Abstract
Background
Suboptimal asthma control during pregnancy may impact perinatal outcomes. US guidelines recommend questionnaires to assess asthma control including the Asthma Control Test (ACT).
Objective
To validate telephone administration of a modified version of the ACT during pregnancy.
Methods
MotherToBaby Pregnancy Studies (2011–2013) enrolled 159 pregnant women with asthma. Participants were interviewed by telephone at intake, at approximately gestational weeks 20 and 32, and postpartum. The ACT was modified to address dyspnea specifically due to asthma; the modified version is the Pregnancy ACT (p-ACT). Women answered p-ACT and guideline-based asthma impairment questions, and reported asthma course changes and exacerbations. Possible p-ACT scores ranged from 5–25; higher score indicated better control. Reliability, criterion validity, construct validity, prospective validity, and responsiveness were assessed.
Results
Cronbach’s alpha for internal consistency was similar across time points, 0.84–0.90. P-ACT score varied by impairment; e.g., at intake, the mean score was 23.2 for well-controlled versus 13.7 for very poorly controlled asthma. P-ACT score change between interviews differed by asthma course; e.g., women reporting that their asthma was much better at week 20 than at intake had a mean score increase of 4.7; women reporting that their asthma was a little worse had a mean decrease of 1.3. Lower p-ACT score was associated with previous exacerbations, whereas intake p-ACT was not associated with future exacerbations during pregnancy.
Conclusions
The p-ACT demonstrated good internal consistency, varied in the expected direction by impairment level, and was responsive to changes in asthma course. Telephone administration of the p-ACT is reliable and valid for assessing asthma control during pregnancy.
Keywords: Asthma, Asthma Control Test, pregnancy, questionnaire, telephone-based, validation study
Introduction
In the United States (US), asthma affects approximately 9% of pregnant women.1,2 Asthma is associated with an increased risk for a number of adverse perinatal outcomes including preeclampsia, antepartum hemorrhage, preterm birth, low birthweight, and small for gestational age.3–7 Risks for adverse outcomes are highest among women with poorly controlled or more severe asthma.4,5 Effective management of asthma may reduce the risk for pregnancy-related complications.5,6,8 To optimize maternal and offspring health, the treatment goal for pregnant women with asthma is to control manifestations of the disease.9 Close monitoring of asthma control is critical because asthma course may be variable during pregnancy; asthma course improves in approximately one-third of women, whereas it worsens in another third.10,11 The National Asthma Education and Prevention Program recommends using validated questionnaires to assess asthma control including the Asthma Control Test (ACT).12
The ACT is a simple, patient-based questionnaire that measures asthma control and was first described in the literature in 2004.13,14 It comprises five questions regarding frequency of asthma symptoms, asthma impact on functioning, and rescue medication use during the past four weeks.13 Scores range from 5 (poor control) to 25 (complete control).14 A score of 20 or higher reflects well-controlled asthma, whereas a score of 15 or less reflects asthma that is very poorly controlled.14 Studies have demonstrated validity of the ACT against specialists’ ratings of asthma control and other asthma control and symptom tools in patients receiving specialist care, patients new to specialist care, and in a more general population of asthma patients.13–15 The ACT was originally validated as a self-administered paper-and-pencil questionnaire, and a subsequent study demonstrated comparability with telephone interview administration.16 The ACT is one of two asthma control instruments designated as a core measure for National Institutes of Health-initiated clinical research in adults.17
The ACT may also be a valuable tool for the clinical management of pregnant women with asthma and for use in observational and interventional studies of asthma during pregnancy. However, there is a need for validation of the tool specifically in pregnant women. For example, the ACT contains a question about dyspnea, which can occur during pregnancy independent of asthma, potentially impacting the ACT score. A validation study of the ACT in pregnant women with asthma was conducted recently in Brazil using the Portuguese version of the ACT.18 This study demonstrated that the ACT, administered through in-person interview to 40 pregnant women, discriminated between controlled and uncontrolled asthma and was responsive to symptom improvements during pregnancy. However, it is critical to confirm the validity of the ACT for pregnant women in different and larger study populations.
