Abstract
Leishmaniasis is a neglected public health problem caused by the protozoan species belonging to the genus Leishmania affecting mostly the poor populations of developing countries. The causative organism is transmitted by female sandflies. Cutaneous, mucocutaneous, and visceral clinical manifestations are the most frequent forms of leishmaniasis. Chemotherapy still relies on the use of pentavalent antimonials, amphotericin B, paromomycin, miltefosin and liposomal amphotericin B. However, the application of these drugs is limited due to low efficacy, life-threatening side effects, high toxicity, induction of parasite resistance, length of treatment and high cost. Given the fact that antileishmanial vaccines may not become available in the near future, the search for better drugs should be continued. Natural products may offer an unlimited source of chemical diversity to identify new drug modules. New medicines should be less toxic or non-toxic, safe, more efficient, less expensive and readily available antileishmanial agents, especially for low-income populations. In the present review, special focus is on medicinal plants used against leishmanaiasis. The bioactive phytocompounds present in the plant derivatives including the crude extracts, essential oils, and other useful compounds can be a good source for discovering and producing new antileishmanial medicines.
Key Words: Leishmaniasis, Treatment, Pentavalent antimonials, Plant extracts
Introduction
Some parasitic diseases including toxoplasmosis, Chagas disease, giardiasis, babesiosis and leishmaniasis occur in animals and can transmit to human beings (Rahbari et al., 2008 ▶; Heidarpour Bami et al., 2010 ▶; Mosallanejad et al., 2011 ▶; Esch and Petersen, 2013 ▶). Leishmaniasis is located in the ninth place of the global burden of disease among individual infectious diseases. This disease is regarded as a protozoan zoonotic disease affecting approximately 12 million people in 88 (Hosseininejad et al., 2012 ▶; Sarkar et al., 2013 ▶; Machado et al., 2014 ▶; Mohapatra, 2014 ▶) or 98 countries of the world, according to various resources (Calla-Magarinos et al., 2013 ▶; Chandrasekaran et al., 2013 ▶; Rodrigues et al., 2014 ▶) with 2 million new cases annually (1.5 million cutaneous and 0.5 million visceral leishmaniasis) and mortality rate of 60,000 (Mohapatra, 2014 ▶; Salehabadi et al., 2014 ▶). Leishmaniasis is caused by various species of the protozoa of the genus Leishmania (Table 1) (WHO, 2010 ▶; Hatam et al., 2013 ▶; Salehabadi et al., 2014 ▶). Cutaneous leishmaniasis (CL) is the most common form of the disease, among other forms such as visceral (VL) and mucocutaneous (MCL) leishmaniasis. With the advent of the human immunodeficiency virus (HIV), leishmaniasis is rapidly becoming an opportunistic infection in AIDS patients (Diro et al., 2014 ▶). On the other hand, an ideal vaccine is not available to control the disease. The standard drugs for leishmaniasis are pentavalent antimonials including sodium stibogluconate (Pentostam®) and meglumine antimoniate (Glucantime) (Diro et al., 2014 ▶; Jabini et al., 2014 ▶). However, serious side effects including arthralgias, myalgias, leukopenia, pancreatitis, liver problems, cardiotoxicity and cardiac arrhythmia in the patients, prolonged treatment time, and increased parasite resistance (Chakravarty and Sundar, 2010 ▶; Lage et al., 2013 ▶; Diro et al., 2014 ▶). Therefore, alternative drugs to the antimonials such as amphotericine B, pentamidine, paromomycin, and miltefosine have been recommended, but they also come with some side effects such as toxicity, high cost, drug resistance and therapeutic failure (Machado et al., 2012 ▶; Wiwanitkit, 2012 ▶; Lage et al., 2013 ▶). Given the above mentioned reasons, development of new, less toxic and more cost-effective drugs with greater efficacy as well as more accessible alternative therapeutic strategies which could become available for low-income populations to treat the disease has become a necessity (Lage et al., 2013 ▶). Since victims of leishmaniasis are generally poor, lengthy treatment using expensive drugs with related costs is far beyond the means of such families (Oryan et al., 2014 ▶). Therefore, many patients seek for herbal therapy which is cheaper and readily available. However, most of the herbs have still not been evaluated scientifically. In recent years, there has been growing interest in alternative natural products and plant compounds for the treatment of leishmaniasis (Alviano et al., 2012 ▶; Garcia et al., 2012 ▶; Ogeto et al., 2013 ▶; Chouhan et al., 2014 ▶). Anti-leishmanial activity of some plants has been attributed to the presence of the compounds such as alkaloids, chalcones, triterpenoids, naphthoquinones, quinones, terpenes, steroids, lignans, saponins, and flavonoids (Lage et al., 2013 ▶; Sifaoui et al., 2014 ▶). Essential oils and extracts of a large number of plants have been shown to be effective against different species of Leishmania (Rodrigues et al., 2013 ▶; Monzote et al., 2014a ▶). This review attempts to provide information regarding Leishmania and the resultant disease, some information about the available drugs, and recent developments in the phytosciences to treat leishmaniasis. Indeed, the current study mostly highlights a range of plant formulations exhibiting leishmanicidal activity.
Table 1.
Leishmania species causing human disease, reservoir hosts and vectors
| Leishmania species | Infection | Geographical location | Reservoir | Vector |
|---|---|---|---|---|
| L. donovani | VL, PKDL | Old world, expect Europe | Humans, rodents | Phlebotomus argentipes, P. orinntalis, P. martini |
| L. infantum | VL, CL, ML | New world for VL, old world for CL and ML | Rodents, dogs, foxes, jackals | P. pcrniciosufi, P. arias, Lutzomyia longipalpis |
| L. chagasi | VL | New world | Foxes, dogs, opossums | L. longipalpis |
| L. major | CL, ML | Old world | Rodents, gerbils | P. papatasi, P. duboscqi |
| L. tropica | CL, ML, VL | Old world | Humans | P. sergenti |
| L. aethiopica | CL, DCL, MCL | Old world | Hyraxes | P. longipes, P. pedifer |
| L. mexicana | CL, DCL, MCL | New world | Forest rodents | L. olmeca |
| L. amazonensis | CL, DCL | New world | Forest rodents | L. flaviscutellata |
| L. braziliensis | CL, MCL | New world | Forest rodents, peridomestic anteaters | Lutzomyia spp. |
| L. guyanemis | CL, MCL | New world | Sloths aboreal anteaters | L. umbratilis |
| L. panamensis | CL, MCL | New world | Sloths | L. trapidoictal |
| L. peruviana | MCL | New world | Dogs | L. verrucarurn, L. pvmenis |
VL: Visceral leishmaniasis; PKDL: Post-kala azar dermal leishmaniasis; P: Phlebotomus; ML: Mucosal leishmaniasis; CL: Cutaneous leishmaniasis; L: Lutzomyia; DCL: Diffuse cutaneous leishmaniasis; MCL: Mucocutaneous leishmaniasis
Main forms of leishmaniasis
Leishmaniasis is the most common neglected tropical disease (NTD) in countries with poor socioeconomic conditions (Neris et al., 2013 ▶; Oryan et al., 2014 ▶). It is a major public health concern in the tropical and subtropical countries throughout the old (Africa, Asia, Southern Europe) and new world (Latin America) (Daneshbod et al., 2011 ▶; de Medeiros et al., 2011 ▶; Monzote, 2011 ▶). Leishmaniasis is characterized by a spectrum of clinical manifestations ranging from ulcerative skin lesions in the site of the sandfly bite (CL), to the more progressive MCL and VL (Neris et al., 2013 ▶; Sachdeva et al., 2014 ▶) depending on the parasite species, the host-parasite interaction, the occurrence of co-infections, and the immunological status of the host (de Medeiros et al., 2011 ▶). Generally, the proven vectors are species of sandflies of the genus Phlebotomus and Lutzomyia in the new world and the old world, respectively (Nekouie et al., 2006 ▶; Parvizi and Amirkhani, 2008; Alidadi and Oryan, 2014). The most common form of leishmaniasis is CL or oriental sore which is caused by many Leishmania species such as L. major, L. tropica, and L. aethiopica in the old world and L. mexicana, L. venezuelensis, L. amazonensis, L. braziliensis, L. panamensis, L. guyanensis and L. peruviana in the new world (Table 1) (Oryan et al., 2008 ▶; WHO, 2010 ▶; Alavi-Naini et al., 2012 ▶; Oryan et al., 2013a ▶, b ▶). CL is presented as papules, crusted nodules, plaques, or ulcerative nodular lesions on the face, hands, and/or feet which self-heal within a few months but may leave scars (Asgari et al., 2007 ▶; Oryan et al., 2007 ▶; Shirian et al., 2014 ▶). Diffuse cutaneous leishmaniasis (DCL) is regarded as a chronic, progressive variant of CL that is manifested by disseminated non-ulcerative skin lesions (Alidadi and Oryan, 2014 ▶). The etiologic agent associated with DCL is L. aethiopica and L. amazonensis in the old and new world, respectively (Alviano et al., 2012 ▶; Lindoso et al., 2012 ▶). Mucosal leismaniasis or MCL leads to extensive destruction of naso-oral and pharyngeal or laryngeal regions causing difficulty in eating and increases the risk of the secondary infections (Daneshbod et al., 2011 ▶; Alviano et al., 2012 ▶; Shirian et al., 2012a ▶; Oryan et al., 2013a ▶). Its main causative organism is L. aethiopica in the old world and L. braziliensis, L. Mexicana, L. amazonensis and L. panamensis in the new world. In addition, species such as L. infantum, L. major and L. tropica have been isolated from some cases of mucosal leishmaniasis (Shirian et al., 2012a ▶, b ▶). This form is transmitted by the forest sandfly Lutzomyia spp. and its reservoir hosts include rodents and dogs (Shirian et al., 2012b ▶; Shirian et al., 2013 ▶).
VL, also called kala-azar, is the most severe amongst various forms of leishmaniasis (Wiwanitkit, 2012 ▶; Diro et al., 2014 ▶). VL is mostly caused by L. donovani, L. infantum, L. chagasi and occasionally L. tropica multiplying in the reticuloendothelial system (WHO, 2010 ▶; Hosseininejad et al., 2012 ▶; Khan et al., 2014 ▶). The disease varies from an asymptomatic infection to a fatal condition (Diro et al., 2014 ▶). VL is characterized by prolonged fever, weakness, fatigue, severe cachexia and, with advancement of the disease, by the enlargement of spleen and liver, termed hepatomegaly and splenomegaly (hepatosplenomegaly), progressive anemia, and sometimes bleeding and blackening of the skin that gives the disease its common name (Prajapati et al., 2011b ▶; Alviano et al., 2012 ▶; Jain and Jain, 2013 ▶; Sachdeva et al., 2014a ▶). This disease is usually lethal due to the secondary infections, severe anemia and organ failure if left it untreated (Alviano et al., 2012 ▶). Dogs are considered as the main reservoir hosts for this form of the disease (Hosseininejad et al., 2012 ▶). Post kala-azar dermal leishmaniasis (PKDL) is characterized by the appearance of non-ulcerative cutaneous lesions containing the parasites in the period after successful treatment of VL and recovery from it (Lindoso et al., 2012 ▶; Oryan et al., 2014 ▶).
Life cycle of Leishmania
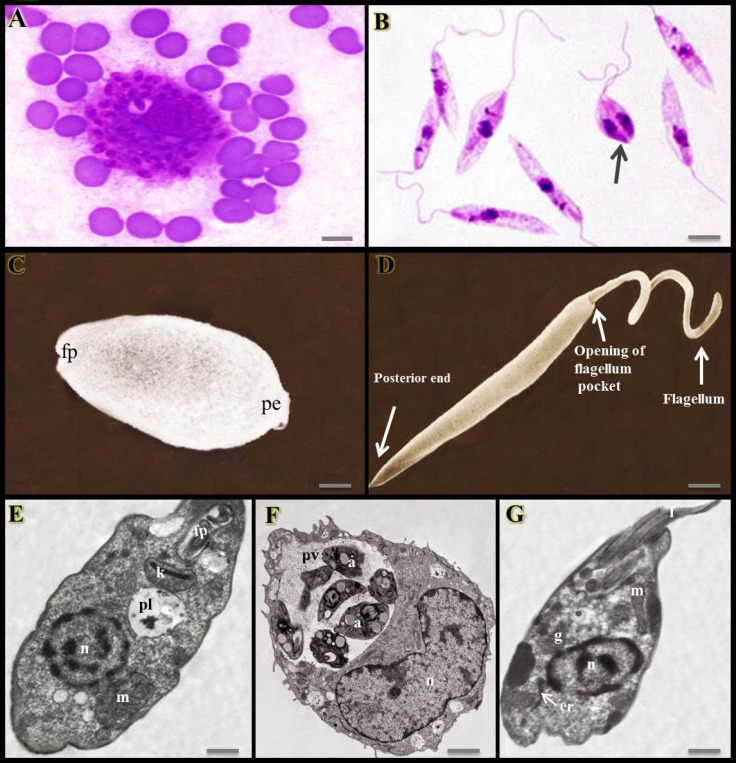
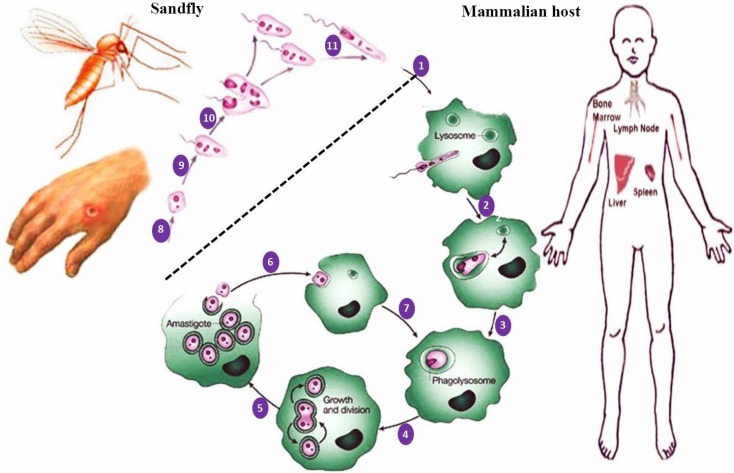
Leishmania is found in two forms or stages (Figs. 1A-G): amastigotes which are intracellular, spherical, without flagellum or with an internalized flagellum, non-motile and reside and multiply within the phagolysosomes of phagocytic cells such as macrophages of the vertebrate host. The other form, termed promastigotes that present inside the sandflies are extracellular, elongated, spindle-shape, flagellated and motile (Mishra et al., 2009 ▶; Monzote, 2011 ▶; Alviano et al., 2012; Esch and Petersen, 2013; Mohapatra, 2014). The life cycle of Leishmania takes place in two hosts, the mammalians and sandflies (Fig. 2) (Motazedian et al., 2006 ▶). The adult female blood-sucking sandfly bites an individual infected with Leishmania and thereby the parasite is ingested within the host’s blood (Motazedian et al., 2006 ▶; Parvizi and Amirkhani, 2008 ▶). Inside the gut of the insect, the amastigotes are transformed into promastigote form. They live extracellularly in the alimentary tract and then migrate to the saliva of the sandfly, until it releases promastigotes from its proboscis into the bite site via biting the next person. Inside the human host, the promastigotes invade macrophages which transform back into the amastigote form (Motazedian et al., 2006 ▶; Alviano et al., 2012 ▶; Aslan et al., 2013 ▶; Esch and Petersen, 2013 ▶). The amastigotes multiply inside the macrophages by binary fission and may lyse the cell and spread within the host. The parasite mainly affects the reticuloendothelial system including spleen, liver, bone marrow and lymphoid tissues (Mishra et al., 2009 ▶; Alviano et al., 2012 ▶; Esch and Petersen, 2013 ▶; Mohapatra, 2014 ▶).
