Abstract
In spite of extensive scientific knowledge about the neurobiological systems and neural pathways underlying addictions, only limited progress has been made to reduce the population-level incidence of addictions by using prevention and treatment programs. In this area of research the translation of basic neuroscience of causal mechanisms to effective interventions has not been fully realized. In this article we describe how an understanding of the effects of early adverse experiences on brain and biological development may provide new opportunities to achieve impact at scale with respect to reduction of addictions. We propose four categories of new knowledge that translational neuroscience investigations of addictions should incorporate to be successful. We then describe a translational neuroscience-informed smoking cessation intervention based on this model.
Keywords: Translational Neuroscience, Addictions, Early Adversity
Introduction
Addictions are an interesting conundrum. On one hand, we know a great deal about the underlying neural circuitry of addictions—specifically in regions associated with reward and self-control [1]. Intriguingly, the same brain regions and circuits appear to be common causal pathways in individuals with addictions [2] regardless of whether their predilection involves drugs and alcohol, shopping, pornography, or other appetitive behaviors. On the other hand, in spite of these common causal models, addictions remain one of the most pernicious and costly psychiatric and public health problems, both in our society and globally. Although evidence-based addiction treatments are available, the scope of the problem is vast and has been only marginally diminished by these treatments. Moreover, the addictions prevention field provides numerous examples of widely implemented programs that have proven, under the scrutiny of empirical research, to be inert [3–5], or worse, iatrogenic [6]. Clearly, this is an area in which the basic neuroscientific knowledge about causal pathways has not been sufficiently translated into efficacious prevention and treatment programs that can be widely scaled to ultimately reduce the incidence of addictions.
It should be noted that the lack of successful translation from basic scientific knowledge to interventions that produce impact at scale is by no means unique to addictions research. There are many other examples of mental health problems (e.g., depression) and of physical health problems (e.g., obesity) about which a great deal is understood in terms of root biological correlates and causes, but nevertheless, only limited progress has been made in the use of this information to reduce the population-level incidence of the phenomenon. However, it is also important to note that the absence of effective translation of “cause-to-cure” to address public health problems is not ubiquitous. In medicine, for instance, great strides have been made in the treatment and prevention of many diseases. Certain forms of cancer and heart disease are some of the most noteworthy areas in which breakthrough treatments have been recently discovered, but many other historical examples exist as well (e.g., the use of antibiotics to treat infection, polio and measles vaccines).
This article includes a discussion about ways in which basic neuroscientific research—in particular, investigations of the effects on neurobiological development of early adverse experiences, such as neglect, abuse, and severe economic disadvantage—can be effectively leveraged in the context of translational research to increase the impact and scalability of interventions to treat addictions, and thereby to ultimately reduce the incidence of addictions. A necessary qualifying statement must first be made: Although we propose strategies for using translational neuroscience to continue to move the field of addictions research forward, this is in no way intended to invalidate the considerable effort and progress that has been made in recent decades in the understanding of and treatment of addictions. To the contrary, translational neuroscience depends critically on continued attention to this very challenging area of science, human behavior, and public policy.
Neurobiological Effects of Early Adversity: Implications for Translational Neuroscience-informed Addictions Interventions
Extensive scientific evidence reveals that experience shapes the architecture of the developing brain [7,8]. This is especially true early in life, when there is both vast proliferation and pruning of neurons as a result of early interactions with caregivers [9]. Consistent and nurturing care by a parent or parent figure, in particular, facilitates healthy neural development; this in turn is associated with positive psychosocial adjustment and physical health. In contrast, early stressful experiences have the potential to dramatically alter the structure and function of developing neurobiological systems and of neural regions and pathways, with lifelong negative consequences for overall health and specifically for mental health [10].
Two domains of findings in recent years in the science of early adversity are particularly relevant for addictions research. First, studies have documented that the experience of traumatic events, such as physical abuse, can have a lasting impact; research has also shown that the absence of early supportive care (i.e., neglect), which is far more common, can represent a “toxic stressor” [11,12]. Adverse early experiences, including both trauma and neglect, have been documented to affect several key brain regions, including the lateral prefrontal and superior temporal cortices, the dorsal anterior cingulate cortex, and the amygdala and hippocampus [13,14], as well as the connectivity among them [15]. Second, many of these systems, especially those that have a more protracted course of development, show plasticity in the context of psychosocial interventions [16].
