Abstract
The present work was conducted to compare the occurrence of Escherichia coli possessing virulence and ESBL genes in backyard and farmed poultry. Three hundred and sixty samples from the poultry kept in backyard system and 120 samples from the farmed birds were collected from West Bengal, India. Among the E. coli isolates of backyard poultry (O2, O10, O25, O55, O60, O106, UT), none of them possessed any of the Shiga toxin genes and eight E. coli isolates (8/272; 2.9%) harboured eaeA gene alone. Whereas among the E. coli isolated from the farmed poultry (O17, O20, O22, O102, O114, O119, rough, UT), four isolates (4/78, 5.1%) harboured stx1/stx2 gene and 11 isolates (11/78, 14.1%) possessed eaeA gene. None of the E. coli isolates from the backyard poultry harboured any studied ESBL gene. Whereas 29.4% of E. coli isolates from the farmed poultry were found to possess the ESBL genes.
Key Words: Backyard, ESBL, Poultry, STEC, West Bengal
Introduction
Intestinal pathotypes of Escherichia coli may cause diarrhoea in human and animals (Samanta, 2013 ▶). Among them, food associated outbreaks in human was observed with Shiga toxin-producing E. coli (STEC) and Enteropathogenic E. coli (EPEC) (Newell et al., 2010 ▶).
Further, transmission of antimicrobial resistant E. coli such as extended-spectrum β-lactamase (ESBL) producers in the human food chain from the chicken is a well recognized phenomenon (Hammerum and Heuer, 2009 ▶).
The poultry ranks first as a source of food borne infection in human (Batz et al., 2011 ▶). There are reports of STEC prevalence in farmed poultry (Schouten et al., 2005 ▶; Dipineto et al., 2006 ▶) and such information from backyard poultry is scanty. Hence the purpose of the present work was to compare the occurrence of STEC and EPEC and possession of major ESBL genes in backyard and farmed poultry.
Materials and Methods
Three hundred and sixty samples (cloacal swabs, feed, drinking water, utensil swabs, litter, soil, dried manure, eggs) for bacteriological analysis from the backyard poultry were collected from four agro-climatic zones of West Bengal, India (Terai, New Alluvial, Coastal, Red Laterite) during the period of July-September, 2011. The majority of the birds were young (1-4 weeks), female and apparently healthy. Further, 30 cloacal swabs were collected from the birds kept in organized poultry farm in the same agro climatic zone (n=120). The majority of the birds were young (1-4 weeks), female and apparently healthy.
The standard technique was followed for preparation of the samples and isolation of E. coli as described earlier (Samanta et al., 2014a ▶).
The DNA from all E. coli isolates was extracted as per the earlier method (Samanta et al., 2014a ▶). All isolates including positive control were subjected to m-PCR for detection of stx1, stx2, eae and ehxA genes (Paton and Paton, 1998 ▶). The m-PCR was performed in a gradient thermocycler (Eppendorf ProS, Germany). All the reagents and oligo nucleotide primers were procured from Genetix Biotechnology Asia Private Limited, India. The STEC strain possessing all the four genes supplied by CAU was used as positive control and sterile distilled water was used as negative control.
All the E. coli isolates having the Shiga toxin/intimin/enterohaemolysin genes were sent for O-serogrouping to Central Research Institute, HP, India.
All the E. coli strains including controls were subjected to PCR for detection of major ESBL (blaCTX-M, blaTEM, blaSHV) genes (Samanta et al., 2014b ▶). The E. coli strain (CAU, India) possessing ESBL genes and the sterile distilled water was used as positive and negative control, respectively.
Results
Among the 272 E. coli isolates from the backyard poultry examined by m-PCR, none (0/272) of them possessed any of the Shiga toxin (stx1/stx2) or enterohaemolysin genes (ehxA). However, eight E. coli isolates (8/272; 2.9%) were found to harbour eaeA gene alone (Table 1) and they belonged to O2, O10, O25, O55, O60, O106, and untypeable (UT) serogroups (Table 1).
Table 1.
