Abstract
The present investigation was conducted to study the effects of experimental Clostridium perfringens type D enterotoxaemia in teddy goats. Clinical signs started to appear after 30 min of experimental infection like anorexia, diarrhea, dehydration, frothing and dyspnea. Gross lesions consisted of severe congestion in tissues of varying intensity with enlarged mesenteric lymph nodes while histological examination revealed edema of lungs, kidney, and lymph nodes and to some extent in brain along with hemorrhages in lungs and intestines. Clostridium perfringens type D carrying alpha and epsilon toxin genes were amplified with amplicon size about 247 bp and 665 bp, respectively. Human erythrocytes showed the highest hemolysis, 68%, followed by mice, 57%, against culture supernatants. The percentage of hemolysis was significantly higher at 37°C as compared to 25°C except for rabbit and dog.
Key Words: Clostridium perfringens type D, Enterotoxaemia ELISA, Alpha and Epsilon genes, Hemolysis
Introduction
Clostridium perfringens, a Gram-positive, spore forming, anaerobic micro-organism is classified into five types, A, B, C, D and E, based on the synthesis of four major lethal toxins, alpha, beta, epsilon and iota (Zerbini and Ossiprandi, 2009; Sayeed et al., 2010 ▶). The disease is characterized by fever, diarrhea, tympany, respiratory and nervous system signs with various degrees in sheep and goat. Clinical signs, postmortem examination and histopathological preliminary changes help to diagnose the disease. Other tests, i.e. mice inoculation and serum neutralization tests are also used but are undesirable due to high cost, complexity and are considered inhumane (Babe et al., 2012 ▶; Hadimli et al., 2012 ▶).
We previously reported the variation of distribution of alpha and epsilon toxin genes in different organs of lambs being the highest in duodenal tissues (Nasir et al., 2013a ▶). The present study was carried out in goats by experimental administration of C. perfringens type D inoculum, isolated from field sample to study the clinico-pathological lesions. Amplification of alpha and epsilon toxin genes of C. perfringens type D from infected and non infected tissues was performed and hemolytic pattern of erythrocytes of different species at different temperatures was also recorded. These findings will help to diagnose the disease under field conditions along with evaluation of pathogenicity on the basis of hemolytic reaction of erythrocytes in different species.
Materials and Methods
Study area and animals
The study was conducted at University of Veterinary and Animal Sciences, Lahore, Pakistan. Goats of teddy breed were divided into infected (n=6) and control group (n=4). There was no history of vaccination against enterotoxaemia neither in animals nor to their dam. These animals were also kept under similar environmental conditions. Conventionally reared Albino Swiss mice weighing 25 ± 5 g were used for mouse tests.
The project and animals used for the experimental purposes were approved by “The Advance Study and Research Board” of University of Veterinary and Animal Sciences, Lahore, Pakistan.
Preparation and inoculation of inoculum
Clostridium perfringens type D was isolated from field outbreak and initial identification was confirmed by studying the morphogical, biochemical characteristics and mice inoculation test (Effat et al., 2007 ▶). The organism was further confirmed by indirect ELISA (Koc and Gökce, 2007 ▶). The experimental dose was calculated as colony forming units (CFU) per ml in a standard spread technique (Tortora et al., 2010 ▶). We adopted a new approach (right para midline) to C. perfringens type D intraduodenally (Nasir et al., 2013b ▶). Two hundred milliliters of 20% solution of corn flour in 0.85% saline was injected in the abomasum of all animals by general anesthesia. Then, approximately 150 ml inoculums of C. perfringens type D with 4.6 × 108 - 5.7 × 108 CFU/ml was administered per animal of infected group (n=6) intraduodenally.
Clinico-pathological observation of animals
Clinical findings were observed and postmortem examination was performed on all the animals slaughtered after 30 h post infection (PI) or that died during this period of the experiment. Intestinal contents, pieces of liver, lungs, lymph node and kidney showing lesions were collected and fixed in 10% formalin. Paraffin embedding technique and haematoxylin and eosin staining methods were used for histopathological studies (Bancroft and Gamble, 2008 ▶). Scoring of lesions was recorded as described by Mubashar (2010) ▶.
Isolation of organism in different tissues and identification of toxins by ELISA
Tissues including duodenum, liver, kidney and lungs were collected from all the animals kept in experimental groups. Isolation of C. perfringens type D was performed as described by Effat et al. (2007) ▶. The alpha and epsilon bacterial toxin of the organism were confirmed by indirect ELISA (Koc and Gökce, 2007 ▶).
