Abstract
A common cause of peripheral nerve injury is trauma. The positive effect of antioxidants on the improvement of nerve regeneration has currently become a focus of attention. In this experiment, the effect of intraperitoneal administration of ubiquinone (CoQ10) on an acute experimentally sciatic nerve crush was studied in a rat model. Forty-five male Wistar rats, weighing between 160-180 g were used. The rats were randomly divided into two experimental groups (n=20). Each group was further subdivided into four subgroups of five animals each. Functional studies confirmed the faster recovery of regenerated axons in the treatment group compared to the un-treated group (P<0.05). Morphometric indices of the regenerated fibers showed the number and diameter of the myelinated fibers to be significantly higher in the treatment group than the un-treated group (P<0.05). Intraperitoneal administration of CoQ10 (10 mg/kg/day) in the early inflammatory stage of sciatic nerve crush was found to improve nerve regeneration.
Key Words: Ubiquinone (coenzyme Q10), Peripheral nerve regeneration, Crush
Introduction
Nerve trauma is the most common form of nervous system trauma encountered in human clinical practices. Nerve injury is also a significant cause of functional morbidity (Hart et al., 2004 ▶). In domestic animals, trauma is considered as a common cause of peripheral nerve injury. Mechanical injury can induce temporary or permanent motor and/or sensory deficits (Summers et al., 1995 ▶).
Recent studies show the positive effect of antioxidants on the improvement of peripheral nerve regeneration. The neuroprotective effect of ascorbic acid (Shokouhi et al., 2005 ▶) and vitamin E (Morani and Bodhankar, 2008 ▶) has been reported to repair crush injuries in rats.
Coenzyme Q10 (CoQ10) or ubiquinone is a vitamin or vitamin-like substance found naturally in mitochondria. CoQ10 carries out important biochemical functions in cells, the best known being an essential cofactor in mitochondrial oxidative phosphorylation, necessary for ATP production. Hence, CoQ10 acts as a mobile electron carrier transferring electrons from complex I or II to complex III (Crane, 2001 ▶). CoQ10 is also well known as an endogenous lipid soluble antioxidant acting as a primary scavenger of free radicals. CoQ10 protects cells both directly by preventing lipid peroxidation and indirectly by regenerating other antioxidants such as ascorbate and α-tocopherol in both mitochondria and membranes (Forsmark-Andree et al., 1997 ▶; Beyer et al., 1985 ▶; Shults and Haas, 2005 ▶).
The neuroprotective effect of CoQ10 as a potent antioxidant and oxygen derived free radicals scavenger in rat cerebral acute injuries and ischemia has been reported (Ostrowski, 2000 ▶). Later reports maintained that CoQ10 prevented secondary injury during experimental spinal cord injuries in rats (Kerimoglu et al., 2007 ▶).
Direct injury to peripheral nerves can cause an increase in free oxygen radicals, which lead to tissue damage (Westling, 1984 ▶). Prevention of secondary injury due to free oxygen radicals can probably enhance nerve healing. Otherwise, clinical outcomes of peripheral nerve injuries are often suboptimal (Isaacs, 2010). Hence, optimizing nerve regeneration requires an adherence to well-established basic principles of evaluation and treatment. The aim of the present study was to evaluate possible effects of the intraperitoneal administration of CoQ10 on an experimentally induced acute sciatic nerve crush in a rat model. Assessment of nerve regeneration was based on functional (walking track analysis) and histomorphometric criteria, 7, 14, 57 and 90 days after surgery.
Materials and Methods
Study design
A total of 45 male Wistar rats, weighing between 160-180 g were used in this experiment. The rats were randomly divided into two experimental groups (n=20). Each group was further subdivided into four subgroups of five animals each. Five rats were used as a sham operation group (normal control). During the entire experiment, the rats were housed in individual plastic cages at an ambient temperature of about 24°C and a humidity of 60-70%, with a natural light-dark cycle. The rats were handled on a regular daily basis for 2 weeks prior to the study in order to acclimatize them with the testing area and experiments. The rats had free access to standard rodent laboratory food and tap water.
