Abstract
Two methods were used to distinguish airborne engineered nanomaterials from other airborne particles in a facility that produces nano-structured lithium titanate metal oxide powder. The first method involved off-line analysis of filter samples collected with conventional respirable samplers at each of seven locations (six near production processes and one outdoors). Throughout most of the facility and outdoors, respirable mass concentrations were low (<0.050 mg m−3) and were attributed to particles other than the nanomaterial (<10% by mass titanium determined with inductively coupled plasma atomic emission spectrometry). In contrast, in a single area with extensive material handling, mass concentrations were greatest (0.118 mg m−3) and contained up to 39% +/− 11% lithium titanium, indicating the presence of airborne nanomaterial. Analysis of the filter samples collected in this area by transmission electron microscope and scanning electron microscope revealed that the airborne nanomaterial was associated only with spherical aggregates (clusters of fused 10–80 nm nanoparticles) that were larger than 200 nm. This analysis also showed that nanoparticles in this area were the smallest particles of a larger distribution of submicrometer chain agglomerates likely from welding in an adjacent area of the facility. The second method used two, hand-held, direct-reading, battery-operated instruments to obtain a time series of very fine particle number (<300 nm), respirable mass, and total mass concentration, which were then related to activities within the area of extensive material handling. This activity-based monitoring showed that very fine particle number concentrations (<300 nm) had no apparent correlation to worker activities, but that sharp peaks in the respirable and total mass concentration coincided with loading a hopper and replacing nanomaterial collection bags. These findings were consistent with those from the filter-based method in that they demonstrate that airborne nanoparticles in this facility are dominated by "incidental" sources (e.g., welding or grinding), and that the airborne "engineered" product is predominately composed of particles larger than several hundred nanometers. The methods presented here are applicable to any occupational or environmental setting in which one needs to distinguish incidental sources from engineered product.
Keywords: engineered, exposure, health, incidental, nanoparticle, respirable
INTRODUCTION
Inhalation of “incidental” nanoparticles (byproducts of combustion or hot processes) has been associated with adverse health effects that range from myocardial infarction to decrements of lung function among asthmatics.(1, 2) As “engineered” nanomaterials (materials designed with at least one dimension <100 nm) increasingly enter the workplace,(3) they may pose some of these same inhalation hazards as well as hazards different from incidental nanoparticles.(4) For example, toxicity studies have shown that inhalation of carbon nanotubes are capable of inducing inflammation, granulomas, and fibrosis in the lung(5, 6, 7) and inducing DNA damage in lung fibroblasts.(8)
Hundreds of companies in the U.S. manufacture products that incorporate engineered nanomaterials with widely varying composition.(3) For example, nanocomposites (polymers with 1–10% by mass carbon nanotubes, carbon nanofibers, or nanoclays) are used in sporting goods, aerospace parts, and automobile parts.(9) Automobile body panels made from nanocomposites are considerably lighter, more durable, and more mar resistant compared with those made from aluminum or steel.(10) Estimates suggest that 2 million workers will be employed in nanotechnology industries by 2020.(11)
A way to distinguish airborne engineered nanomaterials from incidental particles would be of practical value to the environmental health and safety (EHS) professional when assessing workplace inhalation risks. Each engineered nanomaterial will likely have a different toxicity from other engineered nanomaterials and the incidental nanoparticles that they may coincide with. Moreover, conventional methods used to assess risk from airborne particles that rely on the mass of particles per unit volume of air (e.g., respirable mass concentration) are frequently ill suited for assessing nanoparticle exposures(12) because nanoparticles weigh relatively little compared with larger particles that contribute most to mass concentration.(13) Moreover, adverse health effects from exposure to nanoparticles have been found to be more closely related to particle number or surface area concentration than to particle mass concentration.(14) These health effects may differ substantially depending on the size, morphology, composition (both bulk and surface), and concentration of airborne particles.(15)
Analysis of particles collected on filter media by transmission electron microscopy (TEM) or scanning electron microscopy (SEM) is one method to distinguish airborne engineered nanomaterials from incidental particles. The National Institute for Occupational Safety and Health (NIOSH) prescribes this type of analysis to distinguish among different types of airborne asbestos(16) and, in the agency's first effort to advise EHS professionals on assessing an inhalation hazard of a nanomaterial, recommends SEM analysis of filter samples to distinguish nanosized titanium dioxide from larger particles.(17) Alternatively, real-time particle monitoring together with workplace activity logs might be used to distinguish engineered from incidental particles by size. Activity-based monitoring has been used in occupational settings to identify determinants of exposure to respirable mass concentrations(17) and to a limited extent for number concentrations.(18) NIOSH included both microscopy and real-time monitoring in their guidance document for EHS professionals on assessing an inhalation hazard of a nanomaterial.(19)
In this work, two complementary methods were used to distinguish airborne engineered nanomaterials from incidental particles in a facility that produces nano-structured lithium titanate metal oxide powder. The first method used off-line analysis of filter samples by SEM and TEM to obtain definitive identification of various particle size, composition, and morphology. The second method relied on activity-based aerosol monitoring as a lower cost alternative to filter-based sampling. These methods offer practical solutions to assess airborne nanoparticle exposure risk, which in turn will support development of informed protective standards for the rapidly expanding field of nanotechnology.
