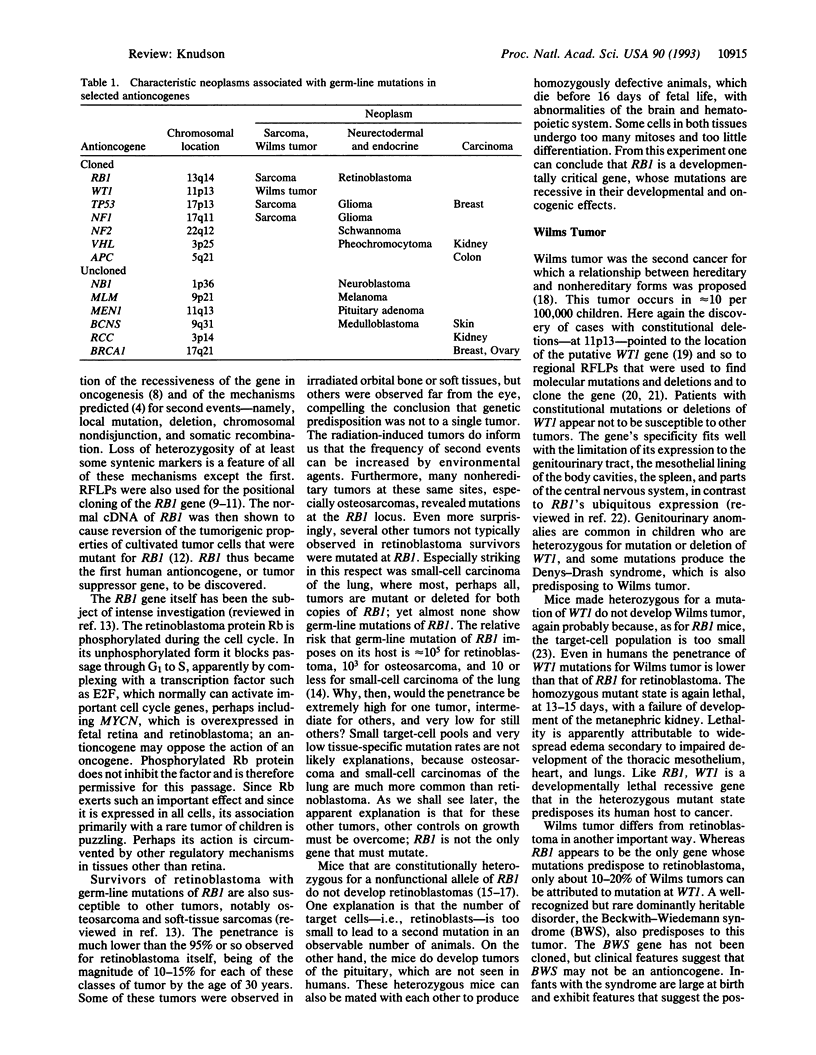
Abstract
The antioncogenes, or tumor suppressor genes, as negative regulators of cell division, stand in contrast to oncogenes. For most human cancers, the more frequently mutated genes are the antioncogenes, the principal exception being the leukemias and lymphomas. Persons heterozygous for germ-line mutations in antioncogenes are strongly predisposed to one or more kinds of cancer, and most dominantly inherited cancer is attributable to such heterozygosity. Seven antioncogenes have been cloned through the study of these persons, and several others have been mapped. An eighth one was mapped and cloned through the investigation of tumors and is not yet known in hereditary form. Three dominantly inherited forms of cancer are not attributable to mutations in antioncogenes. The corresponding nonhereditary forms of most cancers generally reveal abnormalities of the same antioncogenes that are found in the hereditary forms but may also show additional ones. Some cancers, especially the embryonal tumors of children, have a small number of antioncogene mutations; some others, such as most sarcomas, have more, and the common carcinomas have the most, reflecting a hierarchy of controls over growth of stem cell populations. Still more members of this gene category remain to be mapped and cloned through the study of cancer families and of tumors. The genes that have been cloned act at diverse points in the signal transduction pathway in cells, from the outer cell membranes to sites of gene transcription, in some cases as negative regulators of oncogene expression.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Arps S., Rodewald A., Schmalenberger B., Carl P., Bressel M., Kastendieck H. Cytogenetic survey of 32 cancers of the prostate. Cancer Genet Cytogenet. 1993 Apr;66(2):93–99. doi: 10.1016/0165-4608(93)90234-d. [DOI] [PubMed] [Google Scholar]
- Bale S. J., Dracopoli N. C., Tucker M. A., Clark W. H., Jr, Fraser M. C., Stanger B. Z., Green P., Donis-Keller H., Housman D. E., Greene M. H. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome 1p. N Engl J Med. 1989 May 25;320(21):1367–1372. doi: 10.1056/NEJM198905253202102. [DOI] [PubMed] [Google Scholar]
- Ballester R., Marchuk D., Boguski M., Saulino A., Letcher R., Wigler M., Collins F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990 Nov 16;63(4):851–859. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- Biegel J. A., White P. S., Marshall H. N., Fujimori M., Zackai E. H., Scher C. D., Brodeur G. M., Emanuel B. S. Constitutional 1p36 deletion in a child with neuroblastoma. Am J Hum Genet. 1993 Jan;52(1):176–182. [PMC free article] [PubMed] [Google Scholar]
- Bova G. S., Carter B. S., Bussemakers M. J., Emi M., Fujiwara Y., Kyprianou N., Jacobs S. C., Robinson J. C., Epstein J. I., Walsh P. C. Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res. 1993 Sep 1;53(17):3869–3873. [PubMed] [Google Scholar]
