Abstract
Mammalian cells activate DNA damage response pathways in response to virus infections. Activation of these pathways can enhance replication of many viruses, including herpesviruses. Activation of cellular ATM results in phosphorylation of H2AX and recruits proteins to sites of DNA damage. We found that varicella-zoster (VZV) infected cells had elevated levels of phosphorylated H2AX and phosphorylated ATM and that these levels increased in cells infected with VZV deleted for ORF61 or ORF63, but not deleted for ORF67. Expression of VZV ORF61, ORF62, or ORF63 alone did not result in phosphorylation of H2AX. While BGLF4, the Epstein-Barr virus homolog of VZV ORF47 protein kinase, phosphorylates H2AX and ATM, neither VZV ORF47 nor ORF66 protein kinase phosphorylated H2AX or ATM. Cells lacking ATM had no reduction in VZV replication. Thus, VZV induces phosphorylation of H2AX and ATM and this effect is associated with the presence of specific VZV genes in virus-infected cells.
Keywords: varicella-zoster virus, ATM, H2AX, DNA damage, herpesvirus, herpes simplex virus
Introduction
DNA damage is induced by numerous stresses to the cell, including ionizing radiation, ultraviolet (UV) radiation, cytotoxic drugs, telomere shortening, programmed breaks (e.g. V(D)J recombination), class switch recombination, and replication stress associated with virus infections (Bonner et al., 2008; Burhans and Weinberger, 2007). Cells respond to DNA damage by activating checkpoint pathways that delay the progression through the cell cycle, promote DNA repair, or induce cell repair (Garner and Constanzo, 2009). Double-stranded DNA breaks are sensed by the MRN complex which results in recruitment and phosphorylation of the tranducers of the DNA damage response, the PI3-kinase-like kinases: ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related (ATR), and DNA-dependent protein kinase (DNA-PK) (reviewed in Weitzman et al., 2010). ATM is phosphorylated at serine 1981 (Bakkenist and Kastan, 2003; Lee and Paull, 2005; Carson et al., 2003; Uziel et al., 2003) which in turn phosphorylates many downstream proteins involved in coordinating cell cycle arrest, DNA repair and apoptosis such as H2AX, Mdc1, Nbs1, Chk2, 53BP1, and p53 (Bartek amd Lukas 2003).
H2AX is a member of the histone family which interacts with eukaryotic DNA and helps to regulate transcription. H2AX is phosphorylated on serine 139 to form γ-H2AX at sites of double-stranded DNA breaks, where proteins involved in DNA repair accumulate (Rogakou et al., 1999, Bonner et al., 2008). Phosphorylation of H2AX also occurs with other stimuli including single-stranded DNA breaks induced by ultraviolet radiation and with the normal progression through the cell cycle (Ichijima et al., 2005).
Virus replication presents the host cells with foreign DNA, including free DNA ends and unusual DNA structures. Thus, virus replication induces DNA damage responses which can result in apoptosis as a host defense mechanism against infection (Chaurushiya and Weitzman, 2009). Viruses have evolved a variety of mechanisms to counteract DNA damage responses to enhance replication and persistent infection. Many DNA viruses initially activate the DNA damage response pathway and then subsequently inhibit it (reviewed in Chaurushiya and Weizman, 2009; Turnell and Grand, 2012; Weitzman et al. 2010). Adenovirus proteins E1B and E4 inhibit the MRN complex (Stracker et al. 2002), while herpes simplex virus (HSV) UL12 associates with the MRN complex and likely facilitates its activity (Balasubramanian et al. 2010). Kaposi sarcoma associated herpesvirus (KSHV) vIRF1 (Shin et al., 2006) and Epstein-Barr virus (EBV) LMP1 (Ma et al. 2011) inhibit ATM, while HSV ICP0 (Li et al. 2008) and cytomegalovirus IE1 (Castillo et al. 2005) activate ATM. EBV BGLF4 (Li et al. 2011) and KSHV v-cyclin (Koopal et al., 2007) activate H2AX.
The human alphaherpesvirus consist of HSV-1, HSV-2, and varicella-zoster virus (VZV). These viruses cause lytic infections in epithelial cells and latent infections in neurons of sensory ganglia. HSV and VZV have similar mechanisms of replication in the cell and the seven proteins required for origin-dependent replication of HSV DNA (Challberg, 1986) have orthologs in VZV. HSV and VZV infection of cells brings linear viral DNA with free ends into the cell that resemble double-stranded DNA breaks, as well as nicked viral DNA which has single-stranded DNA breaks. Thus, HSV and VZV infection would be expected to result in activation of the DNA damage response. HSV infection of cells results in replication compartments which contain both viral proteins (ICP8, viral DNA polymerase [Bush et al., 1991; Quinlan et al., 1984]) as well as host cell proteins important for the DNA damage response (Nbs1, Mre11, Rad50, and phosphorylated ATM [Gregory and Bachenheimer, 2008; Lilley et al., 2005; Shirata et al., 2005]). γH2AX accumulates in areas surrounding HSV replication complexes (Wilkinson and Weller, 2006). HSV infection results in phosphorylation of other proteins involving the DNA damage response including Nbs1, Chk2, 53BP1, and p53 (Gregory and Bachenheimer, 2008; Lilley et al., 2005; Shirata et al., 2005). While many studies have evaluated the interaction of HSV with DNA damage responses, none have evaluated VZV. Therefore, we studied the effects on VZV infection on activation of cellular proteins involved in the DNA damage response.