There is also a need to validate the ACT for telephone administration in pregnancy. A telephone-based approach in the clinical setting may be desirable for monitoring asthma during pregnancy while requiring fewer office visits. In the research setting, telephone administration may be essential for feasibility of study data collection. The objective of the current study was to validate telephone interview administration of a modified ACT, the Pregnancy ACT (p-ACT©, QualityMetric) for use during pregnancy.
Methods
Recruitment and Study participants
This validation study was part of the MotherToBaby Asthma Medications in Pregnancy Study. This study used three methods to recruit participants residing in the U.S. or Canada who spoke English or Spanish:
MotherToBaby is a service of the Organization of Teratology Information Specialists (OTIS) that provides evidence-based information regarding medications and other exposures during pregnancy and breastfeeding. Pregnant women with self-reported asthma who called the toll-free MotherToBaby telephone service before 20 weeks’ gestation were invited to particpate in the study.
Pregnant women with asthma were recruited through direct to health care provider promotion.
Pregnant women with asthma were recriuted through direct to consumer awareness activites on the internet.
Pregnant women who reported that they had asthma and agreed to participate in the study before 20 weeks’ gestation were enrolled. Women did not receive incentives to participate in this study. This study was approved by the University of California, San Diego Institutional Review Board.
Data collection
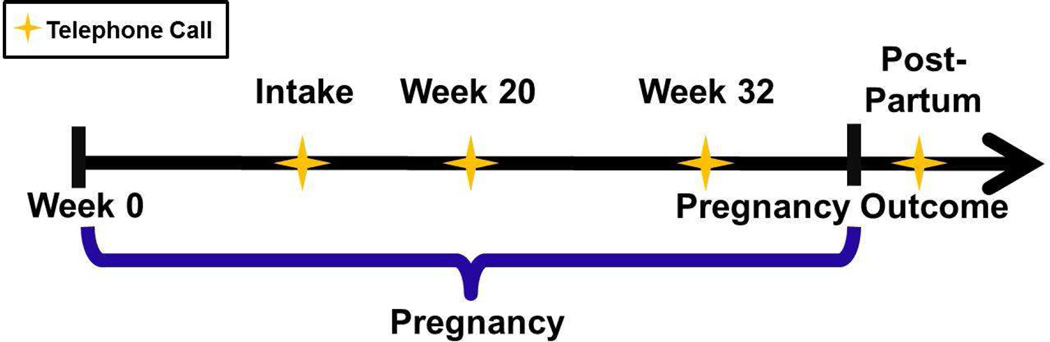
Trained MotherToBaby interviewers collected data from participants through telephone calls at up to four time points as illustrated in Figure 1 and described in detail below. The p-ACT was translated into Spanish, and interviews were conducted in Spanish for the three monolingual Spanish speakers who enrolled in the study. All other interviews were conducted in English. Interviewers administered the p-ACT. The p-ACT contains the five questions from the original ACT with a modification of question two. This question was changed to “During the past 4 weeks, how often have you had shortness of breath due to your asthma?” “Due to your asthma” was added to exclude symptoms of dyspnea caused by pregnancy (Online Repository Figure 1). Additionally, interviewers administered questions related to asthma impairment (Online Repository Figure 2) based on the National Asthma Education and Prevention Program guidelines.12 Although we initially intended to include a question on the frequency of rescue therapy use, this was inadvertently omitted from the guideline based questions. Participants were asked about changes in asthma course between phone calls using the following question: Since your prior phone call, which of the following describes the course of your asthma: “much better”, “a little better”, “stayed the same”, “a little worse”, or “much worse”. Exacerbations were defined as self-report of any overnight hospitalizations or emergency room visits, unscheduled physician visits, or oral corticosteroid use because of asthma symptoms.
Intake telephone call. This call occurred near the time of study enrollment, i.e., at any gestational week between the recognition of pregnancy and 20 completed weeks’ gestation. The interviewers collected information regarding demographics, comorbidities, and medication use during pregnancy, administered the p-ACT and the guideline-based asthma impairment questions for the previous 4 weeks, and asked participants if they had experienced any exacerbations since becoming pregnant.