Fig. 1.
Two stages of Leishmania parasite. (A) Intracellular and non-motile stage called amastigote within the macrophages, (B) Extracellular and motile form called promastigote possessing a flagellum. The promastigotes multiply by binary division (arrow), giemsa stain (×1,000). Scanning electron micrograph of (C) leishmanial amastigote (fp: Flagellum pocket, and pe: Posterior end) and (D) promastigote, (scale bars = 1 µm). (E) Transmission electron micrograph of a mastigote. The flagellum is completely enclosed within the flagellar pocket. n: Nucleus, m: Mitochondrion, pl: Phagolysosome, k: Kinetoplast, and fp: Flagellar pocket. (F) A macrophage containing large parasitophorous vacuole filled with leishamnial amastigotes. n: Nucleus of macrophage, a: Amastigotes, and pv: Parasitophorous or parasite-containing vacuole. (G) TEM of a promastigote showing different organelles. er: Endoplasmic reticulum, n: Nucleus, g: Golgy apparatus, m: Mitochondrion, and f: Flagellum, (scale bars = 15 µm) (Asaln et al., 2013; Hatam et al., 2013
Fig. 2.
Leishmanial infection in sandfly and mammalian hosts. (1) Delivery of promastigotes into human skin by the biting sandfly and attachment of promastigotes to a macrophage by phagocytosis; (2) entry of promastigote into the phagosome of macrophage; (3) fusion of the phagosome containing a promastigote with lysosomes in an infected macrophage and production of phagolysosome; (4) differentiation of promastigote into amastigote within the phagolysosome of the macrophage and then multiplication of amastigotes by binary division; (5) continuing proliferation of amastigotes and rupture of heavily infected macrophages and release of amastigotes; (6) either phagocytosis of the released amastigotes by new macrophages and (7) phagocytosis of amastigotes and formation of phagolysosome and the cycle continues; (8) ingestion of macrophages containing amastigotes by sandfly following a blood meal taken from the infected and/or reservoir host; (9-11) development of promastigotes in the sandfly vector (transformation of amastigotes into promastigotes in sandfly’s midgut; division of promastigotes in midgut and migration to proboscis of sandfly; taking a blood meal from the host by sandfly; and then repetition of the cycle (1)
Diagnosis of leishmaniasis
Laboratory diagnosis of the disease is achieved by the demonstration of the parasites in amastigote form, in materials obtained from the patient including skin lesions in the CL cases, and in the spleen (as the most reliable sample), bone marrow and lymph node aspirates in the cases with VL (Khan et al., 2014 ▶). The smears are stained with giemsa/leishman stains for the presence of amastigotes inside the macrophages. The aspirates can be inoculated into BALB/c mice which are genetically susceptible to Leishmania, and, as a result, they will become infected over time (Khan et al., 2014 ▶; Shirian et al., 2014 ▶; Yamamoto et al., 2014 ▶). For this purpose, the use of methods such as splenic puncture, aspiration of bone marrow, fine-needle biopsy of lymph nodes and culture is helpful in the cases of VL (Alidadi and Oryan, 2014 ▶; Khan et al., 2014 ▶). However, these procedures are risky and invasive. There are several serological tests including direct agglutination test, complement fixation tests, enzyme-linked immunosorbent assay (ELISA), and indirect immunofluorescent antibody test (Daneshbod et al., 2011 ▶; Khan et al., 2014 ▶; Shirian et al., 2012b ▶). However, they are unable to distinguish past and current infections. Moreover, cross-reaction of antibodies against Leishmania may occur with those of other diseases including trypanosomiasis and toxoplamosis (Mehrabani et al., 2011 ▶; Shirian et al., 2014 ▶). Other methodologies include cytology and culture of the aspirates, histopathologic examination and immunohistochemistry (Mehrabani et al., 2007 ▶, 2011; Daneshbod et al., 2011 ▶; Khan et al., 2014 ▶; Shirian et al., 2014 ▶). Molecular assays such as polymerase chain reaction (PCR) assay could be applied for diagnosis of the parasite in the tissues and organs of the patients and also in the blood of asymptomatic healthy persons living in the endemic regions who may serve as parasite reservoirs, sometimes with 100% sensitivity (Abbasi et al., 2013 ▶; Shirian et al., 2014; Sudarshan et al., 2014 ▶).
Control strategies for leishmaniasis
To date, there is no suitable vaccine against Leishmania. However, several vaccine preparations are in more or less advanced stages of testing (Bacon et al., 2013 ▶; Mutiso et al., 2013 ▶). The vaccines evaluated so far include live, live-attenuated (containing the parasites which are infectious but not pathogenic) and killed (with both prophylactic and therapeutic purposes) (Bacon et al., 2013 ▶; Mutiso et al., 2013 ▶; Alidadi and Oryan, 2014 ▶). In addition to these, there are further vaccines such as fractionated/subunit, naked DNA (cloning of the gene encoding the vaccine candidate in a vector with the injection of DNA), recombinant and synthetic (containing the recombinant DNA-derived antigens and peptides), non-protein antigens (such as Leishmania lipophosphoglycin), anti-sandfly saliva components (indirect enhancement anti-parasite immunity) and adjuvants for augmenting the effect of vaccination (Bacon et al., 2013 ▶; Mutiso et al., 2013 ▶). Efficient management of the disease is based on early diagnosis and treatment to lower morbidity and prevent mortality with an efficient vaccination, if that is possible (Alidadi and Oryan, 2014 ▶). On the other hand, disease control and prevention is achieved by identification of risk factors of the disease. For this purpose, control of vector and reservoir hosts through the use of insecticides and impregnated bednets can be useful (Votypka et al., 2012 ▶; Coura-Vital et al., 2013 ▶; Diro et al., 2014 ▶). An emerging and extremely serious problem in the field of VL control is its co-infection with HIV. In these conditions, infection with HIV increases the risk of progression of asymptomatic leishmanial infection to clinical VL and vice versa (Alidadi and Oryan, 2014 ▶; Diro et al., 2014 ▶). Immunosuppression, malnutrition, poverty, and generally any factor exposing people to infected sandflies and reservoir hosts may increase the probability of the infection and disease (Votypka et al., 2012 ▶; Coura-Vital et al., 2013 ▶; Alidadi and Oryan, 2014 ▶).
Current chemotherapy for leishmaniasis
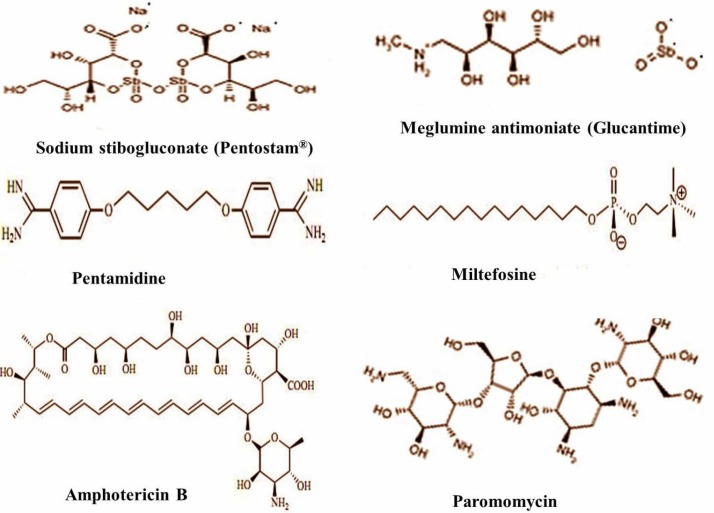
The drug used for treatment of leishmaniasis should be selected based on several factors such as patient characteristics, pharmacogenetical characteristics of the drug, causative Leishmania species, risk factors and geographical area (Lindoso et al., 2012 ▶; Sarkar et al., 2013 ▶; Fernandez et al., 2014 ▶). An ideal drug for treatment of leishmaniasis should provide several requirements for patients including being effective in one or few doses, low cost, not be teratogenic, no side effects, no need for hospitalization, and no induction of resistance (Alviano et al., 2012 ▶). Chemotherapy based on available drugs that are not highly effective and cause serious effects, remains the only treatment available for this disease (Seifert, 2011 ▶; Alviano et al., 2012 ▶). Since the 1950’s ago, pentavalent antimony compounds have been proposed as the first-line drugs for treatment of leishmaniasis at dose of 20-28 mg/kg/day for 28-30 days, intravenously (IV) or intramuscularly (IM) (Jain and Jain, 2013 ▶). The drugs currently used for treatment of leishmaniasis are still based on pentavalent antimonials which are available in two formulations, sodium stibugluconate (SSG, Pentostam®, C12H38O26Sb) and meglumine antimoniate (Glucantime®, C14H29O10N2Sb), that target the amastigotes of both CL and VL (Alviano et al., 2012 ▶; Dastgheib et al., 2012 ▶; Lindoso et al., 2012 ▶; Jabini et al., 2014 ▶; Mohapatra, 2014 ▶; Ribeiro et al., 2014 ▶). The antimonials mechanism of action is not still clear. However, these drugs inhibit the activity of the glycolytic and oxidative pathways of the fatty acids for the reduction of ATP in the amastigotes (Lindoso et al., 2012 ▶). In addition, it has been stated that the pentavalent form utilizes thiols from the parasite and the cell surface of the host and is reduced into the trivalent which is a more active and toxic form inside the macrophages (Alviano et al., 2012 ▶; Lindoso et al., 2012 ▶; Mohapatra, 2014 ▶). Thiol metabolism and its high intracellular levels play prominent roles in developing antimonial resistance. Thiol molecule increases the oxidative stress within the macrophages preventing antioxidant formation and reduction of pentavalent antimonials to trivalent form (Jain and Jain, 2013 ▶; Mohapatra, 2014 ▶). Inhibition of fatty acid oxidation and induction of apoptosis and also induction of the DNA topoisomerase enzyme are other mentioned mechanisms (Alviano et al., 2012 ▶). Nevertheless, pentavalent antimonials have drawbacks and side effects including anorexia, vomiting, dizziness, arthralgia, myalgia, fever, parenteral administration, high cost and long course of treatment (Fernandez et al., 2014 ▶; Gamboa-Leon et al., 2014 ▶). These drugs have limitations for pregnant women, the elderly, and individuals with cardiac, renal and hepatic diseases due to toxicity for these organs and pancreas (Neves et al., 2011 ▶; Sen and Chatterjee, 2011 ▶; Lindoso et al., 2012 ▶). Moreover, increased parasite resistance and treatment failures associated with the use of these compounds in leishmaniasis patients have been reported (Chakravarty and Sundar, 2010 ▶; Valadares et al., 2012; Jain and Jain, 2013 ▶). Despite the side effects of pentavalent antimonials and the prolonged need for daily parenteral injection (daily IM or IV injection), they are used for a long time (Salehabadi et al., 2014 ▶). When the presence of restrictions or treatment failure with these drugs occur, alternative drugs named the second-choice drugs should be used. The alternative drugs to pentavalent antimony compounds include amphotericin B, pentamidine and paromomycin (Prajapati et al., 2011a ▶, b ▶; Valadares et al., 2012; Wiwanitkit, 2012 ▶). The two-dimensional chemical structure related to the available chemotherapeutic agents against leishmanisis is presented in Fig. 3.
Fig. 3.
Two-dimensional structure of drugs most commonly used against leishmaniasis
Amphotericin B (fungizone, C47H73NO17, AmbB), a polyene antifungal agent, is extracted from the filamentous bacteria called Streptomyces nodusus. This compound is administered as the second line drug for the treatment of patients with leishmaniasis and acts on amastigotes and also promastigotes, at dose of 0.75-1 mg/kg for 15 days, IM (Lindoso et al., 2012 ▶; Jain and Jain, 2013 ▶). It exists in four forms namely, deoxycholate amphotericin B, amphotericin colloidal dispersion (Amphocil), amphotericin B lipid complex (Abeclet) and liposomal amphotericin B (AmBisome) (Monzote, 2011 ▶; Mohapatra, 2014 ▶). AmpB tends to and targets sterols such as ergosterol and cholesterol which are important for parasite membrane constitution. It binds to cholesterol and forms pores in the cell membrane of the parasite leading to cell death due to increased permeability and the leakage of cellular content. Moreover, it may lead to generation of oxygen free radicals causing damage to cell, followed by cell death (Jain and Jain, 2013 ▶; Mohapatra, 2014 ▶). Despite the therapeutic property of AmpB, its resistance may occur due to high frequency of its use, but by the unknown mechanism. It is believed that a molecular mechanism may be involved in the development of the drug resistance (Mohapatra, 2014 ▶). Deoxycholate amphotericin B is associated with more severe effects including nausea, vomiting, fever, anemia, hapokalemia, cardiotoxicity and nephrotoxicity (Lindoso et al., 2012 ▶; Neris et al., 2013 ▶; Gamboa-Leon et al., 2014 ▶). Liposomal amphotericin B is less toxic and better tolerated by the patients in comparison with conventional AmpB, but it is very expensive, exhibits short circulating half-life and reaches higher concentrations in the liver and spleen (Lindoso et al., 2012 ▶; Lage et al., 2013 ▶). Moreover, leishmaniasis has emerged as an opportunistic infection in human immunodeficiency virus-infected patients, making the treatment even more challenging (Bhattacharya et al., 2013 ▶). Given the reports regarding high rates of toxicity of antimonials, AmpB has been introduced as the preferential first line treatment for VL patients co-infected with HIV (Lage et al., 2013 ▶; Diro et al., 2014 ▶). Paromomycin (PM, Humatin, paromomycin sulfate, C23H47O18S) is a broad spectrum antibiotic belonging to the aminoglycoside group extracted from Streptomyces rimosus variety paromomycinus (Lindoso et al., 2012 ▶; Wiwanitkit, 2012 ▶; Mohapatra, 2014 ▶). PM and imiquimod can be applied topically for the treatment of localized CL (Tiuman et al., 2012 ▶). The drug is administrated intramuscularly at dose of 15 mg/kg for 21 days (Jain and Jain, 2013). This drug exerts these effects by inferring with ribosomes and mitochondrial membrane and inhibiting protein synthesis and respiration (Lindoso et al., 2012 ▶; Wiwanitkit, 2012 ▶). To treat CL, three ointments of PM have been developed as follows: PM 15% methylbenzethonium chloride, 12%, PM 15% plus urea 10% and PM with gentamycin 0.5% (Monzote, 2011 ▶). The main side effects of this drug include hepatotoxicity, otoxicity and local pain at the injection site (Lindoso et al., 2012 ▶; Sachdeva et al., 2013 ▶). Nonetheless, low cost, negligible side effects, good efficacy and short duration of administration all have made this drug a candidate for the first line therapy for leishmaniasis patients (Mohapatra, 2014 ▶). Because of the possibility of resistance to monotherapy with PM, newer formulations such as PM loaded with albumin microsphere and PM with liposome have been provided for achieving better outcomes (Wiwanitkit, 2012 ▶). Pentamidine (pentamidine isethionate, C19H24N4O2), a type of dibenzamidine, interferes with the synthesis of Leishmania DNA and by affecting on the mitochondrial membrane leads to the parasite death. This drug is recommended at dose of 4 mg/kg, three times per week (Jain and Jain, 2013 ▶). Although pentamidine is administrated for all forms of leishmaniasis, low efficacy and toxicity have limited its application (Monzote, 2011 ▶). Its other side effects are hypotension, myalgia, hypoglycemia, diabetes mellitus and abscess formation at the injection site (Lindoso et al., 2012 ▶). Miltefosine (hexadecylphosphocholine, Impavido®), an anticancer drug, is orally administered as the second line treatment in VL and PKDL patients who fail to respond to antimonial drugs, at dose of 2.5 mg/kg/day for a total of 28 days (Shakya et al., 2011 ▶; Jain and Jain, 2013 ▶; Fernandez et al., 2014 ▶; Mohapatra, 2014 ▶). The mechanism of action of this drug is not well understood. But, it can bind to the Leishmania cell membrane, become internalized and finally cause cell death (Mohapatra, 2014 ▶). Nevertheless, because of misuse of the drug, its long half-life and probably inactivation of genes responsible for drug uptake are reasons for resistance to this drug. Additionally, failures in treatment and relapse have been observed in some cases treated with this compound (Alviano et al., 2012 ▶; Sachdeva et al., 2013 ▶; Fernandez et al., 2014 ▶). Also, adverse effects including toxicity to the gastrointestinal, hepatic, and renal systems have limited administration of miltefosine. In addition, it is teratogenic and so it should not be used in pregnant women (Lindoso et al., 2012 ▶). In addition, sitamaquine, an 8-amino-quinoline, is the second oral antiparasitic drug used in the treatment of leishmaniasis. It seems that this compound targets succinate dehydrogenase causing oxidative stress in Leishmania. Its application is limited due to hematological toxicity such as methemoglubinemia and hemolysis (Carvalho et al., 2011 ▶). Additionally, it has been demonstrated that cisplatin (cis-diaminedichloro-platinum (II), CDDP), an anti-neoplastic DNA-binding drug, exhibits anti-leishmanial activity in the in vitro tests (Sachdeva et al., 2013 ▶). However, this compound causes nephrotoxicity and hepatotoxicity through the generation of reactive oxygen species (ROS) and induction of lipid peroxidation due to decreased antioxidant defense system (Neris et al., 2013 ▶; Sachdeva et al., 2013 ▶; Sachdeva et al., 2014a ▶, b ▶). Further, two distinct classes of the antifungal drugs including imidazoles (ketoconazole) and triazoles (fluconazole and itraconazole) are used in leishmaniasis especially CL due to the less toxicity compared to pentavalent animonials and their oral administration (Alviano et al., 2012 ▶; Lindoso et al., 2012 ▶). The latter is of slower metabolism rate and less toxic than the former. A randomized clinical trial evaluated and compared the efficacy and tolerability of meglumine antimoniate, pentamidine and amphotericin B in treatment of CL due to L. (Viannia) guyanensis in 186 patients (Neves et al., 2011 ▶). No difference was observed among the groups in relation to sex, age and number or site of lesions. Several adverse effects were found in 40% of patients including 20.3% arthralgia for meglumine group, 35.1% pain and 10.8% induration at the injection site in the pentamidine group and 75.7% from the amphotericin B group refused to continue participating in the study. As none of the drugs mentioned above are free of side effects, the search for alternative therapeutic agents is essential in control and prevention of leishmaniasis (de Medeiros et al., 2011 ▶).
