Abstract
Background
Recent studies have suggested that various cytokines may be important players in the development and progression of chronic pancreatitis (CP) and pancreatic adenocarcinoma (PC).
Aims
We studied endothelial dysfunction and subclinical inflammation in patients with newly diagnosed pancreatic adenocarcinoma and CP.
Methods
A total of 45 patients were included in the present investigation, 27 with CP and 18 with PC. In addition, the study included 13 age- and body weight-matched healthy subjects served as controls. In all subjects, plasma adiponectin, TNF-alfa, interleukin 6 (IL-6), interleukin 1beta (IL-1β), E-selectin, thrombomodulin, adhesion molecules ICAM and VCAM, and endothelin-1 were assessed.
Results
PC and CP patients as compared with controls had significantly greater plasma adiponectin (13,292 and 12,227 vs 5408 ng/ml; p < 0.0003), TNF-alfa (22.1 and 23.1 vs 13 pg/ml; p < 0.0002), and IL-6 (6.6 and 7.3 vs 3.3 pg/ml; p < 0.0001). Moreover, there was significantly higher concentration of ICAM (931 and 492 vs 290 ng/ml; p < 0.005) and VCAM (1511 and 1080 vs 840 ng/ml; p < 0.01) in PC and CP patients. When PC and CP patients with and without diabetes were considered separately, there was no difference in adiponectin, cytokines, and parameters of endothelial dysfunction.
Conclusion
In summary, our data indicate that patients with CP and PC express high levels of several cytokines compared with healthy individuals, especially adiponectin, TNF-α and IL-6. Serum TNF-α and ICAM concentrations coordinately increase in advanced CP. Furthermore, especially in PC subjects, elevated markers of endothelial dysfunction are present. This study provides additional evidence that changes in inflammatory cytokine and adhesion molecules in PC and CP are not likely related to endocrine disorders.
Keywords: Pancreatic adenocarcinoma, Chronic pancreatitis, Inflammation, Cytokines
Introduction
Chronic inflammation is typically associated with many types of human malignancies [1]. Inflammatory bowel diseases provide convincing evidence for the association between chronic inflammation and malignant diseases in colon. Kate et al. [2] showed that proinflammatory cytokines are capable of enhancing the adhesion of human colon carcinoma cells to the microvascular endothelium, most likely by the up-regulation of adhesion molecules to the endothelium. In general, the inflammatory reaction leads to the activation of leukocytes and monocytes with the release of proinflammatory cytokines and reactive oxygen species. Sustained cell proliferation in the milieu of inflammatory cells, elevated levels of growth factors, activated stroma, and DNA-damaging agents promote neoplastic transformations [1]. Chronic pancreatic inflammation is mediated by cytokines, reactive oxygen species, up-regulated proinflammatory pathways, and immune cell infiltrates [3, 4]. Moreover, cytokine levels correlate with phenotypic manifestations of carcinoma and with prognosis both in patients with hematologic malignancies and in patients with solid tumors [5]. Cytokines have been also shown to play a role in different processes in the course of pancreatic carcinoma and chronic pancreatitis (CP), such as cachexia, asthenia, and impaired glucose metabolism. Understanding the biology and pathogenesis of CP and pancreatic carcinoma may help to improve current—largely ineffective—treatment.
Pancreatic adenocarcinoma (PC) is one of the five leading causes of cancer-related deaths. Patients with PC have very poor prognosis—in the first year, more than 90 % patients die, and the 5-year survival is <5 % [6]. At the time of diagnosis, only up to 20 % of patients suffering from PC are eligible for surgical resection. This is largely due to the fact that this type of cancer has rapidly progressive course, gives few symptoms, and has high metastatic potential and low responsiveness to chemotherapy, radiotherapy, and immunotherapy. Histologically, an important element of PC is the intensely desmoplastic stroma, which may be related to the inflammatory response in PC [7]. The inflammatory cell clusters are mainly found in areas of fibrosis. The major determinants of pancreatic fibrosis are the pancreatic stellate cells that function as activated myofibroblasts in PC. These cells have been identified as major source of extracellular matrix proteins but have also been involved in the modulation of local immune reaction by production and secretion of cytokines and chemokines [8]. As mentioned above, cytokines have potent immune regulatory and growth regulatory effects that may be involved in the pathogenesis of pancreatic carcinoma. That is why, modulating inflammatory factors may offer attractive pathway for novel, non-surgical approach in PC treatment.
Aims
We studied endothelial dysfunction and subclinical inflammation in patients with newly diagnosed pancreatic adenocarcinoma (PC) and CP compared to healthy volunteers. Cytokines and adhesion molecules levels were analyzed in relation to disease clinical manifestations and glucose metabolism disturbances.
Materials and Methods
Patients
Patients were enrolled from the Department of Digestive Tract Diseases, Medical University of Lodz and Outpatients Gastroenterology Unit. A total of 45 Caucasian patients were included in the present investigation, 27 with CP (17 men and 10 women, aged 20–68 years) and 18 with newly diagnosed pancreatic cancer (4 men and 14 women, aged 49–81 years). Thirteen healthy, age-, gender-, and BMI-matched subjects were included into the control group.
