Abstract
Background
Elevated levels of aldosterone are a modifiable contributor to clinical worsening in heart failure with reduced ejection fraction (HFrEF). Endothelin-1 (ET-1), which is increased in HFrEF, induces pulmonary endothelial aldosterone synthesis in vitro. However, whether transpulmonary aldosterone release occurs in humans or aldosterone relates to functional capacity in HFrEF is not known. Therefore, we aim to characterize ET-1 and transpulmonary aldosterone levels in HFrEF, and determine if aldosterone levels relate to peak volume of oxygen uptake (pVO2).
Methods
Data from 42 consecutive HFrEF patients and 18 controls referred for invasive cardiopulmonary exercise testing were analyzed retrospectively.
Results
Radial ET-1 levels were higher in HFrEF patients compared to controls (17.5 [11.5–31.4] vs. 11.5 [4.4–19.0] pg/ml, p=0.04). A significant ET-1 transpulmonary gradient (pulmonary arterial [PA] - radial arterial levels) was present in HFrEF (P<0.001), but not in controls (P=0.24). Compared to controls, aldosterone levels were increased in HFrEF patients in the PA (364 [250–489] vs. 581 [400–914] ng/dl, P<0.01) and radial compartments (366 [273–466] vs. 702 [443–1223] ng/dl, P<0.001). Akin to ET-1, a transpulmonary increase in aldosterone concentration was also observed between controls and HFrEF patients at rest (7.5 [−54–40] vs. 61.6 [−13.6–165] ng/dl, P=0.01) and peak exercise (−20.7 [−39.6–79.1] vs. +25.8 [−29.2–109.3] ng/dl, P=0.02). The adjusted pVO2 correlated inversely with aldosterone levels at peak activity in the PA (r=−0.31, P=0.01) and radial artery (r=−0.32, P=0.01).
Conclusions
These data provide preliminary evidence in support of increased transpulmonary aldosterone levels in HFrEF and suggest an inverse relationship between circulating aldosterone and pVO2. Future prospective studies are needed to characterize the functional effects of transpulmonary and circulating aldosterone on cardiac reserve capacity in HFrEF.
Keywords: Aldosterone, exercise, heart failure, cardiac reserve
INTRODUCTION
Elevated circulating levels of the mineralocorticoid hormone aldosterone are observed in left ventricular (LV) systolic dysfunction and contribute to the HFrEF syndrome, in part, by promoting myocardial fibrosis and a hypertrophic vasculopathy in systemic blood vessels that impairs vascular compliance. (1–3) Pharmacological inhibition of the mineralocorticoid receptor, in turn, abrogates the adverse effects of aldosterone on pulmonary and systemic vascular function, and improves cardiac output in experimental models of HF suggesting that hyperaldosteronism may promote impaired exercise tolerance. (4–6) However, the ramifications of maladaptive changes to cardiovascular function mediated by increased aldosterone on the clinical profile of HFrEF patients, including exercise reserve capacity (peak volume of oxygen consumption [pVO2]), are not well established.
Endocrine or paracrine functionality of the lung-pulmonary vascular axis is recognized increasingly as a potential contributor to the pathophysiology of HF. For example, pulmonary arterial levels of the vasoactive peptide endothelin-1 (ET-1) correlate positively with pulmonary artery pressure in patients with HFrEF and are thought to increase pulmonary vascular resistance, (7) while transpulmonary differences in the vasodilator molecule cyclic guanosine monophosphate (cGMP) identify a high-risk HFrEF pathophenotype. (8) Despite these findings highlighting the prognostic and functional significance of pulmonary vasoactive small molecules in HF, our understanding of transpulmonary biochemical profiles and their functional significance in HF remains limited.
We have demonstrated recently that ET-1 induces pulmonary endothelial synthesis of functionally relevant aldosterone in vitro at levels akin to those observed in heart failure, (4) raising the possibility that extra-adrenal aldosterone synthesis may occur in human HFrEF. Thus, in the current study, we investigated the hypothesis that transpulmonary aldosterone release occurs in the setting of increased pulmonary arterial ET-1 and is related to impaired cardiac reserve capacity in HFrEF.
METHODS
Patient population
The study was approved by the Partners Human Research Committee (Number 20100P1704) and complies with the Declaration of Helsinki. Written informed consent for study participation was obtained from all subjects who participated in this study. We analyzed data from 42 consecutive HF patients and 18 controls evaluated at the Massachusetts General Hospital for HFrEF or unexplained dyspnea, respectively, over a 6-year period. All HFrEF patients had an LV ejection fraction (LVEF) ≤0.40 and chronic New York Heart Association functional class II-IV symptoms. Control patients were characterized by exertional dyspnea despite normal LVEF or an evident pulmonary mechanical, cardiovascular, or metabolic limit to exercise. None of the patients investigated had cardiac angina or ECG-based evidence of active myocardial ischemia during exercise.
Cardiopulmonary exercise testing
All patients underwent invasive cardiopulmonary exercise testing (iCPET) according to methods reported previously. (9) Briefly, patients were instructed to fast for at least 6 hrs prior to testing, which generally occurred in the hours between 9AM and 12PM. Pulmonary and systemic arterial catheters were inserted via the internal jugular vein and radial artery, respectively. A uniform approach to standardizing the ‘zero pressure line’ was adopted whereby the axilla was used to estimate the level of the mid-right atrium on all study subjects. Resting first pass radionuclide ventriculography was used to confirm appropriate catheter positioning at the mid right atrium. Additionally, right and left ventricular EF was assessed by first-pass radionuclide ventriculography immediately prior to cycle ergometry testing and during the final minute of incremental exercise. (10) Study participants underwent maximum incremental upright cycle ergometry iCPET (5– 15 Watt/min continuous ramp after an initial 3 min period of unloaded exercise, MedGraphics, St. Paul, MN) with simultaneous hemodynamic monitoring (Witt Biomedical Inc, Melbourne, FL) as previously described. (10,11) End-expiratory hemodynamic measurements were obtained in the upright position while patients were seated on the cycle at rest, and at one-minute intervals during exercise for each of the following: right atrial pressure (RAP), pulmonary arterial pressure (PAP), pulmonary artery wedge pressure (PAWP), and mean systemic arterial pressure (MAP). Direct Fick cardiac outputs (CO) were determined using standard methods, (10) and pVO2 was defined as the average maximum O2 measured over 30 seconds during the final minute of symptom-limited exercise as previously reported. (10,11) All patients exceeded their anaerobic threshold as determined by the V-slope method.