Combination therapy for leishmaniasis
Drug resistance related to monotherapy regimes were found in about 10 to 15% and 50 to 60% of CL and VL patients, respectively (Salehabadi et al., 2014 ▶). Combination therapy of anti-leishmanial drugs is currently considered as one of the most rational and promising approaches that has advantages such as reduced toxicity, synergic effects, limited drug resistance development, low treatment failure rate, and shorter treatment regimens (Gazanion et al., 2011 ▶; Monzote et al., 2011 ▶). For example, combined therapy of botulin derivative BT06 and the betulinic acid derivative AB13 with miltefosine via synergistic interactions has been considered as a promising compound in the field of new alternative therapy for leishmaniasis (Sousa et al., 2014 ▶). In addition, Jabini et al. (2014) ▶ suggested that silymarin in conjunction with glucantime, but not alone, can have promising effects on CL caused by L. major. Combination of the treatment with cryosurgery and an antileishmanial drug such as glucantime can be much more effective than each of the two modalities when used alone (Alavi-Naini et al., 2012 ▶). Nonetheless, two treatment protocol including combined oral azithromycin capsule 10 mg/kg/day and allopurinol, a drug with anti-leishmanial activity, tablet 10 mg/kg/day for two months and IM injection of glucantime 20 mg/kg for 20 days showed low efficacy in the treatment of old world CL. However, combination therapy had a slightly better outcome after a two-month period of follow-up in 86 patients. The authors reported no severe adverse effect in their study (Dastgheib et al., 2012 ▶). Nicotinamide (NAm), known as vitamin B3, is able to exert in vitro anti-leishmanial activity. It has been found that NAm significantly improves the anti-leishmanial activity of trivalent antimony in a synergistic manner while it shows additive activity with amphotericin B and slightly antagonizes pentamidine activity (Gazanion et al., 2011 ▶). In addition, it has been indicated that NAm significantly increases the toxicity of pentavalent antimony against the amastigotes of L. infantum, L. amazonensis and L. braziliensis (Gazanion et al., 2011 ▶).
Other treatment approaches
One method for improving a drug’s anti-leishmanial efficacy is nanonization. This approach is identified as foreign bodies/nanoparticles phagocytozed by the macrophages allowing targeted drug delivery. Application of nanoparticles of amphotericin B (amphotericin B attached to functionalized carbon nanotubes) is another therapeutic modality and the in vivo and in vitro experiments have shown promising effects on promastiges as well as amastigotes of L. Donovani (Prajapati et al., 2011a ▶). Another simple and cost-effective modality for improving the treatment with antimony compounds is thermotherapy. The potential mechanism of action of this method is probably direct destruction of parasites by heat (Alavi-Naini et al., 2012 ▶). In a randomized controlled trial conducted on 382 patients, the effectiveness of a single localized treatment by thermotherapy was compared with intralesional administration of glucantime for 5 days, for the treatment of CL by a six-month period of follow up (Safi et al., 2012 ▶). The treatment rate for the thermotherapy group was 82.5%, while it was 74% in the glutamine group. Consequently, thermotherapy was more efficient and cost-effective, with fewer side effects, shorter therapy duration and better patient compliance than intralesional gluctamine. In a randomized controlled trial (RCT), patients with leishmaniasis due to L. tropica were treated with an initial removal of skin lesions through electrocoagulation using a bipolar high-frequency electrosurgery instrument followed by being kept moist with or without pharmaceutical sodium chlorite (DAC N-055) daily (Jebran et al., 2014 ▶). Electrocoagulation reduced the parasite load and also created a fresh wound bed for producing subsequent granulation tissue and epithelialization. Additionally, sodium chlorite initiated tissue-regeneration and was also cytotoxic against promastigote and amastigote forms of L. tropica. This modality was stated as a highly effective, inexpensive and well-tolerated treatment option for patients with CL due to other Leishmania such as L. major, L. mexicana and L. braziliensis, either the old world or the new world (Jebran et al., 2014 ▶). Also, topical administration of honey can be effective in the treatment of leishmaniasis. It influences the collagen formation, wound contraction, and epithelialization with minimal scar formation and has antimicrobial effects (Oryan and Zaker, 1998 ▶; Alavi-Naini et al., 2012 ▶). It can enhance the speed of wound healing and therefore it can be effectively used in patients with CL (Alavi-Naini et al., 2012 ▶).
Herbal or plant therapy
As previously mentioned, lack of an efficient vaccine and resistance to drugs administered for the treatment of leishmaniasis, coupled with their high cost, parenteral route of administration and toxicity, have been regarded as a great concern especially in endemic areas of developing countries. Hence, there could be no doubt that the search for novel agents having anti-leishmanial or leishmanicidal potency is one of the critical challenges in the field of the current drug discovery program and is of worldwide concern (Guimaraes et al., 2010 ▶; Sen and Chatterjee, 2011 ▶; Iqbal et al., 2012 ▶; Wink, 2012 ▶; Ogeto et al., 2013 ▶; Mahmoudvand et al., 2014 ▶). For this purpose, some researchers have been looking for new alternatives in nature as an important source of drugs used in medicine (de Medeiros et al., 2011 ▶; Rondon et al., 2012 ▶; Wink, 2012 ▶; Manjolin et al., 2013 ▶; Gamboa-Leon et al., 2014 ▶; Sifaoui et al., 2014 ▶). It has been estimated that there are about 250,000 medical plant species in the world. However, the biological activities of only about 6% of them have been screened. Furthermore, only approximately 0.75% medical herbal compounds have been studied in clinical trials (Sen and Chatterjee, 2011 ▶; Jameel et al., 2014 ▶). The major merits of herbal medicine include their low cost, low incidence of serious adverse effects and good efficacy (Garcia et al., 2010 ▶; Wink, 2012 ▶; Sarkar et al., 2013 ▶). Unfortunately, the efficacy of most of these compounds has been shown only in in vitro studies and hence they are still in their initial stages of preclinical trials to be applicable in clinical practice and translation of their results into clinical practice is a neglected field. It is estimated that herbal products are involved directly or indirectly in production of about 25% of medicines (Colares et al., 2013 ▶; Vila-Nova et al., 2013 ▶). Several studies have shown that various plant species possess inhibitory activity against certain types of parasites such as L. major (Ogeto et al., 2013 ▶), L. amazonensis (Guimaraes et al., 2010 ▶; de Medeiros et al., 2011 ▶), L. tropica (Iqbal et al., 2012 ▶), L. aethiopica (Bekele et al., 2013 ▶), L. braziliensis (Yamamoto et al., 2014 ▶), L. mexicana (Gamboa-Leon et al., 2014 ▶), L. infantum (Mansour et al., 2013 ▶; Machado et al., 2014 ▶), L. chagasi (Rondon et al., 2012 ▶) and L. donovani (Sachdeva et al., 2014a, b ▶).
The number of recent in vitro and in vivo animal studies regarding the effectiveness of medicinal plants and plant-derived compounds such as the crude extracts and essential oils against CL and VL caused by different Leishmania species has been summarized in Table 2. In order to evaluate the anti-leishmanial or leishmanicidal activity of a compound, several criteria are considered including MIC, IC50 as well as selectivity index (Mahmoudvand et al., 2014 ▶). MIC is defined as the minimum inhibitory concentration of a compound or drug against the parasite. IC50 is described as the concentration of drug that causes 50% growth inhibition of amastigote or promastigote forms of Leishmania (Monzote et al., 2014a ▶). IC50 is used for macrophages of the host, too. CC50 is the cytotoxicity concentration of drug that results in 50% of mortality of macrophage from usually BALB/c mice (Islamuddin et al., 2012 ▶). An anti-leishmanial drug or natural compound is safe when selectivity index is greater than 10 (Mahmoudvand et al., 2014 ▶). Rondon et al. (2011 ▶) evaluated the leishmanicidal potential of the fractions obtained from the leaves of Aloe vera (aloe), and Ricinus communis (castor) and the seeds of Coriandrum sativum (coriander) against amastigotes and promastigotes of L. infantum. These compounds were compared at different concentrations of 6.25, 12.5, 25, 50, and 100 µg/ml with 40 µg/ml pentamidine and amphotericin B as the positive controls for tests related to promastigotes and amastigotes, respectively. They used three extracts obtained from these plants including ethyl acetate, chloroform, and methanol. All fractions were efficient against L. infantum promastigotes but did not show significant difference with pentamidine. On the other hand, ethyl acetate and chloroform fractions of R. communis and C. sativum methanol extract were the most efficacious against amastigotes with no significant difference with amphotericine B. Amongst these, ethyl acetate fraction of R. communis showed the least toxicity and the best results. This action was attributed to iron-dependent enzymes and membrane lysis of the parasite due to the presence of flavonoids, alkaloids, phytoestrols and saponins. The authors proposed the necessity of undertaking in vivo studies with R. communis ethyl acetate fraction due to its low cytotoxicity as well as high effectiveness against the parasite. As well, 2-substituted quinolone, a medicinal plant derivative, is efficacious against promastigotes and amastigotes of L. infantum, and L. amaznensis (Seifert, 2011 ▶), and more effective than miltefosin against L. donovani (Loiseau et al., 2011 ▶). Recently, Ogungbe et al. (2014) ▶ used 352 phenolic phytochemicals exhibiting antiparasitic activity, including 10 aurones, 6 cannabinoids, 34 chalcones, 20 chromenes, 52 coumarins, 92 flavonoids, 41 isoflavonoids, 52 lignans, 25 quinones, 8 stilbenoids, 9 xanthones, and 3 miscellaneous phenolic compounds along with 24 Leishmania enzymes. It was demonstrated that two aurones (bracterin triacetate and 4,6-dibenzoyl-2-[phenylhydroxymethyl]-3(2H)-benzofuranone), one chalcone (crotaorixin), five coumarins (mammeaA/AA, mammeaB/BA, mammeaB/BA cyclo F, scoparone, and umckalin), six flavonoids (diplacone, quercetin-3’,4’,7-trimethyl ether, cannflavin A, 3’-O-methyldiplacone, 3’-O-methyldiplacol, and 4’-O-methyldiplacone), one isoflavonoid (sophoronol E), three lignans (aristolignin, 3,3’,4,5-tetramethoxy-4’,5’-methylenedioxy-7,7’-epoxy-lignan and 4,5,4’,5’-dimethylenedioxy-3,3’-dimethoxy-7,7’-epoxylignan), and one stilbenoid (machaeriol B) can be considered as promising drugs and are worthy of further investigation for the treatment of leishmaniasis (Ogungbe et al., 2014 ▶). Essential oils or volatile extracts as natural compounds obtained from different parts of plants such as the flowers, leaves, bark, stems, wood, roots, fruits or seeds are commonly used in folk medicine to treat leishmaniasis (Colares et al., 2013 ▶). Essential oils prepared by hydrodistillation are composed of different small hydrophobic molecules such as sesquiterpenes and terpenes, especially monoterpenes diffusing easily across cell membranes and consequently gaining access to intracellular targets (Machado et al., 2012 ▶; Colares et al., 2013 ▶; Machado et al., 2014 ▶). Terpenes are hydrocarbons formed from units of isoprene, can easily penetrate the lipid bilayer of cell membrane and produce changes in the integrity of cell structures and mitochondrial membrane (Colares et al., 2013 ▶). There are several reports on the activity of essential oils on endemic Leishmania species responsible for CL and VL (Machado et al., 2012 ▶; Radulovic et al., 2013; Machado et al., 2014 ▶). Pharmacological application of the essential oils against human parasitic infections is unclear due to the potential risk for toxicity (Machado et al., 2012 ▶). However, several studies have demonstrated that the crude essential oils and their major compounds present low or no toxicity to host cells at the effective concentrations (Rodrigues et al., 2013 ▶). It has recently been reported that essential oils from Artemisina annua (Radulovic et al., 2013 ▶), Croton argyropylloides (de Franca-Neto et al., 2012), Ligustim chuanxiong (Zhang et al., 2012) and Menta villosa (Da Silva et al., 2012) are effective and non-toxic in animal models with leishmaniasis. Machado et al. (2014) ▶ have also demonstrated that essential oils derived from T. capitellatus have anti-leishmanial activity without significant cytotoxicity against macrophages. In addition to the anti-leishmanial activity of these essential oils, they have also shown insecticidal activity, which is extremely important in the control of sandflies and leishmaniasis (Machado et al., 2014 ▶). Moreover, some essential oils derived from plants such as Croton cajucara (red sacaca) have immunomodulatory effects and this makes them useful in the treatment of leishmaniasis (Rodrigues et al., 2013 ▶).