Detection of alpha and epsilon toxin genes by PCR
The DNA was extracted by boiling method as described by Komoriya et al. (2007) ▶. Molecular detection of C. perfringens was performed by PCR amplification of genes (Greco et al., 2005 ▶; Wu et al., 2009). Primers designed were based upon the sequence of target gene and synthesized commercially by GeneBank (Table 1).
Table 1.
Oligonucleotide primers used for amplification of C. perfringens type D alpha and epsilon toxin genes
| Genes | Target primer sequence (5′ to 3′) | TM (°C) | Expected (bp) | References |
|---|---|---|---|---|
| Cpa (F) | TGCTAATGTTACTGCCGTTGATAG | 55.4 | 247 | Greco et al. (2005) |
| Cpa (R) | ATAATCCCAATCATCCCAACTATG | 52.5 | ||
| Etx (F) | GCGGTGATATCCATCTATTC | 50.7 | 665 | Wu et al. (2009) |
| Etx (R) | CCACTTACTTGTCCTACTAAC | 50.2 |
Hemolytic activity of erythrocytes of different species
Hemolytic activity of C. perfringens type D in the erythrocytes of various species was reported as described by Mudenda et al. (2006) ▶ with some modification. Briefly, 1% RBC of different species was prepared in phosphate buffered saline (PBS) solution and supernatant culture of the organism serially diluted followed by addition of 1% (V/V) suspension of erythrocytes. Incubation for 1 h at 25°C and 37°C with shaking was made and centrifuged at 1000 g for 5 min at 4°C. One ml supernatant was taken in cuvette and optic density was measured by a spectrophotometer (UV-Vis Spectrophotometer, Shimadzu, Japan) at 595 nm. For control, 1% RBCs was prepared in distilled water for each species. The percentage of hemolysis was calculated by the formula as described by Hwang et al. (2001).
Statistical analysis
The data obtained was analyzed using ANOVA through SAS 9.1 and means were compared through DMRT.
Results
Clostridium perfringens was microscopically examined as Gram-positive thick rods, biochemical identification showed gas and acid production from glucose, fructose, lactose, sucrose and mannitol was observed. There was a double zone of hemolysis on blood agar. No growth was observed in the aerobic culture. Mouse inoculation test in Swiss Albino mice showed the death within 3 days. Clostridium perfringens type D was confirmed by indirect ELISA based on the determination of alpha and epsilon toxins.
Clinical observation of experimental animals
Reported clinical signs and symptoms after 30 min post inoculation were anorexia, diarrhea, dehydration, frothing and dyspnea. However, no significant difference was recorded in case of tympany, fever, shivering, in-coordination and blindness during specified time intervals. The mean score for each clinical finding at different time was recorded and shown in Table 2. No prominent clinical findings were recorded in animals of control group.
Table 2.
Mean score for each clinical finding at different time intervals (n=6)
| Clinical findings | 10 h | 20 h | 30 h |
|---|---|---|---|
| Anorexia | 1.2 ± 0.3b | 1.7 ± 0.2b | 2.7 ± 0.2a |
| Dehydration | 1.3 ± 0.2b | 2.2 ± 0.3a | 2.8 ± 0.1a |
| Diarrhea | 1.3 ± 0.3b | 2.5 ± 0.3a | 2.8 ± 0.2a |
| Tympany | 0.7 ± 0.3a | 1 ± 0.5a | 1.7 ± 0.5a |
| Fever | 0.3 ± 0.2a | 1 ± 0.4a | 0.3 ± 0.3a |
| Shivering | 0.8 ± 0.4a | 0a | 0.3 ± 0.3a |
| Frothing | 0.5 ± 0.2c | 1.7 ± 0.2b | 2.3 ± 0.2a |
| Dyspnea | 0c | 1.2 ± 0.3b | 2.2 ± 0.1a |
| Incoordination | 0a | 0.5 ± 0.3a | 0.8 ± 0.5a |
| Blindness | 0a | 0.3 ± 0.2a | 0.3 ± 0.3a |
Mean score: 1=mild; up to 2=moderate; 2 and above= severe. Means with the different superscripts in the row for each clinical finding is significantly different (P≤0.05). The significant difference was calculated by Duncan Multiple Range Test (DMRT)
Confirmation of C. perfringens through necropsy and serology
Postmortem examination was carried out after 30 h PI which revealed hemorrhages in intestine, i.e. jejunum ileum and colon along with enlarged mesenteric lymph nodes. Histopathological examination revealed severe congestion, edema, hemorrhage and emphysema while kidney exhibited edema along with increased space of Bowman capsule. Severe congestion, mild edema in some cases and hemorrhage in the brain were also recorded (Figs. 1a-e). A significant percentage of bacterium was recorded in duodenum followed by liver, kidney and lungs. Optical density more than 0.15 for alpha and epsilon toxin was recorded and hence declared positive by ELISA. No significant pathological changes
Fig. 1.