Results
Infection with VZV induces phosphorylation of H2AX in melanoma cells and fibroblasts, but not in U2OS cells
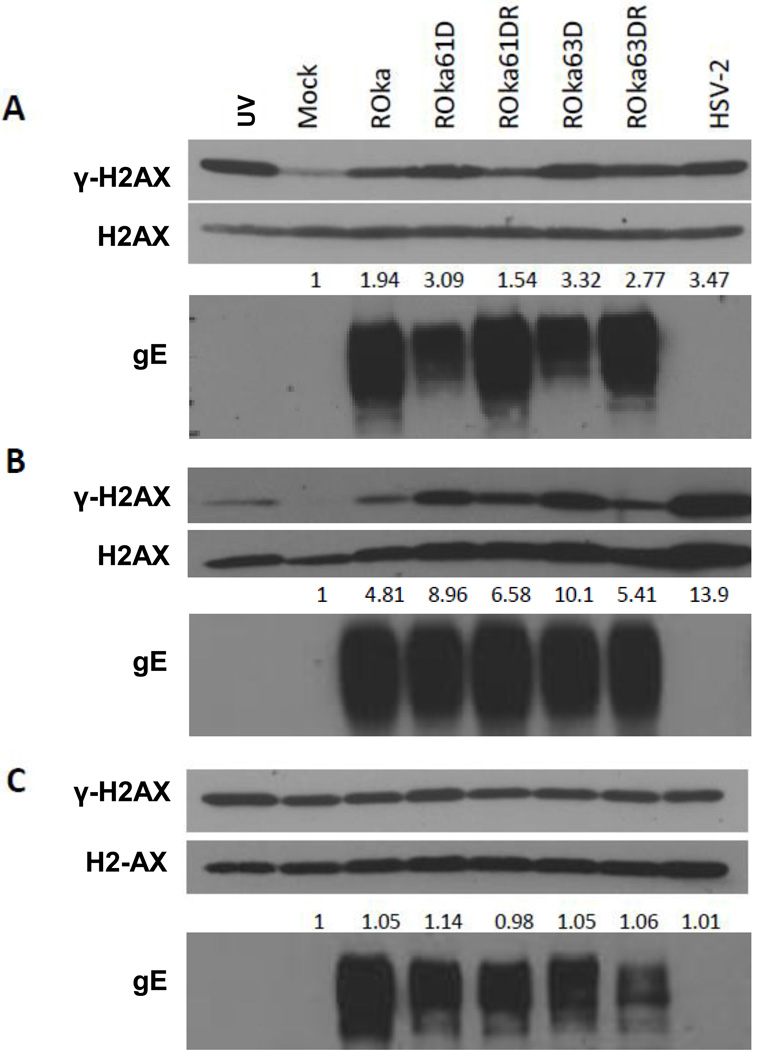
HSV-2 has previously reported to induce phosphorylation of H2AX in Vero cells (Wilkinson and Weller, 2006). Therefore we analyzed the effect of VZV infection on H2AX. We found that VZV, like HSV-2, induced phosphorylation of H2AX in melanoma (Fig. 1A) or human diploid fibroblasts (Fig. 1B); however, the level of total H2AX was unchanged in virus-infected cells. Phosphorylation of H2AX was about 2-fold higher in melanoma cells and 5-fold higher in human diploid fibroblasts infected with cell-associated VZV ROka than in mock-infected cells. UV-irradiated cells (Fig. 1, lane 1) served as a positive control for phosphorylation of H2AX. A similar increase in phosphorylation of H2AX (4-fold) was observed with parental (wild-type) VZV Oka in human diploid fibroblasts compared with mock-infected cells (data not shown). HSV-2 infection also induced phosphorylation of H2AX in melanoma cells and human diploid fibroblasts.
Fig. 1.
Immunoblot of γ-H2AX and H2AX expression in melanoma (MeWo) cells (A), human diploid fibroblasts (MRC-5 cells) (B) and U2OS osteosarcoma cells (C) infected with wild-type VZV, VZV mutants, and HSV-2. MeWo, MRC-5 and U2OS cells were infected with 1 × 106 PFU of cell-associated VZV (ROka, ROka61D, ROka61DR, ROka63D, or ROka63DR) or with HSV-2 at an MOI of 0.5. At 48 hr after infection with VZV (or 24 hr after with HSV-2), the cells were lysed and equivalent amounts of cell lysates were immunoblotted with anti-γ-H2AX, anti-H2AX, and anti-VZV gE antibody. The numbers below the H2AX panels indicate the ratio of intensity of the γ-H2AX and H2AX bands in infected cell lysates divided by the ratio of the intensity of the γ-H2AX and H2AX bands in mock-infected cells. Densitometry was performed using Image J software. UV indicates cells that were treated to 50 mJ/cm2 of UV irradiation and 14 hr later lysates were prepared. The experiments were repeated and similar results were obtained.
High levels of phosphorylated H2AX are induced in melanoma cells and fibroblasts after infection with VZV deleted for ORF61 or ORF63, but not deleted for ORF67
Prior studies showed that immediate-early genes of HSV are important in initiating the DNA damage response in HSV-infected cells (Chenet-Monte C et al. 1986; Johnson et al. 1992; Peat and Stanley, 1986). Therefore we tested two VZV mutants with deletions, one with a deletion in an immediate-early gene (VZV ORF63) and one with a deletion in a gene homologous to the HSV ICP0 immediate-early gene (VZV ORF61). γ-H2AX levels were 1.6-fold higher in melanoma cells infected with ROka61D (deleted for VZV ORF61) compared with ROka, and 1.7-fold higher in melanoma cells infected ROka63D (deleted for VZV ORF63) than ROka (Fig. 1A). Cells infected with rescued viruses (ROka61DR and ROka63DR) had levels of γ-H2AX closer to ROka. Similar increases in γ-H2AX were also observed in fibroblasts infected with ROka61D and ROka63D (Fig. 1B) and in 293T cells (data not shown).
U2OS osteosarcoma cells complement the growth of VZV deleted for ORF61 (ROka61D [Cohen and Nguyen, 1998]) and ORF63 (ROka63D [Ambagala and Cohen, 2007]). Therefore, we determined whether phosphorylation of H2AX might be different in ROka63D or ROka61D–infected U2OS cells than in melanoma cells. Levels of γ-H2AX in U2OS were constitutively elevated in the presence or absence of virus infection and at levels similar to the UV irradiated cells (Fig. 1C); therefore, we could not assess the effect of deletion of ORF61 or ORF63 on γ-H2AX in U2OS cells.
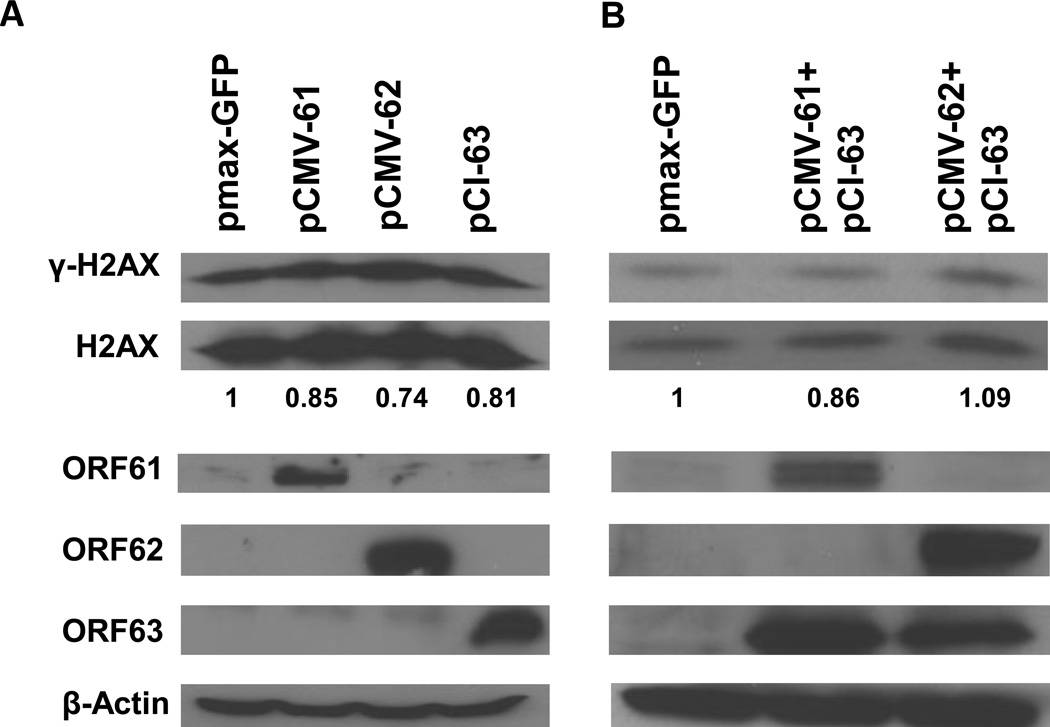
Since deletion VZV ORF61 or ORF63 from VZV increased levels of γ-H2AX, we determined whether ORF61 and ORF63 protein could directly block phosphorylation of H2AX. Melanoma cells were transfected with plasmids expressing VZV ORF61, ORF62, or ORF63, and γ-H2AX was detected on immunoblot. Levels of γ-H2AX were similar in cells expressing VZV ORF61 protein, IE62, or IE63 compared vector control cells (Fig. 2A). Similar results were observed in 293T cells (data not shown). Since ORF61 protein is required for nuclear localization of IE63 in some cell types (Walters et al., 2008), we contransfected cells with plasmids expressing ORF61 and ORF63. Levels of γ-H2AX were comparable in cells expressing both VZV ORF61 protein and IE63, or in cells expressing both IE62 and IE63 compared vector control cells (Fig. 2B). Thus, while VZV deleted for ORF61 or ORF63 induces high levels of phosphorylated H2AX, expression of VZVORF61 or ORF63 alone is unable to inhibit phosphorylation of H2AX in cells.