Table 2.
Antileishmanial activity of plant-derived compounds against CL and VL as tested in vitro and in vivo
| Scientific name | Plant part used | Preparation | Concentration/dose | Organism tested | Way of use | Main outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Against CL ( in vitro and in vivo studies) | |||||||
| Croton pullei var. glabrior | Stem bark | julocrotine, alkaloid isolated from C. pullei | Concentration of 1 mg/ml, IC50: 19.8 µM | Amastigote and promastigotes of L. amazonensis | In vitro | 80% inhibition, significant reduction in the number of amastigotes and morphological changes in promastigotes without any toxicity | Guimaraes et al., 2010 |
| Lippia sidoides Cham. (Alecrim pimenta) | Aerial parts | Essential oil | Concentration of 250-15.6 μg/ml, IC50: 44.38 µg/ml | Amastigotes and promastigotes of L. amazonensis | In vitro | Reduced both amastigote and promastigote survival without cytotoxic effects | de Medeiros et al., 2011 |
| Valeriana wallichii DC | Root | Water, methanol and chloroform extracts | (Promastigotes): 1-500 μg/ml for the extracts and 1-20 μg/ml for the fractions, amphotericin B:0.01-10 μM; (amastigote): 0.001 to 100 μg/ml for the extracts and fractions | Promastigotes of L. donovani and promastigotes and amastigotes of L. major | In vitro | Methanol and chloroform (F3: the most active with IC50 of 3-7 μg/ml for both promastigotes and 0.3 μg/ml against amastigotes of L. major) extracts showed activity against all three options via apoptotic cell death | Ghosh et al., 2011 |
| Aloe vera vs. Tamarix aphylla | Leaf vs. bark | Methanolic extract | 25-100 µg/ml | Promastigotes of L. tropica | In vitro | Especially T. aphylla except for the lowest concentration had high significant effect on parasite motility and with anti-promastigote activity | Iqbal et al., 2012 |
| Cymbopogon citratus and citral | Aerial parts | Essential oil | Concentration: 10-400 µg/ml, and IC50: ranging from 25 to 52 µg/ml and 34 -42 µg/ml, respectively | Promastigotes of L. infantum, L. tropica and L. major | In vitro | both were effective in inhibiting the parasites growth by apoptosis without cytotoxicity induction | Machado et al., 2012 |
| Galipea longiflora Krause | Bark | Quinolinic alkaloid extracts vs. glucantime | 10 µg/ml extract, 14 µg/ml glucantime; in mice, IP: 6.25, 12.5 mg the extract or 25 mg glucantime and 12.5 mg extract with 25 mg the drug | Promastigotes of L. amazonensis | In vitro and in vivo in mouse | The combined therapy was more efficient than individual use, reduced the parasite load and the footpath thickness | Calla-Magarinos et al., 2012 |
| Calophyllum brasiliense | Leaf | Mammea A/BB, coumarin obtained from C. brasiliense | 18 mg/kg/d IM or 0.2% topically vs. 27 mg/kg/d of Glucantime for 30 days | Promastigotes of L. amazonensis | In mouse with CL | Reduced size of skin lesions, similar anti-leishmanial activity to that of 27 mg/kg/d, IM, Glucantime® | Tiuman et al., 2012 |
| Moringa stenopetala | Root | 1,3-dilinoleoyl-2-olein and 1,3-dioleoyl-2-linolein (triglycerides) | Oncentrations: 1.00, 0.33, 0.11, 0.037, 0.012, 0.004, and 0.0013 mg; IC50: 0.079 and 242.5 µg/ml vs. 0.004 and 0.136 µg/kg for amphotericin B and miltefosine | Promastigotes of L. aethiopica | In vitro | Were active against promastigote and amastigote stages of L. aethiopica, but relatively lower than the reference compounds | Bekele et al., 2013 |
| Vanillosmopsis arborea and α-bisabolol | Stem | Essential oil | IC50: 7.35 and 4.95 µg/ml for promastigotes and IC50: 12.58 and 10.70 µg/ml for amastigotes | Promastigotes and amastigotes of L. amazonensis | In vitro | Showed leishmanicidal activity in the absence of obvious cytotoxicity on macrophages | Colares et al., 2013 |
| Curcuma longa Linn. | Spice turmeric | Curcumin, gallium curcumin, indium curcumin, and diacetylcurcumin vs. amphotericin B | 5 µg/ml for all, IC50: 38, 32, 26, 52 and 20 µg/ml respectively | Promastigotes of L. major | In vitro | Indium curcumin was the most effective than others, followed by gallium curcumin | Fouladvand et al., 2013 |
| Piper regnellii var. pallescens | Leaf | Eupomatenoid (a neolignan) | Concentrations of 30.0, 85.0 and 170.0 µM; IC50: | Promastigotes of L. amazonensis | In virto | Induced cell death in the parasite, increased mitochondrial ROS and O2∙production and G0/G1 phase cell cycle arrest | Garcia et al., 2013 |
| Strychnos pseudoquina | Stem bark | Flavonoids of quercetin 3-O-methyl ether and strychnobilavonethyl purified from acetate extract | 0.78-100 µg/ml, and fractions (each one, 50 µg/ml), IC50: 24.9 ± 5.2 µg/ml | Promastigotes of L. amazonensis | In vitro | The ethyl acetate extract and two purified flavonoids showed anti-leishmanial potential with no significant toxicity | Lage et al., 2013 |
| Vitis vinifera L. | Leaf | Ethanolic and aqueous extracts | 50, 25, and 6.25 µg/ml vs. 50, 25, and 6.25 mg/ml ethanolic vs. aqueous extracts, IC50: 0.108 mg/ml | Promastigotes of L. infantum | In vitro | Ethanolic extract was more effective and destructed L. infantum promastigotes due to higher anthocyanin contents | Mansour et al., 2013 |
| Physalis angulata | Stem | Ethanolic extract | 1.2–100 µg/ml; IC50: 5.35 µg/ml and 4.50 µg/ml against L. amazonensis and L. braziliensis promastigotes | Promastigotes of L. amazonensis and L. braziliensis | In vitro | Significantly reduced the parasites, was non-mutagenic and presented a promising anti-leishmanial effect without significant cytotoxicity | Nogueira et al., 2013 |
| Aloe secundiflora | Leaf | Methanolic and water extract | 1-1000 µg/ml, IC50: 279.488 and 42.824 µg/ml for water and methanolic extracts | Premastigotes of L. major | In vitro | Significantly inhibited the growth of L. major vs. amphotericin B | Ogeto et al., 2013 |
| Annona muricata acetogenins, annonacinone and corossolone, and Platymiscium floribundum coumarin scoparone | Stem of P. floribundum and leaves and seeds of A. muricata | One coumarin, scoparone, from P. floribundum and two acetogenins, annonacinone and corossolone, from A. muricata | 100, 50, 25, 12.5 and 6.25 µg/ml | Promastigotes of L. donovani, L. mexicana and L. major | In vitro | L. donovani and L. major were more susceptible to annonacinone and L. mexicana to annonacinone and scoparone, three species had similar susceptibility to corossolone, and corossolone was the most toxic | Vila-Nova et al., 2013 |
| Senna Spectabilis | Flower | Crude ethanolic extract, fractions (dichloromethane and n-butanol) and two major alkaloidal metabolites of (-)- cassine/(-) spectaline | Exctracts and fractions: serial concentrations of 100, 30, 10, 3, 1 and 0.3 µg/ml; Pentamidine and alkaloids: of 100, 30, 10, 3, 1 and 0.3 M | Promastigotes of L. major | In vitro | Fractions and flavonoids exhibited significant leishmanicidal activity than others without toxic effects on murine macrophages | de Albuquerque Melo et al., 2014 |
| Thymus capitellatus Hoffmanns. and Link | Aerial parts | Volatile extract (essential oil) and its compounds (1,8-cineole and borneol) | Concentration from 10 to 400 µg/ml. IC50: ranging from 35 to 62 µg/ml | Promastigotes of L. infantum, L. tropica and L. major | In vitro | Promoted leichmanicidal activity via induction of apoptosis, without significant cytotoxic effects | Machado et al., 2014 |
| Berberis vulgaris and B. barbering (its major principl, an isoquinoline alkaloid) | Root (rich in berberine) | Aqueous, methalonic and chloroform extracts vs. glucantime | IC50: 2.1-26.6 µg/ml; berberine had the least IC50% about 2.1 and 2.9 µg/ml for L. major and L. troica, respectively; CC50: 27.3-362.6 µg/ml | Promastigotes of L. major and L. tropica | In vitro | Were effective in inhibiting promastigotes of both Leishmania with more cytotoxicity by berberine; showed no significant cytotoxicity | Mahmoudvand et al., 2014 |
| Chenopodium ambrosioides | Not available | Essential oil and its components: ascaridole, carvacrol and caryophyllene oxide | 10 µg/kg; ascaridole with the least IC50 for promastigotes, amastigotes and macrophages | Amastigotes and promastigotes of L. amazonensis | In vitro | Were active against both forms the parasite and ascaridole had the better activity; the oil had the greatest selectivity index | Monzote et al., 2014a |
| Arrabidaea chica | Leaf | Five fractions from crude hexanic extract of A. chica | 1-500 µg/ml, IC50: 37.2 and 18.6 µg/ml for L. amazonensis and L. infantum | Promastigotes of L. amazonensis and L. infantum | In vitro | Especially B2 fraction inhibited completely the peptidase activity of promastigotes | Rodrigues et al., 2014 |
| Olea europeaea, 5 olive tree varieties (Limouni, Zarrazi, Dhokkar, Toffehi, Chemlali Tataouine) | Leaf | Ethanol, methanol and mixture extracts | IC50: 2.130-71.570 µg/ml for L. donovani, 17.622-48.94 and 14.661-58.95 µg/ml for L. tropica and L. major, 2.94-16.95 µg/ml for L. amazonensis | Promastigotes of L. donovani, L. amazonensis, L. tropica and L. major | In vitro | All had interesting leishmanicidal activity, the Limouni variety had the highest phenolic content and the strongest activity. L. amazonensis was the most sensitive species | Sifaoui et al., 2014 |
| Bixa orellana | Seed | Essential oil (ishwarane and geranylgeraniol and 71 other compounds in the oil) | 30 mg/kg, IP, 14 days; amphotericin B 1 mg/kg; IC50: 8.5 vs. 0.03 µg/ml for amphotericin B | Amastigote and promastigote forms of L. amazonensis | In vitro and in vivo in BALB/c mouse | Showed activity against amastigote form and ability to control disease progression in mice | Monzote et al., 2014b |
| Tanacetum parthenium (L.) Schultz-Bip | Aerial part | Sesquiterpene lactone-rich dichloromethane fraction | IC50: 2.4±0.76 and 1.76±0.25 µg/ml for promastigotes and amastigotes, respectively, IM | Amastigotes and promastigotes of L. amazonensis | In vitro and in vivo in mouse | Decreased the parasite population with low toxicity, decreased the growth and size of lesions in mice | Rabito et al., 2014 |
| Tridax procumbens alone and in combination with Allium sativum (garlic) | Whole plant and bulbs, respectively | Methanol and aqueous, respectively | 20 mg/kg separately, and 40 mg/kg of the mixture for 2 weeks, IP | Promastigotes of L. mexicana, CL | In mouse with CL | Increase in IgG2/IgG1 ratio and Th1-type immune response with the mixture. The mixture was better at controlling the infection without cytotoxicity | Gamboa-Leon et al., 2014 |
| Berberis vulgaris L. | Root bark | Ethanolic extract | 10% and 20% root bark extract | Promastigotes of L. major | In mouse with CL | Caused 90% and 55% recovery in mice treated with 20% and 10% root extract, respectively | Salehabadi et al., 2014 |
| Baccharis uncinella | Leaf | Oleanolic- and ursolic-containing titerpenic fraction from B. uncinella vs. amphotericin B | 1.0 and 5.0 mg/kg of the fraction (IP) vs. 10.0 mg/kg of amphotericin B; for 5 days | Promastigotes and amastigotes of L. amazonensis and L. braziliensis | In mouse with CL | Decreased lesion size, did not cause alteration in the tissues, a curative effect similar to the drug | Yamamoto et al., 2014 |
| Against VL ( in vitro and in vivo studies) | |||||||
| Lippia sidoides Cham. | Leaf | Thymol- and carvacrol-rich essential oil | IC50: 74.1 and 54.8 µg/ml for thymol- and carvacrol-rich oil | L. chagasi promastigotes | In vitro | Both essential oil showed significant activity, but carvacrol-rich oil was more effective | Farias-Junior et al., 2012 |
| Mangifera indica L. | Leaf | Pertroleum ether, chloroform, and methanol extracts | Concentrations of 3, 5, 10, 15 and 30 µg/ml; IC50: 2.99, 2.91 and 2.74 µg/ml, respectively | Promastigotes of L. donovani | In vitro | All significantly inhibit the growth of the parasite, the methanol extract was the most active followed by chloroform and petroleum extracts | Haldar et al., 2012 |
| Artemisia annua (artemisinin) | Leaf and seed with floral part | n-hexane fractions and seeds vs. pentamidine | All in concentration of 100 µg/ml; IC50: 6.6 and 5.05 µg/ml vs. 1 µg/ml of pentamidine | Promastigotes of L. donovani | In vitro | Showed anti-leishmanial activity via programmed cell death (apoptosis) without cytotoxic effect | Islamuddin et al., 2012 |
| Trichosanthes dioica Roxb. (pointed gourd) | Root | Triterpenoid enriched extract (fraction) | Graded concentrations of 2.5, 5, 10, 20, 40 and 80 µg/ml; IC50: 18.75 µg/ml | Promastigotes of L. donovani | In vitro | Inhibited significantly the growth of L. donovani promastigotes | Bhattacharya et al., 2013 |
| Withanolides (Withania somnifera, Ashwagandha) | Leaf | Two fractions (F5 and F6) from ethanol extract, | 60 and 15 µg/ml, respectively | Promastigotes of L. donovani | In vitro | Induced apoptotic death by the production of ROS from mitochondria and destruction of mitochondrial membrane | Chandrasekaran et al., 2013 |
| Croton Cajucara (red sacaca) | Leaf | Essential oil and 7-hydroxycalamenene | Concentration of 1-1000 μg/ml; IC50: 66.7, 1.37 and 0.01 µg/ml for the oil, the fraction and amphotericin B, respectively | Promastigotes of L. chagasi | In vitro | Caused mitochondrial changes in promastigotes, reduced parasite/macrophage interaction by 52.8%, increased NO production by 80% without any cytotoxicity | Rodrigues et al., 2013 |
| Eryngium foetidum L. | Aerial part | Methanol, n-hexane, ethyl acetate, and 50% methanol, a daucane sesquiterpene | 20 µg/ml; IC50: 7.84 µM | Promastigoes of L. donovani | In vitro | Showed promising growth inhibition without any significant cytotoxicity | Rojas-Silva et al., 2013 |
| Pleumeria pudica | Leaf | Petroleum ether, chloroform and methanol extracts | 3, 5, 10, 15 and 30 µg/ml and IC50: 3.04, 2.98 and 2.93 µg/ml for ether, chloroform and methanol extracts | L. donovani promastigotes | In vitro | All extracts inhibited growth of L. donovani promastigotes; methanol extract was the most active followed by chloroform and petroleum ether extracts | Sarkar et al., 2013 |
| Fumaria parviflora Lam. | Whole plant | Methanolic extract vs. pentamidine | Ranged from 1000 to 1.038 µg/ml; CI50: 5.35 µg/ml | Promastigotes of L. donovani | In vitro | had significant anti-leishmanial activity | Jameel et al., 2014 |
| Millettia species | Stem bark | Water and methanol crude extracts and six fractions of methanol extract | Concentration: 10 mg/ml; IC50: 11.8 μg/ml | Promastigotes of L. donovani | In vitro | dichloromethane fraction showed the best in vitro anti-leishmanial activity, with low cytotoxicity due to high terpenoids and alkaloids levels | Rajemiarimiraho et al., 2014 |
| Withania somnifera (winter cherry) | Whole plant | Aqueous extract | 350 mg/kg, for 15 days, orally, 5 mg/kg cisplatin, for 5 days, IP | Promastigotes of L. donovani | In mouse with VL | Increased anti-leishmanial efficacy of cisplatin, augmented levels of IgG2 over IgG1 and enhanced Th1 type immunity | Sachdeva et al., 2013 |
| Tinospora cordifolia in combination with cisplatin | Whole plant | Pure herb extract (tablet form) | T. cordifolia: 100 mg/kg, 15 days; Cisplatin: 5 mg/kg for 5 days, IP | Promastigotes of L. donovani | In mouse with VL | Enhanced proliferation and differentiation of lymphocytes, induced Th1 immune response and IFN-γ and IL-2, but declined IL-4 and IL-2 levels | Sachdeva et al., 2014a |
| Combination of Asparagus racemosus (shatavari) with cisplatin | Whole plant | Tablet form | A. racemosus: 650 mg/kg for 15 days, oral, and cisplatin: 5 mg/kg for 5 days, IP | L. donovani promastigotes | In mouse with VL | Enhanced protective immune response (IgG) and boosted both cellular and humoral immunity | Sachdeva et al., 2014b |
CL: Cutaneous leishamniasis; VL: Visceral leishmaniasis; ROS: Reactive oxygen species; NO: Nitric oxide; IP: Intraperitoneal; IM: Intramuscular; IC50: Concentration of drug that caused 50% of growth inhibition of amastigote or promastigote form of Leishmania
The antileishmanial activity of some plant extracts has been attributed to flavonoids (Manjolin et al., 2013; Wong et al., 2014). Flavonoids are a group of polyphenolic compounds that naturally present in fruits and vegetables and are known as antioxidants and anticancers with a significant protective effect against membrane damage (Manjolin et al., 2013; Sifaoui et al., 2014). Flavonoids such as catechins are able to form complexes with the parasite cell wall to influence processes requiring cell linking, and hence inhibit the parasite growth (Ogeto et al., 2013). In a research work carried out by Manjolin et al. (2013), it has been shown that dietary flavonoids such as fisetin (the most potent alkaloid), quercetin, luteolin and 7,8-hydroxyflavone with low cytotoxicity characteristics are able to inhibit arginase enzyme from L. amazonensis. Arginase plays a central role in the biosynthesis of polyamine which is very important and essential for protecting the parasite against oxidative stress and ROS produced by the host’s defense system.