Microscopic histopathological lesions (a), lungs showing loss of alveolar structure and edema (b), severe hemorrhage (c), kidney showing edema, increased Bowman space (d), severe congestion and moderate edema in medulla of kidney (e). Brain showing congestion and mild hemorrhage
were found in animals which were kept as control.
Molecular confirmation of alpha and epsilon toxins
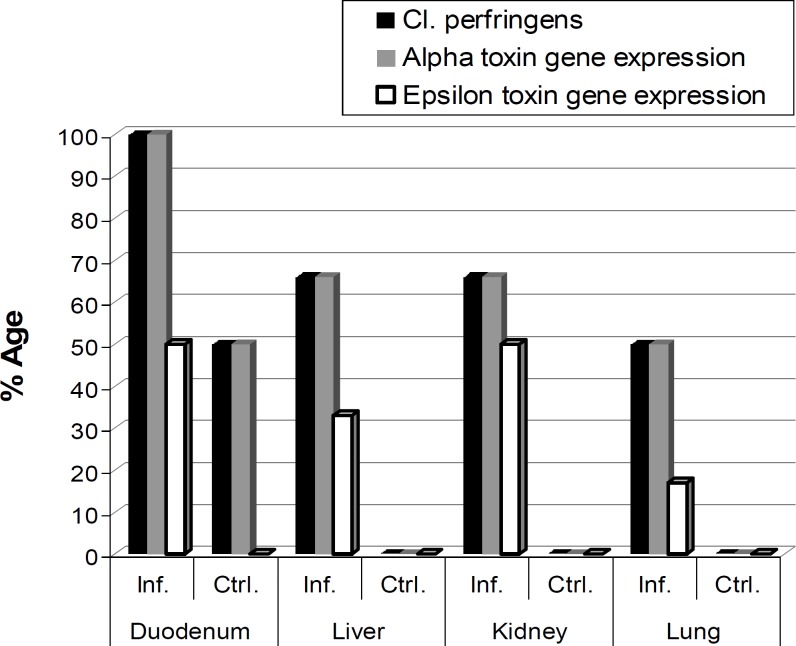
The genes for both toxins were amplified after the extraction of DNA by boiling method. Alpha gene of C. perfringens type D was amplified at annealing temperature 52.2°C with amplicon size 247 bp (Fig. 2) while epsilon at annealing temperature 50.2°C with amplicon size 665 bp (Fig. 3). In infected group, 100% (6/6) amplification for alpha toxin of C. perfringens type D was observed in duodenal scrapings followed by liver, kidney and lungs while for epsilon toxin genes a low percentage was recorded (Fig. 4).
Fig. 2.
Agarose gel electrophoresis of PCR product, C. perfringens type D. Lane 1: 100 bp DNA molecular marker, Lane 2, 4, 5, 6, 7: Cpa (alpha toxin encoding gene) corresponding in size approximately 247 bp. Lane 8: Negative control
Fig. 3.
Agarose gel electrophoresis of PCR product, C. perfringens type D. Lane 1: 100 bp DNA molecular marker, Lane 2, 3, 5, 6, 7: PCR amplified band corresponding to size of the epsilon (ETX) toxin encoding gene about 665 bp can be
Fig. 4.
Isolation of C. perfringens type D from different organs along with amplification of alpha and epsilon toxin genes
Percentage of hemolysis in different species
The percentage of hemolysis was calculated and showed that erythrocytes of human and mice were highly susceptible followed by goat, sheep and guinea pig at 37°C. A significantly lower hemolysis was recorded at 25°C in all species except in rabbits and dog (Fig. 5).
Fig. 5.