Fig. 2.
γ-H2AX levels in cells transfected with plasmids expressing VZV ORF61, ORF62, and ORF63. A. MeWo cells were transfected with 4 ug of plasmid DNAs (pCMV-61, pCMV-62, pCI-63 and pMax-GFP) using Lipofectamine 2000. Two days after transfection, the cells were harvested and lysed, and the clarified lysate was immunoblotted with antibodies to γ-H2AX, H2AX, ORF61 protein, IE62, IE63, and β-actin. The experiment was repeated with similar results. B. MeWo cells were cotransfected with 5 ug of plasmid DNAs (pCMV-61 and pCI63, pCMV-62 and pCI-63 or with pMax-GFP) using Lipofectamine 2000 and processed as described in panel A. The transfection efficiency for melanoma cells was ~20% based on GFP expression. The numbers below the H2AX panels indicate the ratio of intensity of the γ-H2AX and H2AX bands in cells transfected with plasmids expressing VZV proteins divided by the ratio of the intensity of the γ-H2AX and H2AX bands in cells transfected with plasmid expressing GFP. Densitometry was performed using Image J software. The experiment was repeated with similar results.
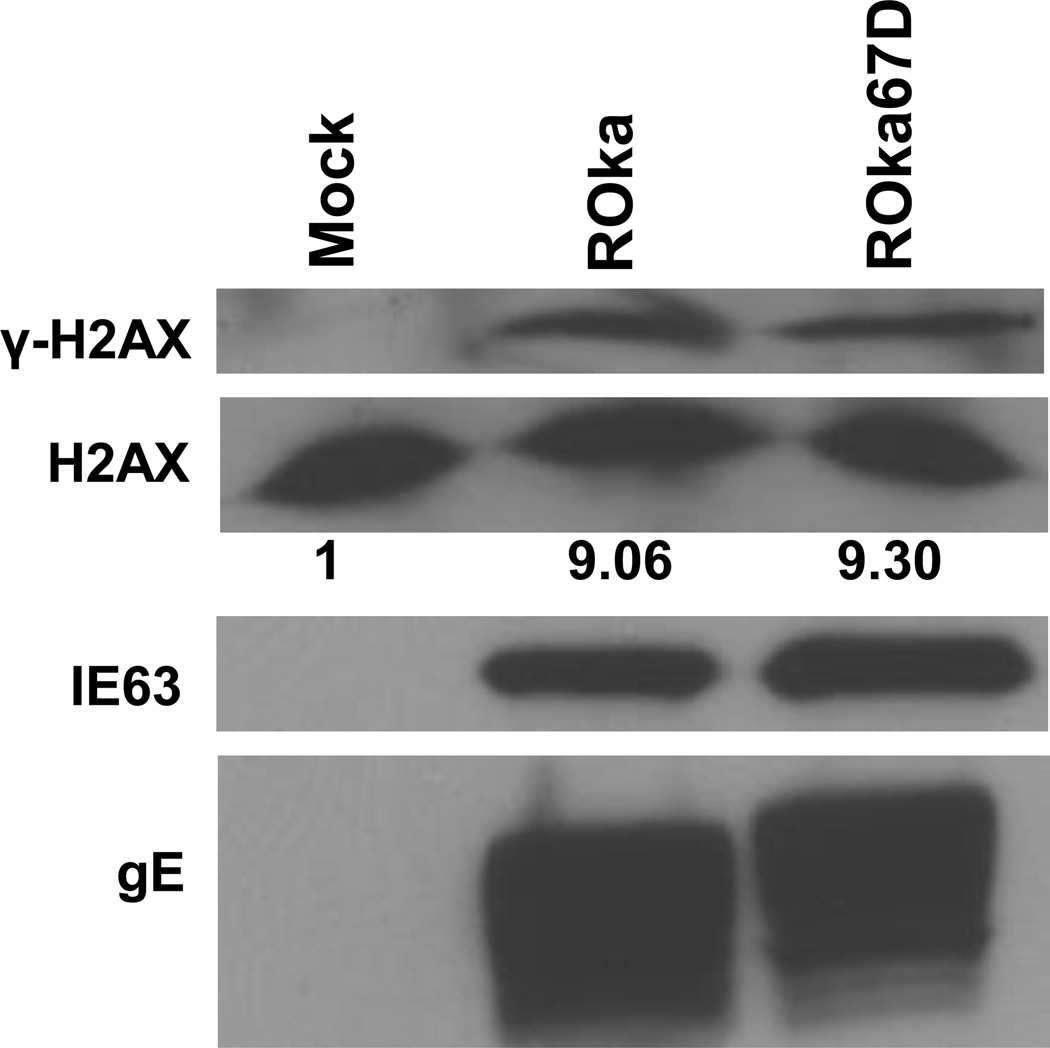
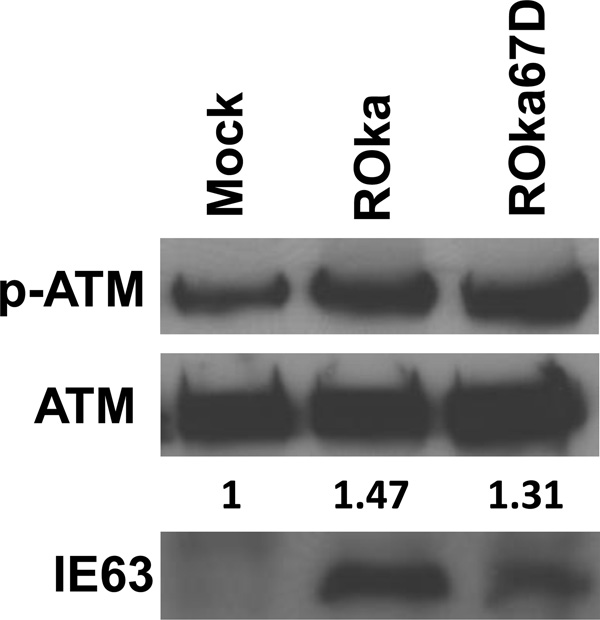
Since the increased level of phosphorylated H2AX in cells infected with ROka61D or ROka63D, relative to wild-type virus, may have been due to a higher titer of inoculum of the mutant viruses used in order to obtain a similar level of infection, we next compared levels of phosphorylated H2AX in cells infected with another VZV mutant. VZV ORF67D is deleted for VZV glycoprotein I, and grows to peak titers about 1 log lower than parental virus which is similar to the reduction in peak titers seen with ROka61D and ROka63D (Cohen et al., 2004; Cohen and Nguyen, 1997; Cohen and Nguyen, 1998). Infection of melanoma cells with ROka67D resulted in similar levels of γ-H2AX as were seen in cells infected with ROka (Fig. 3). Thus, the increased level of phosphorylated H2AX in cells infected with ROka61D or ROka63D was not solely due to impaired growth of the virus in melanoma cells.
