preventive Services Task Force (USPSTF) recommends cervical cancer screening in the general population with periodic cervical cytopathology, or Pap testing, every 3 years for women ages 21 (or 1 year after start of sexual activity, whatever comes first) to 65, or every 5 years after testing negative for HPV screening157. Women who test positive for HIV should start the screening upon diagnosis. The recommended frequency of screening in HIV-infected women is every 6 months in the first year of diagnosis, and annually thereafter, provided cervical cytology tests are normal158. Abnormal findings should be followed up with colposcopy following standard guidelines that include visual inspection with acetic acid (VIA), and treatment of abnormal areas with cryotherapy, laser or conization using loop electrosurgical procedure (LEEP). Compared to women not on cART, women on cART have a decreased incidence of precancerous cervical lesions, increased regression of existing precancerous lesions, decreased recurrence, and increased clearance of oncogenic HPV159,160. Indeed, there is emerging evidence from a cohort of women with relatively preserved CD4+ T-cell counts in the Women’s Interagency HIV Study (WIHS) that HIV infected women with a baseline normal Pap test and negative oncogenic HPV-PCR have rates of subsequent HSIL or cervical cancer that are comparable to the general population161. Nonetheless, currently, the US Department of Health and Human Services (DHHS) Guidelines recommend yearly testing in women with HIV. The integrated use of HPV testing remains controversial, and should not be used in women with abnormal pap tests. Participation in cervical cancer screening programs is low in HIV infected patients with low educational level, depressive symptoms, substance abuse, younger age and smoking, and efforts to increase screening are required162.
In order to decrease cervical cancer incidence in resource-limited settings that do not have established Pap testing programs, “screen and treat” approaches have been evaluated. These strategies utilize VIA or HPV testing to evaluate for the presence of premalignant conditions. When a positive VIA is found or detection of oncogenic HPV is noted, cryotherapy can be applied immediately, offering the opportunity to patients of being screened and treated in a single visit without the need for cytopathology services, colposcopy or biopsy163,164. Both VIA and treat and HPV and treat have been shown to decrease incidence of HSIL at 12 months165. Additional implementation strategies that optimize resources include prioritizing screening of women aged 30–49 years and prioritizing the number of woman tested rather than intensive surveillance in smaller numbers of women157.
Anal Cancer Screening
Anal cancer incidence in HIV-infected MSM is 46 per 100,000, substantially higher than that of the general population166. Women with HIV also have a higher incidence of anal HPV infection compared to HIV negative women167. Risk factors for anal HSIL and associated progression to anal cancer include high-risk HPV infection (most commonly serotype 16) and low CD4+ counts139,168. Based on cervical cancer screening models, routine periodic cytologic examination of anal mucosa with treatment of premalignant lesions is being evaluated in HIV-infected patients to potentially reduce the incidence of anal cancer169. Anal Pap testing demonstrating abnormal cytology is generally followed up with high-resolution anoscopy. While there is no standard therapy, treatment decisions are generally based on the location and grade of the lesion. Current options for HSIL include local treatment with topical immune modulators (e.g., imiquimod)170, topical antiviral agents (e.g., cidofovir)171 or ablative procedures using electrocautery, laser or infrared coagulation172 or surgery. It should be noted, however, that while plausible, treatment of anal dysplasia has not been shown to prevent anal cancer, and current therapies are associated with local morbidity and a high incidence of recurrence. An NCI-funded Anal Cancer/HSIL Outcomes Research Study (ANCHOR) is currently enrolling patients with HIV to undergo anal Pap testing and then be randomized to either therapy (topical or ablative) versus observation to determine whether treatment of HSIL prevents anal cancer in HIV-infected patients and to inform the best practice for anal cancer prevention.
HPV Vaccine
Immunization with HPV virus-like particles composed of the L1 viral protein can induce neutralizing antibodies against HPV173. Antibody responses are directed against type-specific L1 particles of the specific types of HPV utilized in the vaccine, although there is some evidence of development of cross-type neutralizing antibodies174. Currently, there are three vaccines available, a bivalent HPV vaccine (HPV2, Cervarix™), a quadrivalent HPV vaccine (HPV4, Guardasil™) and a nonavalent HPV vaccine (HPV9, Gardasil 9)175, which are prescribed as a series of three injections over 6 months. All protect against HPV 16 and 18. HPV4 also protects against HPV 6 and 11, which cause genital warts, while HPV9 protects against additional oncogenic strains 31, 33, 45, 52 and 58. Vaccines have almost a 100% prevention rate against HPV 16 and 18 infections in non-HIV infected patients and are effective in preventing anogenital diseases, including cervical HSIL176. The HPV4 vaccine has demonstrated efficacy in decreasing anal intraepithelial neoplasia (AIN) by 54.2% in HIV-uninfected MSM ages 16–26, supporting the potential importance of this approach in reducing the risk of anal canal cancer177.
Females are recommended to be vaccinated with HPV2, HPV4 or HPV9 at age 11 or 12; and immunization may begin as early as nine years of age178,179. Males are recommended to be vaccinated with HPV4 or HPV9 starting at the same age. For those not vaccinated earlier, it is recommended for females through age 26 and for males through age 21, although males can be vaccinated trough age 26. Immunization is thought to be most effective in patients never infected with HPV; hence the optimal time of vaccination is prior to sexual debut. For MSM, including HIV infected patients, the recommendation is to consider vaccination with HPV4 or HPV9 through 26 years of age if not previously vaccinated or for individuals who have not completed the 3-dose series180. At the current time, uptake of the HPV vaccine in the US is relatively poor. Vaccination coverage among adolescents ages 13 to 17 was 57.3% for girls and 34.6% for boys in 2013181. A role for HPV vaccine in older subjects is less well established. There is some evidence suggesting HPV4 may have efficacy in the prevention of recurrent anal HSIL in HIV-uninfected MSM older than 18182.
HPV vaccines appear to have reasonably comparable immunogenicity in HIV-infected populations, including children with preserved T-cell function 183, HIV infected women184 or men185 on cART with CD4+ T-cell counts >200 cells/mm3, and a cohort of HIV infected women in Africa (98% with CD4+ T-cell counts > 200 cells/mm3)186. Immunogenicity appears slightly inferior in HIV viremic patients and those with CD4+ T-cell counts less than 200 cells/mm3 187. The Department of Health and Human Services (DHHS) guidelines support use of HPV vaccination in HIV-infected individuals ages 13–26.
Hepatocellular Carcinoma Prevention and Early Detection
Chronic HBV co-infection is more common in HIV-infected patients in the US, and perhaps more importantly, in some parts of sub-Saharan Africa. Furthermore, HIV infection increases the risk of chronic HBV infection87,188. HIV/HBV co-infection was associated with increased liver–related mortality in the pre-cART era compared to infection with either virus alone, especially in patients with decreased CD4+ T-cell counts88. Universal HBV vaccination of infants is currently recommended by the World Health Organization (WHO), and implementation has been shown to reduce the risk of infection by more than 70%189. However, many adults have not received HBV vaccination. Therefore, HIV infected patients should be tested for HBsAg, along with antibodies to HBsAg (anti-HBsAg) and hepatitis B core antigen (anti-HBc) to distinguish between infection and immunity190. In HIV-infected patients lacking serological markers of immunity, vaccination is recommended. Not all HIV infected patients will develop a positive response to the HBV vaccine, and lack of antibody response to HBV vaccination correlates with low CD4+ T-cell counts, HIV viremia, and HCV co-infection. Increasing the number of HBV injections can improve the response rate in HIV-infected persons, as well as increasing the dose from 20 mcg to 40 mcg per dose191. Patients with established co-infection should have HBV DNA viral load and liver function assessed. They should also be started on cART regimens that include agents active against HBV, including lamivudine, emtricitabine, and/or tenofovir, regardless of CD4+ T-cell counts192.