- Brodeur G. M., Sekhon G., Goldstein M. N. Chromosomal aberrations in human neuroblastomas. Cancer. 1977 Nov;40(5):2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Carter B. S., Beaty T. H., Steinberg G. D., Childs B., Walsh P. C. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cawthon R. M., Weiss R., Xu G. F., Viskochil D., Culver M., Stevens J., Robertson M., Dunn D., Gesteland R., O'Connell P. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990 Jul 13;62(1):193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Maandag E. R., van Roon M., van der Lugt N. M., van der Valk M., Hooper M. L., Berns A., te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992 Sep 24;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- Cohen A. J., Li F. P., Berg S., Marchetto D. J., Tsai S., Jacobs S. C., Brown R. S. Hereditary renal-cell carcinoma associated with a chromosomal translocation. N Engl J Med. 1979 Sep 13;301(11):592–595. doi: 10.1056/NEJM197909133011107. [DOI] [PubMed] [Google Scholar]
- Comings D. E. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschner E. E., Lipkin M. Proliferative patterns in colonic mucosa in familial polyposis. Cancer. 1975 Feb;35(2):413–418. doi: 10.1002/1097-0142(197502)35:2<413::aid-cncr2820350217>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Diaz M. O., Ziemin S., Le Beau M. M., Pitha P., Smith S. D., Chilcote R. R., Rowley J. D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller L., Kassel J., Nelson C. E., Gryka M. A., Litwak G., Gebhardt M., Bressac B., Ozturk M., Baker S. J., Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Faulkner K. W., Holmes L. B., Steinfeld A., Abroms I. F. A child with 18q- syndrome and cerebellar astrocytoma. J Pediatr. 1983 Oct;103(4):600–602. doi: 10.1016/s0022-3476(83)80598-8. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Kung F. Sporadic bilateral retinoblastoma and 13q- chromosomal deletion. Med Pediatr Oncol. 1976;2(4):379–385. doi: 10.1002/mpo.2950020404. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gailani M. R., Bale S. J., Leffell D. J., DiGiovanna J. J., Peck G. L., Poliak S., Drum M. A., Pastakia B., McBride O. W., Kase R. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell. 1992 Apr 3;69(1):111–117. doi: 10.1016/0092-8674(92)90122-s. [DOI] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Glover T. W., Stein C. K., Legius E., Andersen L. B., Brereton A., Johnson S. Molecular and cytogenetic analysis of tumors in von Recklinghausen neurofibromatosis. Genes Chromosomes Cancer. 1991 Jan;3(1):62–70. doi: 10.1002/gcc.2870030111. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Lee W. H. Molecular characterization of the retinoblastoma susceptibility gene. Biochim Biophys Acta. 1993 May 25;1155(1):43–61. doi: 10.1016/0304-419x(93)90021-4. [DOI] [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hethcote H. W., Knudson A. G., Jr Model for the incidence of embryonal cancers: application to retinoblastoma. Proc Natl Acad Sci U S A. 1978 May;75(5):2453–2457. doi: 10.1073/pnas.75.5.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino O., Klein-Szanto A. J., Freed J. J., Testa J. R., Brown D. Q., Vilensky M., Yeung R. S., Tartof K. D., Knudson A. G. Spontaneous and radiation-induced renal tumors in the Eker rat model of dominantly inherited cancer. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):327–331. doi: 10.1073/pnas.90.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Ichii S., Horii A., Nakatsuru S., Furuyama J., Utsunomiya J., Nakamura Y. Inactivation of both APC alleles in an early stage of colon adenomas in a patient with familial adenomatous polyposis (FAP). Hum Mol Genet. 1992 Sep;1(6):387–390. doi: 10.1093/hmg/1.6.387. [DOI] [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Knudson A. G., Jr, Meadows A. T., Nichols W. W., Hill R. Chromosomal deletion and retinoblastoma. N Engl J Med. 1976 Nov 11;295(20):1120–1123. doi: 10.1056/NEJM197611112952007. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Retinoblastoma: a prototypic hereditary neoplasm. Semin Oncol. 1978 Mar;5(1):57–60. [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972 Feb;48(2):313–324. [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: neuroblastoma and pheochromocytoma. Am J Hum Genet. 1972 Sep;24(5):514–532. [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G. Stem cell regulation, tissue ontogeny, and oncogenic events. Semin Cancer Biol. 1992 Jun;3(3):99–106. [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. WT-1 is required for early kidney development. Cell. 1993 Aug 27;74(4):679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Larsson C., Skogseid B., Oberg K., Nakamura Y., Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988 Mar 3;332(6159):85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Laureys G., Speleman F., Opdenakker G., Benoit Y., Leroy J. Constitutional translocation t(1;17)(p36;q12-21) in a patient with neuroblastoma. Genes Chromosomes Cancer. 1990 Sep;2(3):252–254. doi: 10.1002/gcc.2870020315. [DOI] [PubMed] [Google Scholar]
- Lavigueur A., Maltby V., Mock D., Rossant J., Pawson T., Bernstein A. High incidence of lung, bone, and lymphoid tumors in transgenic mice overexpressing mutant alleles of the p53 oncogene. Mol Cell Biol. 1989 Sep;9(9):3982–3991. doi: 10.1128/mcb.9.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor K. G., Narayanan R. Persistent expression of the tumor suppressor gene DCC is essential for neuronal differentiation. Cell Growth Differ. 1992 Sep;3(9):609–616. [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Rhabdomyosarcoma in children: epidemiologic study and identification of a familial cancer syndrome. J Natl Cancer Inst. 1969 Dec;43(6):1365–1373. [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Malkin D. p53 and the Li-Fraumeni syndrome. Cancer Genet Cytogenet. 1993 Apr;66(2):83–92. doi: 10.1016/0165-4608(93)90233-c. [DOI] [PubMed] [Google Scholar]
- Martin G. A., Viskochil D., Bollag G., McCabe P. C., Crosier W. J., Haubruck H., Conroy L., Clark R., O'Connell P., Cawthon R. M. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990 Nov 16;63(4):843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Chin K. S., Easton D. F., Thorpe K., Carter C., Liou G. I., Fong S. L., Bridges C. D., Haak H., Kruseman A. C. A linked genetic marker for multiple endocrine neoplasia type 2A on chromosome 10. Nature. 1987 Aug 6;328(6130):527–528. doi: 10.1038/328527a0. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Smith B. A., Thorpe K., Wong Z., Royle N. J., Jeffreys A. J., Ponder B. A. Deletion of genes on chromosome 1 in endocrine neoplasia. Nature. 1987 Aug 6;328(6130):524–526. doi: 10.1038/328524a0. [DOI] [PubMed] [Google Scholar]
- Menon A. G., Anderson K. M., Riccardi V. M., Chung R. Y., Whaley J. M., Yandell D. W., Farmer G. E., Freiman R. N., Lee J. K., Li F. P. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi J., Dobler K. D., Allalunis-Turner J., McKean J. D., Petruk K., Allen P. B., Aronyk K. N., Weir B., Huyser-Wierenga D., Fulton D. Absence of IFNA and IFNB genes from human malignant glioma cell lines and lack of correlation with cellular sensitivity to interferons. Cancer Res. 1990 Jan 15;50(2):278–283. [PubMed] [Google Scholar]