% age hemolysis of erythrocytes of various species to culture supernatants of C. perfringens type D. B: Buffalo, C: Cattle, S: Sheep, G: Goat, H: Horse, D: Dog, CH: Chicken, DK: Duck, Q: Quail, GP: Guinea pig, M: Mice, R: Rabbit, and H: Human
Discussion
Alpha and epsilon toxins of the C. perfringens type D are considered to be the major toxins involved in the disease pathogenesis in animals (Nasir et al., 2013b ▶). The organism was well grown on RCM and colonies caused a double zone of hemolysis on blood agar. Furthermore, C. perfringens fermented the glucose, fructose, lactose and mannitol. Similar results were observed by Javed et al. (2012) ▶. The supernatant culture of organism contained toxins caused the death of mice
and antitoxins coated plates in indirect ELISA confirmed that the death of mice was due to alpha and epsilon toxins of C. perfringens type D. Similarly, C. perfringens toxins were also confirmed through indirect ELISA as described by Koc and Gökce (2007) ▶, which seem be to the test of choice for the determination of toxins in sample.
It was reported from previous study (Uzal and Songer, 2008 ▶; Filho et al., 2009 ▶) that the epsilon toxin of C. perfringens type D caused degenerative changes in vascular endothelium in brain and caused increased capillary permeability, which resulted in edema. However in goats, the least evidence of this lesion exists. This inconsistency in brain lesion is supported by studies of Miyamoto et al. (1998 ▶, 2000); Finnie (2003) ▶ who reported epsilon toxin can possibly interact directly in some cases with neuronal cells without showing edema. The organism causes the sloughing of epithelium of intestine, which results in diarrhea and dehydration. Edema of alveoli has been reported, which suggests the receptors of epsilon toxin on the lung tissues that initiate the process of cellular degeneration. Similar mechanism of action has been suggested in kidney tissues. There is no published literature available regarding the lesions in mesenteric lymph nodes but in current study, severe edema was observed when the tissues of mesenteric lymph nodes were histopathologically observed. So, toxins of C. perfringens also have the affinity of these tissues. The hemorrhages in various organs suggest the hemolytic activity of alpha toxin, which is also confirmed (Stevens and Bryant, 2002 ▶; Islam et al., 2007 ▶).
It was observed that the distribution of C. perfringens type D varies in different tissues, being highest in duodenum followed by liver, kidney and lungs. So, it is clearly indicated that bacterium has tissue tropism for such tissues. These findings were confirmed through the extraction of DNA by boiling method and amplification of alpha and epsilon genes by PCR. Epsilon toxin gene amplified from duodenum of one animal in control group revealed that C. perfringens type D seems to be an uncommon inhabitant. Clostridium perfringens type D has worldwide in distribution but is not a common inhabitant (Miyashiro et al., 2007 ▶). The highest percentage of alpha toxin genes from lambs suspected of enterotoxaemia was also recorded by Hadimli et al. (2012) ▶ which supports our findings. A low percentage of epsilon toxin genes was determined by Wang et al. (2011) ▶ from fecal samples of healthy cattle which lend support to our results. These findings depict amplification of epsilon toxin gene in different tissues which is more difficult than alpha genes suggesting that some factors or tissue inhibitors might be involved for poor efficacy.
Alpha toxin, also called phospholipase C is hemolytic, cytotoxic, changes the vascular permeability (Songer, 1996 ▶; Bunting et al., 1997 ▶) and catalyses the hydrolysis of lecithin and phospholipids in cell membrane (Hale and Stiles, 1999 ▶). The erythrocytes of goat showed the highest hemolysis followed by sheep and cattle among the food animals. Our study correlates to some extent with findings of Tamura et al. (1992) ▶ who used culture supernatants of C. chauvoei and found that the erythrocytes of sheep and cow were highly susceptible as compared to goat. The difference in the results of current studies seems to be associated with the biological activity of hemolysin of C. chauvoei and C. perfringens type D and the test procedure. The RBC of human showed the highest hemolysis while mice were highly sensitive as compared to guinea pig and rabbit among the laboratory animals. Among the chicken, duck and quail RBC, quail exhibited the highest hemolysis. The reasons why hemolytic activity differs in different species may be due to the presence of toxin binding receptors and cell signaling pathway in the erythrocytes. The findings of Mudenda et al. (2006) ▶ lend support to our studies who reported that the existence of toxin binding receptors on the surface of erythrocytes of various species are associated with hemolysis. It is also speculated that the sphingomycin/phospholipids ratio of red cells may be the factor (Ochi et al., 2003 ▶). The percentage of hemolysis was higher at 37°C suggesting that this activity is temperature dependent. Our findings correlates with the observation of Sultan et al. (1999) ▶ who obtained maximum in vitro production and detection of hemolytic toxin of C. perfringens type D at 37°C.