Additionally, approximately one third of HIV-infected patients are co-infected with HCV. Therefore, all HIV-positive persons should also be screened for HCV at time of HIV diagnosis and annually thereafter192. Sensitivity of HCV serology is suboptimal in HIV-infected patients, with 13% of seronegative patients having evidence of HCV upon RNA testing. Therefore, HCV RNA testing is advised in HCV seronegative patients with a history of intravenous drug use, liver function test abnormalities, and/or thrombocytopenia193. Re-infection rates after successful treatment are also high and continuous screening is advisable in some patient populations194. HCV-induced cirrhosis is a well-established risk factor for HCC development, including in the setting of HIV195. In studies performed in the pre-cART era, co-infection of HIV and HCV increased the risk of liver fibrosis and cirrhosis196, while cART decreased progression to cirrhosis in HIV/HCV co-infected patients197. Decreased CD4+ T-cell count (< 200–500 cells/mm3) has been associated with risk for HCC in several small studies198,199, and confirmed in a large retrospective study involving 8,563 veterans co-infected with HIV and HCV. The risk of HCC in co-infected patients was increased compared to patients infected with HCV only. A low CD4+ T-cell count (<200 cells/mm3) and having cirrhosis were also significantly associated with an increased HCC risk200. These studies raise the hypothesis that the use of cART with consequent increase in CD4+ T-cell count and decrease in cirrhosis may be beneficial in preventing HCC. Cumulative HIV viremia has recently been associated with HCC, but this effect was no longer significant after correcting for cirrhosis 152, suggesting cART is most beneficial before cirrhosis develops.
In HIV-infected patients with detectable HCV RNA, HCV therapy decreases liver-related events197 and is generally indicated. Until recently, HCV treatment with pegylated interferon-alfa and ribavirin has been the standard therapy for co-infected patients201. However, new interferon-free regimens employing direct-acting anti-HCV agents have been shown to be highly effective and less toxic than interferon-based regimens, and initial studies of HCV suggest they may be safe and effective in patients with HIV202. Treating clinicians should be aware of the rapidly changing armamentarium of FDA approved drugs for treatment of HCV.
Despite concerns for potential lead and length time bias, as well as marginal cost-effectiveness; surveillance for HCC in high-risk patients is still recommended by some professional organizations and is widely applied203, with the goal of early detection and treatment of small HCC tumors. For HBV carriers, the risk of HCC is not always associated with the development of cirrhosis, whereas the same does not hold true for HCV. Currently surveillance is recommended in cirrhotic HBV carriers, Asian HBV carriers (men over age 40 and women over age 50), HBV carriers with a family history of HCC and HCV, and cirrhotic patients203. It is reasonable to enter patients with HIV and either HBV and/or HCV into HCC surveillance programs if they meet these criteria for high risk. Periodic (6 monthly) liver ultrasound is usually employed to evaluate for nodules. Abnormal findings should be further evaluated by a 4-phase multi-detector computed tomography or a dynamic contrast enhanced magnetic resonance imaging. Specific findings on imaging are highly suggestive of HCC, and equivocal cases require a liver biopsy203.
Smoking cessation interventions
Integration of smoking cessation intervention into the care of patients with HIV is extraordinarily important, as HIV-infected smokers on effective cART lose more life-years to smoking than to HIV complications204. Smoking cessation interventions are likely to decrease the risk of most of the major NADM and have additional health benefits. The most important intervention to increase smoking cessation rates is the assessment and discussion of the topic between patients and health care providers205. Smoking cessation programs tailored to HIV infected patients have also proven to be effective in achieving this goal206,207. Patients with HIV often face significant barriers to cessation that must be recognized and addressed in order to improve the chances of successful abstinence. Such factors include low socioeconomic status, poor access to care and lack of social support, psychiatric disorders, substance and alcohol abuse and low autonomous motivation208. Patients and physicians may find themselves busier dealing with managing HIV/AIDS infectious and associated complications than focusing on smoking cessation and other forms of preventive care209. Motivational interviewing techniques with or without nicotine replacement support significantly reduce smoking rates210. Interventions that include multiple strategies through a longer period of time and that are specifically tailored to this patient population will likely yield a higher rate of smoking cessation211. For example cell-phone interviews with counseling and assessment of smoking status212 have been proposed. Varenicline tartrate, a medication indicated as an aid to smoking cessation treatment was evaluated in a HIV-infected cohort of patients on cART. Most frequently reported adverse events (AEs) were nausea (33%), abnormal dreams (31%), affect lability (19%) and insomnia (19%)213.
Although no grade 3 or 4 AEs were reported, the high frequency of side effects reported might preclude routine use of this medication in HIV patients.
Lung Cancer Screening
The National Lung Cancer Screening Trial showed that low-dose computerized tomography (CT) compared to chest X-ray for early lung cancer detection in patients aged 55–74 with 30+ year smoking histories increased the diagnosis of earlier staged tumors, and improved overall survival214,215. However, the low-dose CT is associated with a high rate of false positive findings, especially in patients with CD4+ T-cell counts less than 200 cells/mm3 who may have other pulmonary disease216, and the specificity of lung cancer screening using low-dose CT in such patients may pose additional challenges. In a prospective lung screening study of 224 HIV-infected patients with a median age of 48 years, median 34 pack-year history, and median CD4+ T-cell count of 400 cells/mm3 (range 217–568), only one lung cancer was found on incident screening with annual low-dose CT for up to 4 years217. Possible explanations for the low rate of lung cancer diagnosis include the lower age of the cohort, selection bias of recruiting “healthier” HIV patients and higher CD4+ T-cell counts. At the present time there is no solid evidence to back up the use of annual low-dose CT chest screening specifically in patients infected with HIV, although consideration of screening for patients over 55 years of age with a 30+ pack-year smoking history is reasonable, extrapolating from the HIV-uninfected population215. For lung cancer screening in HIV-infected populations, definition of appropriate characteristics of patients to include in screening, test characteristics and cost-effectiveness all require further evaluation218.
Skin cancer prevention
The risk for developing most skin cancers appears to be related largely to exposure to ultraviolet (UV) light and patient-specific characteristics (age, ethnicity and skin type). It is therefore reasonable to counsel HIV-infected patients on UV avoidance (excessive sun exposure, avoiding tanning beds) and the use of sunscreen, although these interventions have not been formally studied in HIV-infected populations. Due to the higher recurrence rates of SCC in one study, it may be also advisable to develop a close monitoring schedule after resection of NMSC in HIV-infected patients133.
Aspirin
In persons not infected with HIV, there is strong evidence that long-term (3 or more years) of low dose aspirin use reduces the incidence of cancer. Protective effects are best established for gastrointestinal cancers219,220. While aspirin has not been evaluated for cancer prevention in people with HIV, it has been studied in this population for modulation of cardiovascular risk. HIV patients have a higher risk of cardiovascular disease than the general population221,222. In addition to traditional cardiovascular risk factors, low CD4+ counts and increased inflammation are biomarkers of risk in people with HIV. Specific antiretrovirals may further modulate risk223–227. Interestingly, a pilot study in patients with HIV demonstrated that HIV infected subjects as compared to controls, have increased platelet activation. After treatment with aspirin, HIV patients exhibited decreased markers of T-cell and monocyte activation228. Given the effects of aspirin on immune activation, as well as the effect of aspirin in preventing some common cancers, further evaluation of the effect of aspirin in preventing cancer in this patient population is warranted. Aspirin use for the prevention of cardiovascular outcomes appears to be lower among many HIV-infected populations than that of the general population. There are currently no specific recommendations for aspirin prophylaxis in HIV-infected individuals. This said, given the high risk of cardiovascular events in this population; the evidence that aspirin can prevent certain types of cancer and the lack of recommendations to not use aspirin; it is reasonable to follow guidelines for the use of aspirin prevention of cardiovascular disease in the general population. Like smoking prevention, long-term low-dose aspirin use may have a more global benefit on health that may include cancer prevention.