- Moser A. R., Dove W. F., Roth K. A., Gordon J. I. The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol. 1992 Mar;116(6):1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan L. M., Kwok J. B., Healey C. S., Elsdon M. J., Eng C., Gardner E., Love D. R., Mole S. E., Moore J. K., Papi L. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993 Jun 3;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Neumann H. P., Wiestler O. D. Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991 May 4;337(8749):1052–1054. doi: 10.1016/0140-6736(91)91705-y. [DOI] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Ogawa O., Eccles M. R., Szeto J., McNoe L. A., Yun K., Maw M. A., Smith P. J., Reeve A. E. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms' tumour. Nature. 1993 Apr 22;362(6422):749–751. doi: 10.1038/362749a0. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Buchhagen D. L., Malik K., Sherman J., Nobori T., Bader S., Nau M. M., Gazdar A. F., Minna J. D., Diaz M. O. Homozygous loss of the interferon genes defines the critical region on 9p that is deleted in lung cancers. Cancer Res. 1993 May 15;53(10 Suppl):2410–2415. [PubMed] [Google Scholar]
- Peltomäki P., Aaltonen L. A., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Green J. S., Jass J. R., Weber J. L., Leach F. S. Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993 May 7;260(5109):810–812. doi: 10.1126/science.8484120. [DOI] [PubMed] [Google Scholar]
- Petty E. M., Gibson L. H., Fountain J. W., Bolognia J. L., Yang-Feng T. L., Housman D. E., Bale A. E. Molecular definition of a chromosome 9p21 germ-line deletion in a woman with multiple melanomas and a plexiform neurofibroma: implications for 9p tumor-suppressor gene(s). Am J Hum Genet. 1993 Jul;53(1):96–104. [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Vogelstein B. Tumour suppressor genes. No room at the p53 inn. Nature. 1993 Sep 2;365(6441):17–18. doi: 10.1038/365017a0. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Rainier S., Johnson L. A., Dobry C. J., Ping A. J., Grundy P. E., Feinberg A. P. Relaxation of imprinted genes in human cancer. Nature. 1993 Apr 22;362(6422):747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- Rasheed B. K., Fuller G. N., Friedman A. H., Bigner D. D., Bigner S. H. Loss of heterozygosity for 10q loci in human gliomas. Genes Chromosomes Cancer. 1992 Jul;5(1):75–82. doi: 10.1002/gcc.2870050111. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd The WT1 Wilms tumor gene product: a developmentally regulated transcription factor in the kidney that functions as a tumor suppressor. FASEB J. 1993 Jul;7(10):896–903. [PubMed] [Google Scholar]
- Rouleau G. A., Merel P., Lutchman M., Sanson M., Zucman J., Marineau C., Hoang-Xuan K., Demczuk S., Desmaze C., Plougastel B. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993 Jun 10;363(6429):515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Sherley J. L. Guanine nucleotide biosynthesis is regulated by the cellular p53 concentration. J Biol Chem. 1991 Dec 25;266(36):24815–24828. [PubMed] [Google Scholar]
- Sidransky D., Mikkelsen T., Schwechheimer K., Rosenblum M. L., Cavanee W., Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992 Feb 27;355(6363):846–847. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- Skuse G. R., Kosciolek B. A., Rowley P. T. Molecular genetic analysis of tumors in von Recklinghausen neurofibromatosis: loss of heterozygosity for chromosome 17. Genes Chromosomes Cancer. 1989 Sep;1(1):36–41. doi: 10.1002/gcc.2870010107. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Johnson K. A., Bryan T. M., Hill D. E., Markowitz S., Willson J. K., Paraskeva C., Petersen G. M., Hamilton S. R., Vogelstein B. The APC gene product in normal and tumor cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A., Easton D. F., Evans D. G., Ponder B. A. Allele losses in the region 17q12-21 in familial breast and ovarian cancer involve the wild-type chromosome. Nat Genet. 1992 Oct;2(2):128–131. doi: 10.1038/ng1092-128. [DOI] [PubMed] [Google Scholar]