Mushrooms and their metabolites have been tested for their probable anti-leishmanial potential (Mallick et al., 2014). Mallick et al. (2014) evaluated the anti-leishmanial effect of mushrooms against promastigote and amastigote forms of L. donovani. They used 18 extracts derived from six wild mushrooms (ethanol extracts and water-soluble polysaccharide and polyphenolic fractions). Among these, Astraeus hygrometricus and Tricholoma giganteum induced apoptosis and inhibited the growth of L. donovani promastigotes. On the other hand, water-soluble fractions of A. hygrometricus, Russula laurocerasi, Russula albonigra, Termitomyces eurhizus, Russula delica and polyphenolic fractions of R. laurocerasi could inhibit the proliferation of intracellular amastigotes in macrophages. Regarding intracellular amastigotes, the compounds were non-toxic and induced release of NO and IL-12 in murine cells. They showed that such mushrooms can open the way for further phytochemical and pharmacological investigations towards the search of novel anti-leishmanial compounds (Mallick et al., 2014). A review conducted by Mishra et al. (2009) has reported the leishmanicidal activity of various alkaloids including indole, quinoline, isoquinoline, pyrimidine-carboline, steroidal and diterpene derived from various plants as well as those obtained from marine sources. These alkaloids were from the following origins: indole alkaloids including those obtained from stem bark and leaf of Kopsia griffithii (Apocynaceae), Peschirea australis (Apocynaceae), the bark of Corynanthe pachyceras, (Rubiaceae), stem bark of Aspidosperma ramiflorum (Apocynaceae), and the stem bark of Peschiera van heurkii (Apocynaceae); isoguinoline alkaloids like those isolated from Annona foetida (Annonaceae), the bark of Guatteria foliosa (Annonaceae), the young leaves of plant Guatteria dumetorum (Annonaceae), the stem bark of Rollinia emarginata (Annonaceae), Guatteria sp., and the Unonopsis buchtienii R. E. Fries (Annonaceae); naphthylisoquinoline alkaloids including those derived from leaves and twigs of Ancistrocladus griffithii (Ancistrocladaceae), the roots of Ancistrocladus likoko J. Leonard (Ancistrocladaceae), Ancistrocladaceae sp., and Ancistrocladus tanzaniensis (Ancistrocladaceae); bisbenzylisoquinoline alkaloids such as those obtained from Albertisia papuana (Menispermaceae), Pseudoxandra sclerocarpa (Annonaceae), Gyrocarpus americanus (Hernandiaceae), Caryomene olivasans (Menispermaceae), Limaciopsis loangensis (Menispermaceae), and the stem bark of Guatteria boliviana (Annonaceae); and quinolone alkaloids including those isolated from the plants including Galipea longiflora Krause (Rutaceae), and the bark of Dictyoloma peruviana (Rutaceae). Others included steroidal alkaloids isolated from the leaves of the Bolivian plant Saracha punctata (Solanaceae), and the leaves of Holarrhena curtisii (Apocynaceae), benzoquinolizidine alkaloids derived from Psychotria klugii (Rubiaceae), diterpene alkaloids isolated form the species of genera Aconitum, Delphinium and Consolida (Ranunculaceae), pyrimidine-β-carboline alkaloids obtained from the bark extract of Annona foetida (Annonaceae), leaves of Acanthus illicifolius (Acanthaceae), the marine alkaloids isolated from marine sponges possessing antileishmanial activity like Amphimedon viridis, Acanthostrongylophora sp., Neopetrosia sp., Plakortis angulospiculatus and Pachymatisma johnstonii, and finally the miscellaneous alkaloids from leaves of Acanthus illicifolius (Acanthaceae), and from stem bark of Zanthoxylum chiloperone var. angustifolium (Rutaceae). Saponins are high molecular weight glycosylated plant metabolites with anti-inflammatory, anti-tumor and anti-HIV activity (Makwali et al., 2012). The anti-leishmanial activity of acridine and dinitroaniline herbicides in combination with triterpenoid saponin extract and extracts prepared from roots of Plumbago capensis was investigated against L. major in BALB/c mice (Makwali et al., 2012). The combined therapy eliminated the parasites from the lesions and reduced parasite load in the internal organs so that complete clearance of parasitemia from both sites occurs. The authors demonstrated that the combination therapy with saponin, acriflavine, trifluralin and plumbagin is a promising treatment for the infection with L. major. Sen and Chatterjee (2011) established the mechanism of action of several plant-derived compounds, as follows: plant-derived compounds including chalcones (alter the ultrastructure of parasite mitochondria resulting in inhibition of the parasite respiratory chain), flavonoids (inhibit the parasite DNA synthesis, and induce the production of ROS triggering apoptosis), saponins (able to react with Leishmania membrane, induce a decrease in membrane potential and cause ultimately loss of membrane integrity), quinones (increase the free radicals generation by the parasite and induce apoptotic-like cell death), alkaloids (interfer with the macromolecular biosynthesis and inhibit respiration of amastigotes, modulation of mitogen activated protein kinase, a regulatory enzymes for aoptosis and inflammation, induction apoptotic-like death cell mediated by free radicals and NO production), lignans (interact with macromolecules resulting in cell cycle arrest and prevent the parasite attachment to macrophages and their entry), tannins (increase the NO production in infected macrophages and enhance the expression of cytokines including IL-10, IL-12, TNF-α and IFN-γ), terpenoids (increase the production of NO, induce apoptosis via inhibition of parasite DNA topoisomerase, and inhibit the parasitic growth), and finally oxylipins (inhibit super-oxidase dismutase leading to increased ROS generation and the parasite killing, and enhance generation of NO and TNF-α within macrophages). da Costa et al. (2014) evaluated the activity of hexane and ether acetate extracts of stem wood, root wood and bark from Connarus suberosus Planch (Connaraceae), Neea theifera Oerst. (Nyctaginaceae) and Myrcia linearifolia Cambess. (Myrtaceae) against promastigotes of L. amazonensis at a concentration of 100 µg/ml. Six out of 10 C. suberosus extracts were effective against the parasite and among these the root bark hexane extract had the most activity. The inhibitory concentration index for 50% of the parasites (IC50) ranged from 27.57 to 94.00 µg/ml. N. theifera and M. linearifolia showed no anti-leishmanial activity against L. amazonensis. The root bark ether acetate extract was selected for chemical fractionation resulting in a mixture of rapanone and a new compound called suberonone, a mixture of β-sitosterol and stigmasterol, oleic acid, geranilgeraniol, and two derivatives obtained from the first two mixtures. Several researchers have evaluated anti-leishmanial properties of the most common plants available in their countries. For example, Ribeiro et al. (2014) investigated the in vitro antileishmanial activity of 44 extracts and fractions derived from 16 Brazilian plant species against L. amazonensis. They used plants with minimum inhibitory concentrations (IC50) in Leishmania and their cytotoxic effects on murine macrophages (CC50) for determining the selectivity index (SI) of each extract or fraction. The products that presented higher SI values were used in the treatment of macrophages infected with L. amazonensis. These plants included Bowdichia virgiloides Kunth, Campomanesia lineatifolia Ruiz, Cecropia pachystachya Trecul, Chrysobalanus icaco L, Diospyros hispida, D.C., Dipteryx alata Vog., Syzygium cumini L, Eugenia uniflora L., Hymenaea courbaril L., MS Hymenaea stignocarpa Mart. ex. Hayne, Jacaranda caroba Vell., Jacaranda cuspidifolia Mart., Jacaranda ulei Bureau and K. Schum, and Licania tomentosa Benth. The most potent extracts against L. amazonensis were the hexanic extract of D. alata (IC50 of 59 0.08 µg/ml), the hexanic extract of S. cumini (IC50 of 31.64 µg/ml), the ethanolic and hexanic extracts of leaves of H. courbaril (IC50 of 44.10 µg/ml and 35.84 µg/ml, respectively), the ethanolic extract of H. stignocarpa (IC50 of 4.69 µg/ml), the ethanolic extract of J. caroba (IC50 of 13.22 µg/ml), and the ethanolic extract of J. cuspidifolia leaves (IC50 of 10.96 µg/ml). Extracts obtained from D. alata and J. cuspidifolia presented higher selectivity index, with high leishmanicidal activity and low cytotoxicity in the mammalian cells. Generally, the ethanolic extracts were less effective and more toxic than the hexanoic extracts and buthanolic, dichloromethane ethyl acetate and hexanic fractions in the mammalian cells. Because of the lack of NO production in the treated macrophages, they suggested that this activity of D. alata and J. cuspidifolia is due to mechanisms other than macrophage activation mediated by NO production. Garcia et al. (2012) examined the anti-leishmanial activity of 48 extracts obtained from 46 Cuban plants mainly from leaves but from seeds and roots in concentration between 6.25 and 100 µg/ml against promastigotes and mastigotes of L. amazonensis. Only 4 extracts including Bambusa vulgaris, Hura crepitans, Mangifera indica, and Simarouba glauca exhibited selective activity against the parasite and then were evaluated against intracellular amastigotes of L. amazonensis. Among these, three showed inhibition of growth with IC50 value ≤50 µg/ml. However, M. indica extract showed an IC50 value of 60.1 µg/ml. The IC50 of B. vulgaris, S. glauca and H. crepitans was 41.5 µg/ml, 45.5 µg/ml, and 27.7 µg/ml, respectively. Collectively, among the 46 evaluated plant species, H. crepitans caused the highest inhibition of promastigotes growth (IC50 = 16.4 µg/ml) and showed lower toxicity against host cells (IC50 = 390.5 µg/ml), with an excellent selectivity index equal to 24. In general, the extracts prepared from H. crepitans, B. vulgaris, and S. glauca showed more promising antileishmanial activity against both stages of the parasite. Therefore, the authors suggested identifying and purifying the active compounds of these plants. A study conducted in Saudi Arabia evaluated the in vitro antiprotozoal potential of the methanol extracts of 51 plants and some of their petroleum ether, chloroform, ethyl acetate and aqueous fractions against L. infantum amastigotes and also Plasmodium falciparum, Trypanosoma brucei and T. cruzi (Abdel-Sattar et al., 2010). Among these, only nine crude extracts (18%) showed pronounced anti-leishmanial activity with IC50 from 0.25 to 2.03 μg/ml. Three plants including Euphorbia schimperiana, Tribulus macropterus and Hypoestes forsskalii showed IC50 less than 0.25 μg/ml. The highest activity was associated with the extracts of Solanum villosum, Verbesina encelioides and T. macropterus with IC50 of 0.51, 0.35 and less than 0.25 μg/ml and SI of greater than 125, 99.3 and 87.5, respectively.
Given the high prevalence of CL by L. major in Iran, Kheiri Manjili et al. (2012) assessed the anti-leishmanial potential of the ethanolic extracts of 17 medicinal plants against L. major promastigotes. Four plants including Caesalpinia gilliesii, Satureia hortensis, Carum copticum heirm, and Thymus migricus showed high anti-leishmanial activity with IC50 of 9.76 ± 1.27, 15.625 ± 3.76, 15.625 ± 5.46, and 31.25 ± 15.44 μM, respectively. These plants were toxic against macrophages at concentrations higher than those needed to inhibit the parasite cell growth (IC50, 45.13 ± 3.17, 100.44 ± 17.48, 43.76 ± 0.78, and 39.67 ± 3.29 μM, respectively). Moreover, glucantime inhibited the growth of L. major promastigotes with IC50 = 254 μg/ml without affecting the growth of macrophages. The results obtained from this study revealed the effectiveness of C. gilliesii, S. hortensis, C. copticum heirm, and T. migricus extracts due to the presence of the active compounds which could serve as alternative agents in the control of CL. Although herbal remedies have been used for a long period of time for the treatment of leishmaniasis, a systematic scientific evaluation of the effectiveness of herbal extracts has rarely been performed. A double-blind, randomized, clinical trial carried out by Sattar et al. (2012) investigated the antileishmanial potency of Morinda citrifolia (Noni) stem extract against CL. Among 40 patients, 30% indicated good improvement and 50% exhibited excellent response. In addition, morindicone and morinthone, two major constituents derived from the extract, displayed good antileishmanial activity. Another double-blind randomized clinical trial by Zerehsaz et al. (1999) compared the efficacy of intramuscular administration of meglumine antimonial (glucantime) (85 patients, for 20 days, 15-20 mg/kg/day) with topical herbal extract consisting of a mixture of the pure extracts of Althaea rosa, Althaea officinalis and members of the families Leguminosae, Faliaceae, Malvaceae, and Lythraceae (Z-HE) (86 patients, as a crude paste covered by a dressing, for 5 days) on patients with CL. Complete cure was obtained in 74.4% and 24.1%, partial cure in 11.6% and 14.1% as well as failure in 14.0% and 58.8% in the glucantime group and the Z-HE group, respectively. Moreover, adverse effects due to treatment including urticarial and generalized pruritus only were observed in the glucantime group (Zerehsaz et al., 1999). It was concluded that the mechanism of action could be the enhancement of hosts’ cellular immunity. A thorough investigation on anti-leishamnial activity of the plants present here along with their toxicity, mechanism of action and physiochemical properties for development of an optimum formulation is urgently needed to confirm their efficacy in the treatment of leishamniasis (Jain and Jain, 2013).
