Abstract
Breed-wise standard echocardiographic values in dogs have been reported as there is variation in body and chest conformation which limits the application of data of one breed for other breed. Labrador Retrievers being originated from hunting dogs, might have different echocardiographic values from standard normal range of other dog breeds. So, the present study was aimed to determine the M-mode echocardiographic reference ranges in Labrador Retriever dogs and to evaluate the effect of body weight and gender on these parameters. The data obtained were also compared with that of the other dog breeds. Conscious clinically healthy Labrador Retriever dogs (n=24) of both sexes were made the subject of the study. All the measurements were made from a right parasternal long axis left ventricular outflow tract view and the parameters measured were: left ventricular dimensions, left ventricular function, left ventricular volumes, left atrial and aortic root diameter and mitral valve parameters. Data obtained were also compared with that available for other dog breeds. Significant correlation (P<0.05) with body weight was obtained for some of the left ventricular, atrial and mitral valve parameters, being strong for LAD, AOD, LVIDd, LVIDs, IVSd and IVSs (r>0.5); moderate for LVPWd, LVPWs, EPSS, EF Slope and SV (r=0.3 to 0.5); weak for EDV and ESV (r<0.3). Non-significant effect of gender was seen on all the echocardiographic parameters. However, some of the parameters had a significant breed effect. It is expected that the obtained data will be valuable for the progress of studies on small animal cardiology.
Key Words: Dog, Labrador Retrievers, M-mode echocardiography, Reference values
Introduction
Echocardiography is accepted as the most valuable diagnostic tool in the evaluation of cardiac anatomy and function, as well as in investigating cardiac diseases in small animal cardiology. In addition to qualitative estimation, measurement of cardiac dimensions and calculation of cardiac functional parameters are inevitable to determine cardiac performance of healthy individuals and to diagnose pathological conditions (Foppa et al., 2005 ▶). Echocardiography allows an evaluation of the space relationship between structures, cardiac movement and the blood flow features, the precise and non-invasive diagnosis of cardiac alterations, as well as a follow-up of therapy and to determine the prognosis through direct vision of the cardiac chambers (Gugjoo et al., 2013 ▶). The echocardiographic indices show significant breed variations and it is important to know the normal echocardiographic values for each dog breed considering the influence of body weight on the established echocardiographic values (Boon et al., 1983 ▶; Crippa et al., 1992 ▶; Thomas et al., 1993 ▶; O’Leary et al., 2003 ▶). The aim of the present study was to determine the echocardiographic indices of clinically healthy Labrador Retriever dogs, and correlate them with the body weight and gender. It is expected that the data obtained will be valuable for the progress of studies on small animal cardiology.
Materials and Methods
The present study was conducted in 24 clinically healthy conscious Labrador Retriever dogs (12 males and 12 females), with body weight ranging from 18-31 kg and aged between 16 months to 4 years. The animals were selected through a physical, electrocardiographic and blood (Modified Knott’s technique for Dirofilaria immitis) examination, those showing any clinical sign of cardiomyopathy were excluded. Also, the two-dimensional echocardiography was done before M-mode echocardiographic measurements were made, to rule out any anatomical defect. Animals were shaved on the right side in the region between 3rd to 7th rib and 1-5 cm lateral to sternum and were put in lateral recumbency on the table having a slit in the middle with the side to be examined occupying the slit. Ultrasound coupling gel was applied liberally over the skin of the animal to ensure an intimate contact between skin and the transducer head. M-mode echocardiography was done using a 5 MHz annular array sector transducer (Pie Medicals, The Netherlands). For imaging, the transducer was placed on the dependent side of body through the slit at a point where strongest palpable apex beat was heard. All the measurements were made from a right parasternal long axis left ventricular outflow tract view. Selected prints of the images were taken with the help of a thermoprinter (Mitsubishi, Japan), after freezing the images on screen. All the measurements were made using leading edge method as per the recommendations of the American Society of Echocardiography (Thomas et al., 1993 ▶) from frozen images on the screen.