Characterization of EPEC isolated from backyard birds in different agro-climatic zones in West Bengal, India
| Agro-climatic zone | Serogroup | Genotype |
|||
|---|---|---|---|---|---|
| stx 1 | stx 2 | eaeA | ehxA | ||
| Terai (3) | O2 | - | - | + | - |
| O10 | - | - | + | - | |
| New alluvial (2) | O25 | - | - | + | - |
| O55 | - | - | + | - | |
| Coastal (1) | O60 | - | - | + | - |
| O106 | - | - | + | - | |
| Red laterite (2) | UT | - | - | + | - |
Number in parenthesis indicates the number of positive serogroups
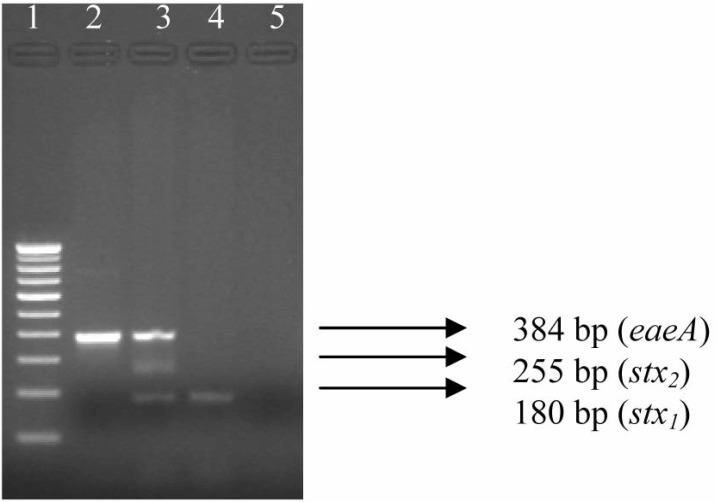
Among the 78 E. coli isolated from the commercial farmed poultry four isolates (4/78, 5.1%) were found to harbour stx1/stx2 gene (Table 2, Fig. 1). Further, 14.1% occurrence of EPEC was detected in healthy farmed poultry (Table 2, Fig. 1). The STEC and EPEC isolates belonged to O17, O20, O22, O102, O114, O119, rough, and untypeable serogroups.
Table 2.
Characterization of STEC/EPEC isolated from commercial poultry in different agro-climatic zones in West Bengal, India
| Agro-climatic zone | Serogroup | Genotype |
|||
|---|---|---|---|---|---|
| stx 1 | stx 2 | eaeA | ehxA | ||
| Terai (7) | O17 | + | - | - | - |
| UT | + | - | - | - | |
| O20 | - | - | + | - | |
| O22 (2) | - | - | + | - | |
| New alluvial (4) | UT | - | + | - | - |
| Rough | - | + | - | - | |
| O119 | - | - | + | - | |
| O114 | - | - | + | - | |
| UT | - | - | + | - | |
| Coastal (3) | O102 | - | - | + | - |
| UT | - | - | + | - | |
| Red laterite (1) | O20 (2) | - | - | + | - |
| Rough | - | - | + | - | |
Number in parenthesis indicates the number of positive serogroups
Fig. 1.
PCR gel documentation showing amplification of different virulence genes of STEC isolated from farmed poultry. Lane 1: 100 bp DNA ladder, Lane 2: Representative isolate possessing eaeA gene alone, Lane 3: A mixed PCR product showing the presence of stx1, stx2, eaeA genes together, Lane 4: Representative isolate possessing stx1 gene only, and Lane 5: Negative control