METHODS
Manufacturing Facility
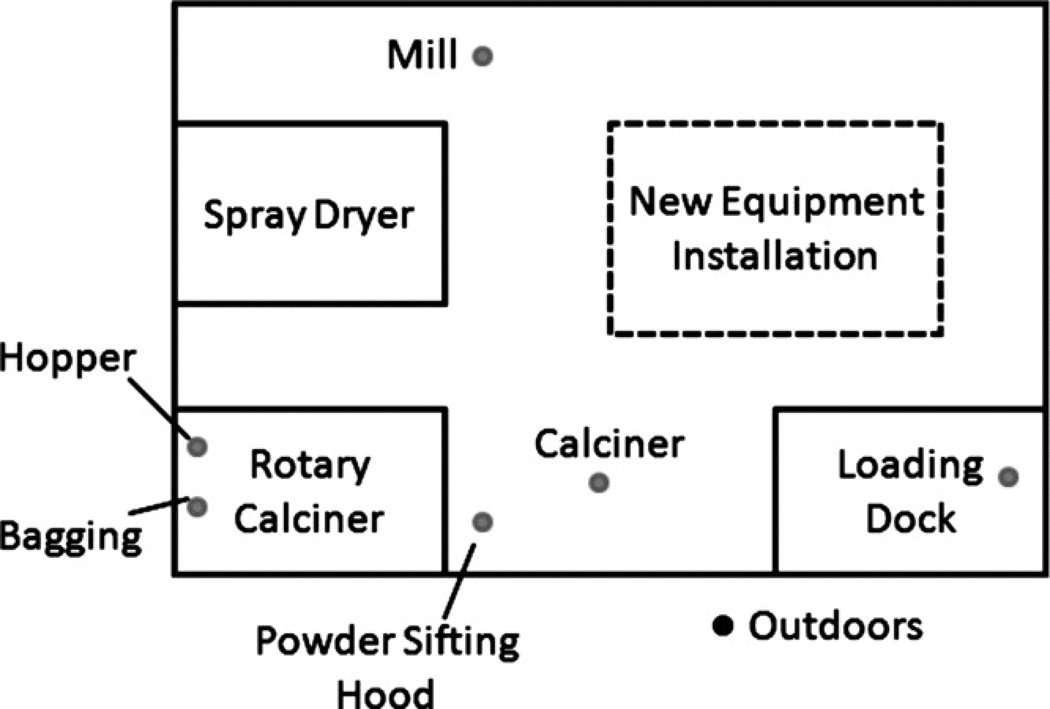
The approximately 60,000-ft2 facility where this work was performed produces lithium titanate metal oxide nanomaterial (the detailed process of making this particular powder is proprietary and the order of the steps cannot be disclosed in this article). This nanomaterial is noted by the company for its high surface area and superior performance in fuel cells. The material was produced in six primary work areas (Figure 1): (1) wet mill, (2) spray dryer, (3) rotary calciner, (4) an open area with a powder sifting hood and a stationary calciner, (5) an area with new equipment installation, (6) and a loading dock. Generally, the areas of the facility were open to each other with partial walls in some places.
FIGURE 1.
Facility layout showing sampling locations (filled dots). The approximately 60,000-ft2 facility produced nano-structured lithium titanate spinel oxide in six primary work areas: (1) wet mill, (2) spray dryer, (3) rotary calciner, (4) an open area with a powder sifting hood and a stationary calciner, (5) an area with new equipment installation, and (6) a loading dock. The rotary calciner area was identified as having a high probability for exposure of workers to the powder product because workers were required every 30 min to dump material from the spray dryer from a 19-L (5-gallon) bucket into a hopper that was located at one end of the rotary calciner.
The rotary calciner area was identified as having a high probability for airborne nanomaterials because workers dumped material every 30 min from a 19-L (5-gallon) bucket into a hopper located at one end of the rotary calciner. They also had to change nanomaterial collection bags at the opposite end of the rotary calciner approximately every 4 hr. When this research was conducted, employees were performing various welding and grinding operations to install new equipment. They also occasionally used a propane-powered forklift to move pallets. The outside air temperature was approximately 85°F (30°C) during the day, so the overhead doors on the loading dock were typically open, allowing circulation of outside air.
Off-Line Filter-Based Sampling
Seven locations were selected for collection of respirable samples: (1) the bagging end of the rotary calciner, (2) the hopper end of the rotary calciner, (3) mill, (4) powder sifting hood, (5) calciner, (6) loading dock, and (7) outside the facility. Samples were not collected in the spray dryer area because it was non-operational when these measurements were taken. At all other locations, three respirable dust samples were collected on consecutive days on polyvinyl chloride (PVC) filters (SKC Inc., Eighty Four, Pa.) for gravimetric analysis. At each location, duplicate respirable dust samples were collected on mixed cellulose ester (MCE) filters (SKC) for analysis by electron microscopy. Samples were collected at breathing zone height with personal sampling pumps (Universal Sampler pumps, model 224-PCXR4; SKC) pulling air through respirable cyclones (GK2.69, BGI Inc., Waltham, Mass.) at 4.0 L/min. A volumetric airflow calibrator (Trical, BGI Inc., Waltham, Mass.) was used to set the flow rate at the beginning of the sampling period and to record the flow rate at the end of the sampling period. Blank samples (four PVC blank filters and two MCE blank filters) were collected for quality control purposes. Collection times averaged 4 hr to yield a sample volume of approximately 1000 L.