Week 20 telephone call. This call occurred during the second trimester at approximately 20 weeks’ gestation. For women who enrolled at 16 weeks’ gestation and beyond, the call occured approximately 4 weeks after enrollment. With the exception of four women, the call was omitted for those who enrolled after 18 week’s gestation. During this call, interviewers collected information regarding medication use, administered the p-ACT and the guideline-based impairment questions for the previous 4 weeks, and asked participants if they had experienced a change in asthma course or an exacerbation since the previous call.
Week 32 telephone call. This call occurred during the third trimester at approximately 32 weeks’ gestation and followed the same format as the 20 week call.
Post-partum telephone call. This call occurred after the pregnancy ended. The interviewers collected information on medication use, administered the p-ACT for the last 4 weeks of pregnancy and asked participants if they experienced any exacerbations between the previous call and delivery.
Figure 1.
Illustration of data collection time points.
Statistical analysis
First, we used Crohnbach’s alpha to test for internal consistency reliability of the five p-ACT items. Good internal consistency was defined as an alpha of 0.8 to 0.9.19 Second, we evaluated criterion validity by comparing mean p-ACT scores at intake, week 20, and week 32 with guideline-based impairment categories of “well-controlled”, “not well-controlled,” and “very poorly controlled” at the same time points using one-way ANOVA.12 Third, we evaluated responsiveness of the p-ACT by comparing the mean p-ACT score change between two calls (i.e., between intake and week 20 and between week 20 and week 32 calls) across the 5 categories of self-reported asthma change status between the same two calls using one-way analysis of variance (ANOVA). Fourth, we evaluated construct validity by comparing mean p-ACT scores according to previous exacerbations (yes versus no) since the beginning of pregnancy for the intake call, since the previous call for week 20 and week 32, or between the previous call and the end of pregnancy for post-partum calls using two sample t-tests. Finally, we investigated predictive validity in two ways. We compared mean p-ACT scores between those who reported subsequent exacerbations before the next call or before the end of pregnancy for the week 32 call versus those who did not. Excluding those missing the postpartum call, we compared the mean p-ACT score at intake between those who had subsequent exacerbations during pregnancy versus those who did not using two-sample t-tests. P-values less than 0.05 were considered to be statistically significant. As a sensitivity analysis for potential violations of normality, we substituted non-parametric tests for the parametric tests used in the primary analysis (i.e., Wilcoxon-Mann-Whitney tests instead of t-tests and Kruskal-Wallis tests instead of ANOVAs).
Results
Maternal and pregnancy characteristics
Between July 2011 and June 2013, a total of 159 women with asthma residing in the U.S. or Canada were enrolled in the study at a mean of 12.4 weeks’ gestation. One study participant who met the gestational age requirement at the time of enrollment later received a revised estimate of gestational age at enrollment of 20.4 weeks. The average maternal age was 31.5 years old. Most cohort members were Non-Hispanic White (83.7%) and had relatively high socioeconomic status (Table 1). Short-acting Beta2-agonists (75.5%) and inhaled corticosteroids (56.6%) were the most commonly used asthma medications during pregnancy in this cohort. Approximately 15% of the women used an oral corticosteroid at least once during pregnancy. The mean p-ACT score at intake was 20.4. In terms of pregnancy outcomes, 87.4% had live births, 4.4% had stillbirths or spontaneous abortions, and 8.2% were lost to follow-up. Among the 145 (91.2%) pregnancies with known gestational age at pregnancy outcome, the average gestational age at outcome was 38.2 weeks.
Table 1.