Fig. 3.
Immunoblot of γ-H2AX and H2AX expression in melanoma (MeWo) cells infected with wild-type VZV or VZV deleted for ORF67 (ROka67D). MeWo cells were infected with ROka or ROka67D and 48 hr after infection, the cells were lysed and equivalent amounts of cell lysates were immunoblotted with anti-γ-H2AX, anti-H2AX, anti-VZV IE63, anti-VZV gE antibody. The numbers below the H2AX panels indicate the ratio of intensity of the γ-H2AX and H2AX bands in infected cell lysates divided by the ratio of the intensity of the γ-H2AX and H2AX bands in mock-infected cells. Densitometry was performed using Image J software. The experiment was repeated with similar results.
ATM is activated in melanoma cells after infection with VZV, and higher levels of activated ATM are observed in cells deleted for ORF61 or ORF63, but not deleted for ORF67
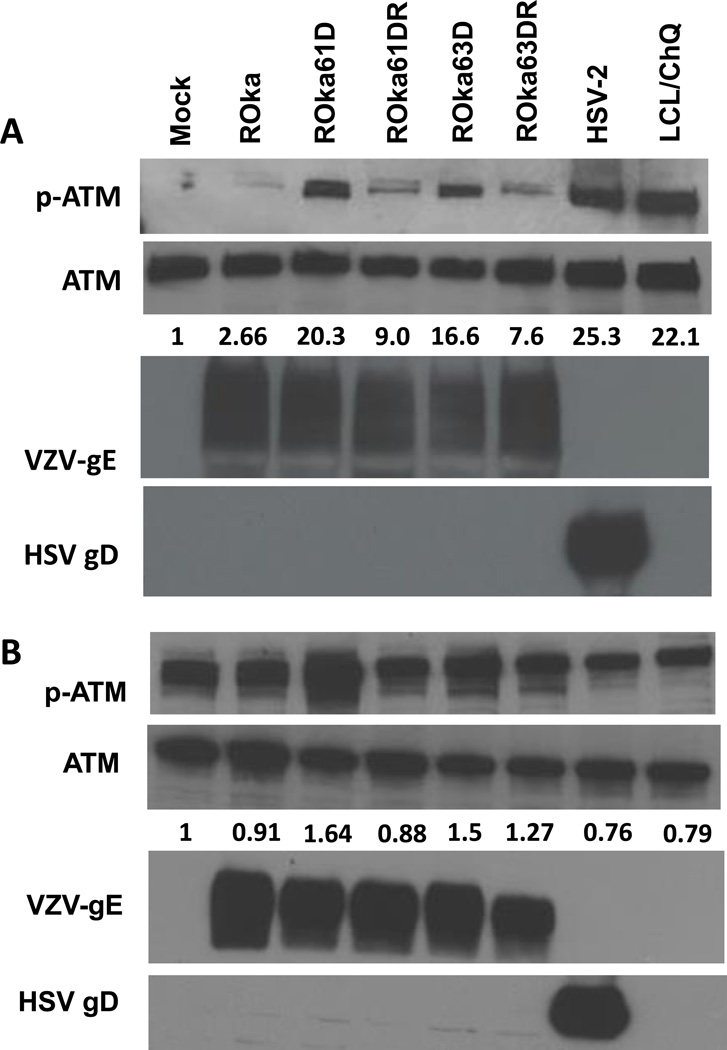
The ATM protein kinase is phosphorylated in response to DNA damage and accumulates at sites of single- and double-stranded DNA breaks (Garner and Costanzo, 2009). Since infection of cells with HSV induces phosphorylation of ATM (Shirata et al., 2005), we determined whether VZV infection also results in phosphorylation of ATM. Infection of melanoma cells with VZV or HSV induced phosphorylation of ATM (Fig. 4A). A chloroquine treated lymphoblastoid cell line served as a positive control for phosphorylation of ATM (Goldstine et al. 2006). Melanoma cells infected with VZV deleted for ORF61 or ORF63 showed a marked increase in phosphorylated ATM compared to cells infected with wild-type VZV, while cells infected with rescued viruses (ROka61DR and ROka63DR) had levels of phosphorylated ATM closer to ROka. Infection of U2OS cells by VZV deleted for ORF61 or ORF63, or by HSV-2 did not result in increased phosphorylation of ATM (Fig. 4B). Melanoma cells infected with VZV deleted for ORF67 did not show an increase in phosphorylated ATM compared with cells infected with parental VZV (Fig. 5).
Fig. 4.
Immunoblot of phosphorylated-ATM and ATM expression in melanoma (MeWo) cells (A) and U2OS osteosarcoma cells (B) infected with wild-type or mutant VZV or HSV-2. Cells were infected with 1 × 106 pfu of cell-associated VZV ROka, ROka61D, ROka61DR, ROka63D, ROka63DR, or infected with HSV-2 at an MOI of 0.5. At 48 hr after infection (or 24 hr after infection in HSV-2), the cells were lysed and equal amounts of clarified lysates were immunoblotted with anti-phosphorylated-ATM (Ser1981), ATM, VZV gE, and HSV gD. The numbers below the ATM panels indicate the ratio of intensity of the phosphorylated ATM and ATM bands in infected cell lysates divided by the ratio of the intensity of the phosphorylated-ATM and ATM bands in mock-infected cells. Densitometry was performed using Image J software. A chloroquine (ChQ) treated lymphoblastoid cell line (LCL) was a positive control for phosphorylation of ATM. The experiments were repeated with similar results.
Fig. 5.
Immunoblot of phosphorylated ATM and total ATM expression in melanoma (MeWo) cells infected with wild-type VZV or VZV deleted for ORF67 (ROka67D). MeWo cells were infected with ROka or ROka67D and 48 hr after infection, the cells were lysed and equivalent amounts of cell lysates were immunoblotted with anti-phosphorylated ATM, anti-ATM, or anti-VZV IE63 antibody. The numbers below the ATM panels indicate the ratio of intensity of the phosphorylated ATM and ATM bands in infected cell lysates divided by the ratio of the intensity of the phosphorylated ATM and ATM bands in mock-infected cells. Densitometry was performed using Image J software. The experiment was repeated with similar results.