Other cancer prevention for incidental cancers
HIV-infected patients are increasingly at risk for developing incidental cancers not associated with HIV infection. It is thus important that they also participate in generally recommended strategies to prevent and screen for cancers, including routine screening for colon cancer and breast cancer. There is emerging evidence that weight control and exercise may reduce the incidence of certain cancers, such as colon, breast, and pancreatic cancer, and this should also be encouraged. Clinicians should be alert for new developments in this area.
Future directions
Anti-inflammatory and immune modulatory approaches
Medical interventions that modulate inflammation may hold particular potential for preventing cancers in HIV-infected patients. Current and planned studies are evaluating strategies that may modulate immune activation in patients with HIV, using agents that may be of particular interest for cancer prevention in this patient population. One example is an ongoing double-blind randomized study of aspirin or placebo with a primary outcome to evaluate changes in sCD14, a marker of immune activation (clinicaltrials.gov NCT02155985). HMG-CoA reductase inhibitors (or statins) are also known to have anti-inflammatory and in vitro antineoplastic properties, such as promoting cell cycle arrest, inducing apoptosis and blocking c-Myc-induced lymphomagenesis, among others 229. Mechanistic studies have shown that the addition of rosuvastatin to tenofovir/emtricitabine and efavirenz decreased the serum mean levels of C-reactive protein and tumor necrosis factor alpha (TNF-a) when compared to anti-retroviral drugs alone20. Interestingly, a case-control study that evaluated HIV-positive patients with and without NHL found that the use of statins was associated with a lower risk of AIDS-related NHL230. A large randomized prospective study in HIV-infected patients, but no cardiovascular indications for statins, is planned comparing pitavistatin combined with cART versus cART alone to primarily evaluate cardiovascular outcomes. However, this trial will also explore incident cancers and may provide additional insights for cancer prevention in HIV-infected patients. Additional immune modulatory agents may also have cancer preventative effects in specific populations 231.
Novel anti-viral approaches
The majority of HIV-associated tumors are caused by other viruses, and this may provide opportunities for developing of additional anti-viral preventive measures in the future. One potential approach would be to develop effective vaccines for EBV, KSHV, HCV, or Merkel cell polyomavirus. For example, a recombinant EBV gp350 vaccine has been developed. While it did not prevent EBV infection in a phase 2 trial, it was found to prevent the development of mononucleosis232. Studies evaluating the use of the anti-gp350 vaccine to modulate of the natural history of EBV lymphoproliferations and prevent EBV-associated tumors, especially Burkitt lymphoma, have been proposed. It is unclear whether vaccine development is economically feasible for KSHV or Merkel cell polyoma virus.
For KSHV-associated malignancies, other anti-viral approaches are attractive. One approach would involve decreasing the transmission of KSHV. KSHV prevalence varies widely among populations. The main route of transmission of KSHV is by saliva, in contrast to other herpesviruses233. Practices that are associated with KSHV transmission include pre-mastication of food given to infants in resource-poor countries, deep kissing, and use of saliva as a lubricant for anal intercourse. It is thus possible that public health measures might be developed to reduce KSHV transmission by these routes.
Additionally, several antiviral drugs are somewhat effective against KSHV replication. In one study performed in the pre-cART era, the administration of systemic ganciclovir for AIDS-associated cytomegalovirus retinitis was found to reduce the incidence of KS234. While chronic ganciclovir is too toxic to be used for cancer prevention, other drugs with anti-KSHV effect may be developed that could be used as preventive therapy. Interestingly, nelfinavir, an HIV protease inhibitor, has recently been shown to inhibit replication of KSHV in vitro235. Furthermore, some HIV protease inhibitors may have anti-angiogenic or anti-tumor properties that may help prevent KS236 and perhaps other tumors as well. While most epidemiologic studies have not demonstrated superiority of protease inhibitor (PI)-based cART over other regimens in preventing KS237,238, a recent large Veteran’s Affair cohort study did show that ritonavir-boosted PI-based cART could reduce the incidence of KS, even after correcting for cumulative HIV viremia and CD4+ T-cell counts167. Differences in studies may be based on the time exposed to a PI-based regimen. Given the need for cART in patients with HIV, further evaluation of the effects of PI-based regimens on prevention of KS and perhaps other malignancies are important, and may inform future guidelines for “what to start” in some HIV infected populations at increased risk of developing cancer.
Conclusion
The initial decrease in ADM after cART was introduced led to an optimism that HIV-associated cancer would become much less of a clinical problem. In fact, the opposite has happened; cancer is now the leading cause of death among HIV-infected patients in a number of studies and it is vital that we consider means for effective prevention. Substantial progress has already been made on several fronts, such as decreased incidence of cancers occurring at low CD4+ T-cell counts with rollout of cART and the development of an effective HPV vaccine and effective antiviral therapy for HBV and HCV. At the same time, many challenges persist, and further improvements in cancer prevention appear highly feasible. Therefore prevention of cancer in HIV-infected patients remains a critically important area for public health interventions and future research.
Acknowledgments
Research Support: This research was supported by the Intramural Research Program, National Cancer Institute (NCI), NIH.