PVC filters were weighed according to NIOSH Method 0600 to determine the mass concentration of the respirable dust. Sampling and analytical limit of detection (LOD) were estimated as three times the standard deviation of the weight gain on four blank filters or 0.02 mg m−3. These filters were then analyzed by inductively coupled argon plasma atomic emission spectroscopy (ICP-AES) for titanium and lithium according to NIOSH method 7300.
Blank values for the ICP-AES were reported by the laboratory to be less than the method detection limit for titanium (0.0181 µg per filter or 0.02 µg m−3 for a sample volume of 1000 L) and for lithium (0.01 µg per filter or 0.01 µg m−3 for a sample volume of 1000 L). These values were used as the LOD because discrete blank values were unavailable due to laboratory policy. The mass of the lithium titanate was estimated from the mass of titanium identified through ICP-AES and the stoichiometry of the lithium titanate (proprietary to the company). This value was divided by the total weight of the mass collected on the filter to determine the percentage of the respirable mass concentration that was composed of lithium titanate. A value of the LOD divided by the square root of 2 was used in statistical analyses for samples below the LOD.(20)
The MCE filters were analyzed by TEM (Hitachi H-7000; Tokyo, Japan) and SEM (Hitachi S-4800 SEM) to assess the morphology and composition of the particles by size. Following Burdett and Rood,(21) MCE filters were collapsed with a mixture of 50% deionized water, 35% dimethylformamide, and 15% glacial acetic acid; etched using a plasma asher; and coated with a carbon layer. A square section of the collapsed filter was placed specimen side up on 3-mm TEM grids and placed in a Jaffe washer filled with dimethylformamide for 2 hr. TEM images were examined to classify particles by shape: (1) large 200-nm to 10-µm spheres, (2) irregularly shaped particles of varying size, and (3) submicrometer particle chains. For each shape class, the range of particle size was then identified by TEM, the general surface morphology by SEM, and chemical composition by TEM with energy dispersive spectrometry (EDS). Specialized software was used to acquire elemental maps of selected particles.
Real-Time Activity-Based Monitoring
Particle number and mass concentrations were related over time with worker activities (filling a hopper and changing a collection bag) that were part of normal operation near a rotary calciner operation. Airborne concentrations were derived from two direct-reading instruments: (1) a condensation particle counter (CPC, model 3007; TSI Inc., Shoreview, Minn.) that measures particle number concentration from 10 nm to 1000 nm; and (2) an optical particle counter (OPC, PDM-1108; Grimm, Ainring, Germany) that measures particle number concentration by size in 15 channels from 300 nm to 20 µm. Following Peters et al.,(22) the very fine particle number concentration (N, particles < 300 nm) was estimated as:
| (1) |
where NCPC is the number concentration indicated by the CPC, and NOPC, i is the number concentration indicated by the OPC for a given channel, i. The first channel of the OPC included particles larger than 300 nm, whereas the fifth channel included particles smaller than 1 µm, which corresponds with the upper detection limit of the CPC. Respirable mass concentration, MR, and the total mass concentration, MT, were estimated from the CPC and OPC as:
| (2) |
| (3) |
where dCPC is the assumed midpoint diameter of the CPC data, ρ is the particle density, SR is a function for the fraction of respirable mass defined by ACGIH,(23) and dmid, i is the midpoint diameter of the OPC ith channel. The midpoint diameter of the CPC was assumed to be 150 nm for this work. Unit density (1 g/cm−3) was assumed for these calculations.
Although an imperfect measure of nanoparticle number concentration because N includes particles from 100 nm to 300 nm, this metric was chosen because the CPC and OPC are relatively affordable within industrial hygiene budgets. Their compact size and battery operation also allows them to be easily transported throughout the facility. Other instruments such as a scanning mobility particle sizer would have provided better size resolution but are considerably more expensive and not easily transportable. In addition, the combined particle size information provided by the CPC and OPC instruments spanned a range of 10 nm to 20 µm, which represented the range of particle sizes expected.
Several other computations were made with the real-time data. The fraction of particles within the 300 nm to 1 µm size range was calculated to assess the magnitude of the correction made in Eq. 1. The OPC number concentration subtracted from the CPC data in Eq. 1 was on average less than 0.9% of the total CPC counts.
Statistical Analysis
The ANOVA procedure was used to test the hypothesis that the mean respirable mass concentrations were the same across locations. Statistical significance was evaluated at the 95% confidence level. Duncan's multiple comparison test was used to identify which locations had different mean values if the null hypothesis was rejected. Statistical tests were carried out in statistical analysis software (Proc GLM; SAS, v. 9.1).
RESULTS
Off-Line Filter-Based Sampling
Table I provides a summary of respirable mass concentration and bulk composition measured throughout the facility. ANOVA showed that the mean of the respirable mass concentrations were not equal between locations (p < 0.001). The Duncan's multiple range test demonstrated that mean respirable mass concentrations were greatest at the bagging end of the rotary calciner (0.118 mg m−3) than at other locations: hopper end of the rotary calciner (0.035 mg m−3), mill (0.025 mg m−3), powder sifting hood area (0.039 mg m−3), and loading dock (0.036 mg m−3). Outside the facility, all respirable mass concentrations were lower than the LOD of 0.030 mg m−3.
TABLE I.