Because many of the brain regions examined in this aforementioned work have also been implicated as playing a causal role in addictions [17,18], this research has potential to guide intervention development and evaluation. For example, it may help provide clues about who is more or less likely to respond to an addiction intervention, based on degree of exposure to early adversity and extent of the impact on underlying neural mechanisms of addiction and their neurodevelopmental trajectory [19]. This research may also enable us to identify markers of intervention effectiveness in terms of specific brain or neurobiological changes that we may observe [20]. However, the process by which such translational work is likely to be most effective requires explication.
What Will Make Translational Neuroscience-informed Addictions Intervention Research Successful?
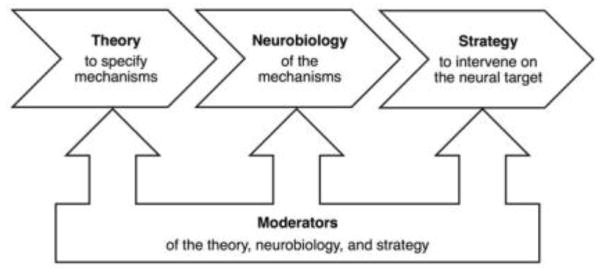
Translational neuroscience could be described as knowledge hungry in that it requires fairly detailed information about the underlying processes that confer risk or perpetuate a disorder. This knowledge falls into four categories, as shown in Figure 1.
Figure 1.
Translational neuroscience requires four kinds of information. A Theory specifies the processes or mechanisms that underlie the relationship between an intervention and an outcome. Neurobiology defines the neural targets that correspond to the mechanisms of intervention action. A Strategy dictates the means to engage with the neurobiological system through psychosocial or other intervention. Moderators indicate relevant individual differences, such as the nature and timing of early adverse experiences that alter the theory, the neurobiological targets, and the strategy to intervene.
First, translational neuroscience requires a theoretical or conceptual model that implicates particular behavioral and/or mental processes and, thereby, neural regions or systems in a disease. The model must specify a mechanism that can be mapped at some level of detail to neural or other biological function systems or structures. These models are similar to mediational models in psychology and related fields insofar as the models propose a relationship between two variables that can be explained by a third “process” variable. However, translational neuroscience models are ideally informed and constrained by current knowledge about how the mediating processes are implemented at a neurobiological level. Therefore, the level of the precision of the theoretical models to be tested in translational neuroscience research is directly predicated upon the quality of the information available about the underlying neural systems associated with the processes implicated in the model.
Following from the theoretical models, the second kind of prerequisite knowledge for translational neuroscience is a mapping between the behaviors and/or mental processes and neurobiological systems. We use the term neurobiological systems inclusively here to refer to not only functional neural activation, but also to structure, connectivity, and resting state properties, as well as psychophysiology, immune and neuroendocrine responses, and so forth. Of utmost importance is information about the nature and timing of the effects of early adverse experiences on neurobiological systems. Increasingly refined knowledge about the brain will feed in to more-specific models of disease, so there will always be a need in translational neuroscience for high-quality, basic neuroscientific information about psychological processes. This requirement speaks directly to the perceived tension between “basic” and “applied” neuroscience research, which we view as a false dichotomy. As has been argued elsewhere [21], progress in translational science will necessarily iterate between testing mechanistic models for intervention and refining those models on the basis of increasingly precise descriptions and constraints of the neural systems.
The third kind of knowledge required by translational neuroscience is a set of theory-derived tools to intervene on the neural systems implicated in the disease. The knowledge base in this area is by far the least progressed in the field’s development. This scarcity is natural given the relative youth of translational neuroscience compared with its neighboring fields of intervention science and cognitive neuroscience. Nonetheless, obtaining ways to manipulate specific neural systems is essential for translational neuroscience to move forward. Reciprocally, developing and empirically evaluating interventions based on neural systems models carries with it potential to move beyond the “black box” intervention models that are so pervasive in the addictions and mental health fields, and move toward greater understanding of precisely “how” interventions work.