Footnotes
The authors have no financial disclosures or conflict of interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011 May 4;103(9):753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg MJ, Abrams DI. AIDS-defining and non-AIDS-defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007 Sep;19(5):446–451. doi: 10.1097/CCO.0b013e3282c8c90d. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg MJ, Leyden W, Warton EM, Quesenberry CP, Jr, Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013 Mar 6;105(5):350–360. doi: 10.1093/jnci/djs529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet F, Burty C, Lewden C, et al. Changes in cancer mortality among HIV-infected patients: the Mortalite 2005 Survey. Clin Infect Dis. 2009 Mar 1;48(5):633–639. doi: 10.1086/596766. [DOI] [PubMed] [Google Scholar]
- 5.When To Start C. Sterne JA, May M, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009 Apr 18;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarchoan R, Tosato G, Little RF. Therapy insight: AIDS-related malignancies--the influence of antiviral therapy on pathogenesis and management. Nat Clin Pract Oncol. 2005 Aug;2(8):406–415. doi: 10.1038/ncponc0253. quiz 423. [DOI] [PubMed] [Google Scholar]
- 8.Morlat P, Roussillon C, Henard S, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS. 2014 May 15;28(8):1181–1191. doi: 10.1097/QAD.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008 Jul 1;123(1):187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 10.Suneja G, Shiels MS, Melville SK, Williams MA, Rengan R, Engels EA. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS. 2013 Jan 28;27(3):459–468. doi: 10.1097/QAD.0b013e32835ad56e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suneja G, Shiels MS, Angulo R, et al. Cancer Treatment Disparities in HIV-Infected Individuals in the United States. J Clin Oncol. 2014 Jun 30; doi: 10.1200/JCO.2013.54.8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002 Feb 9;359(9305):497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 13.Iliyasu Z, Gajida AU, Abubakar IS, Shittu O, Babashani M, Aliyu MH. Patterns and predictors of cigarette smoking among HIV-infected patients in northern Nigeria. Int J STD AIDS. 2012 Dec;23(12):849–852. doi: 10.1258/ijsa.2012.012001. [DOI] [PubMed] [Google Scholar]
- 14.Olsen CM, Knight LL, Green AC. Risk of melanoma in people with HIV/AIDS in the pre- and post-HAART eras: a systematic review and meta-analysis of cohort studies. PLoS One. 2014;9(4):e95096. doi: 10.1371/journal.pone.0095096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996 May 10;272(5263):872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 16.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996 Jun 20;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 17.Mitsuya H, Weinhold KJ, Furman PA, et al. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan L, Engelman A. Retroviral integrase proteins and HIV-1 DNA integration. J Biol Chem. 2012 Nov 30;287(49):40858–40866. doi: 10.1074/jbc.R112.397760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer RA, Schaber MD, Skalka AM, Ganguly K, Wong-Staal F, Reddy EP. HTLV-III gag protein is processed in yeast cells by the virus pol-protease. Science. 1986 Mar 28;231(4745):1580–1584. doi: 10.1126/science.2420008. [DOI] [PubMed] [Google Scholar]
- 20.Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995 Jan 27;267(5197):483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 21.Shiels MS, Engels EA. Increased risk of histologically defined cancer subtypes in human immunodeficiency virus-infected individuals: clues for possible immunosuppression-related or infectious etiology. Cancer. 2012 Oct 1;118(19):4869–4876. doi: 10.1002/cncr.27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piriou E, van Dort K, Nanlohy NM, van Oers MH, Miedema F, van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005 Nov 1;106(9):3166–3174. doi: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 23.Guihot A, Dupin N, Marcelin AG, et al. Low T cell responses to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006 Oct 15;194(8):1078–1088. doi: 10.1086/507648. [DOI] [PubMed] [Google Scholar]
- 24.de Jong A, van Poelgeest MI, van der Hulst JM, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004 Aug 1;64(15):5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 25.Wong HL, Breen EC, Pfeiffer RM, et al. Cytokine signaling pathway polymorphisms and AIDS-related non-Hodgkin lymphoma risk in the multicenter AIDS cohort study. AIDS. 2010 Apr 24;24(7):1025–1033. doi: 10.1097/QAD.0b013e328332d5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer MJ, Micallef IN, Cerhan JR, et al. Elevated serum free light chains are associated with event-free and overall survival in two independent cohorts of patients with diffuse large B-cell lymphoma. J Clin Oncol. 2011 Apr 20;29(12):1620–1626. doi: 10.1200/JCO.2010.29.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vendrame E, Hussain SK, Breen EC, et al. Serum levels of cytokines and biomarkers for inflammation and immune activation, and HIV-associated non-Hodgkin B-cell lymphoma risk. Cancer Epidemiol Biomarkers Prev. 2014 Feb;23(2):343–349. doi: 10.1158/1055-9965.EPI-13-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996 Jun 28;272(5270):1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 29.Widney DP, Gui D, Popoviciu LM, et al. Expression and Function of the Chemokine, CXCL13, and Its Receptor, CXCR5, in Aids-Associated Non-Hodgkin’s Lymphoma. AIDS Res Treat. 2010;2010:164586. doi: 10.1155/2010/164586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003 Nov;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levesque MC, Moody MA, Hwang KK, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009 Jul 7;6(7):e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulbin H, Bode H, Stocker H, et al. Cytokine expression in the colonic mucosa of human immunodeficiency virus-infected individuals before and during 9 months of antiretroviral therapy. Antimicrob Agents Chemother. 2008 Sep;52(9):3377–3384. doi: 10.1128/AAC.00250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankaran S, George MD, Reay E, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008 Jan;82(1):538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008 Oct 1;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaulke CA, Porter M, Han YH, et al. Intestinal epithelial barrier disruption through altered mucosal microRNA expression in human immunodeficiency virus and simian immunodeficiency virus infections. J Virol. 2014 Jun;88(11):6268–6280. doi: 10.1128/JVI.00097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macal M, Sankaran S, Chun TW, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008 Nov;1(6):475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 37.Schuetz A, Deleage C, Sereti I, et al. Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation. PLoS Pathog. 2014 Dec;10(12):e1004543. doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008 Oct 1;22(15):2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 39.Marks MA, Rabkin CS, Engels EA, et al. Markers of microbial translocation and risk of AIDS-related lymphoma. AIDS. 2013 Jan 28;27(3):469–474. doi: 10.1097/QAD.0b013e32835c1333. [DOI] [PubMed] [Google Scholar]
- 40.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008 Aug 4;205(8):1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epeldegui M, Thapa DR, De la Cruz J, Kitchen S, Zack JA, Martinez-Maza O. CD40 ligand (CD154) incorporated into HIV virions induces activation-induced cytidine deaminase (AID) expression in human B lymphocytes. PLoS One. 2010;5(7):e11448. doi: 10.1371/journal.pone.0011448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epeldegui M, Breen EC, Hung YP, Boscardin WJ, Detels R, Martinez-Maza O. Elevated expression of activation induced cytidine deaminase in peripheral blood mononuclear cells precedes AIDS-NHL diagnosis. AIDS. 2007 Nov 12;21(17):2265–2270. doi: 10.1097/QAD.0b013e3282ef9f59. [DOI] [PubMed] [Google Scholar]
- 43.Pasqualucci L, Bhagat G, Jankovic M, et al. AID is required for germinal center-derived lymphomagenesis. Nat Genet. 2008 Jan;40(1):108–112. doi: 10.1038/ng.2007.35. [DOI] [PubMed] [Google Scholar]