Maternal and pregnancy characteristics.
| Characteristics | All Women (N=159) |
|---|---|
| Gestational Week at Enrollment, mean (standard deviation) | 12.4 (4.3) |
| Age, mean (standard deviation) | 31.5 (5.4) |
| Race/Ethnicity, n (%) | |
| Non-Hispanic White | 133 (83.7) |
| Hispanic | 14 (8.8) |
| Asian | 7 (4.4) |
| Non-Hispanic Black | 5 (3.1) |
| Socioeconomic Status*, n (%) | |
| 1 | 58 (36.5) |
| 2 | 60 (37.7) |
| 3 | 25 (15.7) |
| 4 | 6 (3.8) |
| 5 | 9 (5.7) |
| Missing | 1 (0.6) |
| Nulliparous, n (%) | 92 (57.9) |
| Multifetal gestation, n (%) | |
| Singleton | 154 (96.9) |
| Twin | 5 (3.1) |
| Body Mass Index, n (%) | |
| <25 | 84 (52.8) |
| 25–29.9 | 47 (29.6) |
| ≥30 | 28 (17.6) |
| Any Smoking During Pregnancy, n (%) | 11 (6.9) |
| Asthma Medication Use During Pregnancy† n (%) | |
| None Observed | 24 (15.1) |
| Short-Acting Beta-Agonists | 120 (75.5) |
| Inhaled Corticosteroids | 88 (55.4) |
| Long-Acting Beta-Agonists | 56 (35.2) |
| Leukotriene Receptor Antagonists | 26 (16.4) |
| Oral Corticosteroids | 24 (15.1) |
| Short or Long Acting Anticholinergics | 4 (2.5) |
| Inhaled Asthma Medication Not Otherwise Specified | 3 (1.9) |
| Intake Pregnancy Asthma Control Test Score, mean (standard deviation) | 20.4 (4.3) |
| Pregnancy outcome | |
| Live birth | 139 (87.4) |
| Stillbirth or spontaneous abortion | 7 (4.4) |
| Lost to follow up | 13 (8.2) |
| Gestational age at outcome | |
| Mean (standard deviation) | 38.2 (5.4) |
| Missing, n (%) | 14 (8.8) |
Calculated using Hollingshead categories based on maternal and paternal education and occupation; 1, highest; 5, lowest.
Women may be classified in multiple exposure groups due to the use of combination products and the use of multiple medications during pregnancy.
Interview completion and timing
There were 135 (84.9%) women who completed at least three interviews. Fourteen (8.8%) women completed the intake interview only; of these women, 8 were lost to follow up, 4 had stillbirths or spontaneous abortions, and 2 had live births. There were 22 (13.8%) women who were missing the postpartum interview; 13 of these women were lost to follow up, 5 women had stillbirths or spontaneous abortions, and 4 women had live births. Table 2 lists the proportion of women completing all components of each interview and the average timing of their interviews. For example, 86.2% of participants completed the postpartum interview, which occurred on average 5.2 weeks after delivery.
Table 2.
Number of participants, timing of interview, and Cronbach’s alpha at each phone call.
| Phone Call | N Total |
n with Complete Questionnaire Information Available |
% with Complete Questionnaire Information Available |
Mean Gestational Age or Weeks After Delivery§ at time of Phone Call (Standard Deviation) |
Cronbach’s alpha |
|---|---|---|---|---|---|
| Intake | 159 | 157‡ | 98.7 | 12.6 (4.3) | 0.87 |
| Week 20* | 155 | 124 | 80.0 | 21.4 (1.7) | 0.84 |
| Week 32† | 149 | 120 | 80.5 | 33.0 (1.1) | 0.84 |
| Postpartum | 159 | 137 | 86.2 | 5.2 (4.8) | 0.90 |
Excludes women who reported pregnancy outcome ≤20 completed gestational weeks.
Excludes women who reported pregnancy outcome ≤32 completed gestational weeks.
All 159 women have p-ACT information available.
Weeks after delivery is reported for the postpartum interview.
Internal consistency reliability
Cronbach’s alpha for internal consistency was similar across time points. It ranged from 0.84–0.90 (Table 2), which indicates good internal consistency reliability.