Animals were anesthetized by intraperitoneal administration of ketamine hydrochloride 5%, 70 mg kg-1 (Ketaset 5%; Alfasan, Woerden, The Netherlands) and xylazine hydrochloride 2%, 5 mg kg-1 (Rompun 2%, Bayer, Leverkusen, Germany). The left thigh area was then shaved and prepared for surgery. The left sciatic nerve was exposed through skin incision and gluteal muscle splitting. A single crush was applied on the sciatic nerve with a straight Halsted Mosquito hemostat for 30 sec (Bridge et al., 1994 ▶). The jaws of the forceps had already been dressed by a peace of Foley catheter. Indian ink was used as a marker for the accurate sampling of the crush site at the end of experiment. The split muscle and skin incision was closed by 5-0 coated Vicryl and 4-0 Nylon suture, respectively.
In the sham-operation group, the left sciatic nerve was exposed and the incision site was sutured. In the treatment group the rats were treated by intraperitoneal injection of CoQ10 (10 mg/kg of b. wt. I. p. for 4 days consecutively). The rats of the un-treated group were subjected to the same procedure with soybean oil. Coenzyme Q10 (sigmaC9538) was dissolved in soybean oil (sigmaC7381) prior to the injection. The procedures were carried out based on the guidelines of the ethics committee of the International Association for the Study of Pain (Zimmermann et al., 1983 ▶). The University Research Council approved all the procedures.
Functional assessment of nerve regeneration
Walking track analysis was performed 7, 14, 57 and 90 days after surgery based on Bain et al. (1989) ▶. Measurements of the third toe to heel (PL), the first toe to the fifth (TS), and the second toe to the fourth (IT) were made on the experimental side (E) and the contralateral normal side (N) in each rat. The sciatic function index (SFI) in each animal was calculated by the following formula:
SFI = -38.3 × (EPL - NPL)/NPL + 109.5 × (ETS - NTS)/NTS + 13.3 × (EIT - NIT)/NIT - 8.8
SFI generally varies around 0 for normal nerve function; however, values around -100 SFI represent total dysfunction. In the present study, a value of 0 was considered as normal function. The results of the treatment group were compared to those obtained in the un-treated group. SFI had a negative value, with a higher SFI meaning better function of the sciatic nerve.
Histological preparation and quantitative mor-phometric studies
The animals were anesthetized (as described above) and euthanized with transcardial perfusion of a fixative containing 2% paraformaldehyde and 1% glutaraldehyde buffer (pH = 7.4), 7, 14, 57 and 90 days after surgery. The left crushed sciatic nerve was dissected from the surrounding tissues, and a segment of the nerve including the regenerated part was harvested.
The sample was fixed in 2.5% glutaraldehyde (sigma G6403). Specimens were cut in 5-µm-thick sections, postfixed in OsO4 (2%, 2 h), dehydrated through an ethanol series and embedded in Epon. Samples were then stained with toluidine blue (Bancroft and Stevens, 1990 ▶) and examined under light microscopy. The immediate proximal part of the crushed area was included in the microscopic survey to detect possible effects of CoQ10 on nerve degeneration of both un-treated and treatment groups for the first sampling only. Thirty minutes prior to euthanasia, each rat received heparin at a dose of 250 IU intraperitoneally (Ferreira et al., 2001 ▶). Mor-phometrical analysis was carried out using image analyzing software (Image-Pro Express, version 6.0.0.319, Media Cybernetics). Equal opportunity, systematic random sampling and two-dimensional dissector rules were followed to cope with sampling-related, fiber location-related and fiber size-related biases (Geuna et al., 2003 ▶).
Statistical analysis
Statistical analyses were performed with SPSS (SPSS for Windows 11.0: SPSS, Chicago, Illinois). Data are presented as means ± SDs. Model assumptions were evaluated by examining the residual plot. Results were analyzed using a factorial analysis of variance (ANOVA) with two between subject factors. A Bonferroni test for pairwise comparisons was used to examine the effects of time and treatments. The differences were considered significant when P<0.05.