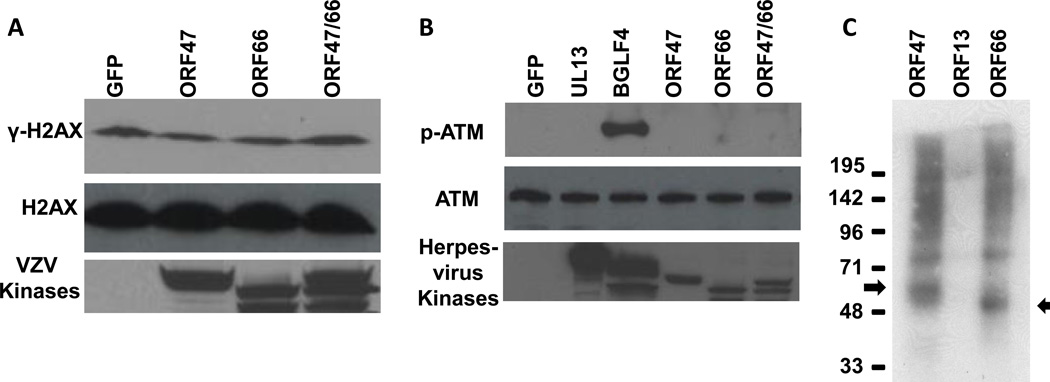
VZV ORF47 and ORF66 protein kinases do not phosphorylate H2AX or ATM
To determine whether phosphorylation of H2AX is induced by VZV protein kinases, we transfected 293T cells with plasmids expressing GFP, VZV ORF47, ORF66, or the combination of ORF47 and ORF66 together and immunoblotted for γ-H2AX , phosphorylated ATM, or the V5 epitope tag that was fused to the viral protein kinases. Although the protein kinases were expressed in the cells, neither individual VZV protein kinase nor the combination of both protein kinases induced phorphorylation of H2AX (Fig. 6A).
Fig. 6.
Phosphorylated H2AX and ATM levels in cells transfected with plasmids expressing VZV ORF47, ORF66, or combined ORF47 and ORF66. MeWo cells were transfected with 5ug of plasmid DNAs containing GFP, carboxyl epitope tagged VZV ORF47, ORF66, ORF47 and ORF66, HSV UL13, or EBV BGLF4 using Lipofectamine 2000. Two days after transfection, the cells were harvested and lysates were immunoblotted with antibodies to (A) γ-H2AX, H2AX, or V5 epitope tag, or (B) phosphorylated ATM, ATM, or V5 epitope tag. (C) Melanoma cells were transfected with plasmids expressing carboxyl V5 epitope-tagged VZV ORF47, ORF66 or ORF13. Two days after transfection, VZV proteins were immunoprecipitated with anti-V5 antibody, washed in protein kinase buffer, incubated with γ32P ATP, separated by electrophoresis, and autoradiography was performed. Long and short arrows indicate VZV ORF47 and ORF66 protein kinases, respectively.
The EBV BGLF4 protein kinase induces phosphorylation of ATM and H2AX (Li et al. 2011; Tarakanova et al. 2007), and its orthologs in HSV and VZV are UL13 and ORF47, respectively. 293T cells were transfected with GFP, HSV UL13, EBV BGLF4, or the two VZV protein kinases. Each of the protein kinases were expressed in transfected 293T cells; however, only EBV BGLF4 and neither HSV UL13 nor the VZV protein kinases induced phosphorylation of ATM (Fig. 6B). As a control, the V5 epitope tagged VZV proteins were immunoprecipitated and protein kinase assays showed that ORF47 and ORF66 proteins could autophosphorylate (while the ORF13 thymidylate synthetase protein could not autophosphorylate), indicating that the epitope tag did not interfere with the kinase activity of the VZV proteins (Fig. 6C).
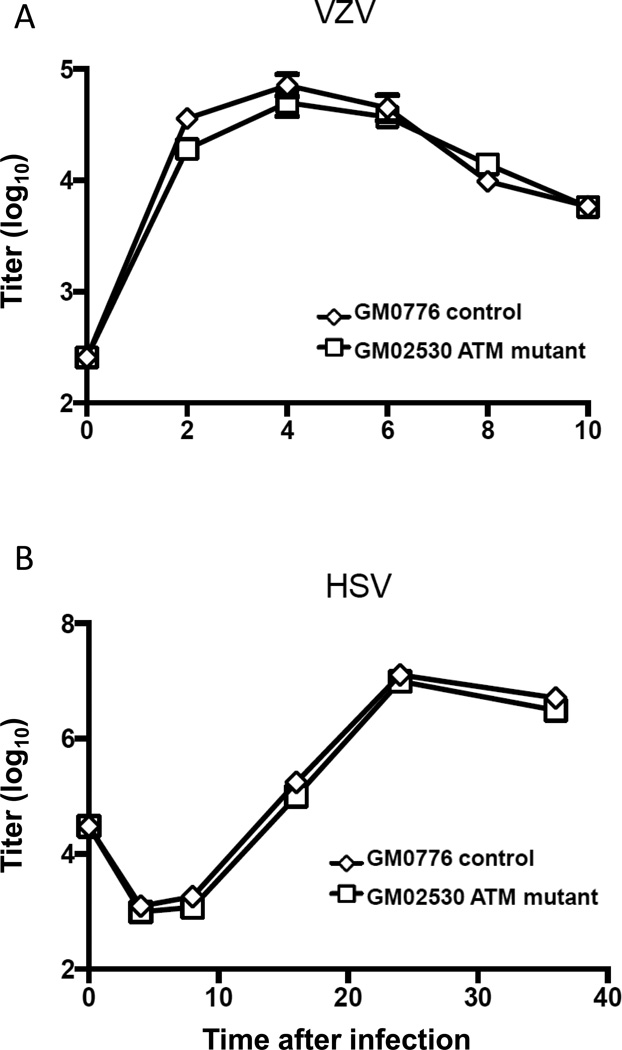
VZV is not impaired for growth in fibroblasts lacking ATM
To determine if ATM has an important role in VZV replication, we infected human fibroblasts from healthy persons or patients with ataxia telangiectasia (with mutations in ATM) with VZV and HSV-2 and measured the titer of virus over time. Growth of VZV (Fig. 7A) or HSV-2 (Fig. 7B) was not impaired in fibroblasts with mutations in ATM compared to wild-type fibroblasts. Similar results were seen with another ATM deficient cell line (data not shown). Thus, ATM is not critical for growth of VZV in vitro.
Fig. 7.
Growth of VZV ROka or HSV-2 in wild-type and ataxia telangiectasia mutant (ATM) fibroblasts. VZV ROka (A) or HSV-2 (B) was used to infect wild-type (GM07667) or ATM mutant (GM02530) fibroblasts for various periods of time. VZV infections were performed with cell-associated virus containing 250 PFU added to 106 cells; HSV infections were performed with 30,000 PFU of cell-free virus added to 3 × 105 cells (MOI 0.1). At various days after infection the monolayers were treated with trypsin and infected cells were titrated onto melanoma cells. Error bars show standard error. The experiment in panel A with VZV was repeated with similar results.