Criterion validity
P-ACT score varied by self-reported level of asthma impairment. For example, at the intake call, the mean score was 23.2 for well controlled asthma, 19.7 for not well controlled asthma, and 13.7 for very poorly controlled asthma (Table 3). The pattern of decreasing p-ACT scores with increasing impairment was observed at the week 20 and 32 calls, although there were only 4 women classified in the very poorly controlled group at week 32.
Table 3.
Mean Pregnancy Asthma Control Test score at each call by guideline-based impairment categories at each call.
| Phone Call | Well Controlled |
Not Well Controlled |
Very Poorly Controlled |
p- value* |
|||
|---|---|---|---|---|---|---|---|
| n | Mean p-ACT Score (SD) |
n | Mean p-ACT Score (SD) |
n | Mean p-ACT Score (SD) |
||
| Intake | 74 | 23.2 (2.2) | 58 | 19.7 (2.3) | 25 | 13.7 (4.6) | <0.01 |
| Week 20 | 75 | 23.4 (2.0) | 38 | 18.9 (3.3) | 11 | 16.3 (3.9) | <0.01 |
| Week 32 | 82 | 23.8 (1.4) | 34 | 19.1 (3.1) | 4 | 14.5 (4.1) | <0.01 |
Abbreviation: SD, standard deviation.
ANOVA.
Responsiveness
The p-ACT score change between interviews differed by asthma course change (Table 4). Women reporting that their asthma was much better at week 20 than at intake had a mean score increase of 4.7, whereas women reporting that their asthma was a little worse had mean decrease of 1.3. Similarly, women reporting that their asthma symptoms were much better at week 32 than week 20 had a mean score increase of 3.6, whereas women reporting that their asthma was a little worse had a mean score decrease of 3.0. There were only 4 women in both comparisons who reported that their asthma was much worse.
Table 4.
Mean Pregnancy Asthma Control Test score change by asthma course change between two calls.
| Change in Asthma Status Between Two Calls |
Intake and 20 Weeks’ Gestation, N=124 |
20 and 32 Weeks’ Gestation, N=103 |
||
|---|---|---|---|---|
| n | Mean p-ACT Score Change* (SD) |
n | Mean p-ACT Score Change† (SD) |
|
| Much Better | 21 | 4.7 (4.4) | 25 | 3.6 (3.6) |
| A Little Better | 20 | 1.4 (4.6) | 6 | 0.8 (1.6) |
| Stayed the Same | 50 | 0.8 (3.3) | 45 | 0.4 (2.3) |
| A Little Worse | 29 | −1.3 (3.8) | 23 | −3.0 (4.0) |
| Much Worse | 4 | −7.3 (4.1) | 4 | −0.3 (3.6) |
ANOVA p-values < 0.01 for both time frames.
Abbreviation: SD, standard deviation.
P-ACT Score at intake call subtracted from p-ACT Score at week 20 call.
P-ACT Score at week 20 call subtracted from p-ACT Score at week 32 call.
Construct validity
At the intake call, mean p-ACT scores were lower among women with an exacerbation earlier in pregnancy compared with women who did not have an exacerbation earlier in pregnancy (15.2 versus 21.1; Table 5). For the other calls, mean p-ACT scores were also lower among women who had exacerbations since the previous asthma assessment, although the comparison was not statistically significant at the week 32 and postpartum calls.
Table 5.
Mean Pregnancy Asthma Control Test score at each call according to whether participants had a previous exacerbation* (since the beginning of pregnancy for the intake call, since the previous call for week 20 and week 32 calls, or between the previous call and the end of pregnancy for the postpartum call).
| Phone Call | Previous Exacerbation |
No Previous Exacerbation |
p- value† |
||
|---|---|---|---|---|---|
| n | Mean p-ACT Score (SD) |
n | Mean p-ACT Score (SD) |
||
| Intake | 18 | 15.2 (6.2) | 140 | 21.1 (3.5) | <0.01 |
| Week 20 | 9 | 17.3 (5.0) | 115 | 21.7 (3.4) | 0.03 |
| Week 32 | 7 | 19.4 (4.3) | 113 | 22.3 (3.2) | 0.13 |
| Postpartum | 7 | 17.0 (6.3) | 130 | 23.0 (3.1) | 0.05 |
Abbreviation: SD, standard deviation.