Respirable Mass Concentration and Percentage of Respirable Mass Concentration Lithium Titanate by Location
| Area / Location | Mass Concentration, mg m−3 Mean ± Std. Dev. |
Percentage Lithium Titanate (%) |
|---|---|---|
| Rotary calciner/bagging | 0.118 ± 0.023 A | 39 ± 11 |
| Rotary calciner/hopper | 0.035 ± 0.006 | <10 B |
| Spray dryer C | — | — |
| Wet Mill | 0.026 ± 0.007 | <LOD |
| Powder sifting hood | 0.039 ± 0.016 | <4 B |
| Loading dock | 0.036 ± 0.015 | <LOD |
| Calciner | 0.028 ± 0.012 | <LOD |
| Outdoors | <LOD | <LOD |
Duncan multiple range test identified mean as statistically different from other means.
One or more sample below LOD for titanium; value reported based on sample(s) >LOD.
No samples available because spray dryer was not operational.
At the bagging end of the rotary calciner, the mass concentration of lithium titanate was estimated to be 0.047 mg m−3±0.010 mg m−3 or 39% ± 11% of the respirable mass concentration. The percentage respirable mass concentration attributed to lithium titanate was 10% at the hopper end of the rotary calciner and 4% at the powder sifting hood.
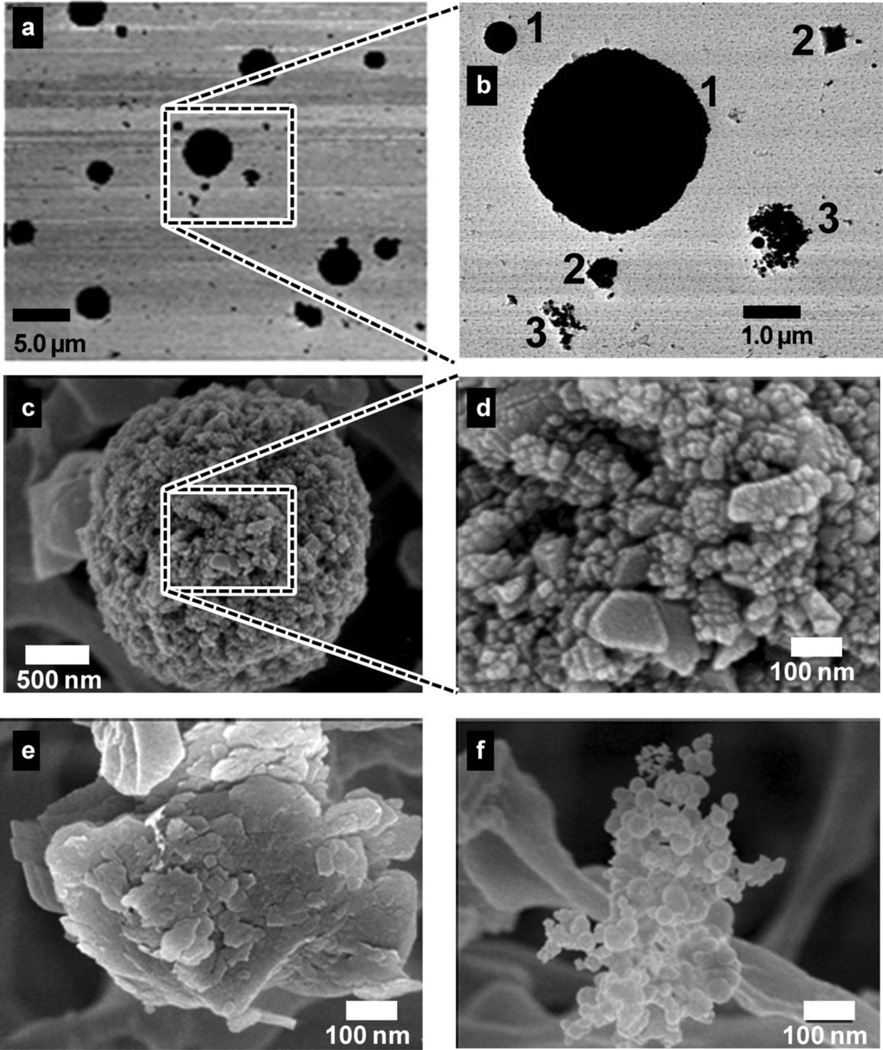
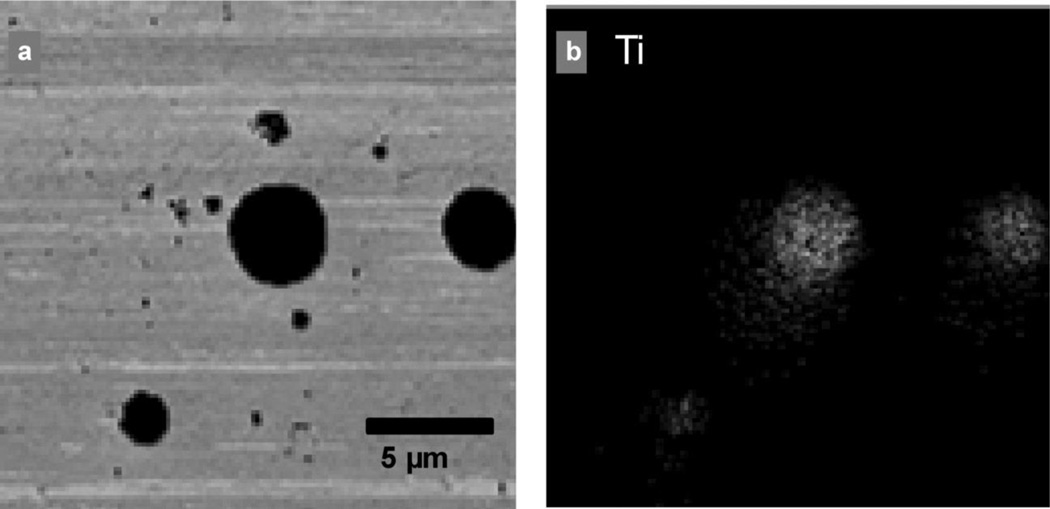
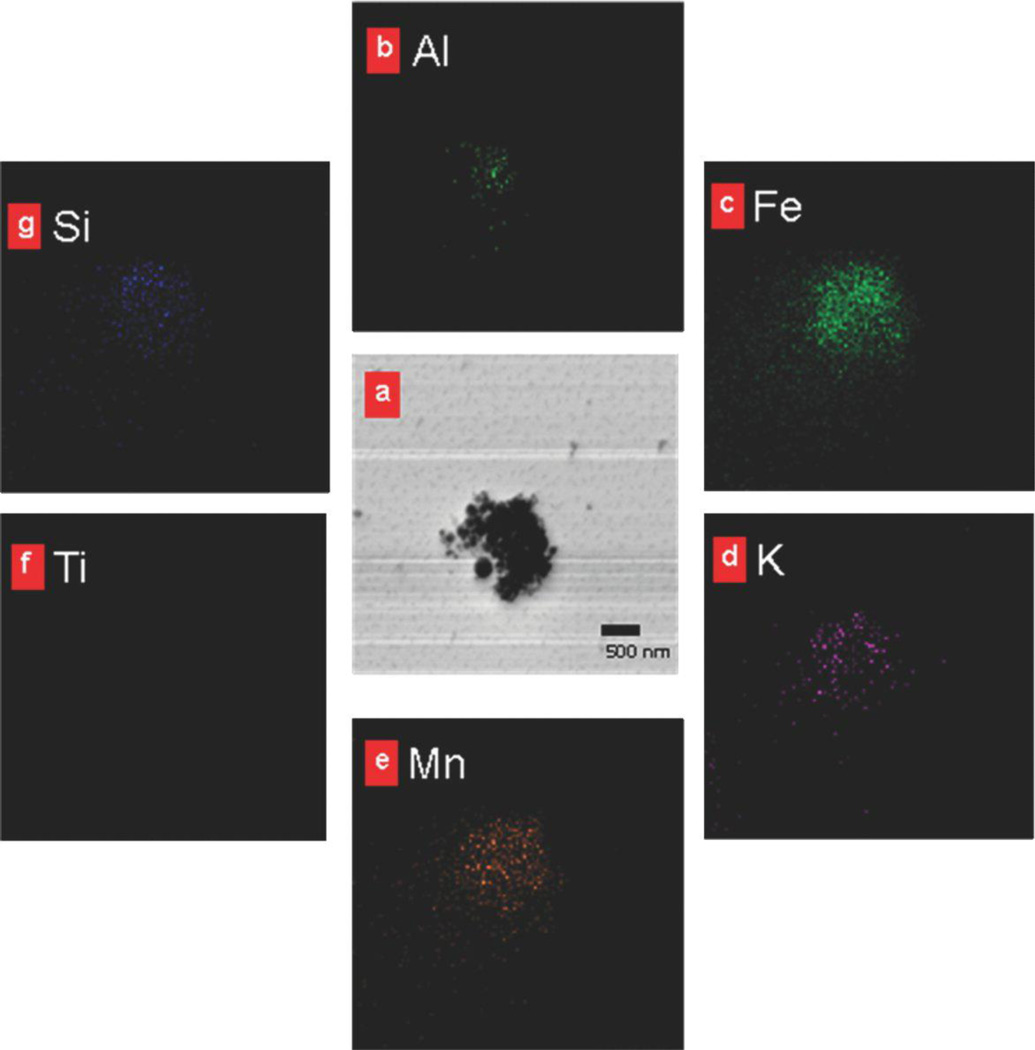
TEM analysis of samples collected at the bagging end of the rotary calciner revealed distinct particle morphologies (Figures 2a and 2b): (1) large 200-nm to 10-µm spheres, (2) irregularly shaped particles of varying size, and (3) submicrometer particle chains. Further analysis with SEM revealed that the spheres were composed of clusters of fused 