Currently, the most direct ways to manipulate neural function are pharmacological [22,23] and surgical [24]. Emerging research is also documenting the impact of less invasive means of stimulating focal areas of the brain to either increase or decrease activation in specific neural regions implicated in disease [25,26]. In addition to these direct approaches to targeting neural systems, a novel and innovative class of psychosocial “brain-training” interventions are beginning to emerge that can target key systems, such as the fear circuitry implicated in anxiety [27] and regions of the frontrostriatal motor planning and implementation network that are involved in inhibitory control [28]; see Bryck and Fisher, 2012, for a summary [29]. These interventions use behavioral or psychosocial means, such as narrowly focused neurocognitive tasks, to engage and thereby alter the function of specific neural systems. Brain-training protocols are grounded in basic cognitive, affective, and social neuroscience research that identifies procedures to elicit activity in the targeted networks. The question of how to develop interventions to target specific neurocognitive systems is critically important for translational neuroscience; we return to it in the final section of this article.
A fourth category of information, and one that applies to each of the three types of knowledge already described, is about moderating, that is, interacting, factors. For instance, are there individual or group differences in the primary relationships specified in the theoretical model? To what extent do these differences allow us to distinguish variations in the impact of the specified interventions? The answers to these questions might strike some as secondary to the questions about the “main effects” of the intervention. However, moderators are of equal importance to main effects for translational research because they directly inform implementation by indicating when and for whom a model applies and for whom a treatment is effective.
We have described in abstract terms how translational neuroscience can proceed once a mechanistic theory, a candidate neural system, and a means to intervene on that system are in hand, and then a set of moderating factors is identified. In the next section, we discuss a specific example of how the translational neuroscience approach is being applied in the domain of cigarette smoking cessation.
Case Study: Using Translational Neuroscience to Inform Cigarette Smoking Cessation
Research on cigarette smoking cessation provides a concrete example of how translational neuroimaging can contribute to preventive interventions that use an experimental and precision medicine approach. Smoking cessation is a good candidate for translational neuroscience because theoretical models implicate craving as a specific mediating process that perpetuates smoking and psychosocial interventions such as cognitive behavioral therapy can reduce craving, but it is difficult to identify how they do so using behavioral research alone. For example, therapies might work by causing a shift in attentional orientation toward drug cues, a change in the emotional potency of the cues, or both; knowing which would help refine existing interventions and build new ones that might not have been apparent based on behavioral data alone. Scientists have now established a set of neurocognitive mechanisms of craving and are actively developing interventions that target it at the neurobiological level. Most relevant to this article, the neurobiology of craving, and more generally of addictions, is known to be affected by early adverse experiences.
The theoretical model guiding our smoking cessation research is that cue-induced craving for cigarettes increases the chances of relapse, so reducing the potency of these cravings by targeting the neurocognitive systems that give rise to cravings will reduce relapse. It has long been established that cue-induced craving is a key proximal risk factor for relapse [30], and that early adversity affects craving and the regulation of craving [31]. The identification of specific neural regions that give rise to cravings, such as the ventral striatum, insula, amygdala, and orbitofrontal cortex [32,33], have given rise to new ways to reduce craving. For example, initial work has shown that directly targeting those regions pharmacologically [34] or with transcranial magnetic stimulation [35] could alter their function and the associated experience of craving. This research provides proof-of-concept for the idea that identifying specific neural systems and targeting them for intervention can be fruitful for cigarette smoking cessation research. Presumably, traditional interventions that reduce smoking such as cognitive behavioral therapy have effects on the brain, but what those effects are and how they relate specifically to craving (e.g., whether they are direct or indirect) is unknown.
Recently, researchers in the field of affective neuroscience have begun to verify that traditional psychosocial interventions reduce craving by altering its neural systems in ways similar to those of pharmacological or stimulation interventions. For example, imagining the negative long-term health consequences of continued smoking reduces activation in craving-related neural regions by increasing activity in effortful control regions, such as the ventrolateral and dorsolateral prefrontal cortices [36]. This result is a breakthrough because it enables scientists to apply the considerable knowledge about the brain systems involved in effortful control gained from cognitive neuroscience to practical contexts. It is known, for example, that cognitive reappraisal of emotional stimuli draws upon the same neural systems as the effortful control induction that Kober and colleagues used [37], and that training in cognitive reappraisal can cause relatively long-lasting changes in those systems [38]. Together, these facts suggest the hypothesis that training in cognitive reappraisal of cigarette craving would alter the function of reappraisal-related brain regions, in turn reducing cigarette craving by influencing its underlying brain systems.
