Abstract
This study was carried out to investigate the effects of dietary putrescine (PUT) on broiler’s response fed low crude protein (CP) diets. A total of 192 male day old chicks were fed with four dietary treatments including two levels of PUT (0 and 0.03%) and two levels of CP (normal and low) with factorial combinations. Weekly growth performance, nutrient digestibility and intestinal morphology (at the age of 21 days) and liver and intestinal tissue polyamines content were measured. As a result of this study lower dietary CP had a significant (P<0.05) lower body weight gain (BWG) and improved protein efficiency ratio (PER). PUT improved energy efficiency ratio (EER) significantly (P<0.05). Dry matter (DM) digestibility was decreased by lower dietary CP whereas 0.03% PUT significantly (P<0.05) increased it. Low CP caused significant (P<0.05) greater calcium digestibility, while this effect was not found when PUT was added. PUT had no effect on intestine villous height and crypt depth. Polyamine content of intestine and liver was influenced by the age of the birds, while PUT had no effects on them. In conclusion, dietary PUT has beneficial effects on EER in chicks fed CP-deficient diet, indicating possible involvement of PUT in energy metabolism. PUT supplementation did not moderate the reduced BWG of the chicks fed low protein. Intestinal and liver polyamine concentration was mainly affected by dietary CP and age of the birds rather than dietary PUT.
Key Words: Broiler, Protein deficiency, Putrescine, Tissue polyamine
Introduction
The polyamines (PA), putrescine (PUT), spermidine (SPD) and spermine (SPM) are essential biological polycations that are considered as growth promoter due to their role in the regulation of normal cell growth and proliferation (Pegg and McCann, 1982). There is strong binding between polyamines and DNA modifies histone acetylation (Hobbs and Gilmour, 2006 ▶). The effects of polyamines on protein synthesis are exerted through their impact on RNA-related functions (Igarashi and Kashiwagi, 2010 ▶). In addition, polyamines are required at both translation initiation and elongation steps of cell division (Guy et al., 2010 ▶).
Polyamine concentration in an organ is associated with organ’s metabolic activity and function (Bardocz et al., 1992 ▶). It is reported that long term feeding of polyamine deficient diets may result in a significant hypoplasia of small intestinal and colonic mucosa (Loser, 2000 ▶). Although Colnago and Jensen (1992) ▶ reported that adding PUT to low protein diets failed to affect any of the performance parameters in broiler, Meziani and Benamouzig (1999) ▶ found that con-centration of intestinal polyamines is associated with the quality and quantity of dietary protein. Moreover, there is evidence showing the relationship between ornithine decarboxylase (ODC, a key enzyme in polyamine biosynthesis) induction and dietary protein content (Murakammi et al., 1983 ▶; Machi et al., 1999 ▶). This information implies that the effect of dietary protein on intestinal cell proliferation is regulated, at least in part, by PA mediation. Thus, the objective of this research was to investigate the effects of dietary PUT supplements in CP-deficient diets on broiler growth performance, nutrient digestibility, intestinal morphology and PA contents of intestinal tissue and liver. This study has been performed in accordance with the ethical standards of the Ethics Committee of Animal Utilization of the University Putra Malaysia (UPM).
Materials and Methods
Experimental birds and diet
A total of 192 male day old broiler chicks of Cobb 500 strain were obtained from a local hatchery and individually weighed, wing banded and allocated to 4 dietary treatments including factorial combination of two CP levels (normal and low) and two PUT levels (0, 0.03%) as shown in Table 1. The mean body weight of the chicks at 1st day was 45.9 ± 0.29 g. The pure PUT was purchased from Merck Company (CAS No. 333-93-7, Darmstadt, Germany). Each treatment had 6 replicates with 8 birds per cage. Analysed content of dietary CP and PA are shown in Table 2. No SPM and SPD were detected in all experimental diets.
Table 1.