10–80 nm nanoparticles (Figures 2c and 2d). These particles are referred to as spherical aggregates in line with the ASTM definition that an aggregate is “a discrete group of particles in which the various individual components are not easily broken apart.”(24) The irregularly shaped particles had amorphous morphology (Figure 2e), whereas the smaller particle chains were either irregular clumps or chains of spherical 5–50 nm nodules (Figure 2f). The submicrometer chains are referred to as chain agglomerates to be consistent with the aerosol literature(13) and the ASTM definition of an agglomerate (“a group of particles held together by relatively weak forces … that may break apart into smaller particles upon processing”).(24) Titanium was present in all of the spherical nanostructured aggregates (Figure 3b) but was not present in the chain agglomerates (Figure 4) or the irregularly shaped particles. The chain agglomerates contained Al, Fe, K, Mn, and small amounts of Si (Figure 4).
FIGURE 2.
Electron microscopy images from filter samples depict particle size and morphology. TEM images a and b show three types of particles of different shape and size: (1) b1, large spherically shaped particles; (2) b2, irregularly shaped particles; and (3) b3, smaller particle chains. SEM images c-f reveal that the larger spherical particles are actually composed of smaller nanoparticles 10–80 nm in size clustered into larger aggregates, c, d. Irregularly shaped particles have an amorphous structure, e. Chain agglomerates are composed of spherical nodules of 5–50 nm in size, f.