Future directions
Some studies have focused on a way to prevent side effects of the drugs effective on leishmaniasis such as cisplatin through the simultaneous supplementation of preventive agents. One of these methods includes induction of antioxidant enzymes with free radicals scavenging activities. The extracts of several plants have been shown to exhibit effective protection against drug (cisplatin)-induced toxicities and ameliorative effect (Sachdeva et al., 2013; Sachdeva et al., 2014a, b). Since infective form, amastigote, is located inside macrophages, the treatment of leishmaniasis is difficult (de Medeiros et al., 2011). It has been demonstrated that synthetic flavonoid dimers can increase intercellular drug accumulation and thereby avert drug resistance via impairment of the ATPase pump (Wong et al., 2014). For example, Wong et al. (2014) examined the in vitro and in vivo anti-amastigote and anti-promastigote activities of flavonoid dimer 39 (39.HCL) compared with well-known anti-leishmanial drugs. For in vivo study, mice acquired CL through subcutaneous inoculation of L. amazonensis promastigotes into the foopad. Then, they received 0.5 or 2.5 mg/kg of 39.HCL dissolved in 50% polyethylene glycol, 28 mg/kg SSG dissolved in 0.9% NaCl from day 16 to 48 post-infection. Compound 39 displayed significant anti-promastigote (IC50 from 0.19 to 0.69 µM) and anti-amastigote (IC50, 0.17 to 2.2 µM) activities towards promastigotes of all species including L. major, L. amazonensis, L. braziliensis, and L. tropica. In mice, 39.HCL reduced thickness of the footpad by 36% and the amastigote load present in the lesions was reduced 20-fold. Importantly, compound 39 was not toxic to peritoneal macrophages. Therefore, the authors introduced flavonoid dimer 39 as a novel safe and efficient leishmanicidal agent against leishmaniasis. Apart from a combination of two or more antileishmanial drugs, researchers are also investigating the effect of immmunotherapy due to immunomedulators obtained from plants in conjunction with anti-leishmanial drugs (Sachdeva et al., 2013). Indeed, the use of products and formulations that stimulate the immune system can be regarded as a promising alternative for the treatment of leishmaniasis (Sachdeva et al., 2013; Sachdeva et al., 2014b). It is necessary to consider the immune response elicited by the treatment as well as effect of therapy on the immune response since the Th1 and Th2 responses play a prominent role in recovery from leishmaniasis and shift from Th2 to Th1 response helps in providing resistance to infection (Chowdhury et al., 2012; Yamamoto et al., 2014). Some studies have demonstrated the benefits of combining conventional treatment with other compounds that can control immune response or they have immunomodulatory property (Calla-Magarinos et al., 2012; Chandrasekaran et al., 2013; Gamboa-Leon et al., 2014). An in vivo study on anti-leishmanial and immunomodulatory activity of Withania somnifera against L. donovani has shown that it is more effective with the combination of cisplatin and W. somnifera compared to the drugs alone (Sachdeva et al., 2013). This inhibitory effect of W. somnifera has been attributed to the presence of withaferin A (steroidal lactone) and withanolide A. Withaferin A can induce Leishamnia-affected cells apoptosis via inhibition of protein kinase C affecting apoptosis (Sachdeva et al., 2013). In addition, W. somnifera due to its hepatoprotective and antioxidant properties can eliminate hepatotoxicity and the liver dysfunction either induced by cisplatin used for the treatment of VL or caused by the parasite itself (Sachdeva et al., 2013). Plant extracts, secondary metabolites or biomolecules can exert immunostimulatory properties (Chouhan et al., 2014). It is believed that the most common mechanism for exerting immunomodulatory activity is the stimulation of NO or ROS production that is principle effector molecule for killing of Leishmania amastigotes (Niranthrin, lignan isolated from aerial parts of Phyllanthus amarus) (Chowdhury et al., 2012). The tritepene fraction containing oleanolic and ursolic acids can modulate immune cells to produce and increase levels of IL-12 and IFN-γ (Yamamoto et al., 2014). Tannins and related compounds kill Leishmania via a NO-mediated mechanism (Chouhan et al., 2014). Increase in cytokines related to Th1 lymphocytes (Berberine chloride) (Saha et al., 2011), decrease in those associated with Th2 cells (Asparagus racemosus) (Sachdeva et al., 2014b) or high IgG2/IgG1 ratio levels (mixture of Tridax procumbens and Allium sativum) (Gamboa-Leon et al., 2014) are regarded as other potential mechanisms for anti-leishmanial, leishmani-cidal or immunomodulatory activities of plant-derived formulations (Chouhan et al., 2014). Leishmanicidal activity of Allium sativum (garlic) has been demonstrated against infection with L. major and L. maxicana, so that it induces a Th1-type response and stimulates INF-γ and NO production in macrophage, and thus prevents the progression of the infection (Gharavi et al., 2011; Gamboa-Leon et al., 2014). To improve the therapeutic efficacy and reduce toxicity, the above mentioned natural molecules can be applied as either scaffold for producing and exploring new immunodrugs or natural immuno-modulators in synergy and in combination with existing drugs (Prajapati et al., 2011a; Jain and Jain, 2013). Targeting of anti-leishmanial drugs to macrophages via drug delivery systems reflects a promising strategy overcoming the drawbacks associated with the current treatment protocols. Various novel drug carriers including liposomes, nanoparticles, carbon nanotubes and microspheres among others may target macrophages in both ways, actively and passively (Jain and Jain, 2013). Liposomes and nanoparticles among others are naturally taken up by macrophages via phagocytosis and hence can target the drugs passively to target site. It is attempted to alleviate high toxicity of the available drugs by the site-specific delivery to macrophage through development of an ideal drug delivery system (Prajapati et al., 2011a; Jain and Jain, 2013). Gold nanoparticles conjugated with quercetin (a widely available herbal flavonoid) have been successfully used against leishmanial infections by Das et al. (2013). They utilized synergistic effect of quercetin and the gold nanoparticles in impairing the oxygen metabolism of the parasite in macrophages. This compound was effective and presented promising results compared to sodium stibogluconate and paramomycin resistant strains. The value of the plants mentioned above and their derivations as drug candidates cannot be fully assessed, since data achieved from in vivo animal model studies and clinical trials have not been greatly generated.
Conclusion
Despite good knowledge on the epidemiology of leishmaniasis, it still remains uncontrollable. Researchers from every corner of the world should devote more attention and time for leishmaniasis, as one neglected protozoan disease with public health importance. In general, identification of the most efficient surveillance system and control strategies to reduce the mortality and morbidity rate, a suitable approach to control vectors, identifying and combatting risk factors, a safe, non-invasive, short and cheap treatment, all should be regarded as the most important approaches for satisfactory control towards complete eradication of leishmaniasis from particular developing countries. The present study shows a wide range of plant extracts exhibiting interesting antileishmanial properties in vitro and in vivo animal experimental studies. But the necessity of generalizing the results obtained from the in vitro and also the in vivo studies on the effectiveness of plant extracts, metabolites or formulations against different Leishmania species to clinical practice should be stressed to validate their activities.
Conflict of interest
Nothing to declare.
References
- Abbasi, I, Aramin, S, Hailu, A, Shiferaw, W, Kassahun, A, Belay, S, Jaffe, C, Warburg, A. Evaluation of PCR procedures for detecting and quantifying Leishmania donovani DNA in large numbers of dried human blood samples from a visceral leishmaniasis focus in Northern Ethiopia. BMC Infect. Dis. 2013;13(153) doi: 10.1186/1471-2334-13-153. doi: 10.1186/1471-2334-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Sattar, E, Maes, L, Salama, MM. In vitro activities of plant extracts from Saudi Arabia against malaria, leishmaniasis, sleeping sickness and Chagas disease. Phytother. Res. 2010;24:1322–1328. doi: 10.1002/ptr.3108. [DOI] [PubMed] [Google Scholar]
- Alavi-Naini, R, Fazaeli, A, O’Dempsey, T. Topical treatment modalities for old world cutaneous leishmaniasis: a review. Prague. Med. Report. 2012;113:105–118. doi: 10.14712/23362936.2015.26. [DOI] [PubMed] [Google Scholar]
- Alidadi, S, Oryan, A. Cutaneous leishmaniasis and the strategies for its prevention and control. Trop. Med. Surg. 2014;2:e114. doi: 10.4172/23299088.1000e114. [Google Scholar]
- Alviano, DS, Barreto, AL, Dias Fde, A, Rodrigues Ide, A, Rosa Mdo, S, Alviano, CS, Soares, RM. Conventional therapy and promising plant-derived compounds against trypanosomatid parasites. Front. Microbiol. 2012;3:283. doi: 10.3389/fmicb.2012.00283. doi: 10.3389/fmicb.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari, Q, Motazedian, MH, Mehrabani, D, Oryan, A, Hatam, GR, Owji, SM, Paykari, H. Zoonotic cutaneous leishmaniasis in Shiraz, Southern Iran: a molecular, isoenzyme and morphologic approach. J. Res. Med. Sci. 2007;12:7–15. [Google Scholar]
- Aslan, H, Dey, R, Meneses, C, Castrovinci, P, Jeronimo, SM, Oliva, G, Fischer, L, Duncan, RC, Nakhasi, HL, Valenzuela, JG, Kamhawi, S. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. J. Infect. Dis. 2013;207:1328–1338. doi: 10.1093/infdis/jis932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon, KM, Hotez, PJ, Kruchten, SD, Kamhawi, S, Bottazzi, ME, Valenzuela, JG, Lee, BY. The potential economic value of a cutaneous leishmaniasis vaccine in seven endemic countries in the Americas. Vaccine. 2013;31:480–486. doi: 10.1016/j.vaccine.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekele, B, Adane, L, Tariku, Y, Hailu, A. Evaluation of antileishmanial activities of triglycerides isolated from roots of Moringa stenopetala. Med. Chem. Res. 2013;22:4592–4599. [Google Scholar]
- Bhattacharya, S, Biswas, M, Haldar, PK. The triterpenoid fraction from Trichosanthes dioica root exhibits in vitro antileishmanial effect against Leishmania donovani promastigotes. Pharmacognosy. Res. 2013;5:109–112. doi: 10.4103/0974-8490.110540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calla-Magarinos, J, Quispe, T, Gimenez, A, Freysdottir, J, Troye-Blomberg, M, Fernandez, C. Quinolinic alkaloids from Galipea longiflora Krause suppress production of proinflammatory cytokines in vitro and control inflammation in vivo upon Leishmania infection in mice. Scand. J. Immunol. 2013;77:30–38. doi: 10.1111/sji.12010. [DOI] [PubMed] [Google Scholar]
- Carvalho, L, Luque-Ortega, JR, Lopez-Martin, C, Castanys, S, Rivas, L, Gamarro, F. The 8-aminoquinoline analogue sitamaquine causes oxidative stress in Leishmania donovani promastigotes by targeting succinate dehydrogenase. Antimicrob. Agents. Chemother. 2011;55:4204–4210. doi: 10.1128/AAC.00520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty, J, Sundar, S. Drug resistance in leishmaniasis. J. Global. Infect. Dis. 2010;2:167–176. doi: 10.4103/0974-777X.62887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran, S, Dayakar, A, Veronica, J, Sundar, S, Maurya, R. An in vitro study of apoptotic like death in Leishmaniadonovani promastigotes by withanolides. Parasitol. Int. 2013;62:253–261. doi: 10.1016/j.parint.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Chouhan, G, Islamuddin, M, Sahal, D, Afrin, F. Exploring the role of medicinal plant-based immuno-modulators for effective therapy of leishmaniasis. Front. Immunol. 2014;5:193. doi: 10.3389/fimmu.2014.00193. doi: 10.3389/fimmu.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, S, Mukherjee, T, Mukhopadhyay, R, Mukherjee, B, Sengupta, S, Chattopadhyay, S, Jaisankar, P, Roy, S, Majumder, HK. The lignan niranthin poisons Leishmania donovani topoisomerase IB and favours a Th1 immune response in mice. EMBO. Mol. Med. 2012;4:1126–1143. doi: 10.1002/emmm.201201316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colares, AV, Almeida-Souza, F, Taniwaki, NN, Souza Cda, S, da Costa, JG, Calabrese Kda, S, Abreu-Silva, AL. In vitro antileishmanial activity of essential oil of Vanillosmopsis arborea (Asteraceae) baker. Evid. Based. Complement. Alternat. Med. 2013;2013:727042. doi: 10.1155/2013/727042. doi: 10.1155/2013/727042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura-Vital, W, Reis, AB, Reis, LE, Braga, SL, Roatt, BM, Aquiar-Soares, RD, Marques, MJ, Veloso, VM, Carneiro, M. Canine visceral leishmaniasis: incidence and risk factors for infection in a cohort study in Brazil. Vet. Parasitol. 2013;197:411–417. doi: 10.1016/j.vetpar.2013.07.031. [DOI] [PubMed] [Google Scholar]
- da Costa, RC, Santana, DB, Araujo, RM, de Paula, JE, do Nascimento, PC, Lopes, NP, Braz-Filho, R, Espindola, LS. Discovery of the rapanone and suberonone mixture as a motif for leishmanicidal and antifungal applications. Bioorg. Med. Chem. 2014;22:135–140. doi: 10.1016/j.bmc.2013.11.044. [DOI] [PubMed] [Google Scholar]
- Daneshbod, Y, Oryan, A, Davarmanesh, M, Shirian, S, Negahban, S, Aledavood, A, Davarpanah, MA, Soleimanpoor, H, Daneshbod, K. Clinical, histopathologic, and cytologic diagnosis of mucosal leishmaniasis and literature review. Arch. Pathol. Lab. Med. 2011;135:478–482. doi: 10.5858/2010-0069-OA.1. [DOI] [PubMed] [Google Scholar]