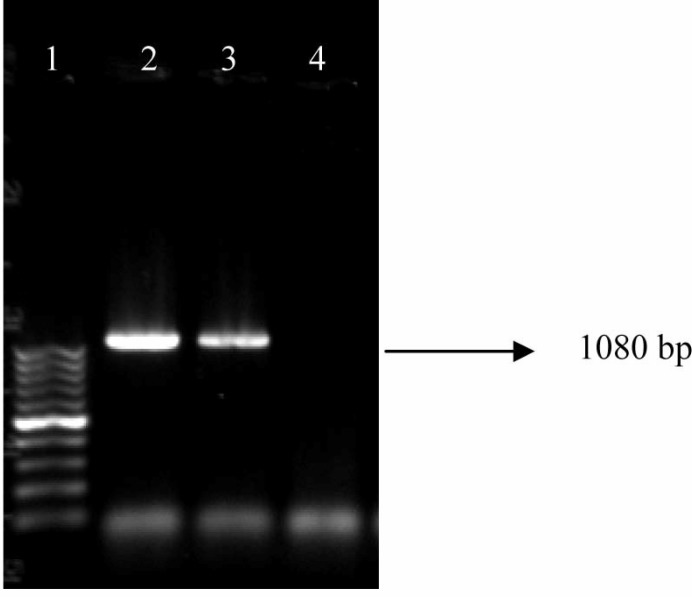
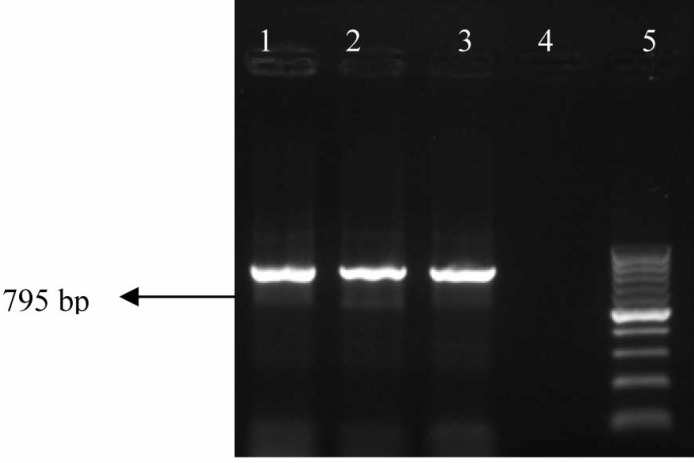
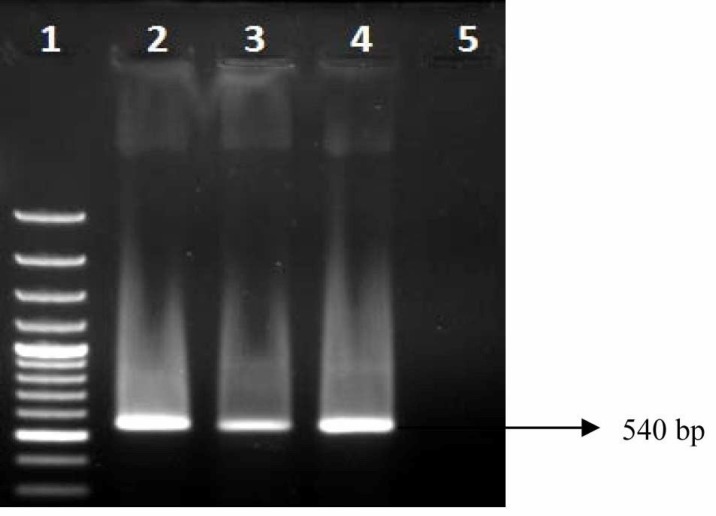
None of the E. coli isolates from the backyard poultry harboured any studied ESBL gene in PCR. Whereas 29.4% of E. coli isolates from the farmed poultry were found to possess none of the ESBL genes in terai and new alluvial zones (Table 3, Figs. 2, 3, 4). In both zones, isolation of blaCTX-M possessing E. coli was highest (43.4%) which was followed by isolation of blaSHV (34.7%) and blaTEM (21.7%) possessing E. coli, respectively (Table 3). None of the ESBL-possessing isolates harboured any gene for Shiga toxins or intimin.
Table 3.
Distribution of major ESBL genes in E. coli isolated from farmed poultry in different agro-climatic zones of West Bengal, India
| Agro-climatic zones | No. of cloacal swabs collected | No. of E. coli isolated | Distribution of studied ESBL genes among the E. coli isolates |
||
|---|---|---|---|---|---|
| bla TEM | bla SHV | bla CTX-M | |||
| Terai | 30 | 19 (63%) | 1 | 2 | 3 |
| New alluvial | 30 | 23 (76%) | 4 | 6 | 7 |
| Coastal | 30 | 17 (56%) | 0 | 0 | 0 |
| Red laterite | 30 | 19 (63%) | 0 | 0 | 0 |
| 5 (21.7%) | 8 (34.7%) | 10 (43.4%) | |||
| Total | 120 | 78 (65%) | 23 (29.4%) | ||
Fig. 2.
PCR gel documentation showing amplification of blaTEM in E. coli isolates from farmed poultry. Lane 1: 100 bp ladder, Lane 2: Representative sample showing positive blaTEM gene, Lane 3: Positive control, and Lane 4: Negative control
Fig. 3.
PCR gel documentation photo showing amplification of blaSHV in E. coli isolates from farmed poultry. Lane 1, 2: Representative sample showing positive blaSHV gene, Lane 3: Positive control, Lane 4: Negative control, and Lane 5: 100 bp ladder
Fig. 4.