Feed compositions of starter and grower diets with different crude protein levels
| Ingredients % | Starter |
|
Grower |
||||||
|---|---|---|---|---|---|---|---|---|---|
| T11 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | ||
| Corn | 47.13 | 47.10 | 49.03 | 49 | 49.7 | 49.67 | 51.9 | 51.87 | |
| Soybean meal | 42 | 42 | 36.01 | 36.01 | 29.5 | 29.5 | 24.6 | 24.6 | |
| Palm oil | 6 | 6 | 6 | 6 | 6.5 | 6.5 | 6.4 | 6.4 | |
| Wheat bran | 0.8 | 0.8 | 4.76 | 4.76 | 10.3 | 10.3 | 13.08 | 13.08 | |
| L-lysin | 0 | 0 | 0.1 | 0.1 | 0 | 0 | 0 | 0 | |
| DL-Methionie | 0.19 | 0.19 | 0.22 | 0.22 | 0.11 | 0.11 | 0.13 | 0.13 | |
| Mono calcium phos. | 1.9 | 1.9 | 1.9 | 1.9 | 1.8 | 1.8 | 1.8 | 1.8 | |
| CaCO3 | 1.6 | 1.6 | 1.6 | 1.6 | 1.7 | 1.7 | 1.7 | 1.7 | |
| Salt | 0.28 | 0.28 | 0.28 | 0.28 | 0.29 | 0.29 | 0.29 | 0.29 | |
| Vitamin permix2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | |
| Mineral permix3 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | |
| Putrescine | 0 | 0.03 | 0 | 0.03 | 0 | 0.03 | 0 | 0.03 | |
| Analysis results | |||||||||
| ME (kcal/kg) | 3132 | 3132 | 3132 | 3132 | 3132 | 3132 | 3132 | 3132 | |
| Crude protein (calculated) (%) | 22 | 22 | 20 | 20 | 18.9 | 18.9 | 16.5 | 16.5 | |
| Ether extract (%) | 7.99 | 7.99 | 8 | 8 | 8.4 | 8.4 | 8.3 | 8.3 | |
| Crude fiber (%) | 4.18 | 4.18 | 4.11 | 4.11 | 4.1 | 4.1 | 4 | 4 | |
| Calcium (%) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Total P (%) | 0.82 | 0.82 | 0.82 | 0.82 | 0.81 | 0.81 | 0.81 | 0.81 | |
| Available P (%) | 0.45 | 0.45 | 0.46 | 0.46 | 0.45 | 0.45 | 0.45 | 0.45 | |
| Arg (%) | 1.57 | 1.57 | 1.38 | 1.38 | 1.17 | 1.17 | 1.01 | 1.01 | |
| Lys (%) | 1.29 | 1.29 | 1.23 | 1.23 | 1 | 1 | 0.89 | 0.89 | |
| Met+Cys (%) | 0.89 | 0.89 | 0.87 | 0.87 | 0.7 | 0.7 | 0.68 | 0.68 | |
| Met. (%) | 0.54 | 0.54 | 0.54 | 0.54 | 0.4 | 0.4 | 0.41 | 0.41 | |
| Thr (%) | 0.87 | 0.87 | 0.77 | 0.77 | 0.66 | 0.66 | 0.58 | 0.58 | |
| Trp (%) | 0.29 | 0.29 | 0.26 | 0.26 | 0.22 | 0.22 | 0.19 | 0.19 | |
| Putrescine4 (%) | 0.06 | 0.09 | 0.06 | 0.09 | 0.06 | 0.09 | 0.06 | 0.09 | |
T1 and T3 were normal and low CP diets, respectively. T2 and T4 were normal and low CP diets supplemented with 0.03% put, respectively.
Supplied per kg of diet: Vitamin A, 1,500 IU; cholecalciferol, 200 IU; vitamin E, 10 IU; riboflavin, 3.5 mg; pantothenic acid, 10 mg; niacin, 30 mg; cobalamin, 10 μg; choline chloride, 1,000 mg; biotin, 0.15 mg; folic acid, 0.5 mg; thiamine 1.5 mg; pyridoxine 3.0 mg.
Supplied per kg of diet: Copper, 8 mg; selenium, 0.15 mg; iron, 80 mg; zinc, 40 mg; manganese, 60 mg; iodine, 0.18 mg.
Putrescine content of corn and soybean meal samples were analyzed and in average PUT content of corn = 1 mg/g and soybean meal = 0.3 mg/g
Table 2.
Analysed content of polyamines and crude protein (CP) of experimental mixed diets, corn and soybean meal
| Treatments1 | CP |
PUT (mg/g) | SPM (nano g/g) | SPD (nano g/g) | ||
|---|---|---|---|---|---|---|
| Starter | Grower | |||||
| T1 | 21.2 | 16.8 | 0.10 | ND2 | ND | |
| T2 | 20.8 | 16.5 | 1.11 | ND | ND | |
| T3 | 18.9 | 15.4 | 0.25 | ND | ND | |
| T4 | 19.1 | 16.0 | 1.66 | ND | ND | |
| Corn | - | - | 0.98 | ND | ND | |
| Soybean meal | - | - | 0.33 | ND | ND | |
T1 and T3 were normal and low CP diets, respectively. T2 and T4 were normal and low CP diets supplemented with 0.03% put, respectively.
ND: Not detectable
Parameters measured
Performance data
Group feed intake (FI) and chicks individual body weight (BW) were measured weekly. Feed conversion ratio (FCR) and body weight gain (BWG) were calculated accordingly. FCR was calculated as daily feed intake/daily BWG. Protein efficiency ratio (PER) and energy efficiency ratio (EER) were calculated as daily BWG (g)/daily protein intake (g) and daily BWG (g)/daily 1000 cal intake, respectively.