- 44.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 45.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995 May 4;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 46.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006 Aug 1;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 47.Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996 Aug;2(8):925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 48.Butler LM, Dorsey G, Hladik W, et al. Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmission. J Infect Dis. 2009 Aug 1;200(3):430–438. doi: 10.1086/600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pauk J, Huang ML, Brodie SJ, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000 Nov 9;343(19):1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- 50.Dukers NH, Renwick N, Prins M, et al. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. American journal of epidemiology. 2000;151:213–224. doi: 10.1093/oxfordjournals.aje.a010195. [DOI] [PubMed] [Google Scholar]
- 51.Wakeham K, Webb EL, Sebina I, et al. Risk factors for seropositivity to Kaposi sarcoma-associated herpesvirus among children in Uganda. J Acquir Immune Defic Syndr. 2013 Jun 1;63(2):228–233. doi: 10.1097/QAI.0b013e31828a7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston C, Orem J, Okuku F, et al. Impact of HIV infection and Kaposi sarcoma on human herpesvirus-8 mucosal replication and dissemination in Uganda. PLoS One. 2009;4(1):e4222. doi: 10.1371/journal.pone.0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aoki Y, Tosato G. HIV-1 Tat enhances Kaposi sarcoma-associated herpesvirus (KSHV) infectivity. Blood. 2004 Aug 1;104(3):810–814. doi: 10.1182/blood-2003-07-2533. [DOI] [PubMed] [Google Scholar]
- 54.Vogel J, Hinrichs SH, Reynolds RK, Luciw PA, Jay G. The HIV tat gene induces dermal lesions resembling Kaposi’s sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- 55.Ensoli B, Gendelman R, Markham P, et al. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature. 1994 Oct 20;371(6499):674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 56.Zhou F, Xue M, Qin D, et al. HIV-1 Tat promotes Kaposi’s sarcoma-associated herpesvirus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3beta signaling pathway. PLoS One. 2013;8(1):e53145. doi: 10.1371/journal.pone.0053145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boshoff C, Endo Y, Collins PD, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278(5336):290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 58.Cesarman E, Nador RG, Bai F, et al. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999 Oct 14;18(42):5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 60.Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79:9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallaher AM, Das S, Xiao Z, et al. Proteomic screening of human targets of viral microRNAs reveals functions associated with immune evasion and angiogenesis. PLoS Pathog. 2013;9(9):e1003584. doi: 10.1371/journal.ppat.1003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamranvar SA, Masucci MG. The Epstein-Barr virus nuclear antigen-1 promotes telomere dysfunction via induction of oxidative stress. Leukemia. 2011 Jun;25(6):1017–1025. doi: 10.1038/leu.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramalingam D, Kieffer-Kwon P, Ziegelbauer JM. Emerging themes from EBV and KSHV microRNA targets. Viruses. 2012 Sep;4(9):1687–1710. doi: 10.3390/v4091687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leucci E, Onnis A, Cocco M, et al. B-cell differentiation in EBV-positive Burkitt lymphoma is impaired at posttranscriptional level by miRNA-altered expression. Int J Cancer. 2010 Mar 15;126(6):1316–1326. doi: 10.1002/ijc.24655. [DOI] [PubMed] [Google Scholar]
- 65.Nagy N, Klein G, Klein E. To the genesis of Burkitt lymphoma: regulation of apoptosis by EBNA-1 and SAP may determine the fate of Ig-myc translocation carrying B lymphocytes. Semin Cancer Biol. 2009 Dec;19(6):407–410. doi: 10.1016/j.semcancer.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Audouin J, Diebold J, Pallesen G. Frequent expression of Epstein-Barr virus latent membrane protein-1 in tumour cells of Hodgkin’s disease in HIV-positive patients. J Pathol. 1992 Aug;167(4):381–384. doi: 10.1002/path.1711670406. [DOI] [PubMed] [Google Scholar]
- 67.Uchida J, Yasui T, Takaoka-Shichijo Y, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science. 1999 Oct 8;286(5438):300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- 68.Xu D, Zhao L, Del Valle L, Miklossy J, Zhang L. Interferon regulatory factor 4 is involved in Epstein-Barr virus-mediated transformation of human B lymphocytes. J Virol. 2008 Jul;82(13):6251–6258. doi: 10.1128/JVI.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mancao C, Hammerschmidt W. Epstein-Barr virus latent membrane protein 2A is a B-cell receptor mimic and essential for B-cell survival. Blood. 2007 Nov 15;110(10):3715–3721. doi: 10.1182/blood-2007-05-090142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baumforth KR, Birgersdotter A, Reynolds GM, et al. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates Up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008 Jul;173(1):195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013 Jul 1;19(13):3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’Souza G, Carey TE, William WN, Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014 Apr 15;65(5):603–610. doi: 10.1097/QAI.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000 Sep 20;92(18):1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 74.Williams AB, Darragh TM, Vranizan K, Ochia C, Moss AR, Palefsky JM. Anal and cervical human papillomavirus infection and risk of anal and cervical epithelial abnormalities in human immunodeficiency virus- infected women. Obstet Gynecol. 1994;83(2):205–211. [PubMed] [Google Scholar]
- 75.Hariri S, Unger ER, Powell SE, et al. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis. 2012 Dec 15;206(12):1878–1886. doi: 10.1093/infdis/jis627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess Cancers Among HIV-Infected People in the United States. J Natl Cancer Inst. 2015 Apr;107(4) doi: 10.1093/jnci/dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000 Feb 23;283(8):1031–1037. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 78.Tugizov SM, Herrera R, Chin-Hong P, et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013 Nov;446(1–2):378–388. doi: 10.1016/j.virol.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konopnicki D, Manigart Y, Gilles C, et al. Sustained viral suppression and higher CD4+ T-cell count reduces the risk of persistent cervical high-risk human papillomavirus infection in HIV-positive women. J Infect Dis. 2013 Jun 1;207(11):1723–1729. doi: 10.1093/infdis/jit090. [DOI] [PubMed] [Google Scholar]
- 80.Steele JC, Mann CH, Rookes S, et al. T-cell responses to human papillomavirus type 16 among women with different grades of cervical neoplasia. Br J Cancer. 2005 Jul 25;93(2):248–259. doi: 10.1038/sj.bjc.6602679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fernandes AT, da Rocha NP, Avvad E, et al. Balance of apoptotic and anti-apoptotic marker and perforin granule release in squamous intraepithelial lesions. HIV infection leads to a decrease in perforin degranulation. Exp Mol Pathol. 2013 Oct;95(2):166–173. doi: 10.1016/j.yexmp.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Sudenga SL, Wiener HW, Shendre A, Wilson CM, Tang J, Shrestha S. Variants in interleukin family of cytokines genes influence clearance of high risk HPV in HIV-1 coinfected African-American adolescents. Hum Immunol. 2013 Dec;74(12):1696–1700. doi: 10.1016/j.humimm.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sudenga SL, Wiener HW, King CC, et al. Dense genotyping of immune-related loci identifies variants associated with clearance of HPV among HIV-positive women in the HIV epidemiology research study (HERS) PLoS One. 2014;9(6):e99109. doi: 10.1371/journal.pone.0099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010 Aug;10(8):550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 85.Tommasino M. The human papillomavirus family and its role in carcinogenesis. Semin Cancer Biol. 2014 Jun;26:13–21. doi: 10.1016/j.semcancer.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev. 2007 Jan-Mar;9(1):25–39. [PubMed] [Google Scholar]
- 87.Benhamou Y. Antiretroviral therapy and HIV/hepatitis B virus coinfection. Clin Infect Dis. 2004 Mar 1;38( Suppl 2):S98–103. doi: 10.1086/381451. [DOI] [PubMed] [Google Scholar]
- 88.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002 Dec 14;360(9349):1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 89.Pineda JA, Romero-Gomez M, Diaz-Garcia F, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005 Apr;41(4):779–789. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 90.Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003 Feb;23(1):47–58. doi: 10.1055/s-2003-37590. [DOI] [PubMed] [Google Scholar]