PCR gel documentation showing amplification of blaCTX-M in E. coli isolates from farmed poultry. Lane 1: 100 bp ladder, Lane 2, 3: Representative sample showing positive blaCTX-M gene, Lane 4: Positive control, and Lane 5: Negative control
Discussion
The present study could not detect any E. coli isolates possessing Shiga toxin genes from the backyard poultry. Similar finding was observed in free range chicken in Spain (Esteban et al., 2008 ▶). No study was apparently available regarding the isolation of EPEC from backyard poultry to compare the present finding. The serogroups such as O2, O55 and UT were detected as EPEC serogroup from the broiler birds in India earlier (Wani et al., 2004 ▶).
The present study detected Shiga toxin producing-E. coli isolates (4/78, 5.1%) from the farmed poultry. Similarly, previous works isolated STEC (O157:H7) from live poultry in other countries (Schouten et al., 2005 ▶; Dipineto et al., 2006 ▶), as well as from carcass of the broilers in Iran (Salehi, 2012 ▶). The occurrence of EPEC (14.1%) in the farmed poultry in the present study was consistent with earlier findings in India (Farooq et al., 2009 ▶).
The serogroups O17, O20, O22, O78, O102, rough and UT, isolated in the present study as STEC, were also earlier isolated as STEC from pigeon in Northern India (Farooq et al., 2009 ▶) and chicken meat in Western India (Zende et al., 2013 ▶). The serogroups O114 and O119 werealso previously reported as EPEC serogroup from chicken in Brazil (Gonzalez et al., 2000 ▶). Comparing the STEC/EPEC isolation status, it seems that the backyard rearing conditions might have an advantageous effect on diminishing STEC shedding as detected earlier (Esteban et al., 2008 ▶).
The present study could not detect any ESBL gene in the E. coli isolates from the backyard poultry probably due to lack of antibiotic use, specially those associated with the studied ESBL genes. Whereas 29.4% of E. coli isolates from the farmed poultry were found to possess none of the studied ESBL genes. Similarly, 33.3% E. coli isolates from the broiler meat was detected to carry blaCTX-M in the UK (Warren et al., 2008 ▶). The possible use of antibiotics in farmed poultry (cephalosporin, ampicillin) coupled with the greater density of birds in intensive farming system might explain such high levels of resistance in the studied zones. A similar kind of comparative study revealed that the E. coli isolates from the backyard poultry were free of phenotypical resistance to tetracycline, streptomycin and sulphonamide in comparison to the E. coli isolates from farmed poultry in Nigeria (Ojeniyi, 1985). Another study revealed the presence of ESBL in E. coli (3/59, 5.08%) in very low proportion in free range birds than the commercial broilers (15/58, 25.8%). The presence of ESBL in free range layer was due to vertical transfer of the resistance gene from the breeder (Obeng et al., 2012 ▶). Further, the STEC/EPEC isolates of the farmed and backyard poultry did not possess any ESBL gene, which is supported by earlier findings except in one study where ESBL-possessing STEC was detected from farmed poultry (Roset et al., 2007).
With respect to STEC shedding and antimicrobial resistance genes the backyard poultry or their products seem to be safer for human consumption than the farmed poultry in the studied zones in India.
Acknowledgements
The amenities provided by Department of Biotechnology, Government of India and Rastriya Krishi Vikash Yojana West Bengal University of Animal and Fishery Sciences are highly appreciated. We are also grateful to the director, Central Research Institute, India for serotyping.