For obtaining the left ventricular images, the cursor was positioned at the level just posterior to the chordae tendinae, perpendicular to the interventricular septum and left ventricular posterior wall. Left ventricular end-diastolic and end-systolic measurements were taken at the largest and at the smallest dimensions between the interventricular septum and the left ventricular posterior wall, respectively. The following parameters were measured from left ventricle-left ventricular internal dimension at diastole (LVDd) and systole (LVDs), left ventricular posterior wall dimensions at diastole (LVPWd) and systole (LVPWs), interventricular septal thickness at diastole (IVSd) and systole (IVSs). With the cursor line positioned perpendicularly over the mitral valve, the two leaflets produced an ‘M’ shaped image in inverse fashion on the M mode. Various points of this M mode mitral valve image were identified and then measurements were recorded which include maximum mitral valve excursion amplitude (DE amplitude), early diastolic posterior motion of mitral valve leaflet (EF slope), time interval of the peak of the A wave and the C point (AC interval) and distance between interventricular septum and mitral valve leaflet at maximum opening (EPSS). For left atrial measurements, cursor was positioned over it and left atrial diameter (LAD) was measured. Aortic root measurements were made on a portion of the sweep with at least one visible valve cusp.
Calculations
Left ventricular volumes like end diastolic volume and end systolic volume were calculated by the Teicholz formulae (Teicholz et al., 1976 ▶):
End diastolic volume (EDV in ml) = 7 (Х) LVDd3/(2.4+LVDd)
End systolic volume (ESV in ml) = 7 (Х) LVDs3/(2.4+LVDs)
The rest of the parameters were calculated using established formulae (Kittleson and Kienle, 1998a ▶; Reece, 2005 ▶).
Stroke volume (SV in ml) = EDV-ESV
Ejection fraction (EF%) = (EDV-ESV) (Х) 100/EDV
Left ventricle fractional shortening (LVFS in %) = (LVDd-LVDs) (Х) 100/LVDd
Statistical analysis
All the data were analysed by SPSS software version 15.0 (SPSS, Inc., Chicago, IL). One way analysis of variance (ANOVA) and Duncan’s multiple range test (DMRT) were used to compare the mean values among different groups based on body weight and gender. Linear regression analysis was done to assess the relationship between echocardiographic parameters and body weight (BW) using GraphPad Prism v.3.0 (GraphPad Software, Inc., California Corp., US). P-values <0.05 were considered significant.
Results
Mean±SE, range values (minimum and maximum values) and co-efficient of variation of all the echocardiographic parameters and body weight are given in Table 1. Fractional shortening in the majority of the animals was above 25%, only 8% of the animals (2/24) had less than 25%. None of the dogs were found to have EF <45%.
Table 1.
Mean±SE, range values and co-efficient of variation of different echocardiographic parameters in Labrador Retrievers
| Parameters (units) | Mean±SE | Range | CV (%) |
|---|---|---|---|
| Body weight (kg) | 23.67±0.46 kg | 18.0-30.0 | - |
| LVIDd (mm) | 37.58±1.05 | 29.4-45.3 | 16.16 |
| LVIDs (mm) | 23.98±0.97 | 14.5-36.8 | 12.48 |
| IVSd (mm) | 9.06±0.37 | 5.6-13.5 | 19.73 |
| IVSs (mm) | 14.47±0.64 | 8.1-20.8 | 15.09 |
| LVPWd (mm) | 8.75±0.26 | 6.2-11.3 | 3.367 |
| LVPWs (mm) | 12.08±0.4 | 9.1-14.7 | 7.144 |
| EDV (ml) | 65.42±3.58 | 26.97-93.82 | 19.75 |
| ESV (ml) | 22.38±2.14 | 5.57-57.35 | 13.29 |
| SV (ml) | 40.61±2.79 | 16.39-64.11 | 6.720 |
| LVFS (%) | 35.89±1.56 | 18.75-49.66 | 0.209 |
| EF (%) | 65.50±2.15 | 45.45-81.35 | 0.743 |
| DE amplitude (mm) | 10.43±0.43 | 6.7-15.6 | 3.863 |
| EF slope (mm/s) | 96.98±3.96 | 57.2-153.2 | 6.211 |
| AC interval (ms) | 171.58±6.87 | 117.0-215.0 | 0.960 |
| EPSS (mm) | 5.20±0.17 | 4.0-7.5 | 4.934 |
| LAD (mm) | 21.93±0.37 | 19.34-26.1 | 38.03 |
| AOD (mm) | 23.63±0.40 | 20.12-28.1 | 25.97 |
Relationship between gender and echo-cardiographic parameters
In the current study, uniformity in body weight in males and females was maintained. Statistically non-significant differences were observed between the gender and echocardiographic parameters (Tables 2 and 3). Left ventricular fractional shortening (LVFS) and ejection fraction (EF) that are commonly used clinical measurements of the left ventricular systolic function were also statistically not different. Therefore, cardiac function in male Labrador Retrievers can be considered similar to females of equal body weight.