- Das, S, Roy, P, Mondal, S, Bera, T, Mukherjee, A. One pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids. Surf. B. Biointerfaces. 2013;107:27–34. doi: 10.1016/j.colsurfb.2013.01.061. [DOI] [PubMed] [Google Scholar]
- Dastgheib, L, Naseri, M, Mirashe, Z. Both combined oral azithromycin plus allopurinol and intramuscular glucantime yield low efficacy in the treatment of old world cutaneous leishmaniasis: a randomized controlled clinical trial. Int. J. Dermatol. 2012;51:1508–1511. doi: 10.1111/j.1365-4632.2012.05610.x. [DOI] [PubMed] [Google Scholar]
- de Albuquerque Melo, GM, Silva, MC, Guimaraes, TP, Pinheiro, KM, da Matta, CB, de Queiroz, AC, Pivatto, M, Bolzani Vda, S, Alexandre-Moreira, MS, Viegas, CJr. Leishmanicidal activity of the crude extract, fractions and major piperidine alkaloids from the flowers of Senna spectabilis. Phytomedicine. 2014;21:277–281. doi: 10.1016/j.phymed.2013.09.024. [DOI] [PubMed] [Google Scholar]
- de Medeiros, Md, da Silva, AC, Cito, AM, Borges, AR, de Lima, SG, Lopes, JA, Figueiredo, RC. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011;60:237–241. doi: 10.1016/j.parint.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Diro, E, Lynen, L, Mohammed, R, Boelaert, M, Hailu, A, van Griensven, J. High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS. Negl. Trop. Dis. 2014;8:e2875. doi: 10.1371/journal.pntd.0002875. doi: 10.1371/journal.pntd.0002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch, KJ, Petersen, CA. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 2013;26:58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias-Junior, PA, Rios, MC, Moura, TA, Almeida, RP, Alves, PB, Blank, AF, Fernandes, RP, Scher, R. Leishmanicidal activity of carvacrol-rich essential oil from Lippia sidoides Cham. Biol. Res. 2012;45:399–402. doi: 10.4067/S0716-97602012000400012. [DOI] [PubMed] [Google Scholar]
- Fernandez, OL, Diaz-Toro, Y, Ovalle, C, Valderrama, L, Muvdi, S, Rodriguez, I, Gomez, MA, Saravia, NG. Miltefosine and antimonial drug susceptibility of Leishmania/Viannia species and populations in regions of high transmission in Colombia. PLoS. Negl. Trop. Dis. 2014;8:e2871. doi: 10.1371/journal.pntd.0002871. doi: 10.1371/journal.pntd.0002871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladvand, M, Barazesh, A, Tahmasebi, R. Evaluation of in vitro antileishmanial activity of curcumin and its derivatives “gallium curcumin, indium curcumin and diacethyle curcumin”. Eur. Rev. Med. Pharmacol. Sci. 2013;17:3306–3308. [PubMed] [Google Scholar]
- Gamboa-Leon, R, Vera-Ku, M, Peraza-Sanchez, SR, Ku-Chulim, C, Horta-Baas, A, Rosado-Vallado, M. Antileishmanial activity of a mixture of Tridaxprocumbens and Alliumsativum in mice. Parasite. 2014;21:15. doi: 10.1051/parasite/2014016. doi: 10.org/10.1051/parasite/2014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, FP, Lazarin-Bidoia, D, Ueda-Nakamura, T, Silva Sde, O, Nakamura, CV. Eupomatenoid-5 isolated from leaves of Piper regnellii induces apoptosis in Leishmania amazonensis. Evid. Based. Complement. Alternat. Med. 2013;2013:940531. doi: 10.1155/2013/940531. doi: 10.1155/2013/940531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, M, Monzote, L, Montalvo, AM, Scull, R. Screening of medicinal plants against Leishmania amazonensis. Pharm. Biol. 2010;48:1053–1058. doi: 10.3109/13880200903485729. [DOI] [PubMed] [Google Scholar]
- Garcia, M, Monzote, L, Scull, R, Herrera, P. Activity of Cuban plants extracts against Leishmania amazonensis. ISRN. Pharmacol. 2012;2012:104540. doi: 10.5402/2012/104540. doi: 10.5402/2012/104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazanion, E, Vergnes, B, Seveno, M, Garcia, D, Oury, B, Ait-Oudhia, K, Ouaissi, A, Sereno, D. In vitro activity of nicotinamide/antileishmanial drug combinations. Parasitol. Int. 2011;60:19–24. doi: 10.1016/j.parint.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Gharavi, M, Nobakht, M, Khademvatan, S, Fani, F, Bakhshayesh, M, Roozbehani, M. The effect of aqueous garlic extract on interleukin-12 and 10 levels in Leishmania major (MRHO/IR/75/ER) infected macro-phages. Iran. J. Public Health. 2011;40:105–111. [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S, Debnath, S, Hazra, S, Hartung, A, Thomale, K, Schultheis, M, Kapkova, P, Schurigt, U, Moll, H, Holzgrabe, U, Hazra, B. Valeriana wallichii root extracts and fractions with activity against Leishmania spp. Parasitol. Res. 2011;108:861–871. doi: 10.1007/s00436-010-2127-0. [DOI] [PubMed] [Google Scholar]
- Guimaraes, LR, Rodrigues, AP, Marinho, PS, Muller, AH, Guilhon, GM, Santos, LS, do Nascimento, JL, Silva, EO. Activity of the julocrotine, a glutarimide alkaloid from Croton pullei var glabrior, on Leishmania (L.) amazonensis. . Parasitol. Res. 2010;107:1075–1081. doi: 10.1007/s00436-010-1973-0. [DOI] [PubMed] [Google Scholar]
- Haldar, N, Basu, S, Bhattacharya, S, Pandey, JN, Biswas, M. Antileishmanial activity of Mangifera indica leaf extracts on the in vitro growth of Leishmania donovani promastigotes. Elixir. Pharmacy. 2012;46:8189–8191. [Google Scholar]
- Hatam, GR, Bahrami, S, Razavi, SM, Oryan, A. Isoenzyme and ultrastructural characterization of Leishmaniatropica axenic amastigotes and promastigotes. Parasitol. Res. 2013;112:643–648. doi: 10.1007/s00436-012-3179-0. [DOI] [PubMed] [Google Scholar]
- Heidarpour Bami, M, Khazraiinia, P, Haddadzadeh, HR, Kazemi, B. Identification of Theileria species in sheep in the eastern half of Iran using nested PCR-RFLP and microscopic techniques. 2010;11:262–266. [Google Scholar]
- Hosseininejad, M, Mohebali, M, Hosseini, F, Karimi, S, Sharifzad, S, Akhoundi, B. Seroprevalence of canine visceral leishmaniasis in asymptomatic dogs in Iran. Iranian J. Vet. Res. 2012;13:54–57. [Google Scholar]
- Iqbal, H, Khattak, B, Ayaz, S, Rehman, A, Ishfaq, M, Naseer Abbas, M, Rehman, H, Waheed, S, Wahab, A. Comparative efficacy of Aloe vera and Tamarix aphylla against cutaneous leishmaniasis. IJBMSP. 2012;2:42–45. [Google Scholar]
- Islamuddin, M, Farooque, A, Dwarakanath, BS, Sahal, D, Afrin, F. Extracts of Artemisia annua leaves and seeds mediate programmed cell death in Leishmania donovani. J. Med. Microbiol. 2012;61:1709–1718. doi: 10.1099/jmm.0.049387-0. [DOI] [PubMed] [Google Scholar]
- Jabini, R, Jaafari, MR, Vahdati Hasani, F, Ghazizadeh, F, Khamesipour, A, Karimi, G. Effects of combined therapy with silymarin and glucantime on leishmaniasis induced by Leishmania major in BALB/c mice. Drug. Res. (Stuttg) 2014;65:119–124. doi: 10.1055/s-0034-1370914. [DOI] [PubMed] [Google Scholar]
- Jain, K, Jain, NK. Novel therapeutic strategies for treatment of visceral leishmaniasis. Drug. Discov. Today. 2013;18:1272–1281. doi: 10.1016/j.drudis.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Jameel, M, Islamuddin, M, Ali, A, Afrin, F, Ali, M. Isolation, characterization and antimicrobial evaluation of a novel compound N-octacosan 7β ol, from Fumaria parviflora Lam. BMC. Complement. Altern. Med. 2014;14:98. doi: 10.1186/1472-6882-14-98. doi: 10.1186/1472-6882-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebran, AF, Schleicher, U, Steiner, R, Wentker, P, Mahfuz, F, Stahl, HC, Mohamad Amin, F, Bogdan, C, Stahl, KW. Rapid healing of cutaneous leishmaniasis by high-frequency electrocauterization and hydrogel wound care with or without DAC N-055: a randomized controlled phase IIa trial in Kabul. PLoS. Negl. Trop. Dis. 2014;8:e2694. doi: 10.1371/journal.pntd.0002694. doi: 10.1371/journal.pntd.0002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, MG, Bhaskar, KR, Kikuchi, M, Salam, MA, Akther, T, Haque, R, Mondal, D, Hamano, S. Com-parison of PCR-based diagnoses for visceral leishmaniasis in Bangladesh. Parasitol. Int. 2014;63:327–331. doi: 10.1016/j.parint.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Kheiri Manjili, H, Jafari, H, Ramazani, A, Davoudi, N. Anti-leishmanial and toxicity activities of some selected Iranian medicinal plants. Parasitol. Res. 2012;111:2115–2121. doi: 10.1007/s00436-012-3059-7. [DOI] [PubMed] [Google Scholar]
- Lage, PS, de Andrade, PH, Lopes Ade, S, Chavez Fumagalli, MA, Valadares, DG, Duarte, MC, Pagliara Lage, D, Costa, LE, Martins, VT, Ribeiro, TG, Filho, JD, Tavares, CA, de Padua, RM, Leite, JP, Coelho, EA. Strychnos pseudoquina and its purified compounds present an effective in vitro antileishmanial activity. Evid. Based. Complement. Alternat. Med. 2013;2013:304354. doi: 10.1155/2013/304354. doi: 10.1155/2013/304354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindoso, JA, Costa, JM, Queiroz, IT, Goto, H. Review of the current treatments for leishmaniases. Res. Rep. Trop. Med. 2012;3:69–77. doi: 10.2147/RRTM.S24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau, PM, Gupta, S, Verma, A, Srivastava, S, Puri, SK, Sliman, F, Normand-Bayle, M, Desmaele, D. In vitro activities of new 2-substituted quinolines against Leishmania donovani. Antimicrob. Agents. Chemother. 2011;55:1777–1780. doi: 10.1128/AAC.01299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, M, Dinis, AM, Santos-Rosa, M, Alves, V, Salgueiro, L, Cavaleiro, C, Sousa, MC. Activity of Thymus capitellatus volatile extract, 1,8-cineole and borneol against Leishmania species. Vet. Parasitol. 2014;200:39–49. doi: 10.1016/j.vetpar.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Machado, M, Pires, P, Dinis, AM, Santos-Rosa, M, Alves, V, Salgueiro, L, Cavaleiro, C, Sousa, MC. Monoterpenic aldehydes as potential anti-Leishmania agents: activity of Cymbopogon citratus and citral on L infantum, L. tropica and L. major. . Exp. Parasitol. 2012;130:223–231. doi: 10.1016/j.exppara.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand, H, Ayatollahi-Mousavi, SA, Sepahvand, A, Sharififar, F, Ezatpour, B, Gorohi, F, Saedi Dezaki, E, Jahanbakhsh, S. Antifungal, antileishmanial, and cytotoxicity activities of various extracts of Berberis vulgaris (Berberidaceae) and its active principle berberine. ISRN. Pharmacol. 2014;2014:602436. doi: 10.1155/2014/602436. doi: 10.1155/2014/ 602436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makwali, JA, Wanjala, FM, Kaburi, JC, Ingonga, J, Byrum, WW, Anjili, CO. Combination and monotherapy of Leishmania major infection in BALB/c mice using plant extracts and herbicides. J. Vector. Borne Dis. 2012;49:123–130. [PubMed] [Google Scholar]
- Mallick, S, Dutta, A, Dey, S, Ghosh, J, Mukherjee, D, Sultana, SS, Mandal, S, Paloi, S, Khatua, S, Acharya, K, Pal, C. Selective inhibition of Leishmania donovani by active extracts of wild mushrooms used by the tribal population of India: an in vitro exploration for new leads against parasitic protozoans. Exp. Parasitol. 2014;138:9–17. doi: 10.1016/j.exppara.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Manjolin, LC, dos Reis, MB, Maquiaveli Cdo, C, Santos-Filho, OA, da Silva, ER. Dietary flavonoids fisetin, luteolin and their derived compounds inhibit arginase, a central enzyme in Leishmania (Leishmania) amazonensis infection. Food. Chem. 2013;141:2253–2262. doi: 10.1016/j.foodchem.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Mansour, R, Haouas, N, Ben Kahla-Nakbi, A, Hammami, S, Mighri, Z, Mhenni, F, Babba, H. The effect of Vitisvinifera L leaves extract on Leishmaniainfantum. . Iran. J. Pharm. Res. 2013;12:349–355. [PMC free article] [PubMed] [Google Scholar]
- Mehrabani, D, Motazedian, MH, Asgari, Q, Hatam, GR, Owji, SM, Oryan, A. Leishmania major in Tatera indica in Estahban, southern Iran: microscopy, culture, isoenzyme and PCR. Pak. J. Med. Sci. 2011;27:734–738. [Google Scholar]
- Mehrabani, D, Motazedian, MH, Oryan, A, Asgari, Q, Hatam, GR, Karamian, M. A search for the rodent hosts of Leishmaniamajor in the Larestan region of southern Iran: demonstration of the parasite in Tateraindica and Gerbillus sp , by microscopy, culture and PCR. . Ann. Trop. Med. Parasitol. 2007;101:315–322. doi: 10.1179/136485907X176445. [DOI] [PubMed] [Google Scholar]
- Mishra, BB, Singh, RK, Srivastava, A, Tripathi, VJ, Tiwari, VK. Fighting against leishmaniasis: search of alkaloids as future true potential anti-Leishmanial agents. Mini. Rev. Med. Chem. 2009;9:107–123. doi: 10.2174/138955709787001758. [DOI] [PubMed] [Google Scholar]
- Mohapatra, S. Drug resistance in leishmaniasis: newer developments. Trop. Parasitol. 2014;4:4–9. doi: 10.4103/2229-5070.129142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzote, L. Antileishmanial patents antileishmanial current drugs and relevant patents. Recent. Pat. Antiinfect. Drug. Discov. 2011;6:1–26. doi: 10.2174/157489111794407868. [DOI] [PubMed] [Google Scholar]
- Monzote, L, Garcia, M, Pastor, J, Gil, L, Scull, R, Maes, L, Cos, P, Gille, L. Essential oil from Chenopodium ambrosioides and main components: activity against Leishmania, their mitochondria and other microorganisms. Exp. Parasitol. 2014a;136:20–26. doi: 10.1016/j.exppara.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Monzote, L, Garcia, M, Scull, R, Cuellar, A, Setzer, WN. Antileishmanial activity of the essential oil from Bixa orellana. Phytother. Res. 2014b;28:753–758. doi: 10.1002/ptr.5055. [DOI] [PubMed] [Google Scholar]
- Mosallanejad, B, Avizeh, R, Razi Jalali, MH, Pourmehdi, M. A study on seroprevalence and coproantigen detection of Toxoplasma gondii in companion cats in Ahvaz area, southwestern Iran. Iranian J. Vet. Res. 2011;12:139–144. [Google Scholar]
- Motazedian, MH, Mehrabani, D, Oryan, A, Asgari, Q, Karamian, M, Kalantari, M. 2006) Life cycle of cutaneous leishmaniasis in Larestan, southern Iran. Iran. J. Clin. Infect. Dis. 1:137–143. [Google Scholar]
- Mutiso, JM, Macharia, JC, Kiio, MN, Ichagichu, JM, Rikoi, H, Gicheru, MM. Development of Leishmania vaccines: predicting the future from past and present experience. J. Biomed. Res. 2013;27:85–102. doi: 10.7555/JBR.27.20120064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekouie, H, Assmar, M, Razavi, MR, Naddaf, SR. A study on Leishmania infection rate among Phlebotomus spp collected from Abardejh district, Iran. . Iranian J. Vet. Res. 2006;7:77–81. [Google Scholar]