Knowledge of the brain systems of craving regulation can also suggest entirely novel interventions that do not share superficial features with existing interventions but operate through the same underlying mechanisms. We showed that the neural effects of inhibitory control in one domain (such as motor control) can unintentionally “spill over” to influence the brain activity in other domains (such as emotion control) [39]. In particular, participants showed decreases in amygdala activity to below-baseline levels when they happened to withhold a button press while they viewed a negative image. The degree of the decrease in emotional reactivity (as measured by amygdala activity) was proportional to the increase in cognitive control system activity involved by the motor control task. It would be possible to design an intervention based on this “inhibitory spillover” principle that leverages the fact that effortful control systems can be invoked by a range of manipulations and need not be specific to cigarette cues.
Formulating the theory of change in terms of underlying neural systems instead of psychological constructs has the additional advantage of highlighting places in the model where moderating factors might come into play. For example, cognitive reappraisal is not equally effective at eliciting activation in the targeted brain regions for all individuals [40], and the nature of the interrelations between the prefrontal and subcortical regions may vary with certain polymorphisms [41]. Baseline neural activity patterns may be a useful diagnostic tool to determine which kinds of interventions work for whom [42]. And knowledge about the timing and type of early adversity can inform which and how reappraisal-related neural systems were affected and, thereby, how and when to best target them for change through intervention [43]. As is the case with mechanistic variables, more-precise knowledge about moderating factors will enable researchers to develop increasingly refined hypotheses and, thereby, increasingly powerful interventions.
Next Steps for Translational Neuroscience Research
The substantial knowledge bases in neuroscience and intervention science have led directly to the establishment and initial success of translational neuroscience. Work in these two fields can and must progress to drive translational neuroscience forward, but we see three topics in neuroscience and intervention, and their overlap, as particularly important for the future of translational neuroscience.
First, as noted previously, the field must continue to develop basic neuroscientific knowledge about disorder-relevant processes. This work should focus on brain mapping to localize the necessary and sufficient regions for cognitive and affective processes and also functional and anatomical connectivity to establish the ways in which the implicated regions relate to one another. Greater recognition is needed among the lay public and research sponsors alike, that basic science continues to play a direct role in fostering interventions that will have broad societal impact.
Second, the field must develop integrated theoretical models that operate on the behavioral, psychological, and neurobiological levels. A vexing issue in the field is how to understand cases in which an intervention has effects at one level but not the other, such as when behavior changes but neural activity does not, or vice versa. These cases reveal gaps in knowledge about the concordance between behavioral, psychological, and neurobiological function and can suggest topics that need further investigation. However, such investigations can progress in parallel with translational efforts. Theoretical models for intervention will always be limited by the current state of scientific knowledge, but that doesn’t mean that intervention is not possible with imperfect information. On the contrary, interventions based on the best-available data can have an important role in testing theory as part of an iterative cycle between theory development and neurobiological hypothesis testing.
And third, the field must continue to develop psychosocial interventions that can alter neurobiological function in targeted regions and networks. Discovery is somewhat haphazard at this preliminary stage in the field’s development because a coherent framework for intervention development is lacking: Given a specific neural system, what is the procedure for creating an intervention that might alter it? A logical starting point is training based on associative learning or classical conditioning. Examples of recent successes based on these approaches include cognitive training to target proactive control in the lateral prefrontal cortex [28] and attention bias modification to target amygdala activation in anxiety [44]. Nonetheless, more-rapid development of neurally targeted interventions will require more-sophisticated frameworks that are grounded in recent advances in neuroscientific knowledge.
Conclusions
We have presented a framework for how translational neuroscience that is focusing on the effects of early adversity might be used to inform addictions intervention. We also described an application of this framework to the area of smoking cessation. Because of the common neural pathways of addiction, similar strategies may prove fruitful for interventions to address issues such as alcohol abuse and overeating. These approaches are needed to move beyond the current scientific knowledge base and to produce meaningful reductions in the incidence of addictions at a population level.
Acknowledgments
The following grant funding is gratefully acknowledged: DA035763 from NIDA to Philip Fisher, HD075716 from NICHD to Philip Fisher, AG048840 from NIA to Elliot Berkman, CA175241 from NCI to Elliot Berkman, and MH078105 from NIMH to Megan Gunnar.
Footnotes
Conflict of Interest
Philip A. Fisher and Elliot T. Berkman declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article doesn’t contain human or animal studies, but we cite several studies of ours that do include human subjects. Informed consent was obtained for all of those studies under approval of the UO IRB.