It has been concluded from clinico-pathological findings that toxins of C. perfringens type D is not only target the alimentary canal but produces the lesions in all body of goats. The distribution of the bacterium varies being the highest in duodenum and amplification of alpha gene seems to be easy as compared to epsilon in different tissues. However, further studies are required to determine the factors involved in multiplication of organism in particular tissues and cell signaling pathway in infected tissues with C. perfringens type D.
Acknowledgment
The authors thank to Higher Education Commission, Islamabad, Pakistan for providing the necessary facilities to achieve this target.
References
- Babe, T, Jafari, B, Bahmanpour, S. Typing of toxigenic isolates of C perfringens by ELISA in Ostrich. . Afr. J. Microbiol. Res. 2012;8:1766–1769. [Google Scholar]
- Bancroft, JD, Gamble, M. Theory and practice of histological techniques. 6th Edn. Philadelphia, PA: Churchill Livingstone Elsevier; 2008. [Google Scholar]
- Black, F, Hwang, JJ, Bulmus, V, Woodward, M. Standard procedure for hemolysis assay by Hoffman group. 2003;5:8–13. [Google Scholar]
- Bunting, M, Lorant, DE, Bryant, AE, Zimmerman, GA, Mcintyre, TM, Stevens, DL, Prescott, SM. Alpha toxin from C perfringens induces proinflammatory changes in endothelial cells. J. Clin. Invest. 1997;100:565–574. doi: 10.1172/JCI119566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effat, MM, Abdullah, YA, Soheir, MF, Ready, MM. Characterization of C Perfringens field isolates, implicated in necrotic enteritis outbreaks on private broiler farms in Cairo, by multiplex PCR. . Afr. J. Microbiol. Res. 2007;1:29–32. [Google Scholar]
- Filho, EJ, Carvalho, AU, Assis, AR, Lobato, FF, Rachid, MA, Carvalho, AA, Ferreira, PM, Nascimento, RA, Fernandes, AA, Vidal, JE, Uzal, FA. Clinicopathologic features of experimental C perfringens type D enterotoxaemia in cattle. . Vet. Pathol. 2009;46:1213–1220. doi: 10.1354/vp.08-VP-0304-U-FL. [DOI] [PubMed] [Google Scholar]
- Finnie, JW. Pathogenesis of brain damage produced in sheep by C perfringens type D epsilon toxin: a review. . Aust. Vet. J. 2003;81:219–221. doi: 10.1111/j.1751-0813.2003.tb11474.x. [DOI] [PubMed] [Google Scholar]
- Greco, G, Madio, A, Buonavoglia, D, Totaro, M, Corrente, M, Buonavoglia, C. C perfringens toxin-types in lambs and kids affected with gastroenteric pathologies in Italy. . Vet. J. 2005;170:346–350. doi: 10.1016/j.tvjl.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Hadimli, HH, Erganis, O, Syin, Z, Aras, Z. Toxinotyping of C perfringens isolates by ELISA and PCR from lambs suspected of enterotoxaemia. Turk. J. Vet. Anim. Sci. 2012;36:409–415. [Google Scholar]
- Hale, ML, Stiles, BG. Detection of C perfringens alpha toxin using capture antibody ELISA. Toxicon. 1999;37:471–484. doi: 10.1016/s0041-0101(98)00179-2. [DOI] [PubMed] [Google Scholar]
- Islam, KBMS, Rehman, MD, Ershaduzzaman, MD, Taimur, MJFA, Song, HJ. Experimental development of caprine enterotoxaemia with C perfringens type D whole culture in natural host and its treatments. . Kor. J. Vet. Serv. 2007;30:219–223. [Google Scholar]
- Javed, S, Rafeeq, M, Tariq, MM, Awan, MA, Rashid, N, Ali, M. Study on in-vitro biochemical growth characterization and assessment of hemolytic toxin of C perfringens type B and D. . Pak. J. Zool. 2012;44:1575–1580. [Google Scholar]
- Koc, R, Gökce, HI. Determination of the toxins and biotypes of C perfringens in diarrhoeic calves in the Kars district of Turkey. . Turk. J. Anim. Sci. 2007;31:207–211. [Google Scholar]