Nutrient digestibility
At the age of 21 days, three groups of chicks per each treatment were randomly selected and caged separately. Each group contained 3 chicks. The feeds were mixed with 0.3% TiO2 as indigestible marker. Samples of excreta and feed were taken after 3 days and kept frozen at -20°C for future analysis. Samples were analysed for gross energy (GE), dry matter (DM), phosphorus (P), calcium (Ca), nitrogen (N) and TiO2 content. GE was measured by adiabatic calorimetric bomb (IKA C 200 bomb calorimeter). N content was analysed by semi-automated kjeldal apparatus (FOSS TECATOR, 2400 Kjeltec Analyzer Unit). Ca content was measured by flame atomic absorption spectrophotometer (Perkin Elmer, A-Analyst 200) based on AOAC, (1990; method 968.08) and P content was analysed according to the method of Galyean (2010) ▶. TiO2 content of feeds and excreta were determined based on the procedures by Myers et al. (2004) ▶. Apparent metabolisable energy (AME) of experimental diets was calculated based on the formula used by Scott et al. (1998) as shown below:
AME = GE diet – (GEexcreta × Markerdiet/Markerexcreta)
Whereas,
mineral digestibility was calculated based on the formula used by Pourreza et al. (2007) as shown below:
Minerals digestibility = 100 – [100 × (%Markerdiet/%Markerfeces) × (%Mineralfeces/%Mineraldiet)]
Villous height and crypt depth
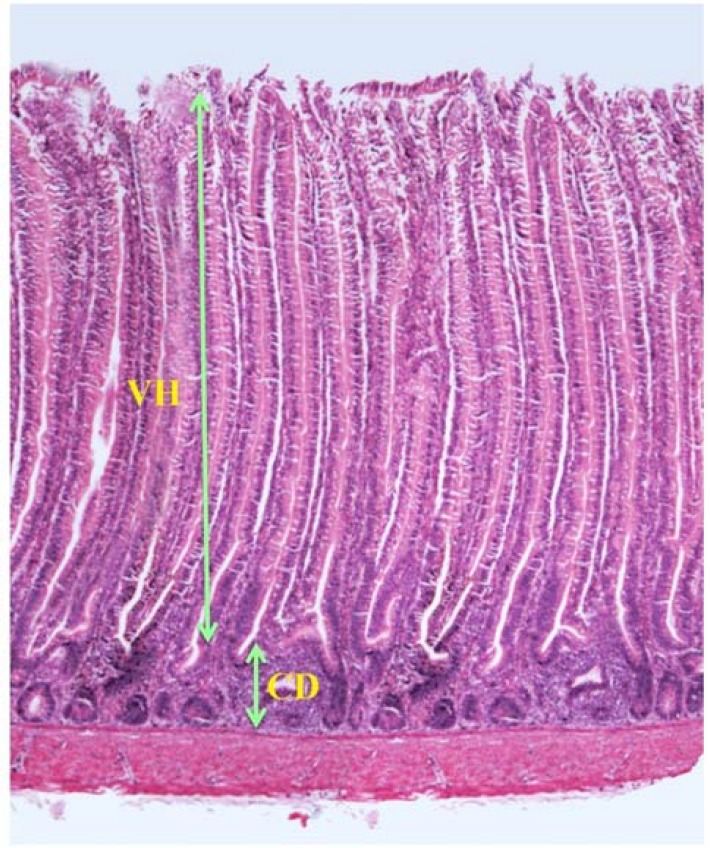
Intestine samples were taken from those chicks used for nutrient digestibility trial at the age of 24 days. From each section of the small intestine (duodenum, jejunum and ileum), a 6-8 cm sample was cut, flushed with distilled water and kept in 10% formalin. Samples were dehydrated for 16 h in a tissue processing machine (Leica ASP 3000, Japan) followed by embedding in paraffin. Samples were cut 4 μm thick and were fixed on the glass slides and dried at 57°C. Haematoxylin and eosin were used for staining, and thereafter mounted with cover slips. In each sample, 8-10 complete villous-crypt structure was examined to measure villous height (VH) and crypt depth (CD) as depicted in Fig. 1. This procedure was done according to the method of Thanh et al. (2009).
Fig. 1.
The length of villous height (VH) and crypt depth (CD) of small intestine in chickens (jejunum of control group)
Polyamine content of liver, intestine and feeds
At the ages of 24 and 42 days, 6 birds per treatment were randomly selected and slaughtered for intestine and liver samples. Intestine was flushed with distilled water and 10 cm from each segment (duodenum, jejunum and ileum) was taken and kept frozen at -20°C, as well as liver samples. Pure PA was purchased from Merck Company (Darmstadt, Germany). The procedure des-cribed by Hwang et al. (1997) ▶ was used for tissue PA analysis using HPLC.
Statistical analysis
Data were analysed by the GLM procedure of SAS software (SAS, 1999) as a 2 × 2 factorial arrangement. Duncan multiple range test was used for comparing means. Non-parametric data (mortality rate and tissue PA content) were analysed using the Chi-Square and Kruskal Wallis Test. The p-value less than 0.05 was considered significant.
Results
Performance
The performance parameters in different ages are shown in Table 3. Low dietary CP significantly (P<0.05) improved PER at the starter and whole period. At the age of 21-42 days, BWG was reduced by low CP diet significantly (P<0.05). EER was improved with 0.03% PUT significantly (P<0.05) in grower period (21-42 days). In the whole period, low CP decreased BWG and improved PER significantly (P<0.05) and PUT increased EER at P=0.06 in low CP group.
Table 3.