Only patients with confirmed (through aspiration biopsy or surgical resection sample) diagnosis of ductal pancreatic adenocarcinoma were included into the study. Among PC patients, six (33 %) underwent surgical tumor resection, whereas 12 (67 %) have unresectable tumors. Among the patients with pancreatic adenocarcinoma, 12 (67 %) had lymph node metastases at the time of the study, eight (44 %) had distant metastases, and five (28 %) had diabetes at the time of the cancer diagnosis for less than 3 years. Diabetes diagnosis was based according to WHO/ADA criteria [9]. Moreover, in eight (44 %) PC patients mean BMI levels was within normal range. Five patients (28 %) had BMI lower than 20 kg/m2, and five had above 25 kg/m2. Clinicopathologic data were gathered retrospectively for each case from the patient’s medical records. Detailed subjects’ characteristics are presented in Table 1.
Table 1.
Baseline characteristics of pancreatic cancer patients
| Pancreatic cancer (n = 18) | |
|---|---|
| Mean age (years ± SD) | 69.61 ± 8.93 |
| Gender (M/F) | 4/14 |
| Primary tumor localization: | |
| Head | 16 (89 %) |
| Body/tail | 2 (11 %) |
| Lymph node metastases | 12 (67 %) |
| Distant metastases | 8 (44 %) |
| Jaundice | 12 (67 %) |
| Diabetes | 5 (28 %) |
| BMI (kg/m2) | 22.18 ± 4.06 |
The diagnosis of CP was based on the following criteria: presence of a typical history of recurrent attacks of pancreatitis and anatomical abnormalities such as calcifications and/or pancreatic ductal irregularities present in pancreatic imaging [10]. In the CP group, 23 with alcohol-related disease and four with idiopathic disease were included. ACP (alcoholic CP) was diagnosed in patients who consumed more than 80 g/day (males) and 40 g/day of alcohol (females) for >5 years before the first symptoms of the disease. ICP (idiopathic CP) was diagnosed when possible etiological factors such as alcohol abuse, metabolic disorders, anatomic, traumatic causes, and positive family history were absent. The mean duration of CP was 6.3 ± 4.51 years. The onset of CP was defined as the initial manifestation—first episode of acute pancreatitis. The Cambridge classification was used to grade severity of CP [10]. In CP group, six patients had indications for surgical intervention and 14 for endoscopic treatment due to intractable pain, pancreatic stones, pseudocysts, common bile stenosis, or suspicion of pancreatic cancer. Characteristics of CP patients are presented in Table 2.
Table 2.
Baseline characteristics of chronic pancreatitis patients
| Chronic pancreatitis (n = 27) | |
|---|---|
| Mean age (years ± SD) | 46.82 ± 10.85 |
| Gender (M/F) | 17/10 |
| Etiology | |
| Alcoholic | 23 (85 %) |
| Idiopathic | 4 (15 %) |
| Duration of CP | |
| <5 years | 18 (67 %) |
| >5 years | 9 (33 %) |
| Smoking | 22 (81 %) |
| Calcifications | 21 (78 %) |
| Diabetes | 12 (44 %) |
| Cambridge classification: | |
| II | 12 (44 %) |
| III | 15 (56 %) |
| Endoscopic treatment | 14 (52 %) |
| Surgical treatment | 6 (22 %) |
| BMI (kg/m2) | 22.18 ± 4.06 |
Smoking was defined as having smoked ≥10 cigarettes per day for ≥2 years. According to this definition, 13 (72 %) of patients with pancreatic adenocarcinoma and 22 (81 %) of CP patients were smokers.
In the controls, blood samples were collected when they had no infection, were not receiving any medications, and did not have any recent (<3 months) history of any acute illnesses. All patients with PC and CP as well as healthy controls provided informed, written consent for the use of their samples, and the study was approved by the Ethics Committee of Medical University of Lodz.
Blood Analysis
At 8 am, fasting blood samples were collected from patients and healthy controls. All blood samples were stored at −20 °C until laboratory workup. None of the samples were previously thawed. The plasma concentrations of adiponectin, TNF-α, interleukin 6 (IL-6), interleukin 1beta (IL-1β), E-selectin, adhesion molecules sICAM-1, and sVCAM-1 have been measured with an enzyme-linked immunoassay (ELISA, R&D Systems, USA). The limit of detection of TNF-α was determined to be 1.6 pg/ml and adiponectin 0.246 ng/ml. The minimum detectable of IL-6 and IL-1β was determined to be, respectively, 0.7 and 1 pg/ml. Endothelin-1 was measured with a sensitivity of 1.0 pg/ml with the intra-assay coefficients of variation 4.6 % and the inter-assay CVs 6.5 %. E-selectin was measured with a sensitivity of 0.009 ng/ml with the intra-assay coefficients of variation 6.6 % and the inter-assay CVs 7.3 %. The minimum detectable of ICAM-1 was 0.096 ng/ml and VCAM-1 was 0.6 ng/ml. The plasma thrombomodulin has been analyzed with ELISA (American Diagnostica Inc.) with the intra-assay coefficients of variation 4.0 % and the inter-assay CVs 5.2 %.
Statistical Analysis
The distribution of categorical variables was assessed with the use of Chi-squared test or—if subgroup numbers were lower than 5—with the exact Fisher test. The differences between the means of continuous variables were assessed with the Student’s t test. Conditional logistic regression models were used for estimating odds ratio and 95 % confidence interval (95 % CI) to evaluate the association of each variable with pancreatic cancer. Linear regression analysis was used to determine correlation coefficients between various parameters. The statistical calculations were carried out using StatView-J 5.0 statistical software (SAS Institute, Cary, NC). Differences with a p value of less than 0.05 were considered to be statistically significant.