Self-report of exacerbations requiring oral corticosteroids, any overnight hospitalizations or emergency room visits because of asthma symptoms, or any unscheduled physician visits because of asthma symptoms.
Two-sample t-test.
Predictive validity
The p-ACT score was not a strong predictor of subsequent exacerbations. There was no evidence of an association between p-ACT score at the intake phone call and having an exacerbation before the week 20 phone call (Table 6). Mean p-ACT scores at the week 20 and 32 calls were not significantly lower among women who had an exacerbation before the subsequent asthma assessment, or before the end of pregnancy, respectively, compared with those who did. Furthermore, p-ACT score at intake was not predictive of having any future exacerbations during pregnancy; intake p-ACT score was 19.1 for women with future exacerbations compared with 20.8 for women without future exacerbations (p=0.16).
Table 6.
Mean Pregnancy Asthma Control Test score at each call according to whether participants had an exacerbation* before the next call (or before the end of pregnancy for the 32 week call).
| Phone Call | Exacerbation Before Next Call |
No Exacerbation Before Next Call |
p-value† | ||
|---|---|---|---|---|---|
| n | Mean p-ACT Score (SD) |
n | Mean p-ACT Score (SD) |
||
| Intake | 9 | 20.4 (6.3) | 115 | 20.6 (4.1) | 0.95 |
| Week 20 | 6 | 18.3 (5.8) | 97 | 21.8 (3.3) | 0.21 |
| Week 32 | 5 | 19.8 (6.1) | 113 | 22.2 (3.2) | 0.43 |
Abbreviation: SD, standard deviation.
Self-report of exacerbations requiring oral corticosteroids, any overnight hospitalizations or emergency room visits because of asthma symptoms, or any unscheduled physician visits because of asthma symptoms.
Two-sample t-test.
Sensitivity analysis
Wilcoxon-Mann-Whitney tests comparing median p-ACT scores according to previous exacerbations at the week 32 and postpartum calls were statistically significant. All other non-parametric tests yielded the same conclusions regarding statistical significance as the parametric tests.
Discussion
In this cohort of pregnant women with well-controlled asthma on average, the p-ACT administered via telephone interview demonstrated good internal consistency, varied by impairment level, and was responsive to changes in asthma course at multiple time points during pregnancy. P-ACT score was associated with previous exacerbations, but p-ACT score was not predictive of future exacerbations. This study suggests that telephone administration of the p-ACT is reliable and valid for assessing asthma control during pregnancy.
The internal consistency reliability was similar to or higher than previous reports that used paper-and-pencil and telephone administration methods from non-pregnant populations.13,14,16,20 That p-ACT score from multiple time points during pregnancy varied by self-reported guideline-based impairment level from well to very poorly controlled asthma is supportive of criterion validity. This finding is consistent with previous studies of the ACT among men and women that utilized asthma specialists’ ratings to classify impairment level.13,14 In the current study, the p-ACT was a responsive measure in the appropriate direction of change in status at each time point. Previous studies also described that ACT scores were responsive to changes in asthma course, although the report from pregnant women was based on only 10 individuals.14,18 Furthermore, the current study provided some evidence supporting construct validity of the p-ACT as lower p-ACT scores were associated with previous exacerbations at most of the assessments. The predictive validity of the p-ACT for exacerbations before the subsequent interview was poor, regardless of the time window examined. A previous study of men and women demonstrated that ACT categories were predictive of emergency hospital care, oral corticosteroid dispensings, and beta-agonist dispensings in the 12 months following ACT assessment.20 Limited predictive ability of the p-ACT in this study may be due to the low number of exacerbations in any one time window, the relatively short follow-up time frame compared with the previous study,20 and the high proportion of women in the study who had well-controlled asthma. We did not compare telephone interview administration of the p-ACT with paper-and-pencil administration of the test. However, telephone interview administration of the ACT is comparable with the paper-and-pencil format administration.16