Discussion
We found that infection of cells with VZV, as well as HSV, induces the phosphorylation of H2AX in melanoma cells and fibroblasts, but not in U2OS cells. U2OS had constitutively high levels of γH2AX that were not increased after virus infection. Unlike many transformed cell lines, U2OS cells have a wild-type p53 and retinoblastoma genes (Yao and Schaffer 1995). U2OS cells are defective either for chromatin-dependent nuclear repression or for a signal transduction pathway that activates nuclear repression in response to virus infection (Hancock et al. 2006). Thus, U2OS cells likely have additional properties that result in constitutive phosphorylation of H2AX.
Infection of melanoma cells and fibroblasts with VZV deleted for VZV ORF61 or ORF63 induced higher levels of γH2AX than infection with wild-type virus. However, transient transfection of cells with either of these viral proteins had no discernable effect on the level of phosphorylated H2AX. These results indicate that ORF61 or ORF63 affect phosphorylation of H2AX only in the context of virus infection. VZV ORF61 and ORF63 have several properties in common including (a) regulation of expression of other viral proteins (Bontems et al., 2002; Moriuchi et al., 1993), (b) importance for optimal virus replication in vitro (Cohen and Nguyen, 1998; Cohen et al., 2004), and (c) complementation of growth of viral mutants in these genes by U2OS cells (Cohen and Nguyen 1998; Ambagala and Cohen 2007). Unlike the increase in γH2AX levels observed in melanoma cells and fibroblasts with the VZV mutants, no increase was observed in U2OS cells.
The increased levels of γH2AX were observed in cells infected with ROka61D or ROka63D compared to the wild-type virus could have been due to their impaired replication in melanoma cells. To obtain a similar level of replication we used a larger infected cell inoculum of ROka61D or ROka63D than for wild-type virus. However, the larger inoculum of the VZV mutant virus infected cells (containing more viral DNA and more free DNA ends) does not alone account for the increased level of γH2AX, since cells infected with ROka67D which is similarly impaired for growth in melanoma cells did not show higher levels of γH2AX than cells infected with parental virus.
Neither the VZV ORF47 or ORF66 protein kinases nor the combination of the two were able to phosphorylate γH2AX or ATM. Previously Li et al. (2011) showed that the EBV BGLF4 protein kinase phosphorylates ATM. Tarakanova et al. (2007) showed that EBV BGLF4 and murine γ-herpesvirus 68 (MHV68) ORF36 protein phosphorylates H2AX. While EBV BGLF4 is the ortholog of VZV ORF47 and HSV UL13, and although the EBV BGLF4 protein kinase shares a number of protein substrates with the HSV UL13 protein kinase, neither VZV ORF47 nor HSV UL13 protein kinases phosphorylated ATM. Phosphorylation of ATM was induced in cells infected with VZV or HSV. Phosphorylated ATM accumulates directly at sites of HSV viral replication (Lilley et al., 2005; Shirata et al., 2005; Gregory and Bachenheimer, 2008).
Growth of VZV and HSV was unchanged in fibroblasts lacking ATM compared with wild-type cells. HSV-1 was impaired for replication in ATM-deficient cells when infected at a low MOI (Lilley et al., 2005), while HSV-2 was not impaired at high or low MOI (Shirata et al. 2005). Since we infected cells with cell-associated VZV, this likely corresponds to a high MOI for the uninfected cell that contacts a virus-infected cell in the inoculum. Thus, the lack of effect on VZV replication in ATM null cells may have been due to the relatively high MOI used. HSV infection of ATM-deficient cells failed to induce phosphorylation of a member of the MRN complex (Nbs1), Chk2, and p53 indicating that the DNA damage response requires activation of ATM in HSV infected cells (Lilley et al., 2005; Shirata et al., 2005). Interestingly, while ATM is not needed for replication of MHV68 in mouse fibroblasts, ATM is required for efficient replication of MHV68 in mouse macrophages (Tarakanova et al., 2007). Thus, while we did not observe a difference in VZV replication in fibroblasts lacking ATM, ATM might be important for VZV replication in other cell types.
In summary, we found that melanoma cells infected with VZV have elevated levels of phosphorylated H2AX and phosphorylated ATM, and that levels of both of these phosphoproteins increased in cells infected with VZV deleted for ORF61 or ORF63, but not deleted for ORF67. Thus, the level of phosphorylation of H2AX and ATM is associated with the presence of specific VZV genes.
Materials and Methods
Cells and viruses
MeWo cells (human melanoma cells provided by Charles Grose, University of Iowa) and MRC-5 cells (human diploid fibroblasts from the American Type Culture Collection, Manassas, VA) were maintained in minimal essential medium and supplemented with 10% fetal bovine serum (FBS). U2OS cells (human osteosarcoma cells from the American Type Culture Collection) were maintained in Dulbecco’s modified Eagle’s medium. Human fibroblasts with a mutation in ATM (GM02530) or from a healthy donor (GM07667) were obtained from the Coriell Institute for Medical Research, Camden, New Jersey and were grown in media recommended by Coriell. Recombinant Oka VZV (ROka) was obtained from cosmid clones derived from the Oka vaccine strain of VZV (Cohen and Seidel, 1993). VZV ROka61D (Cohen and Nguyen, 1998), ROka63D (Cohen et al., 2004), and ROka67D (Cohen and Nguyen, 1997) have ORF61, ORF63, and ORF67 deleted, respectively, and ROka61DR and ROka63DR are rescued viruses that express ORF61 and ORF63 proteins. HSV-2 strain 333 was obtained from Gary Hayward. VZV and HSV-2 were propagated and titrated in MeWo and Vero cells, respectively.
Plasmids and Transient-Transfection Assays
Plasmids pCMV61 (Moriuchi et al., 1994), pCMV62 (Moriuchi et al., 1994), and pCI63 (Ambagala et al., 2009) containing the coding region of VZV ORF61, 62 and 63, respectively, under the control of IE promoter of human cytomegalovirus were used for transfections. Plasmids containing VZV ORF47 and HSV-1 UL13, which have a carboxyl terminal V5 epitope tag from the P and V genes of SV5 paramyxovirus, inserted into the multiple cloning site of pcDNA3.1 (Invitrogen) were described previously (Liu et al., 2008; Liu et al. 2012). Plasmids expressing VZVORF66 and EBV BGLF4, containing carboxyl terminal V5 epitope tags, were constructed similarly. Plasmid pmaxGFP (Amaxa, Gaithersburg, MD) was used as a control for transient transfections. MeWo cells, grown in six-well plates, were transfected with 4 µg of plasmid DNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Two days after transfection, the cells were harvested and lysed, and immunoblots were performed.