Results
Circulating Concentrations of Adiponectin
Plasma levels of adiponectin were significantly higher in PC patients (mean cytokine level: 13,292 ng/ml; range: 9542–22,446 ng/ml) and CP patients (12,227 ng/ml; range: 8674–16,751 ng/ml) as compared with the controls (5408 ng/ml; range: 3933–7799 ng/ml) (p < 0.0003), but there was no difference in adiponectin levels between CP and PC patients (Fig. 1). There was also no relationship between adiponectin plasma level and clinical data, including alcohol and smoking of analyzed PC and CP patients.
Fig. 1.

Analysis of adiponectin serum levels in patients with pancreatic cancer (PC), chronic pancreatitis (CP), and the control group
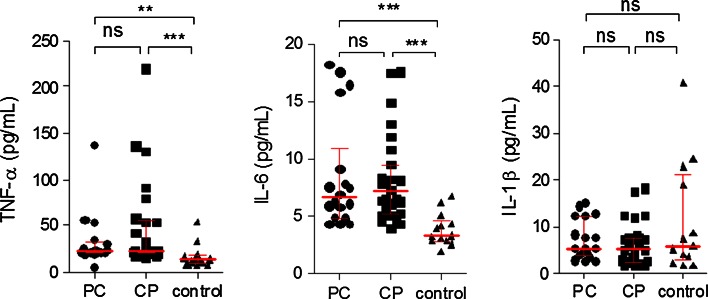
Circulating Concentrations of Inflammatory Cytokines (TNF-α, IL-6, IL-1β)
Plasma levels of TNF-α were significantly higher in PC patients (mean cytokine level: 22.15 pg/ml; range: 20.6–30.1 pg/ml) and CP patients (23.1 pg/ml; range: 19.2–53.6 pg/ml) as compared with the controls (13 pg/ml; range: 9.7–15.2 pg/ml) (p < 0.0002); however, there was no difference in TNF-α levels between CP and PC patients. In PC and CP patients, IL-6 serum levels were significantly higher (6.6 ± 4.89 and 7.3 ± 4.05 pg/ml, respectively,) than in the control group (3.3 ± 1.44 pg/ml) (p < 0.0001).There was no statistically significant difference between PC and CP IL-6 levels. Serum IL-1β level in patients with pancreatic carcinoma and CP did not differ from those in normal individuals (Fig. 2).
Fig. 2.
Analysis of TNF-α, IL-6, and IL-1β levels in sera obtained from patients with pancreatic cancer (PC), chronic pancreatitis (CP), and the control group
Fasting plasma TNF-α was found to be higher in the severe stage of CP (Cambridge 3) (33.2 pg/ml) compared to the moderate CP (Cambridge 2) (20.65 pg/ml), (p < 0.047). There was no other statistically significant association between cytokines serum level and clinicopatolological data of analyzed PC and CP groups.
There was no other statistically significant association between cytokines serum level and alcohol and smoking of analyzed PC and CP groups.
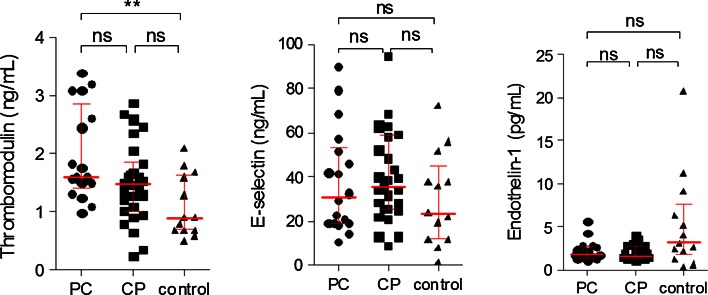
Circulating Concentrations of Endothelial Dysfunction Biomarkers
As shown in Fig. 3, there was a significantly higher concentration of thrombomodulin in the sera of patients with PC (p < 0.05), compared with healthy controls. Moreover, there were no differences between both pancreatic groups and the controls healthy volunteers in E-selectin and endothelin-1 levels (Fig. 3). Concentration of E-selectin in the sera of patients with chronic alcoholic pancreatitis was higher compared with the patients with idiopathic etiology, (38.7 vs 17.4 ng/ml; p = 0.049), but the statistical difference was marginal.
Fig. 3.
Analysis of thrombomodulin, E-selectin, and endothelin-1 levels in sera obtained from patients with pancreatic cancer (PC), chronic pancreatitis (CP), and the control group
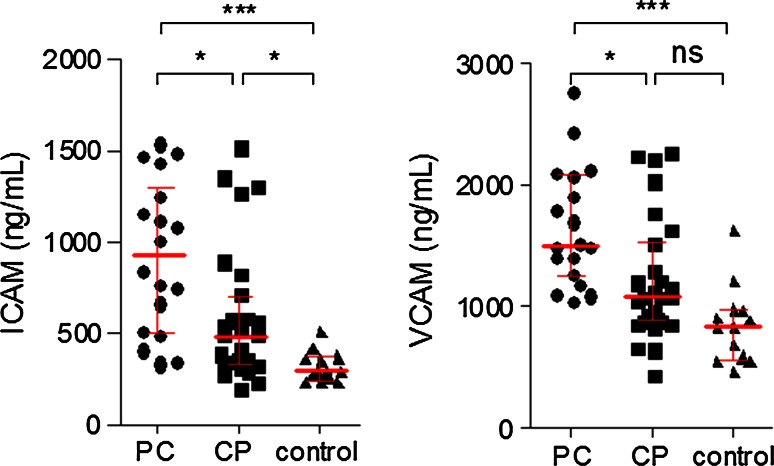
Plasma levels of ICAM were significantly higher in PC patients (mean cytokine level: 931 ng/ml; range: 508–1254 ng/ml) compared with CP patients (492 ng/ml; range 334–712 ng/ml) and control group (290 ng/ml; range 264–368 ng/ml; p = 0.0053) (Fig. 4). Additionally, pancreatic cancer patients who were smokers had significantly higher concentrations of ICAM in the sera (1124 vs 496 ng/ml; p = 0.03), compared with patients who never smoked.