Effects of CP and PUT on broiler performance parameters at different ages
| Age | Treatments | CP1 | PUT (%) |
BWG (g/b/d2) |
BW (g) |
FCR | PER | EER | FI (g/b/d) |
Mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0-21 d | T1 | N | 0 | 32.23 | 677 | 1.69 | 2.70b | 0.19 | 51.33 | 6 |
| T2 | N | 0.03 | 33.72 | 709 | 1.72 | 2.65b | 0.19 | 54.65 | 0 | |
| T3 | L | 0 | 32.03 | 675 | 1.67 | 2.97a | 0.19 | 52.47 | 0 | |
| T4 | L | 0.03 | 32.36 | 669 | 1.68 | 2.95a | 0.19 | 51.69 | 3 | |
| N | 116 | 116 | 24 | 24 | 24 | 24 | 24 | |||
| Pooled SEM | 0.39 | 8.37 | 0.02 | 0.04 | 0.002 | 0.8 | - | |||
| P-value | ||||||||||
| CP | 0.97 | 0.50 | 0.44 | 0.007 | 0.50 | 0.57 | NS | |||
| PUT | 0.73 | 0.82 | 0.62 | 0.60 | 0.63 | 0.44 | NS | |||
| CP × PUT | 0.40 | 0.92 | 0.78 | 0.86 | 0.83 | 0.21 | - | |||
| 21-42 d | T1 | N | 0 | 53.38a | 1320a | 2.18 | 2.65 | 0.17ab | 146.2 | 0 |
| T2 | N | 0.03 | 58.74a | 1277a | 2.12 | 2.73 | 0.18a | 141.04 | 0 | |
| T3 | L | 0 | 51.26b | 1135b | 2.34 | 2.84 | 0.13b | 135.21 | 0 | |
| T4 | L | 0.03 | 51.01b | 1165b | 2.22 | 3.18 | 0.19a | 128.92 | 0 | |
| N | 116 | 116 | 24 | 24 | 24 | 24 | 24 | |||
| Pooled SEM | 0.87 | 18.44 | 0.05 | 0.1 | 0.007 | 16.73 | - | |||
| P-value | ||||||||||
| CP | 0.00 | 0.00 | 0.23 | 0.14 | 0.40 | 0.10 | NS | |||
| PUT | 0.42 | 0.97 | 0.39 | 0.33 | 0.01 | 0.40 | NS | |||
| CP × PUT | 0.12 | 0.89 | 0.78 | 0.54 | 0.08 | 0.93 | - | |||
| 0-42 d | T1 | N | 0 | 42.48ab | 1997a | 2 | 2.55b | 0.18ab | 92.95 | 6 |
| T2 | N | 0.03 | 45.62a | 1991a | 1.97 | 2.58ab | 0.18ab | 93.27 | 0 | |
| T3 | L | 0 | 38.65b | 1822b | 2.05 | 2.75ab | 0.16b | 89.49 | 0 | |
| T4 | L | 0.03 | 39.08b | 1841b | 2 | 2.88a | 0.18a | 86.38 | 3 | |
| N | 116 | 116 | 24 | 24 | 24 | 24 | 24 | |||
| Pooled SEM | 0.52 | 21.93 | 0.02 | 0.5 | 0.003 | 1.82 | ||||
| P-value | ||||||||||
| CP | 0.01 | 0.01 | 0.45 | 0.02 | 0.33 | 0.17 | NS | |||
| Put | 0.88 | 0.93 | 0.50 | 0.43 | 0.06 | 0.70 | NS | |||
| CP × Put | 0.24 | 0.64 | 0.85 | 0.64 | 0.06 | 0.64 | - |
Means with different superscripts in column are significantly different (P<0.05). 1 CP: N: Normal level, L: Low level, and 2 g/b/d: gram/bird/day
Nutrient digestibility
DM digestibility decreased in the treatments of low dietary CP (Table 4). In contrast, PUT significantly (P<0.05) increased the DM digestibility in low CP diets. In the meantime, low CP diet improved Ca digestibility significantly (P<0.05). CP and PUT interaction was significant (P<0.05) on Ca digestibility. At 0% PUT level, low CP had significantly higher (P<0.05) Ca digestibility; however, this effect was not found when PUT was added.
Table 4.
Effects of CP and PUT on nutrient digestibility at the age of 21 days
| CP1 | PUT | DM (%) | N (%) | AME (cal/g) | Ash (%) | Ca (%) | P (%) | |
|---|---|---|---|---|---|---|---|---|
| T1 | N | 0 | 92.71a | 62.66 | 3277 | 52.07 | 50.95b | 54.82 |
| T2 | N | 0.03 | 94.18a | 72.2 | 3197 | 46.34 | 63.92ab | 50.82 |
| T3 | L | 0 | 89.46b | 67.89 | 3761 | 55.26 | 72.53a | 52.39 |
| T4 | L | 0.03 | 92.11a | 69.54 | 2966 | 54.47 | 64.75ab | 51.07 |
| N | 12 | 12 | 12 | 12 | 12 | 12 | ||
| Pooled SEM | 2.11 | 6.29 | 445.07 | 6.93 | 3.08 | 5.28 | ||
| P-value2 | ||||||||
| CP | 0.00 | 0.72 | 0.58 | 0.20 | 0.01 | 0.72 | ||
| PUT | 0.02 | 0.14 | 0.08 | 0.49 | 0.73 | 0.46 | ||
| CP × PUT | 0.45 | 0.28 | 0.14 | 0.60 | 0.04 | 0.79 |
Intestine villous height and crypt depth
Low CP had significant lower (P<0.05) VH in duodenum and jejunum (Table 5). Addition of PUT in diet had no significant (P>0.05) effect on VH and CD in any segments of small intestine.