- 91.Feitelson MA, Lee J. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 2007 Jul 18;252(2):157–170. doi: 10.1016/j.canlet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 92.Tsai WL, Chung RT. Viral hepatocarcinogenesis. Oncogene. 2010 Apr 22;29(16):2309–2324. doi: 10.1038/onc.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gelu-Simeon M, Sobesky R, Haim-Boukobza S, et al. Do the epidemiology, physiological mechanisms and characteristics of hepatocellular carcinoma in HIV-infected patients justify specific screening policies? AIDS. 2014 Jun 19;28(10):1379–1391. doi: 10.1097/QAD.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 94.Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981 Oct 29;305(18):1067–1073. doi: 10.1056/NEJM198110293051807. [DOI] [PubMed] [Google Scholar]
- 95.Tornesello ML, Buonaguro L, Tatangelo F, Botti G, Izzo F, Buonaguro FM. Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. Genomics. 2013 Aug;102(2):74–83. doi: 10.1016/j.ygeno.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 96.Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009 Apr;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bourcier V, Winnock M, Ait Ahmed M, et al. Primary liver cancer is more aggressive in HIV-HCV coinfection than in HCV infection. A prospective study (ANRS CO13 Hepavih and CO12 Cirvir) Clin Res Hepatol Gastroenterol. 2012 Jun;36(3):214–221. doi: 10.1016/j.clinre.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 98.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007 Jun;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 99.Higgs MR, Lerat H, Pawlotsky JM. Hepatitis C virus-induced activation of beta-catenin promotes c-Myc expression and a cascade of pro-carcinogenetic events. Oncogene. 2013 Sep 26;32(39):4683–4693. doi: 10.1038/onc.2012.484. [DOI] [PubMed] [Google Scholar]
- 100.Milward A, Mankouri J, Harris M. Hepatitis C virus NS5A protein interacts with beta-catenin and stimulates its transcriptional activity in a phosphoinositide-3 kinase-dependent fashion. J Gen Virol. 2010 Feb;91(Pt 2):373–381. doi: 10.1099/vir.0.015305-0. [DOI] [PubMed] [Google Scholar]
- 101.de Sanjose S, Benavente Y, Vajdic CM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008 Apr;6(4):451–458. doi: 10.1016/j.cgh.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003 Oct;3(10):733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 103.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996 Oct 18;274(5286):430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 104.Belinsky SA, Liechty KC, Gentry FD, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006 Mar 15;66(6):3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- 105.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010 Jan 19;17(1):89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shiels MS, Pfeiffer RM, Hildesheim A, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013 Dec 18;105(24):1871–1880. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller A, Brooks GD, McLeod L, Ruwanpura S, Jenkins BJ. Differential involvement of gp130 signalling pathways in modulating tobacco carcinogen-induced lung tumourigenesis. Oncogene. 2014 Apr 14; doi: 10.1038/onc.2014.99. 0. [DOI] [PubMed] [Google Scholar]
- 108.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005 Mar 16;97(6):425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 109.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012 May 15;26(8):1017–1025. doi: 10.1097/QAD.0b013e328352d1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2011 Mar 1;117(5):1089–1096. doi: 10.1002/cncr.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mani D, Haigentz M, Jr, Aboulafia DM. Lung cancer in HIV Infection. Clin Lung Cancer. 2012 Jan;13(1):6–13. doi: 10.1016/j.cllc.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010 Oct 5;153(7):452–460. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shiels MS, Katki HA, Freedman ND, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. 2014 Nov;106(11) doi: 10.1093/jnci/dju294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chaturvedi AK, Caporaso NE, Katki HA, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010 Jun 1;28(16):2719–2726. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pine SR, Mechanic LE, Enewold L, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011 Jul 20;103(14):1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guiguet M, Boue F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009 Dec;10(12):1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 117.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010 Nov 15;116(22):5306–5315. doi: 10.1002/cncr.25311. [DOI] [PubMed] [Google Scholar]
- 118.Hou W, Fu J, Ge Y, Du J, Hua S. Incidence and risk of lung cancer in HIV-infected patients. J Cancer Res Clin Oncol. 2013 Nov;139(11):1781–1794. doi: 10.1007/s00432-013-1477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA, Study HACM. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007 Jun 20;99(12):962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 120.Beachler DC, Abraham AG, Silverberg MJ, et al. Incidence and risk factors of HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma in HIV-infected individuals. Oral Oncol. 2014 Dec;50(12):1169–1176. doi: 10.1016/j.oraloncology.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009 Aug 19;101(16):1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Persson EC, Shiels MS, Dawsey SM, Bhatia K, Anderson LA, Engels EA. Increased risk of stomach and esophageal malignancies in people with AIDS. Gastroenterology. 2012 Oct;143(4):943–950. e942. doi: 10.1053/j.gastro.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Zanet E, Berretta M, Benedetto FD, et al. Pancreatic cancer in HIV-positive patients: a clinical case-control study. Pancreas. 2012 Nov;41(8):1331–1335. doi: 10.1097/MPA.0b013e31824a0e40. [DOI] [PubMed] [Google Scholar]
- 124.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993 May 20;328(20):1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 125.Riedel DJ, Gebo KA, Moore RD, Lucas GM. A ten-year analysis of the incidence and risk factors for acute pancreatitis requiring hospitalization in an urban HIV clinical cohort. AIDS Patient Care STDS. 2008 Feb;22(2):113–121. doi: 10.1089/apc.2007.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo JJ, Jang R, Louder A, Cluxton RJ. Acute pancreatitis associated with different combination therapies in patients infected with human immunodeficiency virus. Pharmacotherapy. 2005 Aug;25(8):1044–1054. doi: 10.1592/phco.2005.25.8.1044. [DOI] [PubMed] [Google Scholar]
- 127.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007 Jul 7;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 128.Hampton T. Skin cancer’s ranks rise: immunosuppression to blame. JAMA. 2005 Sep 28;294(12):1476–1480. doi: 10.1001/jama.294.12.1476. [DOI] [PubMed] [Google Scholar]
- 129.Crum-Cianflone N, Hullsiek KH, Satter E, et al. Cutaneous malignancies among HIV-infected persons. Arch Intern Med. 2009 Jun 22;169(12):1130–1138. doi: 10.1001/archinternmed.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ducloux D, Carron PL, Racadot E, et al. CD4 lymphocytopenia in long-term renal transplant recipients. Transplant Proc. 1998 Sep;30(6):2859–2860. doi: 10.1016/s0041-1345(98)00843-4. [DOI] [PubMed] [Google Scholar]
- 131.Lobo DV, Chu P, Grekin RC, Berger TG. Nonmelanoma skin cancers and infection with the human immunodeficiency virus. Arch Dermatol. 1992 May;128(5):623–627. [PubMed] [Google Scholar]
- 132.Maurer TA, Christian KV, Kerschmann RL, et al. Cutaneous squamous cell carcinoma in human immunodeficiency virus-infected patients. A study of epidemiologic risk factors, human papillomavirus, and p53 expression. Arch Dermatol. 1997 May;133(5):577–583. [PubMed] [Google Scholar]
- 133.Hausauer AK, Maurer T, Leslie KS, Parvataneni R, Stuart SE, Chren MM. Recurrence after treatment of cutaneous basal cell and squamous cell carcinomas in patients infected with human immunodeficiency virus. JAMA Dermatol. 2013 Feb;149(2):239–241. doi: 10.1001/2013.jamadermatol.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harwood CA, Surentheran T, Sasieni P, et al. Increased risk of skin cancer associated with the presence of epidermodysplasia verruciformis human papillomavirus types in normal skin. Br J Dermatol. 2004 May;150(5):949–957. doi: 10.1111/j.1365-2133.2004.05847.x. [DOI] [PubMed] [Google Scholar]