Sample processing
All patients underwent elective cardiac catheterization immediately prior to testing, and, thus, had confirmed nil per os dietary status (with the exception of adherence to baseline oral medication use) for ≥6 hrs prior to sample collection. Whole blood samples from the pulmonary arterial (PA) and radial compartments were collected in the upright position at rest and during peak exercise (i.e., final minute of symptom-limited exercise) via the PA catheter or radial artery catheter, respectively. Following acquisition, samples were centrifuged immediately at 1200 RPM for 10 min at 4 °C. The plasma was collected and immediately stored at −80 °C as reported previously. (12) Aldosterone levels were analyzed by immunoassay (Cayman; detection limit 21 pg/ml, mean intra-assay variation=10.7%, inter-assay variation 15.4%) by an experienced laboratory scientist who was blinded to the patients’ clinical data, according to methods reported previously. (12) Similarly, plasma ET-1 levels were analyzed by immunoassay according to manufacturers instructions (Cayman; lower detection limit 7.8 pg/ml, mean intra-assay variation=10%, inter-assay variation 10%).
A total of 480 plasma samples were assayed for aldosterone (N=240) or ET-1 (N=240). Aldosterone levels were recorded for all plasma samples and included in each of the analyses. However, a technical complication prevented the completion of the immunoassay of ET-1 in one HFrEF patient and 4 control patients, and an insufficient quantity of plasma was available for repeat analysis. Overall, data from 96% of all possible aldosterone/ET-1 samples were available for analysis. Of these, all (100%) were included in the analyses. All sample measurements and analyses were performed with knowledge of the sample number only, without any associated clinical data.
Statistical analyses
All statistical analyses were performed using Origin 9.1 (Northampton, MA) or the STATA 10.0 software package (StataCorp LP, College Station, TX). Categorical variables are reported as frequencies with percentages. The Wilk-Shapiro test was used to assess the normality of distribution of the data. All continuous, normally distributed data are reported as mean ± SEM unless otherwise indicated, whereas non-normally distributed data are reported as median (interquartile range). Group differences in normally distributed baseline characteristics were analyzed using the Student t test. For clinical characteristics, comparisons between the groups for continuous variables were performed using an unpaired 2-sample t test. For comparison of differences in biochemical data by vascular compartment between rest and exercise within the same subject, a paired 2-sample t test was used. Differences in biochemical data between groups for data not normally distributed (non-parametric data) were analyzed using the Mann-Whitney test. Univariate logistic regression analyses were used to assess the relationship between aldosterone and pVO2, which are presented as Spearman correlation coefficients. Statistical significance was defined as p<0.05.
RESULTS
Patient demographics
Baseline clinical characteristics, exercise performance data, and pharmacotherapeutic profile for controls (N=18; 7 males) and HFrEF patients (N=42; 20 males) are provided in Tables 1 and 2. Compared to HFrEF patients, controls did not differ significantly by age (57 vs. 51, P=0.13), body mass index (BMI) (28 ± 0.8 vs. 29 ± 1.2 kg/m2, P=0.70), or resting heart rate (74 ± 1.9 vs. 75 ± 3.0 beats/min, P=0.74), although a trend toward higher hemoglobin levels was observed (11 ± 0.7 vs. 13 ± 0.4 gm/dL, P=0.07). A 1:2 ratio of spironolactone:eplerenone dose equivalents was used to convert all mineralocorticoid receptor antagonist doses to spironolactone equivalents. We observed a narrow dose range among study subjects who were taking mineralocorticoid receptor antagonists (N=17, range 12.5–25 mg daily, mean dose 18.7 mg daily).
Table 1.
Patient demographics and exercise performance data
| Control (N=14) | HFrEF (N=42) | P Value | |
|---|---|---|---|
| Male (%) | 7 (39) | 20 (47) | 0.60 |
| Age (yr)(range) | 51 [18–79] | 57 [23–82] | 0.17 |
| BMI (kg/m2) | 29 (1.2) | 28 (0.8) | 0.70 |
| Hgb (gm/dL) | 13 (0.4) | 11 (0.7) | 0.07 |
| pVO2 (ml/kg*min) | 25 (1.7) | 12 (0.5) | <0.001 |
| pVO2 Max (% predicted) | 94 (2.5) | 47 (2.6) | <0.001 |
| Resting HR (BPM) | 75 (3.0) | 74 (1.9) | 0.74 |
| Maximum HR (BPM) | 154 (3.9) | 115 (3.6) | <0.001 |
| O2 saturation (rest) | 97% (2) | 97% (2) | 0.12 |
| O2 saturation (peak) | 97% (4) | 96% (2) | 0.87 |
| Maximum SBp (mmHg) | 185 (6.3) | 153 (5.5) | <0.01 |
| Maximum DBp (mmHg) | 90 (2.8) | 75.7 (2.5) | <0.01 |
| Resting RVEF (%) | 51 (1.8) | 39 (1.6) | <0.001 |
| Peak RVEF (%) | 54 (2.2) | 38 (1.6) | <0.001 |
| Resting LVEF (%) | 64 [62–67] | 32 [29–41] | <0.001 |
| Peak LVEF (%) | 67 [64–71] | 36 [27–42] | <0.001 |
| FEV-1 % predicted | 101 (15) | 71 (21) | <0.001 |
| Comorbidities (%) | |||
| Hypertension | 7 (35) | 29 (70) | |
| Diabetes | 0 (0) | 10 (23) | |
| Hyperlipidemia | 3 (15) | 31 (74) | |
| Stroke | 0 (0) | 2 (4) | |
| Prior MI | 0 (0) | 42% | |
| Prior/Active Tobacco Use | 6 (30) | 22 (52) | |
| Atrial fibrillation | 0 (0) | 1 (3) | |
| Ventricular pacing | 0 (0) | 3 (7) | |
Clinical characteristics were collected from heart failure patients with reduced ejection fraction (HFrEF) or unexplained dyspnea (controls) referred for invasive cardiopulmonary exercise testing. Age is reported as mean (range). For normally distributed data, results are reported as mean (SE). For data not distributed normally, results are reported as median [interquartile range]. rest, value acquired at rest; peak; value acquired at peak exercise; BMI, body mass index; Hgb, hemoglobin level; pVO2, peak volume of oxygen consumption; HR; heart rate; BPM, beats per minute; SBp systolic blood pressure; DBp, diastolic blood pressure; RVEF, right ventricular ejection fraction; LVEF, left ventricular ejection fraction; FEV-1, forced expiratory volume in 1 second; MI, myocardial infarction.