Table 2.
Mean±SE and range values of different echocardiographic parameters in males
| Parameters (units) | Mean±SE | Range |
|---|---|---|
| Body weight (kg) | 23.50±1.04 | 18.0-30.0 |
| LVIDd (mm) | 38.39±1.71 | 29.4-45.3 |
| LVIDs (mm) | 23.90±1.68 | 14.5-36.8 |
| IVSd (mm) | 9.20±0.52 | 5.6-12.5 |
| IVSs (mm) | 15.05±1.00 | 8.1-20.8 |
| LVPWd (mm) | 8.86±0.39 | 6.8-11.3 |
| LVPWs (mm) | 12.50±0.62 | 9.4-14.7 |
| EDV (ml) | 65.12±6.35 | 26.97-93.82 |
| ESV (ml) | 22.21±3.93 | 5.57-57.35 |
| SV (ml) | 42.72±4.36 | 21.4-64.11 |
| LVFS (%) | 37.70±2.47 | 18.75-49.66 |
| EF (%) | 67.35±3.43 | 48.87-81.35 |
| DE amplitude (mm) | 10.45±0.54 | 7.8-14.1 |
| EF slope (mm/s) | 96.65±5.18 | 57.2-117.6 |
| AC interval (ms) | 175.33±9.25 | 117.0-215.0 |
| EPSS (mm) | 5.12±0.23 | 4.0-6.5 |
| LAD (mm) | 21.61±0.51 | 19.34-25.1 |
| AOD (mm) | 23.54±0.65 | 20.62-28.1 |
Table 3.
Mean±SE and range values of different echocardiographic parameters in females
| Parameters (units) | Mean±SE | Range |
|---|---|---|
| Body weight (kg) | 23.83±0.84 | 18-27 |
| LVIDd (mm) | 37.25±1.14 | 30.4-42.6 |
| LVIDs (mm) | 24.56±0.82 | 21.0-30.8 |
| IVSd (mm) | 8.94±0.55 | 6.0-13.5 |
| IVSs (mm) | 13.88±0.80 | 10.2-15.3 |
| LVPWd (mm) | 8.64±0.36 | 6.2-10.3 |
| LVPWs (mm) | 11.67±0.53 | 9.1-14.6 |
| EDV (ml) | 63.64±6.64 | 36.09-84.12 |
| ESV (ml) | 22.01±2.04 | 14.5-32.27 |
| SV (ml) | 39.63±3.36 | 16.39-51.85 |
| LVFS (%) | 34.49±1.93 | 21.75-45.11 |
| EF (%) | 63.64±2.65 | 45.45-76.95 |
| DE amplitude (mm) | 10.35±0.67 | 6.7-15.6 |
| EF slope (mm/s) | 97.17±6.31 | 66.8-153.2 |
| AC interval (ms) | 167.83±10.46 | 117.0-215.0 |
| EPSS (mm) | 5.30±0.26 | 4.5-7.5 |
| LAD (mm) | 22.25±0.55 | 19.4-26.1 |
| AOD (mm) | 23.71±0.48 | 20.12-25.01 |
Correlation between body weight and echo-cardiographic parameters
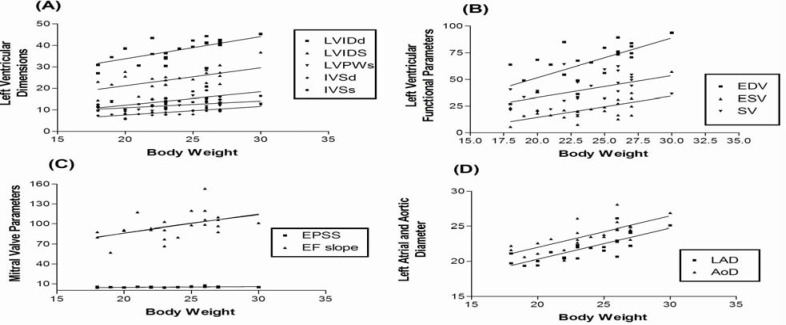
A correlation was documented between the majorities of M-mode echocardiographic measurements with body weight. Linear correlation of different echocardiographic parameters with body weight ranged between 0.183 and 0.796 (Table 4, Fig. 1), being strong (r>0.5) for LAD, AOD, LVIDd, LVIDs, IVSd and IVSs; moderate (r=0.3 to 0.5) for LVPWd, LVPWs, DE amplitude, EPSS, EF Slope and SV; weak (r<0.3) for EDV and ESV. However, statistically non-significant linear correlation with body weight was observed for LVPWd, DE amplitude, LVFS and EF.