FIGURE 3.
Titanium content of a group of particles. a, TEM image; b, corresponding elemental map for titanium. X-rays characteristic of an element, in this case titanium, are emitted upon electron excitation as the microscope's electron beam rasters the sample. The elemental map indicates the relative count rate of characteristic X-rays released at each point on the sample. Lithium cannot be detected with EDS due to its low-energy X-rays that cannot pass through the detector's protective beryllium window. Therefore, the nanomaterial produced in this facility was identified by the presence of titanium, which was found only in the spherical agglomerates.
FIGURE 4.
Chemical composition of a chain agglomerate particle. a, TEM image. b-g, corresponding elemental maps for several elements show that the chain agglomerates do not contain titanium but do contain elements common to welding fume (e.g., Mn).
Real-Time Activity-Based Monitoring
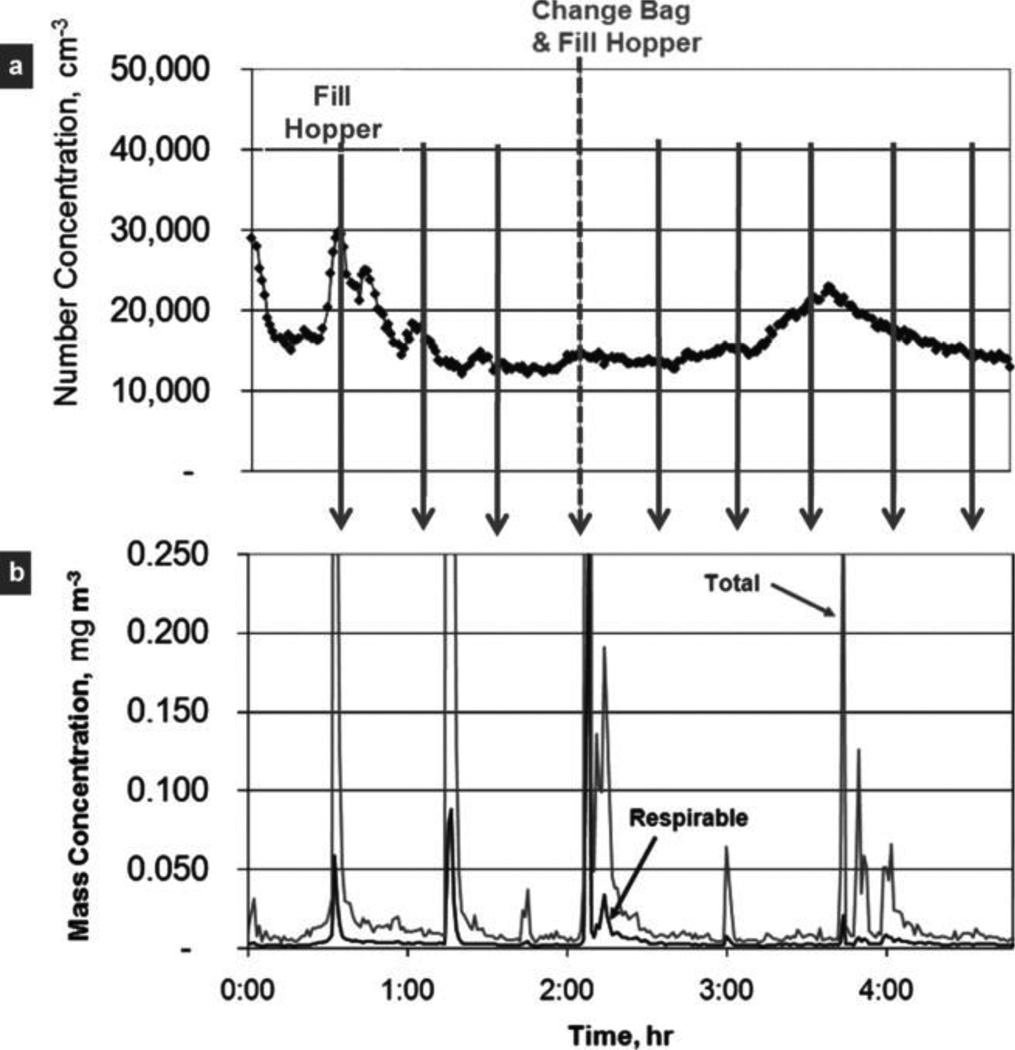
Figure 5 presents results from one of five activity-based monitoring events. Worker activities in the rotary calciner room (loading a hopper and replacing collection bags) were unrelated to the very fine particle number concentrations (Figure 5a). In contrast, worker activities coincided with the respirable and total mass concentrations (Figure 5b). Similar results were obtained for the other four activity-based monitoring events. After the study was complete, the authors became aware that the time of activity was reported by workers to only the nearest half hour, which possibly explains the minor inconsistencies in correlation of mass concentration peaks with logged activities (Figure 5b).
FIGURE 5.
Sample results from real-time, activity-based monitoring. Worker production activities are identified with arrows and labeled at the top of the figure. a, very fine particle number concentrations (<300 nm) were not related to worker activities indicating the presence of other sources of small particles within the facility. b, respirable and total mass concentrations were strongly related to changing a nanomaterial collection bag and filling the hopper.