Immunoblots and Antibodies
MeWo, MRC-5 and U2OS cells in 6 well plates were inoculated with VZV-infected cells containing 1 × 106 PFU of ROka, ROka61DR, or ROka63DR virus or with HSV-2 at an MOI of 0.5. For infections with VZV mutants impaired for growth (ROka61D, ROka63D, ROka67D) the amount of infected cells used was titrated so that the level of infection (as measured by gE) would be similar to parental virus as described previously (Hoover et al., 2006). VZV- or HSV-infected cells were harvested after 48 or 24 hrs after infection, respectively. Cells were lysed in either 1% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, T. J. Baker, Phillipsburg, NJ) in Tris-buffered saline (19.98 mM Tris, 136 mM NaCl, pH 7.4) or in RIPA buffer (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.5% sodium dodecyl sulfate (SDS) supplemented with complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) for 30 min on ice. After centrifugation at 17,900 × g the supernatant was boiled for 5 min in 1× SDS-polyacrylamide gel electrophoresis loading buffer. Equivalent amounts of cell lysates (5 cm2 of infected cells for blotting cellular proteins and 2 cm2 for viral proteins) were separated on 6% or 4% to 20% Tris-glycine SDS-polyacrylamide gels (Invitrogen), transferred to nitrocellulose membranes (Whatman, Sanford, ME) and incubated with antibodies to H2AX (GeneTex, Irvine, CA), γ-H2AX-Ser139 (Millipore, Billerica, MA), phosphorylated ATM-Ser1981 (Epitomics, Burlingame, CA) or ATM (Cell Signaling Technology, Beverly, MA), VZV ORF61, ORF62, or VZV ORF63 protein (Ng et al., 1994) or β-actin (Sigma-Aldrich, St. Louis, MO). After incubation with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antibodies (Thermo Scientific, Rockford, IL), immunoreactive bands were visualized by SuperSignal West Pico or Dura Chemiluminescent Substrate (Thermo Scientific). The intensity of bands was quantified using NIH Image J software (http://rsb.info.nih.gov/ij/).
Immunoprecipitations and Kinase Assays
HEK293T cells in 6 well plates were transfected with 2 µg of plasmids expressing VZV ORF47, ORF66, or ORF13 (Liu et al., 2008) and 2 days later, the cells were lysed in RIPA buffer (0.01 M Tris HCl [pH 7.4], 0.15 M NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% SDS) with complete protease inhibitors (Clontech) and 1 mM sodium vanadate to inhibit phosphatases. Immune complexes were immunoprecipitated using 20 ul anti-V5 agarose beads (Invitrogen) for 1 hr at 4°C. After washing twice in RIPA buffer and twice in protein kinase buffer (Cell Signaling Technology), the immune complexes were resuspended in 50 ul of protein kinase buffer with 1 mM spermidine (Sigma) and 1 µCi of γ32P ATP. The immune complexes were incubated at 30°C for 1 hr and washed twice with kinase buffer and twice with RIPA buffer, and resuspended in 50 µl SDS protein gel loading solution (Quality Biological) containing 10% β-mercaptoethanol, boiled for 5 min and subjected to electrophoresis on a 4%-20% PAGE gel. The gel was dried and autoradiography was performed.
Measurement of virus replication
Human fibroblasts in 25 cm2 flasks were infected with 250 PFU of cell-associated VZV or 30,000 PFU cell-free of HSV (MOI of 0.1). At various times after infection, the VZV monolayers were treated with trypsin and serial dilutions of infected cells were used to infect melanoma cells. One week later the plates were stained with crystal violet and the number of plaques was counted. Flasks containing HSV-infected cells were scraped, freeze thawed three times and aliquots were used to infect Vero cells. After 1 hr, the media was replaced with media containing 0.5% human immunoglobulin (Baxter Healthcare Corporation, Westlake Village, CA), and plaques were stained and counted 2 days later.
Acknowledgments
This study was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases. Takenobu Yamamoto was supported by a JHIF (Japan Herpesvirus Infection Forum) Scholarship Award in Herpesvirus Infections Research. We thank Drs. Paul Kinchington (University of Pittsburgh, Pittsburgh, PA) for antibody to VZV proteins, and Anthony Davis for help with Image J analysis software.
References
- Ambagala AP, Bosma T, Ali MA, Poustovoitov M, Chen JJ, Gershon MD, Adams PD, Cohen JI. Varicella-zoster virus immediate-early 63 protein interacts with human antisilencing function 1 protein and alters its ability to bind histones h3.1 and h3.3. J. Virol. 2009;83:200–209. doi: 10.1128/JVI.00645-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambagala AP, Cohen JI. Varicella-Zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J. Virol. 2007;81:7844–7851. doi: 10.1128/JVI.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Balasubramanian N, Bai P, Buchek G, Korza G, Weller SK. Physical interaction between the herpes simplex virus type 1 exonuclease, UL12, and the DNA double-strand break-sensing MRN complex. J Virol. 2010;84:12504–12514. doi: 10.1128/JVI.01506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, Lukas J. DNA repair: Damage alert. Nature. 2003;421:486–488. doi: 10.1038/421486a. [DOI] [PubMed] [Google Scholar]
- Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontems S, Di Valentin E, Baudoux L, Rentier B, Sadzot-Delvaux C, Piette J. Phosphorylation of varicella-zoster virus IE63 protein by casein kinases influences its cellular localization and gene regulation activity. J Biol Chem. 2002;277:21050–21060. doi: 10.1074/jbc.M111872200. [DOI] [PubMed] [Google Scholar]
- Burhans WC, Weinberger M. DNA replication stress, genome instability and aging. Nucleic Acids Res. 2007;35:7545–7556. doi: 10.1093/nar/gkm1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush M, Yager DR, Gao M, Weisshart K, Marcy AI, Coen DM, Knipe DM. Correct intranuclear localization of herpes simplex virus DNA polymerase requires the viral ICP8 DNA-binding protein. J Virol. 1991;65:1082–1089. doi: 10.1128/jvi.65.3.1082-1089.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson CT, Schwartz RA, Stracker TH, Lilley CE, Lee DV, Weitzman MD. The Mre11 complex is required for ATM activation and the G2/M checkpoint. EMBO J. 2003;22:6610–6620. doi: 10.1093/emboj/cdg630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo JP, Frame FM, Rogoff HA, Pickering MT, Yurochko AD, Kowalik TF. Human cytomegalovirus IE1-72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response. J Virol. 2005;79:11467–11475. doi: 10.1128/JVI.79.17.11467-11475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg MD. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986;83:9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurushiya MS, Weitzman MD. Viral manipulation of DNA repair and cell cycle checkpoints. DNA Repair. 2009;8:1166–1176. doi: 10.1016/j.dnarep.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenet-Monte C, Mohammad F, Celluzzi CM, Schaffer PA, Farber FE. Herpes simplex virus gene products involved in the induction of chromosomal aberrations. Virus Res. 1986;6:245–260. doi: 10.1016/0168-1702(86)90073-0. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Cox E, Pesnicak L, Srinivas S, Krogmann T. The varicella-zoster virus open reading frame 63 latency-associated protein is critical for establishment of latency. J. Virol. 2004;78:11833–11840. doi: 10.1128/JVI.78.21.11833-11840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Nguyen H. Varicella-Zoster Virus glycoprotein I is essential for growth of virus in Vero cells. J. Virol. 