Fig. 4.
Analysis of ICAM and VCAM levels in sera obtained from patients with pancreatic cancer (PC), chronic pancreatitis (CP), and the control group
Plasma levels of VCAM in PC patients (mean cytokine level: 1511 ng/ml; range 1278–2090 ng/ml) were significantly different from those with CP (1080 ng/ml; range 886–1530 ng/ml) and control group (840 ng/ml; range 596–976 ng/ml), though no differences in VCAM levels were found between CP patients and healthy volunteers (Fig. 4).
Discussion
Several cytokines, chemokines, and their receptors have been analyzed in cancer patients and animal models. These molecules of inflammation have multiple functions ranging from mediation of inflammation, immune function, angiogenesis, and ultimately metastasis [11]. Additionally substantial evidence supports a role of chronic subclinical inflammation and activation of the immune system in the pathogenesis of insulin resistance and endothelial dysfunction in the development of type 2 diabetes [12, 13]. Chronic inflammatory states are associated with many types of human malignancies, but its role in pancreatic cancer is not fully understood. Previous correlative studies in patients with pancreatic cancer have emphasized the potential role of inflammatory chemokines in tumor resistance to therapy [14].
Adiponectin is a protein that is produced largely by white adipose tissue (20). It can greatly affect wide range of disorders, including cardiovascular diseases, type 2 diabetes, metabolic syndrome, and rheumatoid arthritis [15]. Adiponectin has been found to have a protective role in several types of malignancies in vivo, particularly those related to the obesity [16–18]. In the present study, plasma level of adiponectin in PC patients was significantly higher than in the control group. This finding is in accordance with a previous case–control study that showed that adiponectin was significantly elevated in 72 patients with PC as compared to control subjects [19]. Similarly in Dalamaga et al. [20] study demonstrated high adiponectin levels, which were independently associated with PC. Moreover, they documented the presence of both adiponectin receptors (AdipoR1 and AdipoR2) in PC tumor tissue. In contrast, another study reported that low prediagnostic levels of circulating adiponectin were associated with elevated risk of pancreatic cancer [21]. Recently, results from a case–control study nested within European Prospective Investigation into Cancer and Nutrition were published [22]. They observed a nonsignificant decrease in PC risk with increasing adiponectin level, but after evaluation of additional factors, they concluded that decreased PC risk may be restricted to never smoking women, whereas smokers tended to have an increase in risk. One explanation for the different findings regarding adiponectin levels are changes in the metabolic profile over time. In healthy subjects decreased adiponectin levels can predict an increase in PC risk, but during PC development and in advanced cancer stage, adiponectin levels can elevate rapidly. Generally, low adiponectin is associated with obesity and they predict PC. During advanced stages of the disease, adiponectin elevate rapidly either to compensate for insulin resistance and/or in response to loss of weight. Additionally, increased adiponectin level in PC patients could also be compensatory response to the inflammation, which is characteristic for this type of cancer [23]. Adiponectin is generally considered as an anti-inflammatory agent although there have been studies also reporting proinflammatory properties [24, 25].
In the current study, the levels of adiponectin were similar in patients with pancreatic adenocarcinoma and CP and were higher than in the control group. These results are consistent with previous findings showing that adiponectin concentrations were similar among patients with benign and malignant disease (autoimmune pancreatitis, CP, pancreatic cancer, and intraductal papillary mutinous tumors of the pancreas) [26]. However, our finding regarding elevated serum adiponectin level in pancreatic cancer and CP cases is in disagreement with a study of Chang et al. [19]. They found that the median level of adiponectin for PC was significantly higher than those for CP and suggested that adiponectin could be useful in the differential diagnosis of PC and CP.
In our study, patients with pancreatic adenocarcinoma and chronic alcoholic pancreatitis had elevated circulating levels of IL-6 and TNF-α, but not IL-1β compared with the levels in healthy individuals. Our results are in agreement with other studies, which reported elevated TNF-α serum levels in patients with different types of cancers compared with normal subjects [14, 27, 28]. Recently, Błogowski et al. also found that patients with pancreatic adenocarcinoma had significantly higher systemic TNF-α level than did individuals diagnosed with other pancreatic malignancies [29]. In contrast, Ebrahimi et al. [5] reported no difference in TNF-α serum levels between PA patients and healthy controls.