DISCUSSION
Two methods were used to distinguish airborne engineered nanomaterials from incidental particles in an occupational setting. In the first method using off-line analysis of particles collected onto filters, the greatest respirable mass concentrations with a large percentage of nanomaterial were identified in an area with extensive nanomaterial handling. Thus, this area was identified as having the greatest potential for airborne nanomaterial exposure. Further analysis of filter samples collected in this area by TEM and SEM showed that only spherical aggregates larger than 200 nm contained titanium and were thus positively identified as engineered and distinct from the other particle types that were incidental to production. The presence of elements such as Mn in particles with chain agglomerate morphology suggests that these particles were fume from welding. The irregularly shaped particles may have come from grinding or possibly from activity outside the facility.
A major strength of this microscopy-based method is the ability to evaluate the important particle properties of physical size, morphology, and composition. Detailed data of these types are critical for designing appropriate toxicity tests that are relevant to environmental and occupational monitoring, since it is increasingly clear that particle size, morphology and composition (both bulk and surface), and concentration all affect the toxicity of nanomaterials.(25, 26) Although computer automation is implemented to speed sample throughput and to collect data on many more particles, electron microscopy is inherently slow, costly, and lacking in standard methods for analyzing nanoparticles. Thus, at least in the short term, this microscopy-based method may be impractical for EHS compliance or routine assessments.
These findings from activity-based monitoring were consistent with those derived from filter-based sampling. The fact that the number concentrations of very fine particles were unrelated to worker activity (Figure 5a) suggests that the nanoparticles present in the rotary calciner area were incidental to production of the nanomaterial. The further finding that respirable and total mass concentration were related to worker activity (Figure 5b) suggests that the particles associated with nanomaterial production were quite large. Although respirable mass concentration includes all particles that collect on a filter <4 µm, it is dominated by the largest particles because mass is dependent on particle diameter cubed. From these results, it can be inferred that the airborne engineered nanomaterials were larger than nanosize in this area.
The activity-based monitoring provided information about the work environment that was unavailable from microscopy-based analysis: time-series data revealed that handling was the primary source of airborne nanomaterial. With this information, the EHS professional would be wise to follow the precautionary principle “when in doubt about the hazard, prevent as much potential exposure as reasonably possible.”(27) Based on the authors' observations, the EHS director for the facility (Maher) implemented reduced handling of the nanomaterial, increased ventilation, and nearly complete enclosure of the process. A minimum recommendation is to use personal protective equipment when handling material to reduce inhalation exposure. However, personal protective equipment would not prevent subsequent inhalation exposure from resuspension of settled nanomaterial, such as that deposited on a worker's clothes.
The Occupational Safety and Health Administration (OSHA) requires that gravimetric sampling be conducted to show that respirable mass concentrations of particles not otherwise specified by other standards are below 3 mg m−3. This standard applies to “biologically inert, insoluble, or poorly soluble particles” and is based on their ability to overload the clearance mechanisms of the respiratory system simply by their physical presence, not their toxicity. In this study, respirable mass concentrations were well below the standard, ranging from less than the limit of detection of 0.030 mg m−3 to 0.118 mg m−3. However, compliance with this standard does not ensure worker protection because the airborne nanomaterial may have toxic properties. The company that owns this facility is engaged in research to evaluate the toxic properties of the lithium titanate nanomaterial produced in this facility.
Due to limitations of the workplace studied here, the authors were unable to evaluate the effectiveness of their methods for airborne engineered nanomaterials that are truly nanosized (i.e., <100 nm). It is expected that activity-based monitoring holds promise for identifying areas in workplaces with elevated concentrations of nanosized engineered nanomaterials. As any activity-based monitoring, however, there is an inherent ambiguity in one's ability to attribute peak concentrations to a single source. Characterization of sources by particle size, composition, and morphology would help to resolve this ambiguity but adds substantial expense to a study and was outside the scope of the current work. There are also difficulties with accurately monitoring activities, such as the minor inconsistencies in correlation of mass concentration peaks with logged activities (Figure 5b), which the authors attributed to their reliance on worker-recorded time activity with only 30-min time resolution.
Single-particle characterization by microscopy offers perhaps the most definitive method to distinguish airborne engineered nanomaterials from incidental particles. Making these methods economical for routine EHS use will probably require integration of computer-controlled microscopy with advanced algorithms to sort particles by size, morphology, and composition. However, microscopy becomes increasingly more difficult as particle size becomes smaller, below several hundred nanometers, particularly for composition and to a lesser extent for size and morphology.
CONCLUSIONS
In summary, two methods (analysis of filter samples by electron microscopy and activity-based monitoring) can be used to distinguish airborne engineered nanomaterials from incidental particles. In this study, both methods allowed the authors to conclude for the process being evaluated that the production of engineered nanomaterial liberated relatively large particles and the nanoparticles observed were not associated with the production. Although time-intensive and costly, microscopic analysis provides detailed compositional and structural information that is critical in the design of environmentally relevant toxicological studies. On the other hand, activity-based monitoring provides information on how particles enter and move through the workplace, which is central to developing strategies to reduce inhalation exposures. It is therefore recommended that both complementary methods be considered when developing protocols for assessing inhalation hazards from nanomaterials. Exposure information derived from these methods, combined with appropriate toxicity tests, can ensure that informed standards are implemented that will protect the estimated 2 million workers who will be employed in the nanotechnology industry by 2020.(11)
Acknowledgments
The authors greatly appreciate the collaboration of Altair Nanotechnologies, Inc., who have taken a very proactive stance in evaluating the potential hazards of nanomaterials in their facilities. Supported by NIH R01 OH008806, NIH P30 ES05605, and CDC K01 OH009255.