Table 2.
Selected pharmacotherapies for controls and heart failure with reduced left ventricular ejection fraction (HFrEF) patients
| Controls (%) | HFrEF (%) | |
|---|---|---|
| Loop or thiazide diuretic | 3 (17) | 34 (81) |
| β-receptor antagonist | 3 (17) | 30 (71) |
| Aspirin | 5 (28) | 27 (64) |
| ACE-inhibitor | 2 (11) | 21 (50) |
| Mineralocorticoid Receptor Antagonists | 0 (0) | 17 (40) |
|
HMG-CoA Reductase Inhibitor (Statin) |
2 (11) | 17 (40) |
| ARB | 1 (6) | 8 (19) |
| Calcium channel antagonist | 4 (22) | 6 (14) |
| Non-statin lipid therapy | 0 (0) | 6 (12) |
| Nitroglycerin | 0 (0) | 5 (12) |
| Insulin | 0 (0) | 4 (10) |
| Metformin | 0 (0) | 2 (5) |
| Glitazone | 0 (0) | 1 (2) |
Pharmacotherapy data was collected from HFrEF patients (N=42) or unexplained dyspnea (controls)(N=18) referred for invasive cardiopulmonary exercise testing. ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
At least moderate valvular heart disease was present in 16% of HFrEF patients while 8% of patients had evident left ventricular hypertrophy. Serum creatinine levels for the HFrEF group were 1.4 ± 0.1g/dl. HFrEF patients relative to controls were characterized by decreased resting right ventricular EF (39 ± 1.6 vs. 51 ± 1.8 %, P<0.001), LVEF (32 [29–41] vs. 64 [62–67] %, P<0.001), and cardiac output (4.0 ± 0.1 vs. 6.0 ± 0.3 l/min, P<0.001). Invasively assessed resting cardiopulmonary hemodynamic measurements were also increased significantly in HFrEF patients compared to controls for mPAP (24 ± 1.3 vs. 14 ± 0.4 mmHg, P<0.001), pulmonary vascular resistance (2.9 ± 0.2 vs. 1.6 ± 0.1 Wood units, P<0.001), and PAWP (13 ± 1.2 vs. 4.8 ± 0.4 mm Hg, P<0.001).
Patient exercise hemodynamics and functional performance
To determine if exercise cardiac reserve capacity (i.e., peak VO2 and cardiopulmonary hemodynamics) corresponded to baseline resting cardiac function data, differences in iCPET variables measured at peak exercise were analyzed between groups (Table 3). Compared to controls, the blood pressure and heart rate responses to exercise were attenuated significantly in HFrEF patients, which was associated with significant differences between groups for mPAP (33 ± 1.5 vs. 44 ± 1.7 mmHg, P<0.001), PAWP (20 ± 1.4 vs. 27 ± 1.5 mmHg, P<0.01), cardiac output (CO) at peak exercise (15 ± 0.6 vs. 7.7 ± 0.5 l/min, P<0.001), as well as diminished CO augmentation from rest to exercise (ΔCO) (+9.3 ± 0.5 vs. +3.6 ± 0.4 l/min, P<0.001).
Table 3.
Patient cohort cardiopulmonary hemodynamics measured at rest and at peak exercise during invasive cardiopulmonary exercise testing.
| Control (N=14) | HFrEF (N=42) | P Value | |
|---|---|---|---|
| Resting CO (L/min) | 6.0 (0.3) | 4.0 (0.1) | <0.001 |
| Peak CO (L/min) | 15 (0.6) | 7.7 (0.5) | <0.001 |
| ΔCO (L/min) | 9.3 (0.5) | 3.6 (0.4) | <0.001 |
| Resting mRAP (mmHg) | 2.2 (0–3.0) | 4.0 (0.5) | <0.05 |
| Peak mRAP (mmHg) | 8.7 (0.7) | 11.6 (1.1) | 0.10 |
| Resting mPAP (mmHg) | 14 (0.5) | 24 (1.3) | <0.001 |
| Peak mPAP (mmHg) | 33 (1.5) | 44 (1.7) | <0.001 |
| ΔmPAP (mmHg) | 19 (1.3) | 18 (1.4) | 0.80 |
| Resting PAWP (mmHg) | 4.9 (0.3) | 13 (1.3) | <0.001 |
| Peak PAWP (mmHg) | 20 (1.4) | 27 (1.5) | <0.01 |
| ΔPAWP (mmHg) | 15 (1.5) | 14 (1.5) | 0.39 |
| Resting PVR (W.U.) | 1.6 (0.1) | 2.9 (0.2) | <0.001 |
| Peak PVR Exercise (W.U.) | 0.9 (0.1) | 2.3 (0.2) | <0.001 |
| ΔPVR (W.U.) | −0.7 (0.1) | −0.6 (0.2) | 0.60 |
Cardiopulmonary hemodynamics were measured invasively in heart failure patients with reduced ejection fraction (HFrEF) or unexplained dyspnea (controls) referred for cardiopulmonary exercise testing. For normally distributed data, results are reported as mean (SEM). For data not distributed normally, results are reported as median (interquartile range). CO, cardiac output; mRAP, mean right atrial pressure; mPAP, mean pulmonary artery pressure; PAWP, end-expiratory pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; W.U., Wood unit; Δ, difference between values measured at peak exercise and rest.
The functional relevance of abnormal cardiopulmonary hemodynamics in HFrEF was assessed next. Compared to controls, HFrEF patients demonstrated lower pVO2 (25 ± 1.7 vs. 12 ± 0.5 ml/kg*min, P<0.001) and pVO2 expressed as percent of predicted for age, sex, and weight (pVO2%) (94 ± 2.5 vs. 47 ± 2.6 %, P<0.001). Moreover, a significant positive correlation was observed between pVO2% and CO (r=+0.84, P<0.00001), and pVO2% and ΔCO (r=+0.82, P<0.00001).