In our study, we observed significantly elevated IL-6 serum levels in patients with pancreatic adenocarcinoma, similar to previous studies [5, 14, 30, 31]. Additionally, plasma levels of IL-6 in patients with CP were significantly higher than those in control group, with no differences between CP patients and PC patients. IL-6 exerts pleiotropic functions on various cells that control inflammatory diseases as well as models of inflammation-associated cancer [32]. In pancreatic cancer, IL-6 increases macrophage recruitment and pancreatic tumor initiation. The tumor-associated macrophages exert their effect in part by increasing fibrosis in pancreatic adenocarcinoma PC [7].
In our study, we could not establish significant relation between TNF-α and IL-6 serum levels and several clinical factors of studied patients, probably due to relatively limited number of patients. For example Dima et al. showed that elevated serum levels of TNF-α correlated with tumor grade and overall survival in patients with PC. In their studies, however, no significant association was observed between tumor size, nodal status, and level of TNF-α [14]. Karayiannakis et al. [33] found elevated serum level of TNF-α in patients with metastatic PC compared with non-metastatic disease. It is worth noting that we excluded from this study all patients with obvious confounding clinical conditions known to raise IL-6 level such as acute inflammatory activity in the pancreas or abnormal liver function. Some authors have suggested that high IL-6 serum levels may also relate to high bilirubin levels [34]. In some studies, increased TNF-α levels are associated with weight loss in patients with pancreatic adenocarcinoma and gastrointestinal cancer but in our study serum TNF-α levels did not differ between cachectic and non-cachectic PC patients [33, 35].
Some data indicate that proinflammatory interleukins may participate in the development of insulin resistance in men by either suppressing insulin receptors tyrosine kinase activity or reducing transmembrane glucose transporters expression [32, 36]. In animal model, local IL-6 production in the pancreas was induced by endothelial cells and proinflammatory cells infiltrating the pancreatic islets [37]. In autoimmune forms of diabetes, conflicting data on IL-6 serum levels have been reported. Some groups reported lower levels of serum IL-6 in children with type 1 diabetes, while others have found normal or even increased levels of IL-6 [38]. Circulating IL-6 levels have been reported to be elevated in subjects with impaired glucose tolerance or type 2 diabetes [32, 39]. Therefore, we hypothesize that increased plasma IL-6 and TNF-α in pancreatic diseases may trigger disturbances in glucose homeostasis. In our previous study, we showed plasma IL-6 to be elevated in patients with CP compared to the healthy controls; however, we did not characterize CP patients for glucose tolerance [30]. In present study, a correlation has not been found between TNF-α, IL-6, IL-1β, and endocrine dysfunction in CP and PC patients. The endocrine dysfunction requires multiple cytokines interacting with or augmenting each other effect in both groups of patients. Hansen et al. [40] evaluated IL-6 in 16 patients with CP with and without diabetes during an oral glucose tolerance test. They showed that patient with CP and secondary DM in contrast to patients with CP and NGT exhibit elevated plasma levels of IL-6, suggesting that increased plasma levels of IL-6 are consequences of the diabetic state, rather than playing primary roles in the development of diabetes. Another study suggested that serum levels of IL-6 are dependent on the stage of the disease. In particular, high IL-6 levels were noted in early phases of type 1 diabetes indicating that this cytokine may play a role in destruction of islet cells. With regard to type 2 diabetes, IL-6 levels in serum were found to be elevated in many patients with this disease and could be used as predictors for atherosclerosis development [32].
Okada et al. [31] have also suggested a high diagnostic accuracy of IL-6 serum concentration assessment in differential diagnosis between CP and pancreatic adenocarcinoma. They reported that IL-6 serum levels were detectable only in 54.5 % patient with PA, 8 % with CP, and 4 % of healthy volunteers. In our study, there was no difference between IL-6 serum levels in PA and CP patients. According to the results observed by other authors IL-6 serum level may be elevated in CP patients [30]. In contrast, the study of Shah et al., which analyzed cytokine profile in CP patients receiving antioxidant therapy (ANTICIPATE trial), showed low values of IL-6 levels [41]. The results of published studies suggested that cytokine levels measured in blood may not necessarily reflect their activity at the pancreatic parenchymal level [41].
We measured also another important mediator of inflammation, IL-1β in patients with CP and pancreatic cancer. Interleukin 1beta is known as a promoter of tumor angiogenesis, invasiveness, and metastasis [14]. We found that IL-1β was not significantly increased in the sera of patients with PC compared with those of patients with CP or healthy individuals.