This study has some limitations to consider. First, this study compared the p-ACT with patient-reported impairment level. Consequently, errors in the p-ACT may not be independent of errors in self-reported asthma status. A more objective standard would be valuable, such as spirometry and specialist’s rating of asthma control. In the previous ACT validation study conducted in pregnant women, asthma control level was assessed using spirometry and clinical evaluation by an obstetrician trained to manage pregnant women with asthma.18 Second, asthma assessment information was missing for phone calls after the intake in 14%-20% of participants. The p-ACT would appear to be a more or less effective tool if the p-ACT was more or less associated with impairment level, changes in asthma course, previous exacerbations, or future exacerbations among women with missing information than women who had information available. For example, if women with very poorly controlled asthma at week 20 did not participate in the week 32 assessment because of an asthma exacerbation, the evidence supporting predictive validity for week 32 would be weakened by this missing information. Third, the intake call occurred on average during gestational week 12 and may only approximate asthma control measures early in pregnancy. It is possible that some women’s asthma control may have worsened or improved during pregnancy by the time of the intake call, and an earlier measure of asthma control may be a stronger or weaker predictor of exacerbations. Fourth, we did not compare the p-ACT with the original ACT to determine whether adding “due to your asthma” in question two resulted in different scores. Finally, the cohort was primarily comprised of women who were white and had older maternal age, high socio-economic status, and relatively well-controlled asthma. Among low-income women, asthma morbidity during pregnancy has been reported to be higher in black women than in white women.21 The results may have less generalizability if the p-ACT performs differently among pregnant women with less well-controlled asthma, in non-white women, or in women of lower socioeconomic status.
This was the largest validation study to date of the ACT among pregnant women, and it was the first conducted in English and Spanish and administered over the telephone. A major strength of the study was that p-ACT was measured at multiple time points during pregnancy so that women did not have to recall their symptoms months after delivery, and conclusions regarding the tool’s validity apply across the entire pregnancy. Additionally, interview completion was high; approximately 5 out of 6 women who enrolled in the study completed at least 3 interviews. On average, interviews were conducted at the pre-specified time points. Furthermore, women included in this study were from a broad geographic area as opposed to a single clinic.
Building on the previous study from Brazil, these data suggest that telephone administration of the p-ACT is reliable and valid for assessing asthma control during pregnancy.18 Telephone interview administration of the p-ACT to pregnant women performed well in terms of reliability, criterion validity, responsiveness, and construct validity as related to past exacerbations. It was not a strong predictor of future exacerbations in the following weeks, which may be due to the short time frame and relatively low-risk composition in this cohort. The inability to confirm predictive validity for exacerbations does not substantially reduce the importance of the demonstrated reliability and validity of the p-ACT as a measure of asthma impairment during pregnancy. Similarly, the initial validation studies of the ACT did not evaluate this type of predictive validity.13,14 A larger study is needed to demonstrate the predictive validity of the p-ACT, and p-ACT score measured even earlier in pregnancy should be investigated as a potential clinical assessment tool for predicting exacerbations during pregnancy. Furthermore, future p-ACT validation studies should be conducted among women with lower socio-economic status and greater racial/ethnic diversity. Finally, future studies should evaluate whether using the p-ACT improves asthma control during pregnancy by signaling inadequately controlled asthma that requires additional interventions.
Supplementary Material
Highlights box.
1. What is already known about this topic?
The Asthma Control Test (ACT) is a patient-based validated tool for the assessment of asthma control.
2. What does this article add to our knowledge?
Telephone interview administration of the Pregnancy ACT (p-ACT) is a valid tool for assessing asthma control throughout pregnancy.
3. How does this study impact current management guidelines?
This study suggests that telephone interview administration of the p-ACT may be clinically useful for tracking asthma control during pregnancy.
Acknowledgments
Sources of Funding:
This study was supported by a research contract with Glaxo Smith Kline (Chambers) and by a grant from the Agency for Healthcare Research and Quality R18HS018474 (Chambers). Glaxo Smith Kline had no role in the study design, in the data collection, in the analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. Kristin Palmsten was supported by a career development award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health K99HD082412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ACT
Asthma Control Test
- p-ACT
Pregnancy Asthma Control Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest:
Michael Schatz receives research funding from GlaxoSmithKline.