Plasma endothelin-1
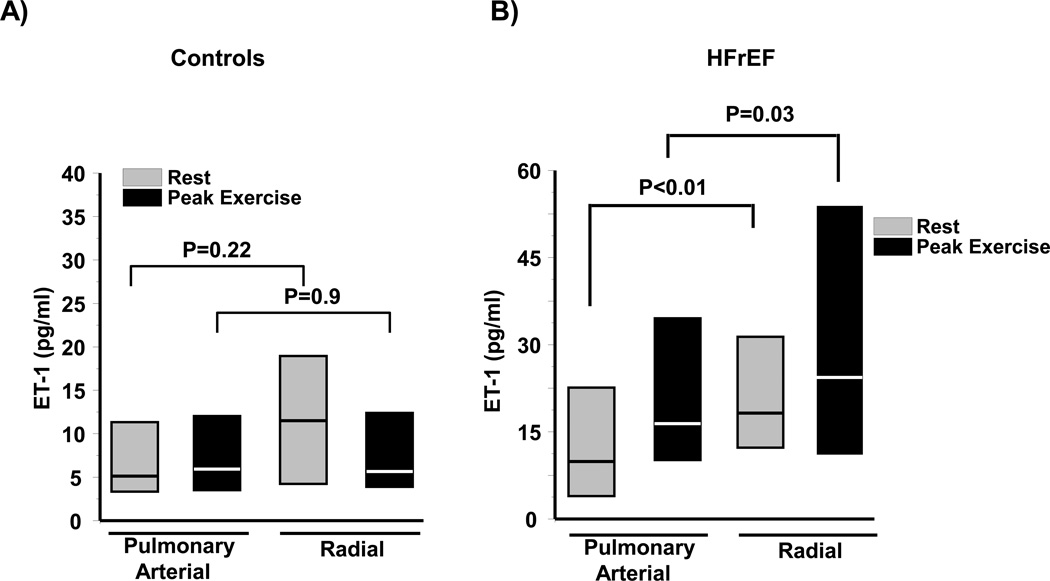
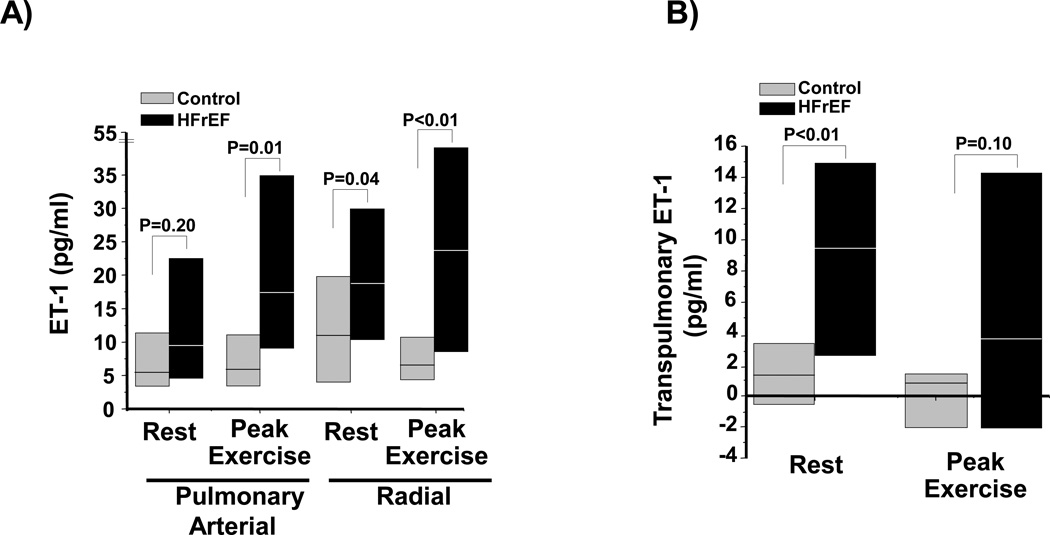
In controls, we observed no significant difference between PA and systemic (radial) plasma ET-1 levels at rest (5.2 [3.4–11.3] vs. 11.5 [4.4–19.0] pg/ml, P=0.22) or at peak exercise (6.1 [3.7–11.9] vs. 6.4 [4.2–11.8] pg/ml, P=0.9) (Figure 1A). By contrast, in HFrEF patients a significant increase in ET-1 levels between the PA and radial compartments was observed at rest (8.7 [5.0–20.6] vs. 17.5 [11.5–31.4] pg/ml, P<0.01) and at peak exercise (17.1 [9.0–35.0] vs. 24.7 [9.8–53.0] pg/ml, P=0.03) (Figure 1B). We investigated if patient volume status influenced these findings, but observed no significant correlation between right atrial pressure and ET-1 levels in the PA or radial artery at rest (r=−0.17, P=0.98 and r=−0.13, P=0.34, respectively) or at peak exercise (r=0.00, P=0.98 and r=−0.05, P=0.67, respectively). Between group comparisons demonstrated that during exercise, ET-1 levels were higher in HFrEF patients than controls in the PA (P=0.01) and radial artery (P<0.01), as well as in the radial artery at rest (P=0.04)(Figure 2A). These findings corresponded to a significant increase in the transpulmonary ET-1 gradient in HFrEF compared to controls at rest (9.4 [2.8–15.3] vs. 1.4 [−0.4–27] pg/ml, P<0.01), and a directionally similar trend in transpulmonary gradient between these groups at peak exercise (3.6 [−2.2–14.1] vs. 0.8 [−1.9–1.7] pg/ml, P=0.10)(Figure 2B).
Figure 1. Plasma endothelin-1 (ET-1) levels measured in controls and heart failure patients with reduced left ventricular ejection fraction (HFrEF).
Plasma from the pulmonary or radial artery harvested from (A) controls (N=14) or (B) HFrEF patients (N=41) at rest and peak exercise during invasive cardiopulmonary exercise testing was analyzed for ET-1 levels. Data are presented as median (interquartile range).
Figure 2. Transpulmonary ET-1 levels in controls and paitients with heart failure with reduced left ventricular ejection fraction (HFrEF).
(A) Plasma from the pulmonary or radial artery harvested from controls (N=14) or HFrEF patients (N=41) at rest and peak exercise during invasive cardiopulmonary exercise testing was analyzed for endothelin-1 (ET-1) levels. (B) The transpulmonary gradient was calculated by the difference in ET-1 levels between the radial and pulmonary arterial samples for each patient. Data are presented as median (interquartile range).