Contributor Information
Philip A. Fisher, Email: philf@uoregon.edu.
Elliot T. Berkman, Email: berkman@uoregon.edu.
References
*Of importance
**Of outstanding importance
- 1.Koob GF. 2009 Dynamics of neuronal circuits in addiction: Reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(S1):S32. doi: 10.1055/s-0029-1216356. http://dx.doi.org/10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–560. doi: 10.1038/nn1452. http://dx.doi.org/10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 3**.Ennett ST, Tobler NS, Ringwalt CL, Flewelling RL. How effective is drug abuse resistance education? A meta-analysis of Project DARE outcome evaluations. Am J Public Health. 1994;84(9):1394–1401. doi: 10.2105/ajph.84.9.1394. http://dx.doi.org/10.2105/AJPH.84.9.1394This meta-analysis of the widely implemented national prevention program DARE showed that effect sizes were null to very small. More targeted approaches are called for to prevent drug abuse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moos RH. Iatrogenic effects of psychosocial interventions for substance use disorders: Prevalence, predictors, prevention. Addiction. 2005;100(5):595–604. doi: 10.1111/j.1360-0443.2005.01073.x. http://dx.doi.org/10.1111/j.1360-0443.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 5.Werch CE, Owen DM. Iatrogenic effects of alcohol and drug prevention programs. J Stud Alcohol. 2002;63(5):581–590. doi: 10.15288/jsa.2002.63.581. [DOI] [PubMed] [Google Scholar]
- 6**.Dishion TJ, McCord J, Poulin F. When interventions harm: Peer groups and problem behavior. Am Psychol. 1999;54(9):755. doi: 10.1037//0003-066x.54.9.755. http://dx.doi.org/10.1037/0003-066X.54.9.755This essay describes a number of prevention programs that have produced negative outcomes. Consideration is given to the qualities of the interventions that explain these harmful effects. [DOI] [PubMed] [Google Scholar]
- 7.Fox SE, Levitt P, Nelson CA., III How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. http://dx.doi.org/10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. http://dx.doi.org/10.1001/jama.2009.754This is one of the most concise yet comprehensive accounts of how experiences build brain architecture. There is also useful information about how adverse experiences impact health and well-being. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RA. Development in the first years of life. Future Child. 2001:21–33. http://dx.doi.org/10.2307/1602807. [PubMed]
- 10.Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. http://dx.doi.org/10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 11.Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81(1):357–367. doi: 10.1111/j.1467-8624.2009.01399.x. http://dx.doi.org/10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- 12**.Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Dev Psychobiol. 2009;51(1):14–23. doi: 10.1002/dev.20333. http://dx.doi.org/10.1002/dev.20333This study shows that the stress-hormone, cortisol, is blunted in individuals with high levels of early adversity, in contrast to many other studies showing that acute stressors produce elevated cortisol. The researchers found that it was specifically neglect that was associated with low cortisol, and not experiences of trauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. http://doi.org/10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 14.Tomalski P, Johnson MH. The effects of early adversity on the adult and developing brain. Curr Opin Psychiatr. 2010;23(3):233–238. doi: 10.1097/YCO.0b013e3283387a8c. http://doi.org/10.1097/YCO.0b013e3283387a8c. [DOI] [PubMed] [Google Scholar]
- 15.Teicher MH, Anderson CM, Ohashi K, Polcari A. Childhood maltreatment: Altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiat. 2014;76(4):297–305. doi: 10.1016/j.biopsych.2013.09.016. http://doi.org/10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelazo PD, Carlson SM. Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Dev Perspectives. 2012;6(4):354–360. http://doi.org/10.1111/j.1750-8606.2012.00246.x. [Google Scholar]
- 17.Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. http://dx.doi.org/10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaser D. Child abuse and neglect and the brain—a review. J Child Psychol Psyc. 2000;41(01):97–116. http://dx.doi.org/10.1017/S0021963099004990. [PubMed] [Google Scholar]
- 19*.Gunnar MR, Fisher PA. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Dev Psychopathol. 2006;18(03):651–677. http://dx.doi.org/10.1017/S0954579406060330. [PubMed] [Google Scholar]
- 20.Bruce J, Gunnar MR, Pears K, Fisher PA. Early adverse care, stress neurobiology, and prevention science: Lessons learned. Prev Sci. 2013;14(3):247–256. doi: 10.1007/s11121-012-0354-6. http://dx.doi.org/10.1007/s11121-012-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham WA. In defense of brain mapping in social and affective neuroscience. Soc Cognition. 2010;28:717–722. http://dx.doi.org/10.1521/soco.2010.28.6.717. [Google Scholar]