Results
Recovery of sciatic nerve function
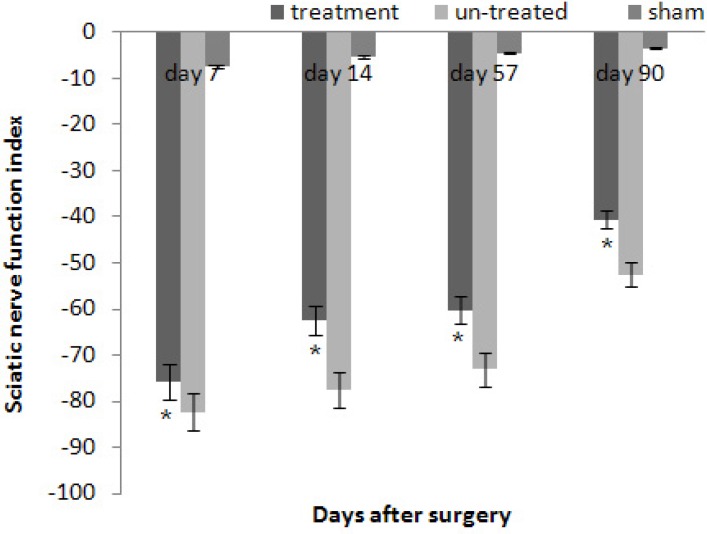
Preoperatively, the SFI of rats in both groups was approximately zero, indicating normal function. After the crush injury, the SFI decreased to approximately -100, indicating complete loss of function. As indicators of functional recovery of the regenerated nerves, SFI values were recorded at 7, 14, 57 and 90 days after surgery. Statistical analyses of the obtained data showed that nerve function recovery was significantly greater (P<0.05) in the treatment group (-75.8 ± -2.32, -62.4 ± -3.19, -60.3 ± -2.43, -40.6 ± -3.41) than the un-treated group (-82.3 ± -2.38, -77.4 ± -2.64, -73.1 ± -2.28, -52.5 ± -3.19) at 7, 14, 57 and 90 days after surgery (Fig. 1).
Fig. 1.
Diagrammatic representation of effects on SFI. Intra peritoneal administration of CoQ10 (treatment group n=5) gave better results in the functional recovery of the sciatic nerve as compared to the un-treated animals (n=5). Data are presented as means ± SD. * P<0.05 vs. the un-treated group
Histologic and morphometric findings
Table 1 shows quantitative morphometric analyses of the regenerated nerves for each of the experimental groups. Statistical analyses showed that the treatment group showed significantly larger numbers of nerve fibers, fiber diameters, and axon diameters compared to those of the un-treated group, 7, 14, 57 and 90 days after surgery (P<0.05). However, this value for myelin thickness was found between animals 7 and 57 days after surgery (P<0.05).
Table 1.
Morphometric analyses of regenerated nerves for experimental groups (mean±D(
| Groups |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | Sham | Treatment (crush + CoQ10) |
Un-treated (crush + soybean oil) |
||||||
| Parameters | 90 | 7 | 14 | 57 | 90 | 7 | 14 | 57 | 90 |
| No. of fibers | 8024 ±385 |
3356 ±167* |
3754 ±238* |
3854 ±178* |
6797 ±211* |
2935 ±157 |
3289 ±137 |
3187 ±148 |
6211 ±226 |
| Diameter of fibers (µm) | 12.01 ±0.01 |
2.59 ±0.33* |
4.48 ±0.53* |
8.49 ±0.42* |
9.75 ±0.92* |
2.16 ±017 |
3.36 ±0.47 |
6.56 ±0.49 |
7.72 ±0.89 |
| Diameter of axon (µm) | 7.06 ±0.02 |
2.24 ±0.28* |
3.63 ±0.43* |
4.87 ±0.59* |
5.36 ±0.24* |
2.15 ±0.19 |
2.52 ±0.37 |
3.53 ±0.62 |
3.68 ±0.63 |
| Myelin thickness (µm) | 2.53 ±0.01 |
0.15 ±0.01* |
0.53 ±0.02 |
2.37 ±0.25* |
2.33 ±0.46 |
0 | 0.47 ±0.02 |
1.59 ±0.11 |
2.42 ±0.52 |
| G-ratio | 0.59 | 0.87 | 0.81 | 0.57 | 0.56 | 0.99 | 0.75 | 0.54 | 0.49 |
P<0.05 vs. un-treated group. G-ratio: Ratio of axon diameter to fiber diameter
A factorial ANOVA with two between-subjects factors (group-time) showed an interaction across time in both treatment and un-treated groups. The fiber number showed significant increase in animals between 7 and 14 days (P<0.05). Significant differences were observed in fiber diameter throughout the study period and in axon diameter between day 57 and 90 (P<0.05). The mean thickness of myelin sheath did not show statistical difference between 57 and 90 days (P>0.05). Ratio of axon diameter to fiber diameter (G-ratio) was calculated in each group to show the effects of time and treatment on the maturation of nerve fibers.