1997;71:6913–6920. doi: 10.1128/jvi.71.9.6913-6920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Nguyen H. Varicella-Zoster Virus ORF61 deletion mutants teplicate in cell culture, but a mutant with stop codons in ORF61 reverts to wild-type virus. Virology. 1998;246:306–316. doi: 10.1006/viro.1998.9198. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Seidel KE. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro . Proc. Natl. Acad. Sci. USA. 1993;90:7376–7380. doi: 10.1073/pnas.90.15.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner E, Costanzo V. Studying the DNA damage response using in vitro model systems. DNA Repair. 2009;8:1025–1037. doi: 10.1016/j.dnarep.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Goldstine JV, Nahas S, Gamo K, Gartler SM, Hansen RS, Roelfsema JH, Gatti RA, Marahrens Y. Constitutive phosphorylation of ATM in lymphoblastoid cell lines from patients with ICF syndrome without downstream kinase activity. DNA Repair (Amst) 2006;5:432–443. doi: 10.1016/j.dnarep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gregory DA, Bachenheimer SL. Characterization of mre11 loss following HSV-1 infection. Virology. 2008;373:124–136. doi: 10.1016/j.virol.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock MH, Corcoran JA, Smiley JR. Herpes simplex virus regulatory proteins VP16 and ICP0 counteract an innate intranuclear barrier to viral gene expression. Virology. 2006;352:237–252. doi: 10.1016/j.virol.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Hoover SE, Cohrs RJ, Rangel ZG, Gilden DH, Munson P, Cohen JI. Downregulation of varicella-zoster virus (VZV) immediate-early ORF62 transcription by VZV ORF63 correlates with virus replication in vitro and with latency. J Virol. 2006;80:3459–3468. doi: 10.1128/JVI.80.7.3459-3468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijima Y, Sakasai R, Okita N, Asahina K, Mizutani S, Teraoka H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem Biophys Res Commun. 2005;336:807–812. doi: 10.1016/j.bbrc.2005.08.164. [DOI] [PubMed] [Google Scholar]
- Johnson PA, Miyanohara A, Levine F, Cahill T, Friedmann T. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol. 1992;66:2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopal S, Furuhjelm JH, Järviluoma A, Jäämaa S, Pyakurel P, Pussinen C, Wirzenius M, Biberfeld P, Alitalo K, Laiho M, Ojala PM. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog. 2007;3:1348–1360. doi: 10.1371/journal.ppat.0030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. Erratum in: Science 308: 1870. [DOI] [PubMed] [Google Scholar]
- Li H, Baskaran R, Krisky DM, Bein K, Grandi P, Cohen JB, Glorioso JC. Chk2 is required for HSV-1 ICP0-mediated G2/M arrest and enhancement of virus growth. Virology. 2008;375:13–23. doi: 10.1016/j.virol.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhu J, Xie Z, Liao G, Liu J, Chen MR, Hu S, Woodard C, Lin J, Taverna SD, Desai P, Ambinder RF, Hayward GS, Qian J, Zhu H, Hayward SD. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe. 2011;10:390–400. doi: 10.1016/j.chom.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley CE, Carson CT, Muotri AR, Gage FH, Weitzman MD. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA. 2005;102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fitzgerald K, Kurt-Jones E, Finberg R, Knipe DM. Herpesvirus tegument protein activates NF-kappaB signaling through the TRAF6 adaptor protein. Proc Natl Acad Sci U S A. 2008;105:11335–11339. doi: 10.1073/pnas.0801617105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li Q, Dowdell K, Fischer ER, Cohen JI. Varicella-zoster virus ORF12 protein triggers phosphorylation of ERK1/2 and inhibits apoptosis. J Virol. 2012;86:3143–3151. doi: 10.1128/JVI.06923-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Yang L, Xiao L, Tang M, Liu L, Li Z, Deng M, Sun L, Cao Y. Down-regulation of EBV-LMP1 radio-sensitizes nasal pharyngeal carcinoma cells via NF-κB regulated ATM expression. PLoS One. 2011;6:e24647. doi: 10.1371/journal.pone.0024647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi H, Moriuchi M, Straus SE, Cohen JI. Varicella-zoster virus (VZV) open reading frame 61 protein transactivates VZV gene promoters and enhances the infectivity of VZV DNA. J. Virol. 1993;67:4920–4295. doi: 10.1128/jvi.67.7.4290-4295.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H, Straus SE, Cohen JI. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology. 1994;200:297–300. doi: 10.1006/viro.1994.1190. [DOI] [PubMed] [Google Scholar]
- Ng TI, Keenan L, Kinchington PR, Grose C. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J. Virol. 1994;68:1350–1359. doi: 10.1128/jvi.68.3.1350-1359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peat DS, Stanley MA. Chromosome damage induced by herpes simplex virus type 1 in early infection. J Gen Virol. 1986;67:2273–2277. doi: 10.1099/0022-1317-67-10-2273. [DOI] [PubMed] [Google Scholar]
- Quinlan MP, Chen LB, Knipe DM. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984;36:857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YC, Nakamura H, Liang X, Feng P, Chang H, Kowalik TF, Jung JU. Inhibition of the ATM/p53 signal transduction pathway by Kaposi’s sarcoma-associated herpesvirus interferon regulatory factor 1. J Virol. 2006;80:2257–2266. doi: 10.1128/JVI.80.5.2257-2266.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirata N, Kudoh A, Daikoku T, Tatsumi Y, Fujita M, Kiyono T, Sugaya Y, Isomura H, Ishizaki K, Tsurumi T. Activation of ataxia telangiectasia-mutated DNA damage checkpoint signal transduction elicited by herpes simplex virus infection. J Biol Chem. 2005;280:30336–30341. doi: 10.1074/jbc.M500976200. [DOI] [PubMed] [Google Scholar]
- Stracker TH, Carson CT, Weitzman MD. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature. 2002;418:348–352. doi: 10.1038/nature00863. [DOI] [PubMed] [Google Scholar]
- Tarakanova VL, Leung-Pineda V, Hwang S, Yang CW, Matatall K, Basson M, Sun R, Piwnica-Worms H, Sleckman BP, Virgin HW. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe. 2007;1:275–286. doi: 10.1016/j.chom.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnell AS, Grand RJ. DNA viruses and the cellular DNA-damage response. J Gen Virol. 2012;93:2076–2097. doi: 10.1099/vir.0.044412-0. [DOI] [PubMed] [Google Scholar]
- Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MS, Kyratsous CA, Wan S, Silverstein S. Nuclear import of the varicella-zoster virus latency-associated protein ORF63 in primary neurons requires expression of the lytic protein ORF61 and occurs in a proteasome-dependent manner. J Virol. 2008;82:8673–8686. doi: 10.1128/JVI.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DE, Weller SK. Herpes simplex virus type I disrupts the ATR-dependent DNA-damage response during lytic infection. J Cell Sci. 2006;119:2695–2703. doi: 10.1242/jcs.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman MD, Lilley CE, Chaurushiya MS. Genomes in conflict: maintaining genome integrity during virus infection. Ann Rev Microbiol. 2010;63:61–81. doi: 10.1146/annurev.micro.112408.134016. [DOI] [PubMed] [Google Scholar]
- Yao F, Schaffer PA. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 1995;69:6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]