- 135.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008 Feb 22;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lanoy E. Epidemiology, risk factor and screening for melanoma and other skin cancers. Rev Prat. 2014 Jan;64(1):31–36. [PubMed] [Google Scholar]
- 137.Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF, Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS. 2009 Jan 28;23(3):385–393. doi: 10.1097/QAD.0b013e3283213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flegg PJ. Potential risks of ultraviolet radiation in HIV infection. Int J STD AIDS. 1990 Jan;1(1):46–48. doi: 10.1177/095646249000100111. [DOI] [PubMed] [Google Scholar]
- 139.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011 Dec;20(12):2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Biggar RJ. Survival after cancer diagnosis in persons with AIDS. Journal of acquired immune deficiency syndromes. 2005;39(3):293. doi: 10.1097/01.qai.0000164033.02947.e3. [DOI] [PubMed] [Google Scholar]
- 141.Bruyand M, Thiebaut R, Lawson-Ayayi S, et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin Infect Dis. 2009 Oct 1;49(7):1109–1116. doi: 10.1086/605594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zoufaly A, Stellbrink HJ, Heiden MA, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis. 2009 Jul 1;200(1):79–87. doi: 10.1086/599313. [DOI] [PubMed] [Google Scholar]
- 143.Chao C, Leyden WA, Xu L, et al. Exposure to antiretroviral therapy and risk of cancer in HIV-infected persons. AIDS. 2012 Nov 13;26(17):2223–2231. doi: 10.1097/QAD.0b013e32835935b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trkola A, Dragic T, Arthos J, et al. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996 Nov 14;384(6605):184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 145.Silverberg MJ, Neuhaus J, Bower M, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS. 2007 Sep 12;21(14):1957–1963. doi: 10.1097/QAD.0b013e3282ed6338. [DOI] [PubMed] [Google Scholar]
- 146.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human Immunodeficiency Virus Transmission at Each Step of the Care Continuum in the United States. JAMA Intern Med. 2015 Feb 23; doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 147.Kowalkowski MA, Mims MA, Day RS, Du XL, Chan W, Chiao EY. Longer duration of combination antiretroviral therapy reduces the risk of Hodgkin lymphoma: A cohort study of HIV-infected male veterans. Cancer Epidemiol. 2014 Aug;38(4):386–392. doi: 10.1016/j.canep.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hidalgo-Tenorio C, Rivero-Rodriguez M, Gil-Anguita C, et al. Antiretroviral therapy as a factor protective against anal dysplasia in HIV-infected males who have sex with males. PLoS One. 2014;9(3):e92376. doi: 10.1371/journal.pone.0092376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chiao EY, Hartman CM, El-Serag HB, Giordano TP. The impact of HIV viral control on the incidence of HIV-associated anal cancer. J Acquir Immune Defic Syndr. 2013 Aug 15;63(5):631–638. doi: 10.1097/QAI.0b013e3182968fa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008 Apr 23;22(7):841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009 Oct 7; doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 152.Kowalkowski MA, Day RS, Du XL, Chan W, Chiao EY. Cumulative HIV viremia and non-AIDS-defining malignancies among a sample of HIV-infected male veterans. J Acquir Immune Defic Syndr. 2014 Oct 1;67(2):204–211. doi: 10.1097/QAI.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Albini L, Calabresi A, Gotti D, et al. Burden of non-AIDS-defining and non-virus-related cancers among HIV-infected patients in the combined antiretroviral therapy era. AIDS research and human retroviruses. 2013 Aug;29(8):1097–1104. doi: 10.1089/aid.2012.0321. [DOI] [PubMed] [Google Scholar]
- 154.Flagg EW, Datta SD, Saraiya M, et al. Population-based surveillance for cervical cancer precursors in three central cancer registries, United States 2009. Cancer Causes Control. 2014 May;25(5):571–581. doi: 10.1007/s10552-014-0362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Massad LS, Seaberg EC, Wright RL, et al. Squamous cervical lesions in women with human immunodeficiency virus: long-term follow-up. Obstet Gynecol. 2008 Jun;111(6):1388–1393. doi: 10.1097/AOG.0b013e3181744619. [DOI] [PubMed] [Google Scholar]
- 156.Fruchter RG, Maiman M, Sedlis A, Bartley L, Camilien L, Arrastia CD. Multiple recurrences of cervical intraepithelial neoplasia in women with the human immunodeficiency virus. Obstet Gynecol. 1996 Mar;87(3):338–344. doi: 10.1016/0029-7844(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 157.Moyer VA Force USPST. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012 Jun 19;156(12):880–891. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 158.Kaplan JE, Benson C, Holmes KH, Brooks J, Pau A, Masur H. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, The National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58( RR4):1–198. [PubMed] [Google Scholar]
- 159.Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010 Mar;201(5):681–690. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Adler DH, Kakinami L, Modisenyane T, et al. Increased regression and decreased incidence of human papillomavirus-related cervical lesions among HIV-infected women on HAART. AIDS. 2012 Aug 24;26(13):1645–1652. doi: 10.1097/QAD.0b013e32835536a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Keller MJ, Burk RD, Xie X, et al. Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA. 2012 Jul 25;308(4):362–369. doi: 10.1001/jama.2012.5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fletcher FE, Vidrine DJ, Tami-Maury I, et al. Cervical cancer screening adherence among HIV-positive female smokers from a comprehensive HIV clinic. AIDS Behav. 2014 Mar;18(3):544–554. doi: 10.1007/s10461-013-0480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mwanahamuntu MH, Sahasrabuddhe VV, Blevins M, et al. Utilization of cervical cancer screening services and trends in screening positivity rates in a ‘screen-and-treat’ program integrated with HIV/AIDS care in Zambia. PLoS One. 2013;8(9):e74607. doi: 10.1371/journal.pone.0074607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huchko MJ, Sneden J, Sawaya G, et al. Accuracy of visual inspection with acetic acid to detect cervical cancer precursors among HIV-infected women in Kenya. Int J Cancer. 2014 May 29; doi: 10.1002/ijc.28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Denny L, Kuhn L, Hu CC, Tsai WY, Wright TC., Jr Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst. 2010 Oct 20;102(20):1557–1567. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 166.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012 May;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 167.Kowalkowski MA, Kramer JR, Richardson PR, Suteria I, Chiao EY. Use of Boosted Protease Inhibitors Reduces Kaposi Sarcoma Incidence Among Male Veterans With HIV Infection. Clin Infect Dis. 2015 Jan 13; doi: 10.1093/cid/civ012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008 May 20;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 169.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV- positive men. JAMA. 1999;281(19):1822–1829. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 170.Kreuter A, Potthoff A, Brockmeyer NH, et al. Imiquimod leads to a decrease of human papillomavirus DNA and to a sustained clearance of anal intraepithelial neoplasia in HIV-infected men. J Invest Dermatol. 2008 Aug;128(8):2078–2083. doi: 10.1038/jid.2008.24. [DOI] [PubMed] [Google Scholar]
- 171.Stier EA, Goldstone SE, Einstein MH, et al. Phase IIA trial of 1% topical cidofovir for treatment of high-grade perianal squamous intraepithelial neoplasia in HIV-infected men and women (AMC046). 12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies; April 27, 2010, 2010; Bethesda, MD. [Google Scholar]
- 172.Stier EA, Goldstone SE, Berry JM, et al. Infrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: an AIDS malignancy consortium pilot study. J Acquir Immune Defic Syndr. 2008 Jan 1;47(1):56–61. doi: 10.1097/QAI.0b013e3181582d93. [DOI] [PubMed] [Google Scholar]
- 173.Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001 Feb 21;93(4):284–292. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 174.Kemp TJ, Safaeian M, Hildesheim A, et al. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix((R)) Vaccine. 2012 Dec 17;31(1):165–170. doi: 10.1016/j.vaccine.2012.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.FDA News Release.