Transpulmonary aldosterone levels are increased in HFrEF patients
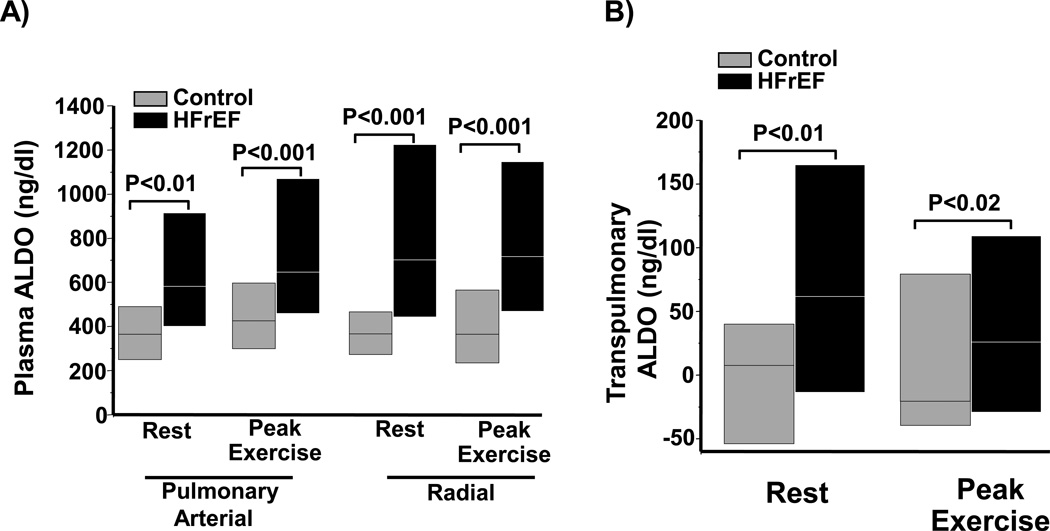
Based on our prior findings demonstrating that ET-1 stimulates aldosterone synthesis in pulmonary endothelial cells in vitro, (8) we next explored whether elevated ET-1 levels observed in HFrEF patients correspond to directionally similar differences in plasma aldosterone levels. Compared to controls, resting plasma aldosterone levels were increased significantly in HFrEF patients in the PA (364 [250–489] vs. 581 [400–914] ng/dl, P<0.01) and radial compartments (366 [273–466] vs. 702 [443–1223] ng/dl, P<0.001), which paralleled findings at peak exercise from the PA (425 [299–596] vs. 646 [459–1069] ng/dl, P<0.001) and radial artery (364 [234–565] vs. 717 [469–1146] ng/dl, P<0.001)(Figure 3A). We next investigated whether renal function or pharmacotherapeutic status could account for differences in aldosterone levels between groups. No significant correlation was observed between serum creatinine level and PA or radial aldosterone levels at rest (r=0.15, P=0.38 and r=0.16, P=0.33, respectively) or at peak exercise (r=0.13, P=0.44 and r=0.17, P=0.29, respectively), nor were significant differences identified in aldosterone levels across vascular compartments at rest or peak activity among HFrEF patients according to mineralocorticoid receptor antagonist treatment status (Table 4). Moreover, we found no significant difference in pulmonary arterial aldosterone levels between HFrEF patients with prior coronary artery bypass graft (CABG) surgery (N=17) compared to the no CABG group (N=25)(p=0.27) or between patients with (N=17) and without (N=25) previous percutaneous interventions for coronary artery disease (p=0.91).
Figure 3. A transpulmonary increase in aldosterone is observed in heart failure patients with reduced left ventricular ejection fraction (HFrEF) but not controls.
(A) Plasma from the pulmonary or radial artery harvested from controls (N=18) or HFrEF patients (N=42) at rest and peak exercise during invasive cardiopulmonary exercise testing was analyzed for aldosterone levels. (B) The transpulmonary gradient was calculated by the difference in aldosterone (ALDO) levels between the radial and pulmonary arterial samples for each patient. Data are presented as median (interquartile range).
Table 4.
Plasma aldosterone levels according to mineralocorticoid receptor antagonist therapy status.
| HFrEF Patients | |||
|---|---|---|---|
| Aldosterone levels (ng/dl) | −MR Antagonist Therapy (N=25) |
+MR Antagonist Therapy (N=17) |
P Value |
| Resting PA | 558.0 [391.5–558.0] | 700.5 [451.9–9–902.5] | 0.44 |
| Resting Radial | 568.5 [411.0–904.8] | 732.0 [606.0–1217] | 0.51 |
| Peak PA | 594.0 [457.5–1146] | 712.5 [513.0–1036] | 0.65 |
| Peak Radial | 699.0 [471.6–904.8] | 735.0 [465.0–1180] | 0.77 |
Plasma radial and pulmonary arterial (PA) aldosterone levels acquired at rest or during peak exercise (peak) patients with heart failure preserved ejection fraction (HFrEF) referred for cardiopulmonary exercise testing were analyzed according to mineralocorticoid receptor (MR) antagonism use.
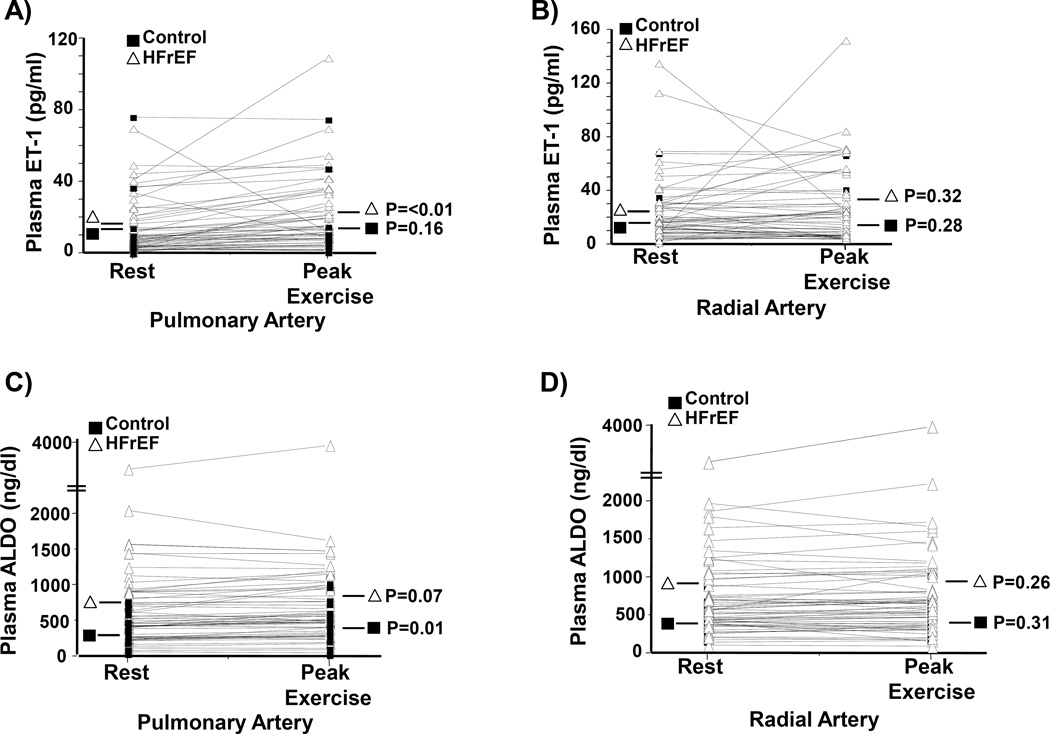
However, akin to our observations for ET-1, a transpulmonary (radial artery level – PA level) increase in plasma aldosterone concentration was also observed between controls and HFrEF patients at rest (7.5 [−54–40] vs. 61.5 [−13.6–165] ng/dl, P=0.01) and peak exercise (−20 [−39.6–79.1] vs. +25.8 [−29.2–109.3] ng/dl, P=0.02) (Figure 3B). Within patient changes between rest and exercise for PA and radial ET-1 and aldosterone levels are provided in Figure 4.