- Komoriya, T, Hashimoto, A, Shinozaki, A, Inoue, M, Kohno, H. Study on partial purification of α-toxin produced from obligate anaerobe Cperfringens. . Report of the Research Institute of Industrial Technology, Nihon University. 2007;88:1–13. [Google Scholar]
- Miyamoto, O, Minami, J, Toyoshima, T, Nakamuru, T, Masada, T, Nagao, S, Negi, T, Itano, T, Okabe, A. Neurotoxicity of C perfringens epsilon-toxin for the rat hippocampus via glutamanergic system. Infect. Immunol. 1998;66:2501–2508. doi: 10.1128/iai.66.6.2501-2508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, O, Sumitami, K, Nakamur, U, Yamagani, T, Miyatal, S, Itano, S. Clostridium perfringens epsilon toxin causes excessive release of glutamate in the mouse hippocampus. FEMS. Microbiol. Lett. 2000;189:109–113. doi: 10.1111/j.1574-6968.2000.tb09215.x. [DOI] [PubMed] [Google Scholar]
- Miyashiro, S, Nassar, AFC, Del, FC, Cabral, AD, Silva, M. C perfringens types A and D associated with enterotoxaemia in an 18-month goat. . J. Venom. Anim. Toxins Incl. Trop. Dis. 2007;13:885–893. [Google Scholar]
- Mubashar, R. Studies on pathogenesis of Mgallisepticum in white leg horn layers [Ph.D. Thesis] Pakistan: University of Veterinary and Animal Sciences Lahore; 2010. [Google Scholar]
- Mudenda, HB, Ombe, BMH, Kohda, T, Mukamoto, M, Kozaki, S. Purfication and sensitivity of C chauvoei hemolysin to various erythrocytes. . Comp. Immunol. Microbiol. Infect. Dis. 2006;29:263–268. doi: 10.1016/j.cimid.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Nasir, AA, Younus, M, Rehman, MU, Lateef, M, Khaliq, SA, Ahmad, I, Abbas, M. Hematological and some biochemical alterations in sheep experimentally infected with C perfringens type D infection. . J. Anim. Pl. Sci. 2013b;23:1553–1558. [Google Scholar]
- Nasir, AA, Younus, M, Rehman, MU, Lateef, M, Rashid, A, Ahmad, R, Abbas, M. Molecular detection of C perfringens type D alpha and epsilon toxin genes from various tissues in lambs. . Pak. Vet. J. 2013a;33:492–495. [Google Scholar]
- Ochi, S, Oda, M, Nagahama, M, Sakurai, J. C perfringens alpha-toxin-induced hemolysis of horse erythrocytes is dependent on Ca2+ uptake. . Biochim. Biophys. Acta. 2003;1613:79–86. doi: 10.1016/s0005-2736(03)00140-8. [DOI] [PubMed] [Google Scholar]
- Sayeed, S, Li, J, McClane, BA. Characterization of virulence plasmid diversity among C perfringens type B isolates. Infect. Immun. 2010;78:495–504. doi: 10.1128/IAI.00838-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer, JG. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, DL, Bryant, AE. The role of Clostridial toxins in the pathogenesis of gas gangrene. Clin. Infect. Dis. 2002;35:93–100. doi: 10.1086/341928. [DOI] [PubMed] [Google Scholar]
- Sultan, B, Muhammad, K, Javed, S, Ali, Z. In vitro production and detection of hemolytic toxin of C perfringens type D. . Int. J. Agric. Biol. 1999;1:56–58. [Google Scholar]
- Tamura, Y, Kijma, M, Hamamoto, K, Yoshimura, H. Partial characterization of the Hemolysin produced by C chauvoei. . J. Vet. Med. Sci. 1992;54:777–778. doi: 10.1292/jvms.54.777. [DOI] [PubMed] [Google Scholar]
- Tortora, GJ, Funke, BR, Case, Cl. Microbiology an introduction. 10th Edn. Sanfrancis Co: Pearsons, Education, Inc; 2010. [Google Scholar]
- Uzal, FA, Songer, JG. Diagnosis of C perfringens intestinal infections in sheep and goats. . J. Vet. Diagn. Invest. 2008;20:253–265. doi: 10.1177/104063870802000301. [DOI] [PubMed] [Google Scholar]
- Wang, G, Zhou, J, Zheng, F, Lin, G, Cao, X, Gong, X, Changqing, Q. Detection of different genotypes of Clostridium perfringens in feces of healthy dairy cattle from China using Real-Time Duplex PCR essay. Pak. Vet. J. 2011;31:120–124. [Google Scholar]