Table 4.
Body weight correlation of different echocardiographic parameters in Labrador Retrievers
| Parameters (units) | Regression (y) | Coefficient of determination (r2) | Correlation | P-value |
|---|---|---|---|---|
| LVIDd (mm) | 1.050 (x) + 12.747 | 0.423 | 0.651 | 0.0006 |
| LVIDs (mm) | 0.844 (x) + 4.266 | 0.362 | 0.602 | 0.0019 |
| IVSd (mm) | 0.395 (x) - 0.291 | 0.473 | 0.687 | 0.0002 |
| IVSs (mm) | 0.624 (x) - 0.304 | 0.407 | 0.638 | 0.0008 |
| LVPWd (mm) | 0.146 (x) + 5.301 | 0.133 | 0.364 | NS (0.0801) |
| LVPWs (mm) | 0.309 (x) + 4.768 | 0.245 | 0.495 | 0.0139 |
| LAD (mm) | 0.449 (x) + 11.3 | 0.639 | 0.796 | 0.0001 |
| AOD (mm) | 0.447 (x) + 13.04 | 0.547 | 0.736 | 0.0001 |
| DE amplitude (mm) | 0.254 (x) + 4.486 | 0.149 | 0.386 | NS (0.0621) |
| EPSS (mm) | 0.111 (x) + 2.588 | 0.183 | 0.428 | 0.0369 |
| EF slope (mm/s) | 2.86 (x) + 29.234 | 0.224 | 0.469 | 0.0207 |
| LVFS (%) | -0.2326 (x) + 41.4 | 0.0095 | -0.097 | 0.6513 |
| EDV (ml) | 3.762 (x) - 23.61 | 0.4730 | 0.246 | 0.0002 |
| ESV (ml) | 2.008 (x) - 25.74 | 0.3767 | 0.282 | 0.0014 |
| SV (ml) | 2.060 (x) - 8.13 | 0.2340 | 0.484 | 0.0166 |
NS= Non-significant
Fig. 1.
Regression lines for various echocardiographic parameters against body weight. (A): Lines for left ventricular internal diameter at end diastole (LVIDd) and systole (LVIDs), left ventricular posterior wall at end systole (LVPWs), interventricular septal thickness at end diastole (IVSd) and systole (IVSs). (B): Lines for end diastolic volume (EDV), end systolic volume (ESV), stroke volume (SV). (C): Lines for highest mitral leaflet excursion from interventricular septum (EPSS), velocity of mitral valve closure (EF Slope). (D): Lines for left atrial diameter (LAD) and aortic root diameter (AOD). Each point represents single data for a particular parameter (P<0.05)
Discussion
Normal echocardiographic parameters have been published for the Cocker Spaniel (Gooding et al., 1986 ▶), English Pointer (Sisson and Schaeffer, 1991 ▶), Pembroke Welsh Corgi, Golden Retriever and Afghan Hounds (Morrison et al., 1992 ▶), Beagle (Crippa et al., 1992 ▶), Greyhound (Page et al., 1993 ▶; Snyder et al., 1995 ▶), Spanish Mastiff (Bayon et al., 1994 ▶), Boxer (Herrtage, 1994 ▶), Irish Wolfhounds (Vollmar, 1999 ▶), Bull Terrier (O’Leary et al., 2003 ▶), Karabash (Kayar and Uysal, 2004 ▶), German Shepherd (Kayar et al., 2006 ▶; Muzzi et al., 2006 ▶), Whippet (Bavegems et al., 2007 ▶), Hungarian dog breeds (Voros et al., 2009 ▶), Indonesian Mongrel dogs (Noviana et al., 2011 ▶) and Border Collies (Jacobson et al., 2013 ▶) but no such information was available for Labrador Retriever dogs. So, the present study aimed to determine the echocardiographic reference ranges in conscious Labrador Retriever dogs of both sexes and to evaluate the effect of sex and body weight on these parameters.