References
- Batz, MB, Hoffmann, S, Glenn-Morris, J. Ranking the risks: the ten pathogen food combinations with the greatest burden on public health. Emerging Pathogens Institute, University of Florida; 2011. http://www.epi.ufl.edu/sites/www.epi.ufl.edu/files/RankingTheRisksREPORT.pdf. [Google Scholar]
- Dipineto, L, Santaniello, A, Fontanella, M, Lagos, K, Fioretti, A, Menna, LF. Presence of Shiga toxin-producing Escherichia coli O157:H7 in living layer hens. Lett. Appl. Microbiol. 2006;43:293–295. doi: 10.1111/j.1472-765X.2006.01954.x. [DOI] [PubMed] [Google Scholar]
- Esteban, JI, Oporto, B, Aduriz, G, Juste, RA, Hurtado, A. A survey of food-borne pathogens in free-range poultry farms. Int. J. Food Microbiol. 2008;123:177–182. doi: 10.1016/j.ijfoodmicro.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Farooq, S, Hussain, I, Mir, MA, Bhat, MA, Wani, SA. Isolation of atypical enteropathogenic Escherichia coli and Shiga toxin 1 and 2f-producing Escherichia coli from avian species in India. Lett. Appl. Microbiol. 2009;48:692–697. doi: 10.1111/j.1472-765X.2009.02594.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez, AGM, Rosa, ACP, Andrade, JRC, Tibana, A. Enteropathogenicity markers in Escherichia coli strains isolated from soft white cheese and poultry in Rio de Janeiro, Brazil. Food Microbiol. 2000;17:321–328. [Google Scholar]
- Hammerum, AM, Heuer, OE. Human health hazards from antimicrobial-resistant Escherichia coli of animal origin. Clin. Infect. Dis. 2009;48:916–921. doi: 10.1086/597292. [DOI] [PubMed] [Google Scholar]
- Newell, DG, Koopmans, M, Verhoef, L, Duizer, E, Aidara-Kane, A, Sprong, H, Opsteegh, M, Langelaar, M, Threfall, J, Scheutz, F, Van der Giessen, J, Kruse, H. Food-borne diseases- the challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010;139:3–15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng, AS, Rickard, H, Ndi, O, Sexton, M, Barton, M. Antibiotic resistance, phylogenetic grouping and virulence potential of Escherichia coli isolated from the faeces of intensively farmed and free range poultry. Vet. Microbiol. 2012;154:305–315. doi: 10.1016/j.vetmic.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Ojeniyi, AA. Comparative bacterial drug resistance in modern battery and free-range poultry in a tropical environment. Vet. Rec. 1985;117:11–12. doi: 10.1136/vr.117.1.11. [DOI] [PubMed] [Google Scholar]
- Paton, AW, Paton, JC. Detection and characterization of Shiga toxigenic Eschertichia coli by using multiplex-PCR assays for stx1, stx2, eaeA, enterohaemorrhagic E coli hlyA, rfbO111 and rfbO157. . J. Clin. Microbiol. 1998;36:598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi, M. Determination of intimin and Shiga toxin genes in Escherichia coli isolates from gastrointestinal contents of healthy broiler chickens in Kerman city, Iran. Comp. Clin. Pathol. 2012;23:175–179. [Google Scholar]
- Samanta, I. 1st Edn. India: New India Publishing Agency; 2013. Veterinary bacteriology; pp. 120–137. [Google Scholar]
- Samanta, I, Joardar, SN, Das, PK, Das, P, Sar, TK, Dutta, TK, Bandyopadhyay, S, Batabyal, S, Isore, DP. Virulence repertoire, characterization and anti-biotic resistance pattern analysis of Escherichia coli isolated from backyard layers and their environment in India. Avian Dis. 2014a;58:39–45. doi: 10.1637/10586-052913-Reg.1. [DOI] [PubMed] [Google Scholar]
- Samanta, I, Joardar, SN, Das, PK, Sar, TK, Bandyopadhyay, S, Dutta, TK, Sarkar, U. Prevalence and antibiotic resistance profiles of Salmonella serotypes isolated from backyard poultry flocks in West Bengal, India. J. Appl. Poult. Res. 2014b;23:536–545. [Google Scholar]
- Schouten, JM, Van de Giessen, AW, Frankena, K, De Jong, MCM, Graat, EAM. Escherichia coli O157 prevalence in Dutch poultry, pig finishing and veal herds and risk factors in Dutch veal herds. Prev. Vet. Med. 2005;70:1–15. doi: 10.1016/j.prevetmed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Wani, SA, Samanta, I, Bhat, MA, Nishikawa, Y. Investigation of Shiga toxin-producing Escherichia coli in avian species in India. Lett. Appl. Microbiol. 2004;39:389–394. doi: 10.1111/j.1472-765X.2004.01586.x. [DOI] [PubMed] [Google Scholar]
- Warren, RE, Ensor, VM, O’Neill, P, Butler, V, Taylor, J, Nye, K, Harvey, M, Livermore, DM, Woodford, N, Hawkey, PM. Imported chicken meat as a potential source of quinolone-resistant Escherichia coli producing extended-spectrum b-lactamases in the UK. J. Antimicrob. Chemother. 2008;61:504–508. doi: 10.1093/jac/dkm517. [DOI] [PubMed] [Google Scholar]
- Zende, RJ, Chavhan, DM, Suryawanshi, PR, Rai, AK, Vaidya, VM. PCR detection and serotyping of enterotoxigenic and shigatoxigenic Escherichia coli isolates obtained from chicken meat in Mumbai, India. Vet. World. 2013;6:770–773. [Google Scholar]