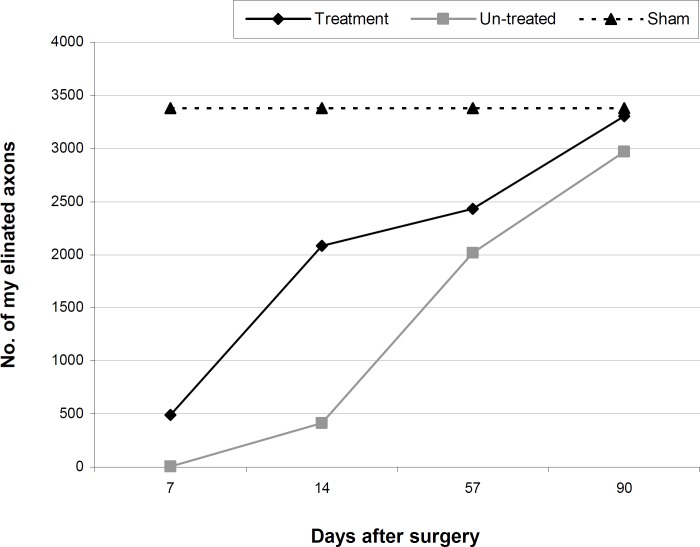
Figure 2 shows the regeneration course feature in the myelinated fibers of the un-treated and treatment groups throughout the experiment. The myelination process of the regenerated fibers’ spiky slope occurred 14 days after surgery in the treatment group. However, this course was slow in the un-treated group, and the smooth curve raise gradually came to the same level by day 57 post-surgery.
Fig. 2.
Diagram of number of myelinated fibers in un-treated (n=5) and treatment (n=5) groups throughout the experiment
In the sham-operation group, the number of myelinated fibers was 3381±283. The number of myelinated fibers was nearly normal by 90 days in the un-treated and treatment groups. However, the size of axons and myelin thickness were larger in the treatment group in comparison to the un-treated group.
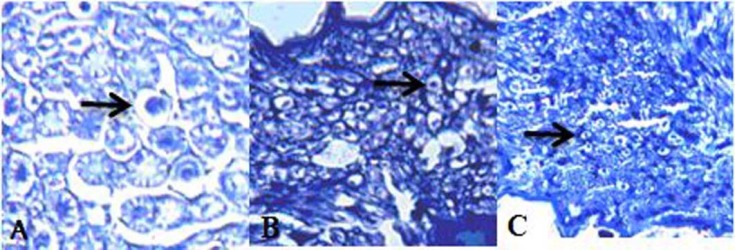
Regenerated sprouts were composed of axons and myelin which were more pronounced in the treatment group in comparison with those of the un-treated animals on day 57 following surgery (Fig. 3).
Fig. 3.
Photomicrograph of a cross-section of rat sciatic nerve (toluidine blue, ×400). A: Sham, the normal histologic appearance and size of axons in normal control group. B and C: 57 days after surgery, note different axon size in treatment and un-treated animals, respectively
Microscopic surveys of the proximal parts of the crushed areas showed more positive effects of CoQ10 on nerve preservation from ascending degeneration in the treatment group compared to that of the un-treated group.
Discussion
The results of the present study showed that intra peritoneal administration of CoQ10 can cause an improvement of peripheral nerve regeneration in the sciatic nerve crush of a rat model.
Sciatic nerve crush is a well-characterized model for studying experimental peripheral nerve regeneration (Bridge et al., 1994 ▶). In this study, we used a modified technique through which the jaws of a Halsted Mosquito hemostat were dressed to impede probable endoneurial scarring and to preserve the nerve’s neurolema.