Table 5.
Effects of CP and PUT on small intestine villi height (VH) and crypt depth (CD) at the age of 21 days (µm)
| Treatments | CP1 | PUT (%) | Duodenum |
|
Jejunum |
|
Ileum |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| VH | CD | VH | CD | VH | CD | |||||
| T1 | N | 0 | 1340a | 170 | 947a | 123 | 397 | 133 | ||
| T2 | N | 0.03 | 1380a | 95 | 930a | 150 | 350 | 52 | ||
| T3 | L | 0 | 1090b | 160 | 833b | 118 | 700 | 51 | ||
| T4 | L | 0.03 | 1025b | 165 | 570b | 41 | 870 | 145 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | ||||
| Pooled SEM | 210 | 39 | 242 | 35 | 156 | 33 | ||||
| P-value | ||||||||||
| CP | 0.02 | 0.63 | 0.02 | 0.32 | 0.25 | 0.49 | ||||
| PUT | 0.91 | 0.21 | 0.39 | 0.96 | 0.72 | 0.66 | ||||
| CP × PUT | 0.81 | 0.38 | 0.10 | 0.42 | 0.62 | 0.49 | ||||
Means with different superscripts in column (a, b) are significantly different (P<0.05).
CP: N: Normal, L: Low
Tissues PA content
Dietary PUT supplementation was not effective (P>0.05) on PA content of intestine and liver tissue at the age of 21 days (Table 6). However, in older age (42 days), PUT increased duodenal SPD significantly (P<0.05). At 42 days of age, duodenal PUT decreased and duodenal SPM increased significantly (P<0.05) in low CP groups. Dietary PUT augmented the duodenal SPD in older age (42 days). SPD content of duodenum, jejunum and ileum increased at older age (42 days). While duodenal SPM and liver PUT content were higher at the age of 21 days as compared with older age (Table 7).
Table 6.
Effects of CP and PUT on polyamine content of duodenum, jejunum + ileum and liver at 21 and 42 days of the age1
| Treatments | CP2 | PUT (%) | Duodenum |
|
Jujenum + ileum |
|
Liver |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PUT (mg/g) |
SPM (ng/g) |
SPD (ng/g) |
PUT (mg/g) |
SPM (ng/g) |
SPD (ng/g) |
PUT (mg/g) |
SPM (ng/g) |
SPD (ng/g) |
||||||
| 21 days | ||||||||||||||
| T1 | N | 0 | 0.30 | 278 | 0 | 0.35b | 230b | 0.00 | 1.76 | 0b | 0 | |||
| T2 | N | 0.03 | 1.30 | 607 | 0 | 0.31b | 157b | 0.00 | 0.37 | 138b | 18 | |||
| T3 | L | 0 | 0.68 | 368 | 0 | 0.55a | 436a | 0.00 | 0.58 | 615a | 0 | |||
| T4 | L | 0.03 | 0.92 | 470 | 0 | 0.74a | 597a | 0.88 | 0.22 | 213a | 0 | |||
| Median | 0.53 | 383 | 0 | 0.41 | 275 | 0 | 0.31 | 116 | 0 | |||||
| P-value | ||||||||||||||
| CP | 0.61 | 0.74 | - | 0.03 | 0.00 | 0.34 | 0.26 | 0.11 | 0.34 | |||||
| PUT | 0.26 | 0.50 | - | 0.70 | 0.68 | 0.34 | 0.25 | 0.47 | 0.34 | |||||
| 42 days | ||||||||||||||
| T1 | N | 0 | 1.79a | 0b | 0b | 0.91 | 991 | 37 | 0.04 | 90 | 0 | |||
| T2 | N | 0.03 | 1.87a | 0b | 93a | 0.69 | 394 | 40 | 0.03 | 90 | 0 | |||
| T3 | L | 0 | 0.66b | 224a | 8b | 1.57 | 566 | 49 | 0.08 | 134 | 0 | |||
| T4 | L | 0.03 | 0.40b | 434a | 12a | 0.91 | 590 | 33 | 0.21 | 36 | 0 | |||
| Median | 1.16 | 0 | 8 | 0.85 | 439 | 37.7 | 0.05 | 69 | 0 | |||||
| P-value | ||||||||||||||
| CP | 0.00 | 0.00 | 0.17 | 0.51 | 0.79 | 0.87 | 0.15 | 0.94 | - | |||||
| PUT | 0.80 | 0.18 | 0.08 | 0.50 | 0.52 | 0.73 | 0.37 | 0.46 | - | |||||
Means with different superscripts in every column (a, b) are significantly different (P<0.05).
Kruskal Wallis Test was used for difference significance. The zero value of PA content means “not detectable”.