It has been reported that gender did not have an important effect on most of the echocardiographic measurements in the majority of the breeds. In line with these findings, non-significant effect of gender on all the echocardiographic parameters was also found in this study. However, there are some reports that showed gender variation in some of the echocardiographic indices. A report on Whippet (Bavegems et al., 2007 ▶) showed a significantly larger value of LVID, LAD and AOD for females compared to males. The explanation was a larger mean heart weight to body weight ratio in female as reported by other researchers (Steel et al., 1976 ▶; Pape et al., 1984 ▶). In other investigations, higher LVPW and FS in German Shepherd dogs (Kayar et al., 2006 ▶), LVPW in Beagle dogs (Crippa et al., 1992 ▶), and AOD and EPSS in Indonesian Mongrel dogs (Noviana et al., 2011 ▶) were reported in males compared to females. In Indonesian Mongrel dogs, however, females had a higher LAD and LAD/AOD compared to males. The absence of an association between all the echocardiographic parameters in the present study with regard to gender is in line with previous findings of other researchers (Bonagura et al., 1985 ▶; Vollmar, 1999 ▶; Muzzi et al., 2006 ▶; Voros et al., 2009 ▶).
Body weight in the present study correlated positively with most of the left ventricular dimensions viz., LVIDd, LVIDs, IVSd, IVSs, LVPWd (P=0.0801) and LVPWs. Non-significant correlation of LVPWd in Afghan Hounds and miniature Poodles, and LVIDd in Pembroke Welsh Corgis and Afghan Hounds, with body weight was reported (Morrison et al., 1992 ▶). For Irish Wolfhounds (Vollmar, 1999 ▶), Beagle (Crippa et al., 1992 ▶) and Greyhound (Page et al., 1993 ▶), the effect of body weight on echocardiographic measurements was reported to be smaller compared to other dog breeds. In English bull Terriers, only LVIDs were reported to be significantly correlated to body weight (Bonagura et al., 1985 ▶). In our study, higher correlation of different left ventricular parameters with body weight was established, as also reported by others (Lombard, 1984 ▶; Bonagura et al., 1985 ▶). This positive correlation between different echocardiographic parameters and body weight may be due to increase in the cardiac size with increase in the body weight of animal. This relative variation in correlation among different reports may be due to specificity of individual breeds. Higher LVIDd in the present study was comparable to that reported for Border Collies (O’Leary et al., 2003 ▶), Whippets (Bavegems et al., 2007 ▶), but lower to that reported for German Shepherd (Kayar et al., 2006 ▶). Lower LVPWd in the present study was found compared to Greyhounds (Page et al., 1993 ▶) and English Bull Terriers (Bonagura et al., 1985 ▶) but similar to the value reported by others (O’Grady et al., 1986 ▶).
Left ventricular dimensions taken in echo-cardiography have quite a significant correlation with the clinical manifestation of cardiac diseases. Left ventricular internal diameter at end diastole and systole is of great help in the direct assessment of cardiomyopathies and indirect assessment of abnormal connections between cardiac chambers and congenital defects. In case of hypertrophic cardiomyopathy, left ventricular internal diameter decreases in both systole and diastole while in dilation cardiomyopathy, both these parameters increase. In left to right shunting patent ductus arteriosus, left ventricular end diastolic diameter increases while end systolic diameter may or may not change (Kittleson and Kienle, 1998 ▶). In any kind of pressure overload on the heart that may result in hypertrophic cardiomyopathy as seen in aortic stenosis, measurement of left ventricular posterior wall thickness becomes a very important diagnostic parameter.
A weak negative correlation between body weight and FS was observed in accordance with the findings of other researchers (Vollmar, 1999 ▶; Kayar and Uysal, 2004 ▶; Kayar et al., 2006 ▶) but in contrast to others (Lombard, 1984 ▶; Jacobs and Mahjoob, 1988 ▶; Sisson and Schaffer, 1991 ▶). Discrepancies in the observations of this parameter may arise as the left ventricular fractional shortening is influenced by many parameters which include preload, afterload and contractility, all of which may act independently or in combination to affect this parameter. Left ventricular fractional shortening in the present study for Labrador Retriever was found almost similar to the values reported (Morrison et al., 1992 ▶) for Afghan hound and Golden Retriever. However, higher values were seen in the present study compared to those reported in Border collies (O’Leary et al., 2003 ▶), Greyhounds (Page et al., 1993 ▶) and Whippets (Bavegems et al., 2007 ▶). As these dog breeds are athletic, their internal dimensions are higher, lowering the need for greater contraction.
Ejection fraction has been demonstrated to be quite constant in mammals ranging in size from a rat to horses (Reece, 2005 ▶). Ejection fraction obtained in this study was well within the range established for mammals and statistically not associated with body weight in compliance with the earlier report (O’Grady et al., 1986 ▶).