In the present study, we used SFI, a comprehensive and reliable test, to evaluate motor function recovery of post-traumatic peripheral nerve regeneration in rats (Varejão et al., 2001 ▶). Accordingly, the greater values in the treatment group compared to the un-treated group throughout the study period indicated that intra peritoneal administration of CoQ10 drastically promoted functional recovery of the regenerated nerves in the treatment group.
In general, quantitative morphometric analyses showed positive effects of intra peritoneal administration of CoQ10 on sciatic nerve regeneration in treated rats.
Significant increases in nerve fiber diameter and myelin thickness from day 14 to day 57 after surgery indicated the maturation of nerve fibers in both experimental groups.
In addition, comparing the spiky slope of the myelinated axonal regeneration curve in the treatment group to the un-treated group can explain the positive effects of CoQ10 on accelerating growth and faster myelination of the regenerating nerve fibers in the early stages of the healing process of sciatic nerve crush.
The diameter of myelinated nerve fibres gradually increases with time. Bridge et al. (1994) ▶ found that the histological recovery of the crushed nerve site occurred 56 days after surgery. In our experiment, the number of myelinated nerve fibers was close to the normal level on day 90 after surgery in both treated and non-treated rats. The process of fiber myelination and increase of myelin sheath thickness showed the additional benefits of the intra peritoneal administration of CoQ10 in early fiber myelination of the regenerated nerves in treated compared to un-treated animals; however, the fiber diameter including myelin thickness and axon diameter was not comparable with the sham operation group.
The role of CoQ10 in nerve tissue regeneration is not well understood. In this research, we studied the effect of CoQ10 on the regeneration of sciatic nerves after crush injury. The results suggest that COQ10 promoted the regeneration of the sciatic nerve after crush injury. Systemic administration of CoQ10 in the early postsurgical period (inflammatory phase) accelerated functional recovery of the regenerated nerve fibers.
As a potent antioxidant and oxygen-derived free-radical scavenger, coenzyme Q10 can stabilize brain cell membranes. The neuroprotective effect of CoQ10 in rat experimental cerebral ischemia has been previously reported (Ostrowski, 2000 ▶). Enzymatic and non-enzymatic mechanisms are involved in maintaining antioxidant properties following CoQ10 treatment. Coenzyme Q10 resumes ATP concentration to a normal level after 24 h in treated rats (Ostrowski, 2000 ▶).
For the treatment group, the positive effect of CoQ10 in preserving nerves from ascending degeneration in the early inflammatory stage of the study could have resulted from the neuroprotective effects of CoQ10. Trauma in the crushed area can induce oxygen-derived free radicals. It seems that CoQ10 by scavenging free radicals prevents lipid peroxidation and stabilizes the cell membrane (Melors and Tappel, 1966 ▶; Koroshetz et al., 1997 ▶).
Coenzyme Q10 has been reported to be effective in decreasing edema and preventing secondary injury in experimental spinal cord injury in rats by decreasing malonialdehyde and increasing superoxide dismutase levels (Kerimoglu et al., 2007 ▶). Although the present study showed the neuroprotective action of intaperitoneal administration of CoQ10 in peripheral nerve injuries in rats, data regarding the molecular mechanisms leading to its neuroprotective action remain to be investigated in depth. The authors have not provided histologic and molecular evidence for the neuroprotective action of CoQ10, which may be considered as a limitation of this study.
In conclusion, the intraperitoneal administration of CoQ10 in early inflammatory stages of sciatic nerve crush was found to improve nerve regeneration.
Acknowledgements
The authors are grateful to K. Amini, Department of Veterinary Pathology, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon SK, Canada, B. Dalir-Naghadeh, Department of Clinical Sciences, Faculty of Veterinary Medicine, Urmia University, Iran, and Mr. Jaafary, Urmia Pathobiology Center for their technical expertise and support.