CP: N: Normal, L: Low
Table 7.
Effects of age on polyamine content of duodenum, jejunum + ileum and liver tissue1
| Age (day) | Duodenum |
|
Jujenum + ileum |
|
Liver |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PUT (mg/g)2 |
SPM (ng/g)3 |
SPD (ng/g) |
PUT (mg/g) |
SPM (ng/g) |
SPD (ng/g) |
PUT (mg/g) |
SPM (ng/g) |
SPD (ng/g) |
|||
| 21 | 0.7 | 415a | 00.00b | 0.48 | 373 | 00.22b | 0.76a | 251 | 3.42 | ||
| 42 | 1.18 | 164b | 28.52a | 1.02 | 635 | 40.22a | 0.09b | 87 | 0 | ||
| Median | 0.76 | 231 | 0 | 0.43 | 302 | 1.32 | 0.14 | 80 | 0 | ||
| P-value4 | 0.19 | 0.3 | 0.05 | 0.09 | 0.19 | 0.00 | 0.00 | 0.21 | 0.32 | ||
Means with different superscripts in every column (a, b) are significantly different (P<0.05).
Kruskal Wallis Test was used for difference significances. The zero value of PA content means “not detectable”.
mg/g= Milligram/gram,
ng/g= Nano gram/gram.
P-value is asymptotic, computed from the asymptotic Chi-square distribution of the test statistic
Discussion
It is reported that small doses of orally administered PUT promoted BWG in broilers (Smith, 1990; Hashemi, 2013 ▶). However, in the current study, no growth improvement was seen due to PUT supplementation. Similarly, Colnago and Jensen (1992) and Devi Priya et al. (2010) ▶ reported that PUT did not change BWG of broiler as compared with the control. Since tissue PA concentration is highly regulated (Heby and Persson, 1990 ▶), the dietary polyamines cannot change the tissue metabolism directly. However, indirect effects of dietary PUT on tissue metabolism is feasible, as we observed EER was altered by PUT supplementation. As a result of the present study, PUT supplementation plays a role in energy metabolism, particularly in protein deficiency situations.
EER and PER are closely related to the energy content of diet (Hosseini-Vashan et al., 2010 ▶). Therefore, the effects of PUT on EER and PER improvement require more study with different dietary energy levels in order to better understand the PUT effects on energy metabolism.
The observations of the present study are in agreement with Ziaei et al. (2011) who suggested that low CP caused lower BWG whereas, FCR and FI were not affected. Similar to Kermanshahi et al. (2011) ▶, in the current study, improvement of PER in Low CP diets have been seen, indicating more N retention. This effect may be due to more activated carrier-mediated transport of amino acids when dietary CP is low. Chicks fed low-protein diets showed remarkably low BW and excreted less N than did chicks fed the high-protein diets (Bregendahl et al., 2002 ▶). In contrast, Kamran et al. (2008) ▶ showed that lowering dietary CP resulted in decreased PER and EER. These contradicting results could be due to differences between digestibilities of individual dietary amino acids.
According to the current study, low CP level decreased DM digestibility and improved Ca digestibility. This finding is in agreement with Kermanshahi et al. (2011) ▶ who reported that increasing the dietary CP reduces Ca retention. However, when PUT is offered, decreasing effect of CP on Ca digestibility was not observed. It is proved that PA are involved in activation of calcium sensing receptors (Quinn et al., 1997) in different tissues, like intestine. In poultry nutrition, the effects of PUT on calcium indices like eggshell quality were reported earlier (Chowdhury and Smith, 2001 ▶, 2002).
Intestinal mucosa development is dependent on luminal polyamine content (Ginty et al., 1989 ▶). However, in the present study dietary PUT had no effect on VH and CD of small intestine at the age of 21 days. In fast growing tissues, like intestinal mucosa, high levels of PA are required. Hashemi (2013) ▶ stated that dietary PUT had significant increasing effect on VH and CD. Similarly, Loser (2000) ▶ reported that long term feeding of polyamine deficient diets would result in a significant hypoplasia of small intestinal and colonic mucosa. Nevertheless, detailed investigation is needed to clarify the exact mechanism and circumstances through which PA may affect VH and CD.
Similar to the results of the current study, Nishimura et al. (2006) ▶ reported that PUT concentration in intestine and liver tissues is higher in the younger age. Holt and Luk (1990) ▶ showed that ileal ODC activity was 66% greater in aging rat, but jejuna ODC activity was slightly increased in young rat. This could be because the higher rate of metabolism at older age, requires greater content of intestinal PA. Polyamine contents in intestine and liver tissues of younger birds were not affected by dietary PUT in the current study. Likewise, Sabater-Molina and Larque et al. (2009) demonstrated that the concentrations of PUT, SPM and SPD in intestinal mucosa of piglet were not affected by dietary PA. It can be concluded that tissue polyamine content is strongly regulated. Additionally, Soda et al. (2009) stated that long-term intake of polyamine-rich foods gradually increases blood polyamine levels in humans and animals. It is suggested that polyamine homeostasis in tissues is substantially independent of dietary supply. Based on the current finding, dietary CP affected intestinal polyamine content. The amino acids provided by the feeding are required for tissue growth. From the other side, the content of PA is closely regulated by the cell according to the state of growth. It is well recognized that high PA levels are required for rapid cell growth, whereas low levels are typical of quiescent cells (Wallace, 1996). Therefore, dietary CP may influence tissue polyamine levels indirectly and through providing individual amino acids.