Several proinflammatory cytokines, acute phase-reactants, and cell adhesion molecules play a pivotal role in chronic subclinical inflammation due to atherosclerosis and type 2 diabetes, but understanding of the interrelations of these molecules is still needed in CP and pancreatic cancer patients. In both groups of patients, acinar atrophy, ductal metaplasia, and a strong stroma reaction by fibroblastic proliferation can be observed. Endothelial dysfunction can be detected by measurement of elevated plasma levels of cellular adhesion molecules (CAMs), including E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1). Cell adhesion molecules, like ICAM-1 or VCAM-1, are known to play an important role in cell–cell interactions, such as leukocyte–endothelium adhesion and subsequent migration of inflammatory cells into the tissue or adhesion-dependent immune responses [42]. Up-regulation of cell adhesion molecules in inflammation or malignancy promotes adhesion of leukocytes to the endothelium and thereby provides the first step for extravascular passage of inflammatory cells. Enhanced VCAM and ICAM expression has been found in human colon, breast, and lung carcinoma cells [43]. On the other hand, some authors also suggested that in solid pseudopapillary tumor, which is a rare neoplasm of pancreas, loss of adhesion complex due to gene mutation results in instability of the complex and dissociation of the tumor cells to form the pseudopapillary pattern [44]. The role of ICAM-1 and VCAM-1 in the adhesion of neutrophils and lymphocytes to endothelium and cells of epithelial origin suggests that they may be important in the immunologic response in CP and pancreatic cancer. In our study, ICAM-1 values in sera of pancreatic cancer and CP patients were significantly elevated compared with the sera patients from control group. These results are consistent with previous findings showing that overexpression of adhesion molecules is characteristic for CP and PC patients [45]. Additionally, we have also demonstrated significantly higher level of VCAM in PC patients compared with CP and controls. Probably increased serum level of IL-6 induces acute phase responses in the liver, modulates macrophage differentiation, induces epithelial cell proliferation and tumor growth, and activates adhesion molecules on endothelial cells [32]. Few clinical studies analyzed different types of adhesion molecule levels in pancreatic diseases. Techezy et al. showed that activated leukocyte cell adhesion molecule (ALCAM) values were significantly elevated in PC patients compared with values of patients with CP and healthy blood donors [46]. Sakamoto et al. analyzed plasma concentrations of angiogenesis-related factors such as platelet endothelial cell adhesion molecule-1, (PECAM-1), in pancreatic and colon cancer and also in hepatobiliary diseases. The concentrations of PECAM-1 were not elevated in patients with PC but were elevated in patients with colon cancer [47].
In our study, the correlation between ICAM-1 and VCAM-1 serum levels in patients with PC with the clinical and pathological parameters revealed mostly no significant findings, which confirms the results of recently published studies. Techezy et al. [46] evaluated the impact of elevated ALCAM levels on patients with PC, and no significant differences were found regarding sex, age, tumor stage, and tumor cell grading, but they do not analyze endocrine insufficiency. Similarly, Sakamoto et al. [47] evaluated the correlation between the plasma concentrations of PECAM-1 and clinical features including age, sex, tumor location, tumor size, TNM stage, lymph node metastasis, and distant metastasis in all the patients with PC. PECAM was significantly elevated only in the older age group of patients—above 60 years. The results of other studies suggested a potential role of adhesion molecules in cancer cell metastasis through its impact on cell migration, intracellular signaling and cell—matrix interaction [48, 49]. In recently published studies, identified adhesion molecules play important function in the induction of chemoresistance in vitro [50]. Additionally, overexpression of adhesion molecules has been previously shown to be a bad prognostic factor for pancreatic adenocarcinoma [48, 51]. Our study has showed elevation of ICAM in patients with jaundice and with lymph node involvement. Although we could not demonstrate the relation between distant metastases and ICAM/VCAM, the expression of these molecules has been previously shown to contribute to metastasis formation and aggressive growth behavior of PC [49].
An additional adhesion molecule, which we analyzed was E-selectin-1 (endothelial leukocyte adhesion molecule-1), is expressed by endothelial cells, which are activated by cytokines released during the inflammatory process and play an important role in neutrophil extravasation into injured tissues. This adhesion molecule interacts with carbohydrate ligands of cell surface and can bind to cell-surface carbohydrate glycoproteins located on several tumors also on pancreatic carcinoma cell lines. Furthermore, E-selectin is thought to play an important role in immunologic reactions. In our study, serum level of E-selectin and endothelin in patients with CP and PC was not different from the control group. Our results are in agreement with results reported by Tempia-Caleira et al. [52]. They demonstrated that many pancreatic adenocarcinomas do not show elevated expression of E-selectin.
In this study, we also found increased level of thrombomodulin in patients with PC compared with CP and controls patients. Results of published studies showed that expression of inhibitors of coagulation, including tissue factor pathway inhibitor, antithrombin III, heparin cofactor II, protein C, free protein S, and thrombomodulin, is decreased in patients with pancreatic carcinoma [53, 54]. Other authors similarly to our results report that circulating levels of thrombomodulin were elevated in patients with pancreatic carcinoma [55]. Future work is needed to establish the exact role of thrombomodulin in patients with PC.
In summary, our data indicate that patients with pancreatic carcinoma and CP express high levels of several cytokines compared with healthy individuals, especially adiponectin, TNF-α, and IL-6. Serum TNF-α and ICAM concentrations coordinately increase in advanced CP. Furthermore, especially in PC subjects, elevated markers of endothelial dysfunction are present. This study provides additional evidence that changes in inflammatory cytokine and adhesion molecules in PC and CP are not likely related to endocrine disorders.
Acknowledgments
No financial relationships with a commercial entity producing healthcare-related products and/or services relevant to this article.
Compliance with ethical standards
Conflict of interest
There were no financial relationships with a commercial entity producing health-care related products and/or services relevant to this article.