- 176.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007 May 10;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 177.Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011 Oct 27;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 178.Saslow D, Castle PE, Cox JT, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007 Jan-Feb;57(1):7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 179.Petrosky E, Bocchini JA, Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015 Mar 27;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 180.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014 Aug 29;63(RR-05):1–30. [PubMed] [Google Scholar]
- 181.Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014 - United States. MMWR Morb Mortal Wkly. 2014 Jul 25;Rep;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 182.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012 Apr;54(7):891–898. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- 183.Levin MJ, Moscicki AB, Song LY, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010 Oct;55(2):197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Toft L, Storgaard M, Muller M, et al. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis. 2014 Apr 15;209(8):1165–1173. doi: 10.1093/infdis/jit657. [DOI] [PubMed] [Google Scholar]
- 185.Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010 Oct 15;202(8):1246–1253. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Denny L, Hendricks B, Gordon C, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine. 2013 Nov 19;31(48):5745–5753. doi: 10.1016/j.vaccine.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 187.Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in HIV-1-Infected Women. Clin Infect Dis. 2014 Apr 9; doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997 Apr;11(5):597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 189.Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13( Suppl 1):S47–49. doi: 10.1016/0264-410x(95)80050-n. [DOI] [PubMed] [Google Scholar]
- 190.Masur H, Kaplan JE, Holmes KK, Service USPH Infectious Diseases Society of A. Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann Intern Med. 2002 Sep 3;137(5 Pt 2):435–478. doi: 10.7326/0003-4819-137-5_part_2-200209031-00002. [DOI] [PubMed] [Google Scholar]
- 191.Potsch DV, Oliveira ML, Ginuino C, et al. High rates of serological response to a modified hepatitis B vaccination schedule in HIV-infected adults subjects. Vaccine. 2010 Feb 10;28(6):1447–1450. doi: 10.1016/j.vaccine.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 192.European AIDS Clinical Society Guidelines. Jun, 2014. version 7.02. [Google Scholar]
- 193.Bharti AR, Letendre SL, Wolfson T, et al. Clinical variables identify seronegative HCV co-infection in HIV-infected individuals. J Clin Virol. 2011 Dec;52(4):328–332. doi: 10.1016/j.jcv.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lambers FA, Prins M, Thomas X, et al. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS. 2011 Nov 13;25(17):F21–27. doi: 10.1097/QAD.0b013e32834bac44. [DOI] [PubMed] [Google Scholar]
- 195.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992–2001. Arch Intern Med. 2004 Nov 22;164(21):2349–2354. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 196.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999 Oct;30(4):1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 197.Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012 Jul 25;308(4):370–378. doi: 10.1001/jama.2012.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Clifford GM, Rickenbach M, Polesel J, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008 Oct 18;22(16):2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 199.Bruyand M, Dabis F, Vandenhende MA, et al. HIV-induced immune deficiency is associated with a higher risk of hepatocarcinoma, ANRS CO3 Aquitaine Cohort, France, 1998–2008. J Hepatol. 2011 Nov;55(5):1058–1062. doi: 10.1016/j.jhep.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 200.Kramer JR, Kowalkowski MA, Duan Z, Chiao EY. The Effect of HIV Viral Control on the Incidence of Hepatocellular Carcinoma in Veterans with Hepatitis C and HIV Coinfection. J Acquir Immune Defic Syndr. 2014 Dec 31; doi: 10.1097/QAI.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002 Sep 26;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 202.Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014 Jul 23–30;312(4):353–361. doi: 10.1001/jama.2014.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bruix J, Sherman M American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011 Mar;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Helleberg M, Afzal S, Kronborg G, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013 Mar;56(5):727–734. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- 205.Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2004;(4):CD000165. doi: 10.1002/14651858.CD000165.pub2. [DOI] [PubMed] [Google Scholar]
- 206.Vidrine DJ, Fletcher FE, Buchberg MK, Li Y, Arduino RC, Gritz ER. The influence of HIV disease events/stages on smoking attitudes and behaviors: project STATE (Study of Tobacco Attitudes and Teachable Events) BMC Public Health. 2014;14:149. doi: 10.1186/1471-2458-14-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Humfleet GL, Hall SM, Delucchi KL, Dilley JW. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine Tob Res. 2013 Aug;15(8):1436–1445. doi: 10.1093/ntr/ntt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shirley DK, Kesari RK, Glesby MJ. Factors associated with smoking in HIV-infected patients and potential barriers to cessation. AIDS Patient Care STDS. 2013 Nov;27(11):604–612. doi: 10.1089/apc.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. Decreased awareness of current smoking among health care providers of HIV-positive compared to HIV-negative veterans. J Gen Intern Med. 2007 Jun;22(6):749–754. doi: 10.1007/s11606-007-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction. 2009 Nov;104(11):1891–1900. doi: 10.1111/j.1360-0443.2009.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moscou-Jackson G, Commodore-Mensah Y, Farley J, DiGiacomo M. Smoking-cessation interventions in people living with HIV infection: a systematic review. J Assoc Nurses AIDS Care. 2014 Jan-Feb;25(1):32–45. doi: 10.1016/j.jana.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vidrine DJ, Marks RM, Arduino RC, Gritz ER. Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine Tob Res. 2012 Jan;14(1):106–110. doi: 10.1093/ntr/ntr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cui Q, Robinson L, Elston D, et al. Safety and tolerability of varenicline tartrate (Champix((R))/Chantix((R))) for smoking cessation in HIV-infected subjects: a pilot open-label study. AIDS Patient Care STDS. 2012 Jan;26(1):12–19. doi: 10.1089/apc.2011.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013 Sep 5;369(10):920–931. doi: 10.1056/NEJMoa1208962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.National Lung Screening Trial Research T. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011 Aug 4;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sigel K, Wisnivesky J, Shahrir S, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS. 2014 Apr 24;28(7):1007–1014. doi: 10.1097/QAD.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hulbert A, Hooker CM, Keruly JC, et al. Prospective CT screening for lung cancer in a high-risk population: HIV-positive smokers. J Thorac Oncol. 2014 Jun;9(6):752–759. doi: 10.1097/JTO.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. [Accessed July 2014];Early Lung Cancer Diagnosis in HIV Infected Population With an Important Smoking History With Low Dose CT: a Pilot Study. (EP48 HIV CHEST) NCT01207986. http://clinicaltrials.gov/ct2/show/NCT01207986.
- 219.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012 Apr 28;379(9826):1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 220.Cuzick J, Thorat MA, Bosetti C, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015 Jan;26(1):47–57. doi: 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Petoumenos K, Reiss P, Ryom L, et al. Increased risk of cardiovascular disease (CVD) with age in HIV-positive men: a comparison of the D:A:D CVD risk equation and general population CVD risk equations. HIV Med. 2014 Nov;15(10):595–603. doi: 10.1111/hiv.12162. [DOI] [PubMed] [Google Scholar]
- 222.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012 May 22;59(21):1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010 Aug 15;51(4):435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 224.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011 Jul;217(1):207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Satchell CS, Cotter AG, O’Connor EF, et al. Platelet function and HIV: a case-control study. AIDS. 2010 Mar 13;24(5):649–657. doi: 10.1097/QAD.0b013e328336098c. [DOI] [PubMed] [Google Scholar]
- 226.Young J, Xiao Y, Moodie EE, et al. The effect of cumulating exposure to abacavir on the risk of cardiovascular disease events in patients from the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2015 Apr 28; doi: 10.1097/QAI.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 227.Friis-Moller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2015 Apr 16; doi: 10.1177/2047487315579291. [DOI] [PubMed] [Google Scholar]
- 228.O’Brien M, Montenont E, Hu L, et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr. 2013 Jul 1;63(3):280–288. doi: 10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005 Dec;5(12):930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 230.Kohl NE, Emini EA, Schleif WA, et al. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sereti I, Estes JD, Thompson WL, et al. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014 Jan;10(1):e1003890. doi: 10.1371/journal.ppat.1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sokal EM, Hoppenbrouwers K, Vandermeulen C, et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis. 2007 Dec 15;196(12):1749–1753. doi: 10.1086/523813. [DOI] [PubMed] [Google Scholar]
- 233.Martin JN. Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 and Kaposi sarcoma. Adv Dent Res. 2011 Apr;23(1):76–78. doi: 10.1177/0022034511399913. [DOI] [PubMed] [Google Scholar]
- 234.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999 Apr 8;340(14):1063–1070. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 235.Gantt S, Carlsson J, Ikoma M, et al. The HIV protease inhibitor nelfinavir inhibits Kaposi’s sarcoma-associated herpesvirus replication in vitro. Antimicrob Agents Chemother. 2011 Jun;55(6):2696–2703. doi: 10.1128/AAC.01295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sgadari C, Barillari G, Toschi E, et al. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002 Mar;8(3):225–232. doi: 10.1038/nm0302-225. [DOI] [PubMed] [Google Scholar]
- 237.Grabar S, Abraham B, Mahamat A, Del Giudice P, Rosenthal E, Costagliola D. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. J Clin Oncol. 2006 Jul 20;24(21):3408–3414. doi: 10.1200/JCO.2005.05.4072. [DOI] [PubMed] [Google Scholar]
- 238.Portsmouth S, Stebbing J, Gill J, et al. A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi’s sarcoma. AIDS. 2003 Jul 25;17(11):F17–22. doi: 10.1097/00002030-200307250-00001. [DOI] [PubMed] [Google Scholar]