REFERENCES
- 1.Peters A, von Klot S, Heier M. Exposure to traffic and the onset of myocardial infarction. N. Engl. J. Med. 2004;351(17):1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 2.McCreanor J, Cullinan P, Nieuwenhuijsen MJ. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007;357(23):2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 3.Thomas K, Aguar P, Kawasaki H. Research strategies for safety evaluation of nanomaterials, Part VIII: International efforts to develop risk-based safety evaluations for nanomaterials. Toxicol. Sci. 2006;92:23–32. doi: 10.1093/toxsci/kfj211. [DOI] [PubMed] [Google Scholar]
- 4.Maynard AD, Aitken RJ, Butz T. Safe handling of nanotechnology. Nature. 2006;444(7117):267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- 5.Shvedova AA, Kisin ER, Mercer R. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 6.Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006;36(3):189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 7.Poland CA, Duffin R, Kinloch I. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 8.Kisin ER, Murray AR, Keane MJ. Single-walled carbon nanotubes: Geno- and cytotoxic effects in lung fibroblast V79 cells. J. Toxicol. Environ. Health A. 2007;70:2071–2079. doi: 10.1080/15287390701601251. [DOI] [PubMed] [Google Scholar]
- 9.Hussain F, Hojjati M, Okamoto M, Gorga RE. Polymer-matix nanocomposites, processing, manufacturing, and application. J. Compos. Mater. 2006;40:1511. [Google Scholar]
- 10.Lloyd SM, Lave LB. Life cycle economic and environmental implications of using nanocomposites in automobiles. Environ. Sci. Technol. 2003;37:3458–3466. doi: 10.1021/es026023q. [DOI] [PubMed] [Google Scholar]
- 11.Schulte P, Geraci C, Zumwalde R, Hoover M, Kuempel E. Occupational risk management of engineered nanoparticles. J. Occup. Environ. Hyg. 2008;5:239–249. doi: 10.1080/15459620801907840. [DOI] [PubMed] [Google Scholar]
- 12.Maynard A, Aitken RJ. Assessing exposure to airborne nanomaterials: current abilities and future requirements. Nanotoxicol. 2007;1:26–41. [Google Scholar]
- 13.Hinds WC. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. 2nd. New York: John Wiley & Sons, Inc.; 1999. pp. 83–90. [Google Scholar]
- 14.Oberdörster G. Toxicology of ultrafine particles: In vivo studies. Philos. Trans. R. Soc. Lond. A. 2000;358:2719–2740. [Google Scholar]
- 15.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Occupational Safety and Health (NIOSH) Method 7402: Asbestos by TEM. In: Schlect PC, O'Connor PF, editors. NIOSH Manual of Analytical Methods (NMAM) 1994. [Google Scholar]
- 17.Ross AS, Teschke K, Brauer M, Kennedy SM. Determinants of exposure to metalworking fluid aerosol in small machine shops. Ann. Occup. Hyg. 2004;48(5):383–391. doi: 10.1093/annhyg/meh042. [DOI] [PubMed] [Google Scholar]
- 18.Yeganeh B, Kull CM, Hull MS, Marr LC. Characterization of airborne particles during production of carbonaceous nanomaterials. Environ. Sci. Technol. 2008;42:4600–4606. doi: 10.1021/es703043c. [DOI] [PubMed] [Google Scholar]
- 19.Approaches to Safe Nanotechnology: An Information Exchange with NIOSH. [Online] [Accessed November 17, 2008]; Available at http://www.cdc.gov/niosh/topics/nanotech/safenano/
- 20.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5:46–51. [Google Scholar]
- 21.Burdett GJ, Rood AP. A membrane filter, direct-transfer technique for the analysis of asbestos fibers or other inorganic particles by transmission electron microscopy. Environ. Sci. Technol. 1983;17:643–648. doi: 10.1021/es00117a004. [DOI] [PubMed] [Google Scholar]
- 22.Peters TM, Heitbrink WA, Evans DE, Slavin TJ, Maynard AD. The mapping of fine and ultrafine particle concentrations in an engine machining and assembly facility. Ann. Occup. Hyg. 2006;50(3):249–257. doi: 10.1093/annhyg/mei061. [DOI] [PubMed] [Google Scholar]
- 23.Maynard AD, Jensen PA. Aerosol measurement in the workplace. In: Baron PA, Willeke K, editors. Aerosol Measurement: Principles, Techniques, and Applications. 2nd. New York: Van Nostrand Reinhold; 2001. pp. 779–799. [Google Scholar]
- 24.ASTM. ASTM Terminology for Nanotechnology. New York: ASTM; 2006. (ASTM E2456-06). [Standard] [Google Scholar]
- 25.Grassian VH, Adamcakova-Dodd A, Pettibone JM, O'Shaughnessy PT, Thorne PS. Inflammatory response of mice to manufactured titanium dioxide nanoparticles: Comparison of size effects through different exposure routes. Nanotoxicology. 2007;1(3):211–226. [Google Scholar]
- 26.Grassian VH, O'Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ. Health Perspect. 2007;115(3):397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monica JC, Heintz ME, Lewis PT. The perils of pre-emptive regulation. Nat. Nanotechnology. 2007;2:68–70. doi: 10.1038/nnano.2007.15. [DOI] [PubMed] [Google Scholar]