Figure 4. Within patient changes in ET-1 or aldosterone (ALDO) levels between rest and exercise.
Differences in levels of ET-1 between rest and exercise were analyzed in plasma harvested from the (A) pulmonary artery and (B) radial artery in controls (N=18) and patients with heart failure with reduced ejection fraction (HFrEF)(N=42). (C, D) Similar analyses were performed in the study cohort for plasma levels of ALDO.
Circulating aldosterone levels associate with cardiac reserve capacity at peak exercise
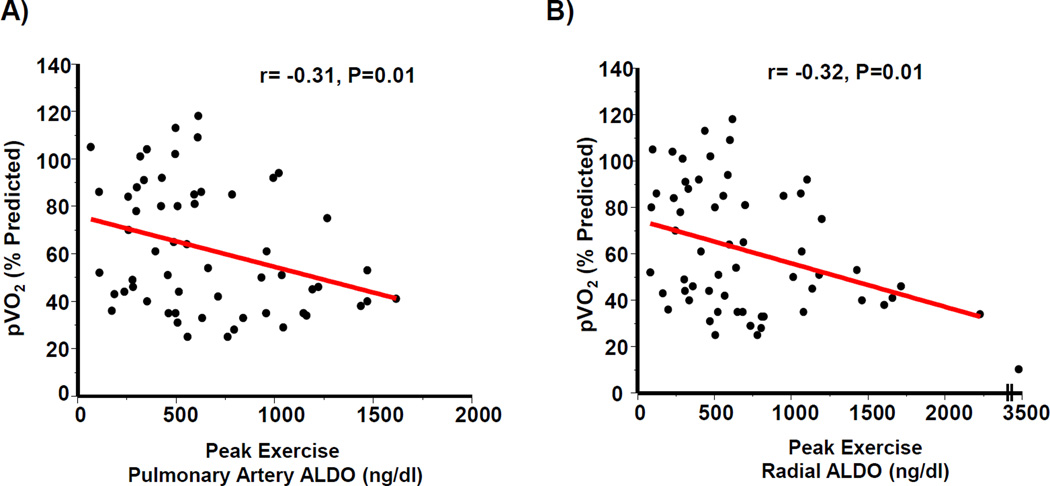
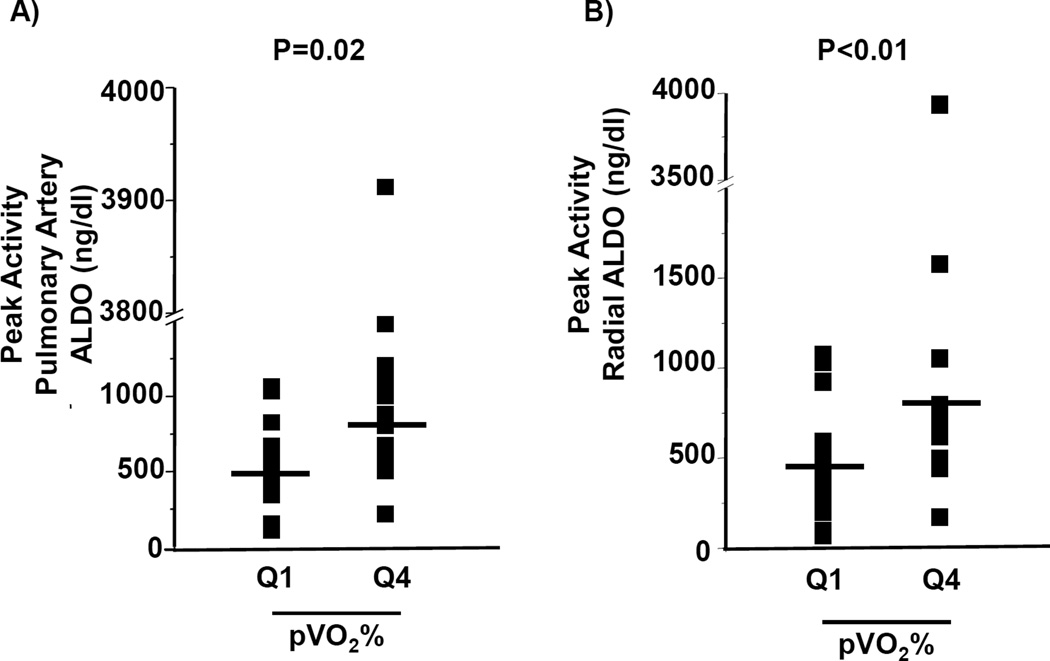
We next investigated the relationship between circulating aldosterone levels and cardiac reserve capacity. After controlling for age, sex, and BMI for the entire cohort, an inverse correlation between pVO2 and aldosterone levels in the PA (r= −0.31, P=0.01) and radial compartments (r= −0.32, P=0.01) was observed (Figure 5). In comparison to patients within the upper quartile for pVO2%, the lowest pVO2% quartile (93 [87–105] vs. 34 [29–35], N=15–16/quartile, P<0.001) demonstrated an increase in the PA and radial aldosterone levels by 1.6-fold (P=0.02) and 1.7-fold (P<0.01), respectively, at peak exercise (Figure 6).
Figure 5. Aldosterone levels correlate inversely with peak volume of oxygen consumption (pVO2).