- Su L. K., Johnson K. A., Smith K. J., Hill D. E., Vogelstein B., Kinzler K. W. Association between wild type and mutant APC gene products. Cancer Res. 1993 Jun 15;53(12):2728–2731. [PubMed] [Google Scholar]
- Su L. K., Kinzler K. W., Vogelstein B., Preisinger A. C., Moser A. R., Luongo C., Gould K. A., Dove W. F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992 May 1;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Thuwe I., Lundström B., Wålinder J. Familial brain tumour. Lancet. 1979 Mar 3;1(8114):504–504. doi: 10.1016/s0140-6736(79)90871-7. [DOI] [PubMed] [Google Scholar]
- Trofatter J. A., MacCollin M. M., Rutter J. L., Murrell J. R., Duyao M. P., Parry D. M., Eldridge R., Kley N., Menon A. G., Pulaski K. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993 Mar 12;72(5):791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Marchuk D. A., Andersen L. B., Letcher R., Odeh H. M., Saulino A. M., Fountain J. W., Brereton A., Nicholson J., Mitchell A. L. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990 Jul 13;249(4965):181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Weith A., Martinsson T., Cziepluch C., Brüderlein S., Amler L. C., Berthold F., Schwab M. Neuroblastoma consensus deletion maps to 1p36.1-2. Genes Chromosomes Cancer. 1989 Nov;1(2):159–166. doi: 10.1002/gcc.2870010209. [DOI] [PubMed] [Google Scholar]
- Weksberg R., Shen D. R., Fei Y. L., Song Q. L., Squire J. Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet. 1993 Oct;5(2):143–150. doi: 10.1038/ng1093-143. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Kao-Shan C. S., Lee E. C., Bunn P. A., Carney D. N., Gazdar A. F., Minna J. D. Specific chromosome defect associated with human small-cell lung cancer; deletion 3p(14-23). Science. 1982 Jan 8;215(4529):181–182. doi: 10.1126/science.6274023. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Knutsen T., Gazdar A., Steinberg S. M., Oie H., Linnoila I., Mulshine J., Nau M., Minna J. D. Nonrandom structural and numerical chromosome changes in non-small-cell lung cancer. Genes Chromosomes Cancer. 1991 May;3(3):168–188. doi: 10.1002/gcc.2870030303. [DOI] [PubMed] [Google Scholar]
- Xu G. F., O'Connell P., Viskochil D., Cawthon R., Robertson M., Culver M., Dunn D., Stevens J., Gesteland R., White R. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990 Aug 10;62(3):599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- Xu W., Mulligan L. M., Ponder M. A., Liu L., Smith B. A., Mathew C. G., Ponder B. A. Loss of NF1 alleles in phaeochromocytomas from patients with type I neurofibromatosis. Genes Chromosomes Cancer. 1992 Jun;4(4):337–342. doi: 10.1002/gcc.2870040411. [DOI] [PubMed] [Google Scholar]
- Yeung R. S., Buetow K. H., Testa J. R., Knudson A. G., Jr Susceptibility to renal carcinoma in the Eker rat involves a tumor suppressor gene on chromosome 10. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8038–8042. doi: 10.1073/pnas.90.17.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]