References
- 1.Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol. 2003;13:317–324. doi: 10.1016/s1047-2797(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 2.Kwon HL, Triche EW, Belanger K, Bracken MB. The epidemiology of asthma during pregnancy: prevalence, diagnosis, and symptoms. Immunol Allergy Clin North Am. 2006;26:29–62. doi: 10.1016/j.iac.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Wen SW, Demissie K, Marcoux S, Kramer MS. Maternal asthma and pregnancy outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2001;184:90–96. doi: 10.1067/mob.2001.108073. [DOI] [PubMed] [Google Scholar]
- 4.Schatz M, Dombrowski MP, Wise R, et al. Spirometry is related to perinatal outcomes in pregnant women with asthma. Am J Obstet Gynecol. 2006;194:120–126. doi: 10.1016/j.ajog.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Enriquez R, Griffin MR, Carroll KN, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007;120:625–630. doi: 10.1016/j.jaci.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 6.Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118:1314–1323. doi: 10.1111/j.1471-0528.2011.03055.x. [DOI] [PubMed] [Google Scholar]
- 7.Mendola P, Laughon SK, Mannisto TI, et al. Obstetric complications among US women with asthma. Am J Obstet Gynecol. 2013;208:127, e1–e8. doi: 10.1016/j.ajog.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombrowski MP, Schatz M ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 90, February 2008: asthma in pregnancy. Obstet Gynecol. 2008;111:457–464. doi: 10.1097/AOG.0b013e3181665ff4. [DOI] [PubMed] [Google Scholar]
- 9.National Heart, Lung, and Blood Institute, National Asthma Education Prevention Program, Asthma Pregnancy Working, Group. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol. 2005;115:34–46. doi: 10.1016/j.jaci.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112:283–288. doi: 10.1067/mai.2003.1516. [DOI] [PubMed] [Google Scholar]
- 11.Valet RS, Dupont WD, Mitchel EF, Hartert TV. Beta-agonist use as an indicator of change in asthma control during pregnancy. Ann Allergy Asthma Immunol. 2009;102:352–353. doi: 10.1016/S1081-1206(10)60345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report. [Retrived July 31, 2015];Full Report. 2007 Availble at: http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf.
- 13.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Schatz M, Mosen DM, Kosinski M, et al. Validity of the Asthma Control Test completed at home. Am J Manag Care. 2007;13:661–667. [PubMed] [Google Scholar]
- 16.Kosinski M, Kite A, Yang M, Rosenzweig JC, Williams A. Comparability of the Asthma Control Test telephone interview administration format with self-administered mail-out mail-back format. Curr Med Res Opin. 2009;25:717–727. doi: 10.1185/03007990802711602. [DOI] [PubMed] [Google Scholar]
- 17.Cloutier MM, Schatz M, Castro M, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012;129:S24–S33. doi: 10.1016/j.jaci.2011.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteiro de Aguiar M, Rizzo JA, de Melo Junior EF, Pires Lins ESLME, Cavalcanti Sarinho ES. Validation of the Asthma Control Test in pregnant asthmatic women. Respir Med. 2014;108:1589–1593. doi: 10.1016/j.rmed.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 19.George D, Mallery P. 11.0 update. 4th ed. Boston (MA): Allyn & Bacon; 2003. SPSS for Windows step by step: A simple guide and reference. [Google Scholar]
- 20.Schatz M, Zeiger RS, Drane A, et al. Reliability and predictive validity of the Asthma Control Test administered by telephone calls using speech recognition technology. J Allergy Clin Immunol. 2007;119:336–343. doi: 10.1016/j.jaci.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Carroll KN, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, Hartert TV. Racial differences in asthma morbidity during pregnancy. Obstet Gynecol. 2005;106:66–72. doi: 10.1097/01.AOG.0000164471.87157.4c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.