Plasma aldosterone levels from controls (N=18) and heart failure patients with reduced left ventricular ejection fraction (HFrEF) (N=42) measured at peak exercise from the (A) pulmonary artery and (B) radial artery were correlated with pVO2 adjusted for age, sex, and body mass index.
Figure 6. Distribution of aldosterone levels among patients with high and low peak volume of oxygen consumption (pVO2) during exercise testing.
Controls (N=42) and heart failure patients with reduced left ventricular ejection fraction (HFrEF) (N=42) were referred for invasive cardiopulmonary exercise testing and stratified by quartile according to pVO2 expressed as a percent of predicted (pVO2%). (A) Pulmonary artery and (B) radial artery aldosterone levels measured at peak exercise are expressed for patients in the lower quartile (Q1; N=15) and upper quartile (Q4; N=16) for pVO2%.
DISCUSSION
In a single institution, retrospective HFrEF cohort analysis, we observed a modest transpulmonary increase in aldosterone levels, and that circulating aldosterone correlated inversely to pVO2. Our results provide empiric evidence in support of prior experimental data indicating that elevated circulating levels of aldosterone occurring in the setting of cardiovascular disease may be due, in part, to extra-adrenal aldosterone synthesis originating from the lung-pulmonary vascular axis. (4, 5, 13) Taken together, findings from this study support future prospective investigations that aim to characterize circulating aldosterone as a previously unrecognized and potentially treatable biochemical indicator of impaired cardiac reserve capacity in HFrEF.
Lung-pulmonary vascular synthesis/metabolism of vasoactive mediators involved in the pathogenesis of cardiopulmonary disease has been proposed previously. For example, a transpulmonary increase in angiotensin II is reported in patients with hypoxic pulmonary hypertension, (14) while directionally similar findings are observed for the ET-1 precursor molecule Big-ET-1 in patients with pulmonary arterial hypertension. (15) In primary left heart disease, fluctuation in levels across the pulmonary circulation of brain natriuretic peptides, atrial natriuretic peptides, cGMP, the anti-oxidant α-tocopherol, as well as intermediaries involved in aldosterone biosynthesis (e.g., the transcription factor c-Fos) have been reported. (4,5,8,16–18) Our current findings are in support of these observations and other experimental data reported previously indicating the potential for extra-adrenal steroidogenesis in lung-pulmonary vascular tissue, (4,19) and add to this field by suggesting that HFrEF is a novel cardiovascular pathophenotype in which transpulmonary aldosterone differences are observed. Identifying increased pulmonary arterial and systemic aldosterone levels as a functionally significant marker or potential mediator of exercise pathophysiology may, in turn, expand the profile of patients for whom aldosterone antagonism therapy is beneficial.
Although a mechanism by which to account for the relationship between aldosterone levels and cardiac reserve capacity was not addressed in the current study, it is noteworthy that in HFrEF patients, exogenous aldosterone administration impairs forearm blood flow and increases systemic vascular resistance, (20–22) which, in turn, are established risk factors for diminished pVO2. (23) Alternatively, adverse LV remodeling (e.g., myocardial fibrosis), disrupted pulmonary vascular-right ventricular function, and/or intravascular plasma volume expansion promote exercise intolerance and are linked to diseases of acquired hyperaldosteronism. Future studies are required to determine the contribution of these or other processes involving aldosterone bioactivity to our observation linking aldosterone levels with cardiac reserve capacity.
Limitations
A number of important confounding factors beyond those inherent to a retrospective study design merit consideration when interpreting our findings. Although observed plasma aldosterone levels are consistent with values published previously for HFrEF patients (24,25) and samples were accessed in patients NPO for >6 hr, the possibility that plasma volume differences, co-morbidities other than heart failure linked to acquired hyperaldosteronism, and/or differences in patients’ pharmacotherapeutic profile influenced our findings can not be excluded. Notwithstanding this, it is notable that moderate or severe renal impairment (e.g., chronic kidney disease stages 3 or 4), which is implicated as an independent risk factor for acquired hyperaldosteronism, was not identified as a comorbidity in any HFrEF patients and aldosterone levels did not correlate with creatinine levels in this study. Furthermore, we choose to compare aldosterone samples in different vascular compartments using a paired analysis strategy in which each patient served as their own control to demonstrate that our novel finding of transpulmonary aldosterone release in HFrEF was unlikely to be a consequence of variability in absolute aldosterone levels observed between patients.
Levels of key aldosterone metabolites required to quantify aldosterone degradation were not measured, and, thus, calculating aldosterone metabolism relative to aldosterone synthesis in each of the studied vascular compartments at rest or during exercise could not be analyzed in this study. Therefore, the possibility remains that differences in pulmonary circulatory aldosterone synthesis as well as metabolism accounted for our aldosterone transpulmonary gradient findings. Along these lines, the effect of sample storage duration on aldosterone or ET-1 levels could not be measured, and, therefore, the possibility that ET-1/aldosterone degradation confounded our study results cannot be excluded.
Use of mineralocorticoid receptor antagonists was reported exclusively in the HFrEF group, although this therapy did not influence aldosterone levels significantly in any vascular compartment at rest or peak activity. This observation is in concert with prior reports in experimental animal models of heart failure demonstrating no effect of this drug class on circulating plasma aldosterone levels. (8)
As an additional limitation, ethical considerations prevent the recruitment of individuals for invasive diagnostic testing in the absence of a clinical indication. On the other hand, analysis of data from our control group characterized by dyspneic patients without evident cardiopulmonary disease may narrow the magnitude of effects observed in this study relative to comparisons between HFrEF patients and a (truly) healthy control group.
Conclusions
In summary, our findings suggest that differences in transpulmonary aldosterone levels may occur in HFrEF, which are not observed in controls. Circulating aldosterone levels also correlate inversely with pVO2, which raises speculation that mineralocorticoid receptor stimulation may be involved in the pathophysiology of impaired cardiac exercise reserve capacity in patients with HFrEF. However, additional studies in larger cohorts are required to confirm transpulmonary aldosterone release, elucidate the factors responsible for this effect in HFrEF patients, and substantiate functional relationship between and aldosterone and pVO2.