The growth performance of birds did not benefit from the addition of PUT in low CP dietary. However, low CP diets caused poor growth performance in birds. It has been found that addition of PUT in the diet improved EER of birds, suggesting involvement of PUT in energy metabolism. Low CP diet improved Ca digestibility and protein efficiency ratio, but lowered DM digestibility and intestinal VH. Liver and intestinal PAs content are influenced significantly by the age and dietary CP, rather than dietary PUT.
Acknowledgment
This research project was supported by Long-Term Research Grant Scheme (LRGS) given by the Ministry of Education, Malaysia.
References
- AOAC . Official methods of analysis. 15th Edn. Arlington, USA: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Bardocz, S, Brown, DS, Grant, G, Pusztai, A, Stewart, JC, Palmer, RM. Effect of the b-adrenoreceptor agonist clenbuterol and phytohaemagglutinin on growth, protein synthesis and polyamine metabolism of tissues of the rat. Br. J. Pharmacol. 1992;106:476–482. doi: 10.1111/j.1476-5381.1992.tb14359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregendahl, K, Sell, JL, Zimmerman, DR. Effect of low-protein diets on growth performance and body composition of broiler chicks. Poult. Sci. 2002;81:1156–1167. doi: 10.1093/ps/81.8.1156. [DOI] [PubMed] [Google Scholar]
- Chowdhury, SR, Smith, TK. Effects of dietary 1,4-diaminobutane (putrescine) on eggshell quality and laying performance of hens laying thin-shelled eggs. Poult. Sci. 2001;80:1702–1709. doi: 10.1093/ps/80.12.1702. [DOI] [PubMed] [Google Scholar]
- Chowdhury, SR, Smith, TK. Dietary interaction of 1,4-diaminobutane (putrescine) and calcium on eggshell quality and performance in laying hens. Poult. Sci. 2002;81:84–91. doi: 10.1093/ps/81.1.84. [DOI] [PubMed] [Google Scholar]
- Colnago, GL, Jensen, LS. Putrescine effects on performance of male broiler chicks fed low-protein diets supplemented with essential amino acids. Poult. Sci. 1992;71:211–214. doi: 10.3382/ps.0710211. [DOI] [PubMed] [Google Scholar]
- Devi Priya, K, Selvaraj, P, Nanjappan, K, Jayachandran, S, Visha, P. Oral supplementation of putrescine and l-glutamine on the growth performance, immunity, intestinal enzymes in the broiler chickens. Tamil. J. Vet. Anim. Sci. 2010;6:250–254. [Google Scholar]
- Galyean, ML. Laboratory procedures in animal nutrition research. Department of Animal and Food Science, Texas Tech University. 2010. www.apps.depts.ttu.edu/afs/home/mgalyean/lab_man.pdf.
- Ginty, DD, Osborne, DL, Seidel, R. Putrescine stimulates DNA synthesis in intestinal epithelial cells. Am. J. Physiol. 1989;257:145–150. doi: 10.1152/ajpgi.1989.257.1.G145. [DOI] [PubMed] [Google Scholar]
- Guy, L, Bercovich, Z, Park, MH, Kahana, C. The role of polyamines in supporting growth of mammalian cells is mediated through their requirement for translation initiation and elongation. J. Biol. Chem. 2010;285:12474–12481. doi: 10.1074/jbc.M110.106419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi, SM. Growth performance and intestinal morphology of broilers fed low protein and low methionine diets supplemented with putrescine [Ph.D. Thesis] 2013. pp. 46–50. [Google Scholar]
- Heby, O, Persson, L. Molecular genetics of poly-amine synthesis in eukaryotic cells. Trends Bio. Chem. Sci. 1990;172:153–158. doi: 10.1016/0968-0004(90)90216-x. [DOI] [PubMed] [Google Scholar]
- Hobbs, CA, Gilmour, SK. Role of polyamines in the regulation of chromatin acetylation. In: Wang, JY, Robert, A, Casero, Jr, editors. Polyamine cell signalling. Totowa, NJ: Humana Press Inc; 2006. pp. 75–89. [Google Scholar]
- Holt, PR, Luk, GD. Aging and intestinal polyamine metabolism in the rat. Exp. Gerontol. 1990;25:173–181. doi: 10.1016/0531-5565(90)90048-7. [DOI] [PubMed] [Google Scholar]
- Hosseini-Vashan, SJ, Golian, A, Motaghinia, Gh, Namvari, M, Hamedi, M. Comparison of growth performance and carcass characteristics of broiler chickens fed diets with various energy and constant energy to protein ratio. J. Anim. Vet. Adv. 2010;9:2565–2570. [Google Scholar]