A positive correlation was established for EDV, ESV and SV and the body weight, in accordance with the other report (O’Grady et al., 1986 ▶). In cases of hypertrophic cardiomyopathy, end systolic volume reduces greatly and may even become zero. End diastolic diameter and volume also reduce significantly in such condition leading to decrease in stroke volume and activation of compensatory renin-angiotensin system (Kahn and Line, 2005 ▶). In myocardial failure, also known as dilation cardiomyopathy, there is an increase in end systolic diameter and volume resulting in compensatory increase in end systolic diameter and volume (Kittleson and Kienle, 1998b ▶). Therefore, measurement of these parameters is of great clinical importance in case of cardiomyopathies.
A positive correlation between body weight and LAD and AOD in dogs was found, similar to the findings of other researchers (Boon et al., 1983 ▶; Lombard, 1984 ▶; Sisson and Schaffer, 1991 ▶; Vollmar, 1999 ▶; Kayar and Uysal, 2004 ▶). In English Bull Terriers, only LAD (long as well as short axis) was significantly correlated to body weight (Bonagura et al., 1985 ▶). However, in German Shepherd dogs, non-significant correlation of LAD/AOD has been reported (Kayar et al., 2006 ▶). AOD in the present study was comparable to that reported for German Shepherd dogs (Kayar et al., 2006 ▶) but was higher than English Bull Terrier (Bonagura et al., 1985 ▶) and Indonesian Mongrel dogs (Noviana et al., 2011 ▶). Unlike ventricles, left atrium increases in size in response to both pressure and volume overload. For confirmatory diagnosis, primary abnormality which is causing the increase in atrial pressure and volume should be identified (Kittleson and Kienle, 1998b ▶). Absolute increase in the left atrial diameter does not confirm left atrial dilation and left atrium to aortic root ratio (LAD/AOD) is a better parameter to assess this abnormality.
Earlier study (Bonagura et al., 1985 ▶) had established the range of left atrium to aortic root (LAD/AOD) between 0.8 and 1.2. In the present study, the value obtained for this ratio was well within the range and almost similar to the values reported by others (Boon et al., 1983 ▶; Kayar et al., 2006 ▶), but lower than that of Border Collies (Jacobson et al., 2013 ▶). Rather than compare absolute measurement in a normogram or with a regression equation, this ratio can be solely relied upon to assess left atrial enlargement. A significant increase in left atrial dimension results in a greater left atrial to aortic root ratio with advancing age, as aortic root significantly diminished with age (Vollmar, 1999 ▶).
In studies on the German Shepherd, Spanish Mastiff and Anatolian Karabash, the mitral valve DE amplitude increased with the increase in body weight (Bayon et al., 1994 ▶; Kayar and Uysal, 2004 ▶; Kayar et al., 2006 ▶), in contrast to the present study. DE amplitude in the present study was smaller to that reported in German Shepherd dogs (Kayar et al., 2006 ▶). Significant association of EF slope with body weight was observed in contrast to that reported by others (Kayar et al., 2006 ▶). Impaired ventricular filling associated with disease status like mitral stenosis, pulmonary hypertension, pericardial restriction and tachycardia result in lower EF slope while augmented blood flow to left side of heart due to congenital abnormalities or some other factors lead to enhanced EF slope (Pipers et al., 1981 ▶).
Mitral valve EPSS in the present study correlated significantly with body weight. Earlier studies done on different breeds by other researchers (Kayar et al., 2006 ▶; Muzzi et al., 2006 ▶; Bavegems et al., 2007 ▶), however, suggested non significant influence of body weight on the value of this parameter. Increase in EPSS with increase in body weight seen in our study may be due to corresponding increase in the LVID and LAD. EPSS in the present study was almost similar to the value reported for the Golden Retriever (Morrison et al., 1992 ▶) but higher than that of Indonesian Mongrel dogs (Noviana et al., 2011 ▶). In left ventricular or left atrial dilation, an increase in the value of this parameter is seen as the mitral valve is pushed more posterior in these conditions (Pipers et al., 1981 ▶). EPSS is only a qualitative indication of the left ventricular function and its normal value might also be seen in presence of severe cardiac disease. It is a simple measurement which, if altered, should alert the examiner to the possibility of cardiac disease (Kirberger, 1981 ▶). Moreover, excessive EPSS correlates well with decreased ejection fraction, although it has been reported that EF may be subnormal while the EPSS remains normal (Massie et al., 1977 ▶). It can be concluded that the present study standardised the reference ranges of different echocardiographic parameters for Labrador Retrievers in terms of intracardiac lumen, valve dimensions, wall thickness and functional indices. Though the number of animals we studied does not seem to be a large population, the clinically important echocardiographic parameters given in our study may be used as breed-specific reference values for evaluating the cardiac function as well as for further research work in small animal cardiology.