References
- Bain, JR, Mackinnon, SE, Hunter, DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989;83:129–136. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- Bancroft, JD, Stevens, A. Theory and practice of histological techniques. 3rd Edn.: Churchill, Livingston; 1990. p. 167. [Google Scholar]
- Beyer, RE, Burnett, BA, Kenneth Cartwright, J, Edington, DW, Falzon, MJ, Kreitman, KR, Kuhn, TW, Ramp, BJ, Rhee, SYS, Rosenwasser, MJ, Stein, M, An LC. Tissue coenzyme Q (ubiquinone) and protein concentrations over the life span of laboratory rat. Mech. Ageing Dev. 1985;32:267–281. doi: 10.1016/0047-6374(85)90085-5. [DOI] [PubMed] [Google Scholar]
- Bridge, PM, Ball, DJ, Mackinnon, SE, Nakao, Y, Brandt, K, Hunter, DA, Hertl, C. Nerve crush injuries- A model for axonotmesis. Exp. Neurol. 1994;127:284–290. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]
- Crane, FL. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001;20:591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- Ferreira, AJ, Santos, RAS, Almeida, AP. Angiotensin-(1-7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001;38:665–668. doi: 10.1161/01.hyp.38.3.665. [DOI] [PubMed] [Google Scholar]
- Forsmark-Andree, CP, Lee, GD, Ernster, L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles, Free Radic. Biol. Med. 1997;22:391–400. doi: 10.1016/s0891-5849(96)00330-9. [DOI] [PubMed] [Google Scholar]
- Geuna, A, Gigo-Benato, D, Rodrigues, AC. On sampling and sampling errors in histomorphometry of peripheral nerve fibers. Microsurg. 2003;23:72–76. doi: 10.1002/micr.10199. [DOI] [PubMed] [Google Scholar]
- Hart, AM, Terenghi, G, Kellerth, JO, Wiberg, M. Sensory neuroprotection, mitochondrial pre-servation and therapeutic potential of N-acetyl-cysteine after nerve injury. Neuroscie. 2004;125:91–101. doi: 10.1016/j.neuroscience.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Isaacs, J. Treatment of acute peripheral nerve injuries: current concepts. J. Hand Surg. Am. 2010;35:491–497. doi: 10.1016/j.jhsa.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Kerimoglu, A, Pasaoglu, O, Kanbak, G, Hanci, V, Ozdemir, F, Atasoy, MA. Efficiency of coenzyme Q10 at experimental spinal cord injury. Ulus Travma Acil Cerrah Derg. 2007;13:85–93. [PubMed] [Google Scholar]
- Koroshetz, WJ, Jenkins, BG, Rosen, BR, Beal, MF. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann. Neurol. 1997;41:160–165. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- Melors, A, Tappel, AL. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J. Biol. Chem. 1966;241:4353–4356. [PubMed] [Google Scholar]
- Morani, AS, Bodhankar, SL. Neuroprotective effect of vitamin E acetate in models of mononeuropathy in rats. Neuroanatomy. 2008;7:33–37. [Google Scholar]
- Ostrowski, RP. Effect of coenzyme Q10 on biochemical and morphological changes in experimental ischemia in the rat brain. Brain Res. Bull. 2000;53:399–407. doi: 10.1016/s0361-9230(00)00406-8. [DOI] [PubMed] [Google Scholar]
- Shokouhi, G, Hadidchi, Sh, Ghorbanihaghjo, A, Rhbani-Noubar, M, Panahi, S, Forouzanfar, MH, Rashtchizadeh, N, Mesgari, M. Neuroprotective effect of ascorbic acid in experimental blunt sciatic nerve injury in rats. The Internet. J. Nutri. Well. 2005;1:1–8. [Google Scholar]
- Shults, CW, Haas, R. Clinical trials of coenzyme Q10 in neurological disorders. Biofact. 2005;25:117–125. doi: 10.1002/biof.5520250113. [DOI] [PubMed] [Google Scholar]
- Summers, BA, Cummings, JF, de Lahunta, A. Veterinary neuropathology. 1st Edn. St Louis: Missouri, Mosby; 1995. pp. 402–423. Year Book. [Google Scholar]
- Varejão, AS, Meek, MF, Ferreira, AJ, Patrício, JA, Cabrita, AM. Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. J. Neurosci. Methods. 2001;108:1–9. doi: 10.1016/s0165-0270(01)00378-8. [DOI] [PubMed] [Google Scholar]
- Westling, G, Johansson, RS. Factors influencing the force control during precision grip. Exp. Brain Res. 1984;53:277–284. doi: 10.1007/BF00238156. [DOI] [PubMed] [Google Scholar]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]