- Hwang, DF, Chang, SH, Shiua, CY, Chai, TJ. High-performance liquid chromatographic determination of biogenic amines in fish implicated in food poisoning. J. Chromatogr. B. Biomed. Sci. Appl. 1997;693:23–30. doi: 10.1016/s0378-4347(97)00067-4. [DOI] [PubMed] [Google Scholar]
- Igarashi, K, Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Kamran, Z, Sarwar, M, Nisa, M, Nadeem, MA, Mahmood, S, Babar, ME, Ahmed, S. Effect of low-protein diets having constant energy-to-protein ratio on performance and carcass characteristics of broiler chickens from one to thirty-five days of age. Poult. Sci. 2008;87:468–474. doi: 10.3382/ps.2007-00180. [DOI] [PubMed] [Google Scholar]
- Kermanshahi, H, Ziaei, N, Pilevar, M. Effect of dietary crude protein fluctuation on performance, blood parameters and nutrients retention in broiler chicken during starter period. Global Vet. 2011;6:162–167. [Google Scholar]
- Loser, C. Polyamines in human and animal milk. Br. J. Nutr. 2000;84:55–58. doi: 10.1017/s0007114500002257. [DOI] [PubMed] [Google Scholar]
- Machi, S, Matsufuji, S, Murakami, Y. Dietary induction of ornithine decarboxylase in male mouse kidney. Biochim Biophys. Acta (BBA) 1999;1472:455–461. doi: 10.1016/s0304-4165(99)00148-8. [DOI] [PubMed] [Google Scholar]
- Meziani, K, Benamouzig, R. Effects of a high soy protein diet on intestinal polyamines and ornithine decarboxylase activity in rats. J. Nutr. Biochem. 1999;10:405–410. doi: 10.1016/s0955-2863(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Murakammi, Y, Noguchi, T, Hayashi, S. Effect of protein quality on dietary induction of hepatic ornithine decarboxylase. J. Nutr. 1983;113:1124–1130. doi: 10.1093/jn/113.6.1124. [DOI] [PubMed] [Google Scholar]
- Myers, WD, Ludden, PA, Nayigihugu, V, Hess, BW. A procedure for the preparation and quantitative analysis of samples for titanium dioxide. Anim. Sci. 2004;82:179–183. doi: 10.2527/2004.821179x. [DOI] [PubMed] [Google Scholar]
- Nishimura, K, Shiina, R, Kashiwagi, K, Igarashiy, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006;139:81–90. doi: 10.1093/jb/mvj003. [DOI] [PubMed] [Google Scholar]
- Pegg, A, McCann, P. Polyaminemetabolism and function. Am. J. Physiol. 1982;243:212–221. [Google Scholar]
- Pourreza, J, Samie, AH, Rowghani, E. Effect of supplemental enzyme on nutrient digestibility and performance of broiler chicks fed on diets containing triticale. Int. J. Poult. Sci. 2007;6:115–117. [Google Scholar]
- Quinn, SJ, Ye, CP, Diaz, R, Kifor, O, Bai, M, Vassilev, P, Brown, E. The Ca2+-sensing receptor: a target for polyamines. Am. J. Physiol. 1997;273:1315–1323. doi: 10.1152/ajpcell.1997.273.4.C1315. [DOI] [PubMed] [Google Scholar]
- Sabater-Molina, M, Larque, E, Torrella, F, Plaza, J, Lozano, T, Munoz, A. Effects of dietary poly-amines at physiologic doses in early-weaned piglets. Nutrition. 2009;25:940–946. doi: 10.1016/j.nut.2009.01.017. [DOI] [PubMed] [Google Scholar]
- SAS . SAS/STAT User’s Guide. Version 6.5th Edn. NC, USA: SAS Institute Inc., Cary; 1999. [Google Scholar]
- Scott, TA, Silversides, FG, Classen, HL, Swift, ML, Bedford, MR. Comparison of sample source (excreta or ileal digesta) and age of broiler chick on measurement of apparent digestible energy of wheat and barley. Poult. Sci. 1998;77:456–463. doi: 10.1093/ps/77.3.456. [DOI] [PubMed] [Google Scholar]
- Smith, TK. Effect of dietaryputrescine on whole body growth and polyamine metabolism. society for experimental biology and medicine; 1990. pp. 332–336. [DOI] [PubMed] [Google Scholar]
- Soda, K, Dobashi, Y, Kano, Y, Tsujinaka, S, Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009;44:727–732. doi: 10.1016/j.exger.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Thanh, NT, Loh, TC, Foo, HL, Hair-Bejo, M, Azhar, BK. Effects of feeding LM combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Br. Poult. Sci. 2009;503:298–306. doi: 10.1080/00071660902873947. [DOI] [PubMed] [Google Scholar]
- Wallace, HM. Pelyamines in human health. Proceeding Nutrition Society. 1996;55:419–431. doi: 10.1079/pns19960039. [DOI] [PubMed] [Google Scholar]
- Ziaei, N, Kermanshahi, H, PileVar, M. Effects of dietary crude protein and calcium phosphorus content on growth, nitrogen and mineral retention in broiler chickens. Afr. J. Biotechnol. 2011;10:13342–13350. [Google Scholar]