Contributor Information
A. Gasiorowska, Phone: +48 42 6786480, Email: anita@sofcom.pl
R. Talar-Wojnarowska, Email: renata.talar-wojnarowska@umed.lodz.pl
A. Kaczka, Email: akaczka@wp.pl
A. Borkowska, Email: anna.borkowska@umed.lodz.pl
L. Czupryniak, Email: bigosik@poczta.onet.pl
E. Małecka-Panas, Email: ewuncia@poczta.onet.pl
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kate M, Hofland LJ, Koetsveld PM, Jeekel J, Eijck CJ. Pro-inflammatory cytokines affect pancreatic carcinoma cell. Endothelial Cell Interact J Pancreas. 2006;7:454–464. [PubMed] [Google Scholar]
- 3.Emmrich J, Weber I, Nausch M, et al. Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion. 1998;59:192–198. doi: 10.1159/000007488. [DOI] [PubMed] [Google Scholar]
- 4.Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res. 2008;149:319–328. doi: 10.1016/j.jss.2007.12.757. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahimi B, Tucker SL, Donghui L, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma. Correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 6.van Grevenstein WM, Hofland LJ, Jeekel J, van Eijck CH. The expression of adhesion molecules and the influence of inflammatory cytokines on the adhesion of human pancreatic carcinoma cells to mesothelial monolayers. Pancreas. 2006;32:396–402. doi: 10.1097/01.mpa.0000220865.80034.2a. [DOI] [PubMed] [Google Scholar]
- 7.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–541. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 11.Gumbs AA. Obesity, pancreatitis, and pancreatic cancer. Obes Surg. 2008;18:1183–1187. doi: 10.1007/s11695-008-9599-3. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 13.Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003;169:203–214. doi: 10.1016/S0021-9150(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 14.Dima SO, Tanase C, Albulescu R, et al. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas. 2012;41:1001–1007. doi: 10.1097/MPA.0b013e3182546e13. [DOI] [PubMed] [Google Scholar]
- 15.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 16.Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa M, Kitayama J, Kazama S, et al. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–472. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 18.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 19.Chang MC, Chang YT, Su TC, et al. Adiponectin as a potential differential marker to distinguish pancreatic cancer and chronic pancreatitis. Pancreas. 2007;35:16–21. doi: 10.1097/MPA.0b013e3180547709. [DOI] [PubMed] [Google Scholar]
- 20.Dalamaga M, Migdalis I, Fargnoli JL, et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case–control study. Cancer Causes Control. 2009;20:625–633. doi: 10.1007/s10552-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao Y, Giovannucci EL, Kraft P, et al. Inflammatory plasma markers and pancreatic cancer risk: a prospective study of five U.S. cohorts. Cancer Epidemiol Biomark Prev. 2013;22:855–861. doi: 10.1158/1055-9965.EPI-12-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grote VA, Rohrmann S, Dossus L, et al. The association of circulating adiponectin levels with pancreatic cancer risk: a study within the prospective EPIC cohort. Int. J. Cancer. 2012;130:2428–2437. doi: 10.1002/ijc.26244. [DOI] [PubMed] [Google Scholar]
- 23.Krechler T, Zeman M, Vecka M, et al. Leptin and adiponectin in pancreatic cancer: connection with diabetes mellitus. Neoplasma. 2011;1:58–64. doi: 10.4149/neo_2011_01_58. [DOI] [PubMed] [Google Scholar]
- 24.Goldfine AB, Kahn CR. Adiponectin: linking the fat cell to insulin sensitivity. Lancet. 2003;362:1431–1432. doi: 10.1016/S0140-6736(03)14727-7. [DOI] [PubMed] [Google Scholar]
- 25.Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci. 2008;114:275–288. doi: 10.1042/CS20070196. [DOI] [PubMed] [Google Scholar]
- 26.Pezzilli R, Barassi A, Corsi MM, et al. Serum leptin, but not adiponectin and receptor for advanced glycation end products, is able to distinguish autoimmune pancreatitis from both chronic pancreatitis and pancreatic neoplasms. Scand J Gastroenterol. 2010;45:93–99. doi: 10.3109/00365520903358907. [DOI] [PubMed] [Google Scholar]
- 27.Nikiteas N, Tzanakis N, Gazouli M, et al. Serum Il-6, TN F alpha and CRP levels in Greek colorectal cancer patients: prognosis implications. World J Gastroenterol. 2005;11:1639–1643. doi: 10.3748/wjg.v11.i11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pftizenmaier J, Vessella R, Higano CS, et al. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97:1211–1216. doi: 10.1002/cncr.11178. [DOI] [PubMed] [Google Scholar]
- 29.Błogowski W, Deskur A, Budkowska M, et al. Selected cytokines in patients with pancreatic cancer: a preliminary report. PLoS One. 2014;9:e97613. doi: 10.1371/journal.pone.0097613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talar-Wojnarowska R, Gasiorowska A, Smolarz B, Romanowicz-Makowska H, Kulig A, Małecka-Panas E. Tumor necrosis factor α and interferon γ genes polymorphisms and serum levels in pancreatic adenocarcinoma. Neoplasma. 2009;1:56–62. doi: 10.4149/neo_2009_01_56. [DOI] [PubMed] [Google Scholar]
- 31.Okada S, Okusaka T, Ishii H, et al. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–15. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- 32.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. 2001;21:1355–1358. [PubMed] [Google Scholar]