Acknowledgment
Authors would like to express their appreciation to the Director of the Institute for providing the necessary facilities.
References
- Bavegems, V, Duchateau, L, Sys, SU, Rick, AD. Echocardiographic reference values in Whippets. Vet. Radiol. Ultrasound. 2007;48:230–238. doi: 10.1111/j.1740-8261.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- Bayon, A, Fernandezdel Palacio, MJ, Montes, AM. M-mode echocardiographic study in growing Spanish Mastiffs. J. Small Anim. Pract. 1994;35:473–479. [Google Scholar]
- Bonagura, JD, O’Grady, MR, Herring, DS. Echocardiography-congenital heart disease. Vet. Clin. North Am.: Small Anim. Pract. 1985;15:1195–1208. doi: 10.1016/s0195-5616(85)50365-4. [DOI] [PubMed] [Google Scholar]
- Boon, JA, Wingfield, WE, Miller, CW. Echo-cardiographic indices in normal dog. Vet. Radiol. Ultrasound. 1983;24:214–221. [Google Scholar]
- Crippa, L, Ferro, E, Melloni, E, Brambilla Cavalletti, E. Echocardiographic parameters and indices in normal Beagle dogs. Lab. Anim. 1992;26:190–195. doi: 10.1258/002367792780740512. [DOI] [PubMed] [Google Scholar]
- Foppa, M, Duncan, BB, Rohde, LE. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? . Cardiovasc. Ultrasound. 2005;3:17–26. doi: 10.1186/1476-7120-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding, JP, Robinson, WF, Meurs, GC. Echocardiographic assessment of left ventricular dimension in clinically normal English Cocker Spaniel. Am. J. Vet. Res. 1986;47:296–300. [PubMed] [Google Scholar]
- Gugjoo, MB, Hoque, M, Saxena, AC, Zama, MMS. Radiographic, electrocardiographic and echo-cardiographic features of dilatation cardiomyopathy in dogs. Indian Vet. J. 2013;90:54–56. [Google Scholar]
- Herrtage, M. Echocardiographic measurements in the normal Boxer. European Soc. Veterinary Intern. Medicine. 4th Annual Congress; 1994. p. 172. [Google Scholar]
- Jacobs, G, Mahjoob, K. Influences of alterations in heart rate on echocardiographic measurements in dogs. Am. J. Vet. Res. 1988;49:548–552. [PubMed] [Google Scholar]
- Jacobson, JH, Boon, JA, Bright, JM. An echocardiographic study of healthy Border Collies with normal reference ranges for the breed. J. Vet. Cardiol. 2013;15:123–130. doi: 10.1016/j.jvc.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Kahn, CM, Line, S. The Merck veterinary manual. 9th Edn. New Jersey: Merck and Co., Inc; 2005. pp. 62–91. [Google Scholar]
- Kayar, A, Gonul, R, Or, ME, Uysal, A. M-mode echocardiographic parameter and indices in the normal German Shepherd Dog. Vet. Radiol. Ultrasound. 2006;47:482–486. doi: 10.1111/j.1740-8261.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- Kayar, A, Uysal, A. Determination of cardiac reference parameters using M-mode and two-dimensional echocardiographic techniques in adult Karabash Dog. Turk. J. Vet. Anim. Sci. 2004;28:39–46. [Google Scholar]
- Kirberger, RM. Mitral valve E point to ventricular septal separation in the dog. J. S. Afr. Vet. Assoc. 1991;62:163–166. [PubMed] [Google Scholar]
- Kittleson, MD, Kienle . Small animal cardio-vascular medicine. 1st Edn. St. Louis: Mosby Inc; 1998a. pp. 402–412. [Google Scholar]
- Kittleson, MD, Kienle . Small animal cardio-vascular medicine. 1st Edn. St. Louis: Mosby Inc; 1998b. pp. 218–230. [Google Scholar]
- Lombard, CW. Normal values of canine M mode echocardiogram. Am. J. Vet. Res. 1984;45:2015–2018. [PubMed] [Google Scholar]
- Massie, BM, Schiller, NB, Ratshin, RA, Parmley, WM. Mitral-septal separation: new echocardiographic index of left ventricular function. Am. J. Cardiol. 1977;44:1332–1338. doi: 10.1016/s0002-9149(77)80215-4. [DOI] [PubMed] [Google Scholar]