- Neris, PL, Caldas, JP, Rodrigues, YK, Amorim, FM, Leite, JA, Rodrigues-Mascarenhas, S, Barbosa-Filho, JM, Rodrigues, LC, Oliveira, MR. Neolignan Licarin A presents effect against Leishmania (Leishmania) major associated with immunomodulation in vitro. Exp. Parasitol. 2013;135:307–313. doi: 10.1016/j.exppara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Neves, LO, Talhari, AC, Gadelha, EP, Silva Junior, RM, Guerra, JA, Ferreira, LC, Talhari, S. A randomized clinical trial comparing meglumine anti-moniate, pentamidine and amphotericin B for the treatment of cutaneous leishmaniasis by Leishmania guyanensis. An. Bras. Dermatol. 2011;86:1092–1101. doi: 10.1590/s0365-05962011000600005. [DOI] [PubMed] [Google Scholar]
- Ogeto, TK, Odhiambo, RA, Shivairo, RS, Muleke, CI, Osero, BO, Anjili, C, Ingonga, JM, Osuga, IM. Antileishmanial activity of Aloe secundiflora plant extracts against Leishmania major. Adv. Life. Sci. Tech. 2013;13:9–18. [Google Scholar]
- Ogungbe, IV, Erwin, WR, Setzer, WN. Antileishmanial phytochemical phenolics: molecular docking to potential protein targets. J. Mol. Graph. Model. 2014;48:105–117. doi: 10.1016/j.jmgm.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Oryan, A, Alidadi, S, Akbari, M. Risk factors associated with leishmaniasis. Trop. Med. Surg. 2014;2:e118. doi: 10.4172/2329-9088.1000e118. [Google Scholar]
- Oryan, A, Mehrabani, D, Owji, SM, Motazedian, MH, Asgari, Q. Histopathologic and electron micros-copic characterization of cutaneous leishmaniasis in Tateraindica and Gerbillus spp infected with Leishmaniamajor. . Comp. Clin. Pathol. 2007;16:275–279. [Google Scholar]
- Oryan, A, Mehrabani, D, Owji, SM, Motazedian, MH, Hatam, G. Morphologic changes due to cutaneous leishmaniosis in BALB/c mice experimentally infected with Leishmaniamajor. J. Appl. Anim. Res. 2008;34:87–92. [Google Scholar]
- Oryan, A, Shirian, S, Tabandeh, MR, Hatam, GR, Kalantari, M, Daneshbod, Y. Molecular, cytological, and immunocytochemical study and kDNA sequencing of laryngeal Leishmaniainfantum infection. Parasitol. Res. 2013a;112:1799–1804. doi: 10.1007/s00436-012-3240-z. [DOI] [PubMed] [Google Scholar]
- Oryan, A, Shirian, S, Tabandeh, MR, Hatam, GR, Randau, G, Daneshbod, Y. Genetic diversity of Leishmaniamajor strains isolated from different clinical forms of cutaneous leishmaniasis in southern Iran based on minicircle kDNA. Infect. Genet. Evol. 2013b;19:226–231. doi: 10.1016/j.meegid.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Oryan, A, Zaker, SR. Effects of topical application of honey on cutaneous wound healing in rabbits. Zentralbl. Veterinarmed. A. 1998;45:181–188. doi: 10.1111/j.1439-0442.1998.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Parvizi, P, Amirkhani, A. Mitochondrial DNA characterization of Sergentomyiasintoni populations and finding mammalian Leishmania infections in this sandfly by using ITS-rDNA gene. Iranian J. Vet. Res. 2008;9:9–18. [Google Scholar]
- Prajapati, VK, Awasthi, K, Gautam, S, Yadav, TP, Rai, M, Srivastava, ON, Sundar, S. Targeted killing of Leishmania donovaniin vivo and in vitro with amphotericin B attached to functionalized carbon nanotubes. J. Antimicrob. Chemother. 2011a;66:874–879. doi: 10.1093/jac/dkr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati, VK, Awasthi, K, Yadav, TP, Rai, M, Srivastava, ON, Sundar, S. An oral formulation of amphotericin B attached to functionalized carbon nanotubes is an effective treatment for experimental visceral leishmaniasis. J. Infect. Dis. 2011b;205:333–336. doi: 10.1093/infdis/jir735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabito, MF, Britta, EA, Pelegrini, BL, Scariot, DB, Almeida, MB, Nixdorf, SL, Nakamura, CV, Ferreira, IC. In vitro and in vivo antileishmania activity of sesquiterpene lactone-rich dichloromethane fraction obtained from Tanacetum parthenium (L ) Schultz-Bip. . Exp. Parasitol. 2014;143:18–23. doi: 10.1016/j.exppara.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Rahbari, S, Nabian, S, Khaki, Z, Alidadi, N, Ashrafihelan, J. Clinical, haematologic and pathologic aspects of experimental ovine babesiosis in Iran. Iranian J. Vet. Res. 2008;9:59–64. [Google Scholar]
- Rajemiarimiraho, M, Banzouzi, JT, Nicolau-Travers, ML, Ramos, S, Cheikh-Ali, Z, Bories, C, Rakotonandrasana, OL, Rakotonandrasana, S, Andrianary, PA, Benoit-Vical, F. Antiprotozoal activities of Millettia richardiana (Fabaceae) from Madagascar. Molecules. 2014;19:4200–4211. doi: 10.3390/molecules19044200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, TG, Chavez-Fumagalli, MA, Valadares, DG, Franca, JR, Lage, PS, Duarte, MC, Andrade, PH, Martins, VT, Costa, LE, Arruda, AL, Faraco, AA, Coelho, EA, Castilho, RO. Antileishmanial activity and cytotoxicity of Brazilian plants. Exp. Parasitol. 2014;143:60–68. doi: 10.1016/j.exppara.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Rodrigues, IA, Azevedo, MM, Chaves, FC, Alviano, CS, Alviano, DS, Vermelho, AB. Arrabidaea chica hexanic extract induces mitochondrion damage and peptidase inhibition on Leishmania spp. Biomed. Res. Int. 2014;2014:985171. doi: 10.1155/2014/985171. doi: 10.1155/2014/985171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, IA, Azevedo, MM, Chaves, FC, Bizzo, HR, Corte-Real, S, Alviano, DS, Alviano, CS, Rosa, MS, Vermelho, AB. In vitro cytocidal effects of the essential oil from Croton cajucara (red sacaca) and its major constituent 7-hydroxycalamenene against Leishmania chagasi. BMC. Complement. Altern. Med. 2013;13:249. doi: 10.1186/1472-6882-13-249. doi: 10.1186/1472-6882-13-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon, FC, Bevilaqua, CM, Accioly, MP, de Morais, SM, de Andrade-Junior, HF, de Carvalho, CA, Lima, JC, Magalhaes, HC. In vitro efficacy of Coriandrum sativum, Lippia sidoides and Copaifera reticulata against Leishmaniachagasi. Rev. Bras. Parasitol. Vet. 2012;21:185–191. doi: 10.1590/s1984-29612012000300002. [DOI] [PubMed] [Google Scholar]
- Rondon, FC, Bevilaqua, CM, Accioly, MP, Morais, SM, Andrade-Junior, HF, Machado, LK, Cardoso, RP, Almeida, CA, Queiroz-Junior, EM, Rodrigues, AC. In vitro effect of Aloe vera, Coriandrum sativum and Ricinus communis fractions on Leishmania infantum and on murine monocytic cells. Vet. Parasitol. 2011;178:235–240. doi: 10.1016/j.vetpar.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Sachdeva, H, Sehgal, R, Kaur, S. Studies on the protective and immunomodulatory efficacy of Withaniasomnifera along with cisplatin against experimental visceral leishmaniasis. Parasitol. Res. 2013;112:2269–2280. doi: 10.1007/s00436-013-3387-2. [DOI] [PubMed] [Google Scholar]
- Sachdeva, H, Sehgal, R, Kaur, S. Tinospora cordifolia as a protective and immunomodulatory agent in combination with cisplatin against murine visceral leishmaniasis. Exp. Parasitol. 2014a;137:53–65. doi: 10.1016/j.exppara.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Sachdeva, H, Sehgal, R, Kaur, S. Asparagus racemosus ameliorates cisplatin induced toxicities and augments its antileishmanial activity by immuno-modulation in vivo. Parasitol. Int. 2014b;63:21–30. doi: 10.1016/j.parint.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Safi, N, Davis, GD, Nadir, M, Hamid, H, Robert, LLJr, Case, AJ. Evaluation of thermotherapy for the treatment of cutaneous leishmaniasis in Kabul, Afghanistan: a randomized controlled trial. Mil. Med. 2012;177:345–351. doi: 10.7205/milmed-d-11-00161. [DOI] [PubMed] [Google Scholar]
- Saha, P, Bhattacharjee, S, Sarkar, A, Manna, A, Majumder, S, Chatterjee, M. Berberine chloride mediates its anti-leishmanial activity via differential regulation of the mitogen activated protein kinase pathway in macrophages. PLoS. One. 2011;6:e18467. doi: 10.1371/journal.pone.0018467. doi: 10.1371/journal.pone.0018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehabadi, A, Karamian, M, Farzad, MH, Namaei, MH. Effect of root bark extract of Berberis vulgaris L on Leishmania major on BALB/c mice. . Parasitol. Res. 2014;113:953–957. doi: 10.1007/s00436-013-3727-2. [DOI] [PubMed] [Google Scholar]
- Sarkar, J, Pal, S, Bhattacharya, S, Biswas, M. In vitro antileishmanial activity of Pleumeria pudica leaf extracts on Leishmania donovani promastigotes. Am-Euras. J. Sci. Res. 2013;8:68–71. [Google Scholar]
- Sattar, FA, Ahmed, F, Ahmed, N, Sattar, SA, Malghani, MA, Choudhary, MI. A double-blind, randomized, clinical trial on the antileishmanial activity of a Morinda citrifolia (Noni) stem extract and its major constituents. Nat. Prod. Commun. 2012;7:195–196. [PubMed] [Google Scholar]
- Shakya, N, Sane, SA, Gupta, S. Antileishmanial efficacy of fluconm and miltefosine in combination with an immunomodulator picroliv. Parasitol. Res. 2011;108:793–800. doi: 10.1007/s00436-010-2230-2. [DOI] [PubMed] [Google Scholar]
- Seifert, K. Structures, targets and recent approaches in anti-leishmanial drug discovery and development. Open. Med. Chem. J. 2011;5:31–39. doi: 10.2174/1874104501105010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, R, Chatterjee, M. Plant derived therapeutics for the treatment of leishmaniasis. Phytomedicine. 2011;18:1056–1069. doi: 10.1016/j.phymed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Shirian, S, Oryan, A, Hatam, GR, Daneshbod, Y. Mixed mucosal leishmaniasis infection caused by Leishmaniatropica and Leishmaniamajor. J. Clin. Microbiol. 2012a;50:3805–3808. doi: 10.1128/JCM.01469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirian, S, Oryan, A, Hatam, GR, Daneshbod, Y. Three Leishmania/L species--L. infantum, L.major, L. tropica--as causative agents of mucosal leishmaniasis in Iran. . Pathog. Glob. Health. 2013;107:267–272. doi: 10.1179/2047773213Y.0000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirian, S, Oryan, A, Hatam, GR, Daneshbod, K, Daneshbod, Y. Molecular diagnosis and species identification of mucosal leishmaniasis in Iran and correlation with cytological findings. Acta Cytol. 2012b;56:304–309. doi: 10.1159/000337450. [DOI] [PubMed] [Google Scholar]
- Shirian, S, Oryan, A, Hatam, GR, Panahi, S, Daneshbod, Y. Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch. Pathol. Lab. Med. 2014;138:235–240. doi: 10.5858/arpa.2013-0098-OA. [DOI] [PubMed] [Google Scholar]
- Sifaoui, I, Lopez-Arencibia, A, Martin-Navarro, CM, Chammem, N, Reyes-Batlle, M, Mejri, M, Lorenzo-Morales, J, Abderabba, M, Pinero, JE. Activity of olive leaf extracts against the promastigote stage of Leishmania species and their correlation with the antioxidant activity. Exp. Parasitol. 2014;141:106–111. doi: 10.1016/j.exppara.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Sousa, MC, Varandas, R, Santos, RC, Santos-Rosa, M, Alves, V, Salvador, JA. Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: synergistic effects with miltefosine. PLoS. One. 2014;9:e89939. doi: 10.1371/journal.pone.0089939. doi: 10.1371/journal. pone.0089939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarshan, M, Singh, T, Singh, AK, Chourasia, A, Singh, B, Wilson, ME, Chakravarty, J, Sundar, S. Quantitative PCR in epidemiology for early detection of visceral leishmaniasis cases in India. PLoS. Negl. Trop. Dis. 2014;8:e3366. doi: 10.1371/journal.pntd.0003366. doi: 10.1371/journal.pntd.0003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiuman, TS, Brenzan, MA, Ueda-Nakamura, T, Filho, BP, Cortez, DA, Nakamura, CV. Intramuscular and topical treatment of cutaneous leishmaniasis lesions in mice infected with Leishmania amazonensis using coumarin (-) mammea A/BB. Phytomedicine. 2012;19:1196–1199. doi: 10.1016/j.phymed.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Vila-Nova, NS, de Morais, SM, Falcao, MJ, Alcantara, TT, Ferreira, PA, Cavalcanti, ES, Vieira, IG, Campello, CC, Wilson, M. Different susceptibilities of Leishmania spp promastigotes to the Annona muricata acetogenins annonacinone and corossolone, and the Platymiscium floribundum coumarin scoparone. Exp. Parasitol. 2013;133:334–338. doi: 10.1016/j.exppara.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votypka, J, Kasap, OE, Volf, P, Kodym, P, Alten, B. Risk factors for cutaneous leishmaniasis in Cukurova region, Turkey. Trans. R. Soc. Trop. Med. Hyg. 2012;106:186–190. doi: 10.1016/j.trstmh.2011.12.004. [DOI] [PubMed] [Google Scholar]
- WHO Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the control of leishmaniases. World Health Organ Tech. Rep. Ser. 2010;949:5–11. [Google Scholar]
- Wink, M. Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules. 2012;17:12771–12791. doi: 10.3390/molecules171112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwanitkit, V. Interest in paromomycin for the treatment of visceral leishmaniasis (kala-azar) Ther. Clin. Risk. Manag. 2012;8:323–328. doi: 10.2147/TCRM.S30139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, IL, Chan, KF, Chen, YF, Lun, ZR, Chan, TH, Chow, LM. In vitro and in vivo efficacy of novel flavonoid dimers against cutaneous leishmaniasis. Antimicrob. Agents. Chemother. 2014;58:3379–3388. doi: 10.1128/AAC.02425-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, ES, Campos, BL, Laurenti, MD, Lago, JH, Grecco Sdos, S, Corbett, CE, Passero, LF. Treatment with triterpenic fraction purified from Baccharis uncinella leaves inhibits Leishmania (Leishmania) amazonensis spreading and improves Th1 immune response in infected mice. Parasitol. Res. 2014;113:333–339. doi: 10.1007/s00436-013-3659-x. [DOI] [PubMed] [Google Scholar]
- Zerehsaz, F, Salmanpour, R, Handjani, F, Ardehali, S, Panjehshahin, MR, Tabei, SZ, Tabatabaee, HR. A double-blind randomized clinical trial of a topical herbal extract (Z-HE) vs systemic meglumine antimoniate for the treatment of cutaneous leishmaniasis in Iran. . Int. J. Dermatol. 1999;38:610–612. doi: 10.1046/j.1365-4362.1999.00727.x. [DOI] [PubMed] [Google Scholar]