Acknowledgments
FUNDING SOURCES
This work was supported by National Institutes of Health (NIH R01 105301 and U01 1252151 to JAL.; R01 HL119154, U10HL110337, and Hassenfeld Clinical Scholar Award to GDL; T32 HL007633 to WMO.; K08HL111207-01A1 to BAM); and American Heart Association (AHA 15GRNT25080016), Pulmonary Hypertension Association, Gilead Research Scholars Fund, Cardiovascular Medical Research and Education Foundation (CMREF) the Klarman Foundation (Boston, MA) at Brigham and Women’s Hospital and CIMIT award to BAM
ABBREVIATIONS
- HFrEF
heart failure with reduced left ventricular (LV) ejection fraction
- pVO2
volume of oxygen consumption at peak exercise
- pVO2%
pVO2 expressed as a percent of predicted
- ET-1
endothelin-1
- iCPET
invasive cardiopulmonary exercise test
- CO
cardiac output
- PA
pulmonary artery
- mPAP
mean pulmonary artery pressure
- PVR
pulmonary vascular resistance
Footnotes
CONFLICTS OF INTEREST
Dr. Maron receives funding from Gilead Sciences Inc. to study pulmonary hypertension. All other authors have nothing to disclose.
REFERENCES
- 1.Leopold JA, Dam A, Maron BA, et al. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nature Med. 2007;13(2):189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCurley A, Pires PW, Bender SB, et al. Direct regulation of blood pressure by smooth muscle mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maron BA, Zhang YY, Handy DE, et al. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem. 2009;284:7665–7672. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron BA, Zhang Y-Y, White K, et al. Aldosterone inactivates the endothelin-B receptor to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126(8):963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maron BA, Oldham WM, Chan SY, et al. Upregulation of steroidogenic acute regulatory protein by hypoxia stimulates aldosterone synthesis in pulmonary artery endothelial cells to promote pulmonary vascular fibrosis. Circulation. 2014;130:168–179. doi: 10.1161/CIRCULATIONAHA.113.007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Susic D, Baragic J, Ahn J, Matavelli L, Frohlich ED. Long-term mineralocorticoid receptor blockade reduces fibrosis and improves cardiac performance and coronary hemodynamics in elderly SHR. Am J Physiol Heart Circ Physiol. 2007;292:H175–H179. doi: 10.1152/ajpheart.00660.2006. [DOI] [PubMed] [Google Scholar]
- 7.Ooi H, Colucci WS, Givertz MM. Endothelin mediates increased pulmonary vascular tone in patients with heart failure: demonstration by direct intrapulmonary infusion of sitaxsentan. Circulation. 2002;106:1618–1621. doi: 10.1161/01.cir.0000034444.31846.f4. [DOI] [PubMed] [Google Scholar]
- 8.Melenovsky V, Al-Hiti H, Kazdova L, et al. Transpulmonary B-type natriuretic peptide uptake and cyclic guanosine monophosphate release in heart failure and pulmonary hypertension: the effects of sildenafil. J Am Coll Cardiol. 2009;54:595–600. doi: 10.1016/j.jacc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Murphy RM, Shah RV, Malhotra R, et al. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation. 2011;124:1442–1451. doi: 10.1161/CIRCULATIONAHA.111.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis GD, Shah R, Shahzad K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 11.Lewis GD, Lachmann J, Camuso J, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 12.Maron BA, Opotowsky AR, Landzberg MJ, Loscalzo J, Waxman AB, Leopold J. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension. Eur J Heart Fail. 2013;15:277–283. doi: 10.1093/eurjhf/hfs173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatakeyama H, Miyamori I, Fujita T, Takeda Y, Takeda R, Yamamoto H. Vascular aldosterone: Biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 1994;269:24316–24320. [PubMed] [Google Scholar]
- 14.Peacock AJ, Matthews A. Transpulmonary angiotensin II formation and pulmonary haemodynamics in stable hypoxic lung disease: the effect of captopril. Respir Med. 1992;86:21–26. doi: 10.1016/s0954-6111(06)80143-5. [DOI] [PubMed] [Google Scholar]
- 15.Morrell NW, Stenmark KR. The renin-angiotensin system in pulmonary hypertension. Am J Respir Crit Care Med. 2013;187:1138–1139. doi: 10.1164/rccm.201210-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkens H, Bauer M, Forestier N, et al. Influence of inhaled iloprost on transpulmonary gradient of big endothelin in patients with pulmonary hypertension. Circulation. 2003;107:1509–1513. doi: 10.1161/01.cir.0000056104.49686.4b. [DOI] [PubMed] [Google Scholar]
- 17.Freire G, Ocampo C, Ilbawai N, Griffin AJ, Gupta M. Overt expression of AP-1 reduces alpha myosin heavy chain expression and contributes to heart failure from chronic volume overload. J Mol Cell Cardiol. 2007;43:465–478. doi: 10.1016/j.yjmcc.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 18.Tsutamoto T, Kanamori T, Wada A, Kinoshita M. Uncoupling of atrial natriuretic peptide extraction and cyclic guanosine monophosphate production in the pulmonary circulation in patients with severe heart failure. J Am Coll Cardiol. 1992;20:541–546. doi: 10.1016/0735-1097(92)90005-8. [DOI] [PubMed] [Google Scholar]
- 19.Hatakeyama H, Miyamori I, Fujita T, Takeda Y, Takeda R, Yamamoto H. Vascular aldosterone. Biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 1994;269:24316–24320. [PubMed] [Google Scholar]
- 20.Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (Lond) 2002;10:425–431. doi: 10.1042/cs1030425. [DOI] [PubMed] [Google Scholar]
- 21.Gunaruwan P, Schmitt M, Sharman J, Lee L, Struthers A, Frenneaux M. Effects of aldosterone on forearm vasculature in treated chronic heart failure. Am J Cardiol. 2005;95:412–414. doi: 10.1016/j.amjcard.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 22.Romagni P, Rossi F, Guerrini L, Quirini C, Santiemma V. Aldosterone induces contraction of the resistance arteries in man. Atherosclerosis. 2003;166:345–349. doi: 10.1016/s0021-9150(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 23.Chiba Y, Maehara K, Yaoita H, Yoshihisa A, Izumida J, Maruyama Y. Vasoconstrictive response in the vascular beds of the non-exercising forearm during leg exercise in patients with mild chronic heart failure. Circ J. 2007;16:922–928. doi: 10.1253/circj.71.922. [DOI] [PubMed] [Google Scholar]
- 24.Sparks RF, Dale SL, Kahn PC, Melby JC. Activation of aldosterone secretion in pulmonary aldosteronism. J Clin Invest. 1969;48:96–104. doi: 10.1172/JCI105978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim PO, MacFadyen RJ, Struthers AD. Is there a role for renin profiling in selecting chronic heart failure patients for ACE inhibitor treatment? Heart. 2000;83:257–261. doi: 10.1136/heart.83.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]