- 34.Bemelmans MA, Gouma DJ, Greve JW, Burman WA. Cytokines, tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology. 1992;15:1132–1136. doi: 10.1002/hep.1840150626. [DOI] [PubMed] [Google Scholar]
- 35.Bossola M, Muscaritoli M, Bellantone R, et al. Serum tumour necrosis factor-alpha levels in cancer patients are discontinuous and correlate with weight loss. Eur J Clin Invest. 2000;30:1107–1112. doi: 10.1046/j.1365-2362.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- 36.Cardellini M, Andreozzi F, Laratta E, et al. Plasma interleukin-6 levels are increased in subjects with impaired glucose tolerance but not in those with impaired fasting glucose in a cohort of Italian Caucasians. Diabetes Metab Res Rev. 2007;23:141–145. doi: 10.1002/dmrr.679. [DOI] [PubMed] [Google Scholar]
- 37.Teros T, Hakala R, Ylinen L, et al. Cytokine balance and lipid antigen presentation in the NOD mouse pancreas during development of insulitis. Pancreas. 2000;20:191–196. doi: 10.1097/00006676-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Dogan Y, Akarsu S, Ustundag B, Yilmaz E, Gurgoze MK. Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediat Inflamm. 2006;1:1–6. doi: 10.1155/MI/2006/59206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller S, Martin S, Koenig W, et al. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute phase proteins but not TNF-α or its receptors. Diabetologia. 2002;45:805–812. doi: 10.1007/s00125-002-0829-2. [DOI] [PubMed] [Google Scholar]
- 40.Hansen M, Nielsen AR, Vilsbøll T, et al. Increased levels of YKL-40 and interleukin 6 in patients with chronic pancreatitis and secondary diabetes. Pancreas. 2012;41:1316–1318. doi: 10.1097/MPA.0b013e31824d9b93. [DOI] [PubMed] [Google Scholar]
- 41.Shah N, Siriwardena AK. Cytokine profiles in patients receiving antioxidant therapy within the ANTICIPATE trial. World J Gastroenterol. 2013;19:4001–4006. doi: 10.3748/wjg.v19.i25.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuehn R, Lelkes PI, Bloechle C, Niendorf A, Izbicki R. Angiogenesis, angiogenic growth factors, and cell adhesion molecules are upregulated in chronic pancreatic diseases: angiogenesis in chronic pancreatitis and in pancreatic cancer. Pancreas. 1999;1:96–103. doi: 10.1097/00006676-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Ali S, Kaur J, Patel KD. Intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and regulated on activation normal T cell expressed and secreted are expressed by human breast carcinoma cells and support eosinophil adhesion and activation. Am J Pathol. 2000;157:313–321. doi: 10.1016/S0002-9440(10)64542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang WW, Stelter AA, French S, et al. Loss of cell-adhesion molecule complexes in solid pseudopapillary tumor of pancreas. Modern Pathology. 2007;20:509–513. doi: 10.1038/modpathol.3800764. [DOI] [PubMed] [Google Scholar]
- 45.Markocka-Maczka K. Concentration of serum soluble forms of ICAM-1 (sVCAM-1) and VCAM-1 (sVCAM-1) in patients with chronic pancreatitis and in patients with pancreatic carcinoma. Wiad Lek. 2003;56:147–151. [PubMed] [Google Scholar]
- 46.Tachezy M, Zander H, Marx AH, et al. ALCAM (CD166) Expression and serum levels in pancreatic cancer. PlosOne. 2012;6:e39018. doi: 10.1371/journal.pone.0039018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakamoto H, Kimura H, Sekijima M, et al. Plasma concentrations of angiogenesis-related molecules in patients with pancreatic cancer. Jpn J Clin Oncol. 2012;42:105–112. doi: 10.1093/jjco/hyr178. [DOI] [PubMed] [Google Scholar]
- 48.Torer N, Kayaselcuk F, Nursal TZ, et al. Adhesion molecules as prognostic markers in pancreatic adenocarcinoma. J Surg Oncol. 2007;96:419–423. doi: 10.1002/jso.20654. [DOI] [PubMed] [Google Scholar]
- 49.Tempia-Caliera AA, Horvath LZ, Zimmermann A, et al. Adhesion molecules in human pancreatic cancer. J Surg Oncol. 2002;79:93–100. doi: 10.1002/jso.10053. [DOI] [PubMed] [Google Scholar]
- 50.Hong X, Michalski CW, Kong B, et al. ALCAM is associated with chemoresistance and tumor cell adhesion in pancreatic cancer. J Surg Oncol. 2010;101:564–569. doi: 10.1002/jso.21538. [DOI] [PubMed] [Google Scholar]
- 51.Li YJ, Ji XR. Relationship between expression of E-cadherin-catenin complex and clinicopathologic characteristics of pancreatic cancer. World J Gastroenterol. 2003;9:368–372. doi: 10.3748/wjg.v9.i2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawada T, Ho JJ, Chung YS, Sowa M, Kim YS. E-selectin binding by pancreatic tumor cells is inhibited by cancer sera. Int J Cancer. 1994;15:901–907. doi: 10.1002/ijc.2910570621. [DOI] [PubMed] [Google Scholar]
- 53.Nakchbandi IA, Lohr JM. Coagulation, anticoagulation and pancreatic carcinoma. Nat Clin Pract Gastrol Hepatol. 2008;8:445–455. doi: 10.1038/ncpgasthep1184. [DOI] [PubMed] [Google Scholar]
- 54.Nitori N, Ino Y, Nakanishi Y, et al. Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2005;11:2531–2539. doi: 10.1158/1078-0432.CCR-04-0866. [DOI] [PubMed] [Google Scholar]
- 55.Lindahl AK, Boffa MC, Abildqaard U. Increased plasma thrombomodulin in cancer patients. Thromb Haemost. 1993;69:112–114. [PubMed] [Google Scholar]