- Morrison, SA, Moise, NS, Scarlett, J. Effect of breed and body weight on echocardiographic values in four breeds of dogs of different somatotypes. J. Vet. Intern. Med. 1992;6:220–224. doi: 10.1111/j.1939-1676.1992.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Muzzi, RAL, Muzzi, LAP, Arauzo, RB, Cherem, M. Echocardiographic indices in normal German Shepherd dog. J. Vet. Sci. 2006;7:193–198. doi: 10.4142/jvs.2006.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noviana, D, Paramitha, D, Wulansari, R. Motion mode and two dimensional echocardiographic measure-ments of cardiac dimensions of Indonesian Mongrel Dogs. Hayati J. Biosci. 2011;18:1–5. [Google Scholar]
- O’Grady, MR, Bonagura, JD, Powers, JD, Herrings, DS. Quantitative cross-sectional echocardiography in the normal dog. Vet. Radiol. Ultrasound. 1986;27:34–49. [Google Scholar]
- O’Leary, CA, Mackay, BM, Taplin, RH, Atwell, RB. Echocardiographic parameters in 14 healthy English Bull Terriers. Aust. Vet. J. 2003;81:535–542. doi: 10.1111/j.1751-0813.2003.tb12881.x. [DOI] [PubMed] [Google Scholar]
- Page, A, Edmunds, G, Atwell, RB. Echo-cardiographic values in Greyhounds. Aust. Vet. J. 1993;70:361–364. doi: 10.1111/j.1751-0813.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Pape, LA, Rippe, JM, Walker, WS. Effect of cessation of training on left ventricular function in racing Greyhounds Serial studies in a model of cardiac hypertrophy. . Basic Res. Cardiol. 1984;67:1725–1729. doi: 10.1007/BF01935812. [DOI] [PubMed] [Google Scholar]
- Pipers, FS, Bonagura, JD, Hamlin, RL, Kittleson, M. Echocardiographic abnormalities of mitral valve associated with left sided heart diseases in dogs. J. Am. Vet. Med. Assoc. 1981;179:580–586. [PubMed] [Google Scholar]
- Reece WO. Duke’s physiology of domestic animals. 12th Edn. New Delhi: Panima Publishing Corporation; 2005. p. 230. [Google Scholar]
- Sisson, D, Schaeffer, D. Changes in linear dimensions of the heart relative to body weight as measured by M-mode echocardiography in growing dogs. Am. J. Vet. Res. 1991;52:1519–1596. [PubMed] [Google Scholar]
- Snyder, PS, Sato, T, Atkins, CE. A comparison of echocardiographic indices of the non-racing healthy greyhound to reference values from other breeds. Vet. Radiol. Ultrasound. 1995;36:387–392. [Google Scholar]
- Steel, JD, Taylor, RI, Davis, PE, Stewart, GA, Salmon, PW. Relationship between heart score, heart weight and body weight in Greyhound dogs. Austr. Vet. J. 1976;52:561–564. doi: 10.1111/j.1751-0813.1976.tb05421.x. [DOI] [PubMed] [Google Scholar]
- Teicholz, LE, Kreulen, T, Herman, MV, Gorlin, R. Problems in echocardiographic volume determina-tions: echocardiographic-angiographic correlations in presence or absence of asynergy. Am. J. Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- Thomas, WP, Gaber, CE, Jacobs, GJ. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. J. Vet. Intern. Med. 1993;7:247–252. doi: 10.1111/j.1939-1676.1993.tb01015.x. [DOI] [PubMed] [Google Scholar]
- Vollmar, AC. Echocardiographic measurements in Irish Wolfhound: reference values for the breed. J. Am. Anim. Hosp. Assoc. 1999;35:271–277. doi: 10.5326/15473317-35-4-271. [DOI] [PubMed] [Google Scholar]
- Voros, K, Hetyey, C, Reiczegel, J, Czirok, GN. M-mode and two dimensional echocardiographic reference values for three Hungarian dog breeds. Acta Vet. Hung. 2009;57:217–227. doi: 10.1556/AVet.57.2009.2.3. [DOI] [PubMed] [Google Scholar]