- 22.Le Foll B, Di Ciano P, Panlilio LV, Goldberg SR, Ciccocioppo R. Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: Preclinical evidence. Curr Drug Targets. 2013;14:768–776. doi: 10.2174/1389450111314070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Morris SE, Rumsey JM, Cuthbert BN. Rethinking mental disorders: The role of learning and brain plasticity. Restor Neurol Neuros. 2014;32:5–23. doi: 10.3233/RNN-139015. http://doi.org/10.3233/RNN-139015A summary of the translational neuroscience approach based in NIMH’s “Research Domain Criteria” (RDoC). The concluding section reviews cognitive, behavioral, and other approaches to interventions that target specific neurocognitive systems. [DOI] [PubMed] [Google Scholar]
- 24.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat. Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. http://doi.org/10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Corazza M, Turovets S, Luu P, Tucker D. Optimization in transcranial electrical neuromodulation based on the reciprocity principle. Brain Stimul. 2015;2(8):403. doi: 10.3389/fpsyt.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): A review. Exp Neurol. 2009;219(1):14–19. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 27.Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, et al. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiat. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. http://dx.doi.org/10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Berkman ET, Kahn LE, Merchant JS. Training-induced changes in inhibitory control network activity. J Neurosci. 2014;34:149–157. doi: 10.1523/JNEUROSCI.3564-13.2014. http://doi.org/10.1523/JNEUROSCI.3564-13.2014Presents evidence that a behavioral training program can alter neural function in the inhibitory control network. This work represents proof-of-concept that highly focused behavioral training programs can affect change in targeted neural networks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Bryck RL, Fisher PA. Training the brain: Practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. Am Psychol. 2012;67:87–100. doi: 10.1037/a0024657. http://doi.org/10.1037/a0024657A review of non-invasive brain-training interventions for children with a focus on programs to increase executive function during critical periods of plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. http://doi.org/10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Andersen SL, Teicher MH. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neurosc Biobehav R. 2009;33(4):516–524. doi: 10.1016/j.neubiorev.2008.09.009. http://doi.org/10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiat. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. http://doi.org/10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 33.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. http://doi.org/10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- 34.Brody A, Mandelkern M, Lee G, Smith E, Sadeghi M, Saxena S, et al. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: A preliminary study. Psychiat Res Neuroim. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. http://doi.org/10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brody AL, Cook IA. Manipulation of cigarette craving with transcranial magnetic stimulation. Biol Psychiat. 2011;70:702–703. doi: 10.1016/j.biopsych.2011.08.001. http://doi.org/10.1016/j.biopsych.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl A Sci. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. http://doi.org/10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. http://doi.org/10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Denny BT, Inhoff MC, Zerubavel N, Davachi L, Ochsner KN. Getting over it: Long-lasting effects of emotion regulation on amygdala response. Psychol Sci. doi: 10.1177/0956797615578863. In press. Initial evidence of the durability of a psychological intervention to increase emotion regulation. This study is a model of how to validate potential interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkman ET, Burklund L, Lieberman MD. Inhibitory spillover: Intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. Neuroimage. 2009;47:705–712. doi: 10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliani NR, Mann T, Tomiyama AJ, Berkman ET. Neural systems underlying the reappraisal of personally craved foods. J Cognitive Neurosci. 2014;26:1390–1402. doi: 10.1162/jocn_a_00563. http://doi.org/10.1162/jocn_a_00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. http://doi.org/10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 42.Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: Neural correlates of breaking the link between craving and smoking. Psychol Sci. 2011;22:498–506. doi: 10.1177/0956797611400918. http://doi.org/10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. http://doi.org/10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, Bar-Haim Y. Neural changes with attention bias modification for anxiety: A randomized trial. Soc Cog Affect Neur. 2014;10:913–920. doi: 10.1093/scan/nsu141. http://doi.org/10.1093/scan/nsu141. [DOI] [PMC free article] [PubMed] [Google Scholar]