Abstract
Serum-free media have been shown to be effective in the expansion of mesenchymal stem cells (MSCs). However, the effects may go beyond cell expansion as the differentiation potentials of the cells may be modified, thus influencing their efficacy for downstream applications. The latter is poorly understood, and this has prompted an evaluation of the influence of a serum-free formulation on the chondrogenic, adipogenic, and osteogenic potential of MSCs. The media consisted of Knockout™ Serum Replacement (KSR) with a cocktail of growth factors coupled with either collagen or fibronectin coatings. Collagen coating was selected as it promoted consistent cellular attachment. When compared against fetal bovine serum (FBS) controls, cell proliferation in the serum-free media was enhanced at passage 1. Similar levels of surface markers were observed in the two groups with a slight reduction in CD90 and CD73 in the serum-free culture at passage 3. The cultures were screened under differentiation conditions and a better maintenance of the chondrogenic potential was noted in the serum-free media with higher expressions of glycoaminoglycans (GAGs) and collagen II. Chondrogenesis was deficient in the FBS group and this was attributed to the inherent inconsistency of animal serum. Adipogenesis was enhanced in the serum-free group with a higher PPARG expression and lipid accumulation. Similar levels of osteogenic mineralization was noted in the FBS and serum-free groups but collagen I gene expression was suppressed in the latter. This was initially observed during expansion. These observations were attributed to the signaling cascades triggered by the cytokines presented in the serum-free formulation and the interaction with the collagen substrate. The serum-free media helps to maintain and enhance the chondrogenic and adipogenic potentials of the MSCs, respectively. This advantage can be exploited for therapeutic applications in cartilage and adipose tissue engineering.
Key words: Mesenchymal stem cells (MSCs), Serum-free media, Chondrogenesis, Adipogenesis
INTRODUCTION
The discovery of mesenchymal stem cells (MSCs) by Friedenstein was a major accomplishment in cellular therapeutics (18). This multipotent cell was initially identified in bone marrow, and the International Society for Cellular Therapy (ISCT) has characterized it as a plastic adherent fibroblast-like clonogenic cell with CD73, CD90, and CD105 surface antigens. MSCs lack CD34 and CD45 human leukocyte markers (31). Recent research has shown that the secretory activity of MSCs can be exploited for therapeutic applications such as the immunosuppression of T cells, antiscarring, angiogenesis, antiapoptosis, and mitosis. Jeon et al. observed that conditioned media from MSCs enhanced the survival of fibroblast cells and the secretion of collagen, elastin, and fibronectin (29). Thus, MSC stimulation could assist skin wound healing.
Bonfield et al. has also demonstrated that MSCs were able to suppress chronic airway inflammation associated with the murine ovalbumin model of asthma and this was attributed to the anti-inflammatory molecular signals expressed by the stem cells (7). The expression of such molecular cues enables MSCs to influence other cell types. The homing and proliferation of hematopoietic stem cells (HSCs) within the bone marrow niche is influenced by MSCs (44). Through cocultures, MSCs were found to support vasculature formation from endothelial progenitor cells (16). These reparative cells were capable of systemic migration and they home in on sites of injury such as myocardial infarction with stromal cell-derived factor (SDF)-1α and integrins forming part of this migratory mechanism (20,46,73). Besides bone marrow, MSCs were found in a range of tissues that include Wharton jelly (12), placenta (60), amniotic fluid (13), adipose tissue (84), and skin dermis (71). The latest literature has even shown that MSCs reside as pericytes in the perivascular location of various tissues (17).
Intensive research was conducted to exploit the osteogenic, adipogenic, and chondrogenic potential of MSCs in regenerative medicine. Osseous regeneration was one of the earliest avenues. Nonhealing segmental bone defects were bridged with MSCs seeded on scaffolds (34,56). Enhanced regeneration at calvarial defects was achieved with porous matrices implanted with MSCs (70). The stem cells can also be harnessed in the gene therapy of osteogenesis imperfecta (61). Wakitani et al. (87) and Lee et al. (36) advocated its use for cartilage repair as MSCs are capable of differentiating into chondrocytes. Moreover, the cell could home in on the cartilage defect to exert a reparative response (36). The need for soft tissue fillers is a constraint in plastic and reconstructive procedures. Cui et al. (11) and Neubauer et al. (53) attempted to resolve this via an MSC-based adipose tissue engineering approach. But stem cells are presented at low frequency even within the bone marrow; hence, monolayer culturing is needed so as to yield significant numbers for therapeutic use. Unfortunately, conventional ex vivo expansion requires fetal bovine serum, which is a worrisome concern.
Fetal bovine serum (FBS) is commonly used in cell cultures but it is a complex mixture with only 200 components identified. The biochemical effects of these molecules remain undefined and the composition varies between serum batches, thus leading to inconsistent outcomes that can only be prevented via tedious prescreening (54). Moreover the presence of viruses, prions, and mycoplasmas presents a safety concern. Animal serum also introduces xenogenic antibodies that can cause severe anaphylactic or arthus-like immune reactions (72). The risk is not completely eliminated via washing as there is active cellular uptake of FBS (59,79). Therefore, substitutes were sought with human serum, platelet lysate, and growth factor formulations. Stute et al. observed enhanced MSC proliferation in autologous human serum when compared to FBS (82). But Lange et al. cautioned that the amount of autologous serum necessary for sufficient cell expansion exceeded the feasible donor amount (35). Moreover, serum collection in the event of serious injury or emergency is impractical (86). Shahdadfar et al. (74) and Turnovcova et al. (86) resorted to human allogenic serum. Although Turnovcova et al. reported improved MSC colony formation (86), Shahdadfar et al. encountered growth arrest and cell death (74). This might be due to the elevated level of hemagglutinins in the pooled sera (74). Platelet derivative is another alternative. MSCs cultured with 5% platelet lysate remained spindled shaped with conserved progenitor markers (86). In addition to that, the colony forming units of fibroblasts (CFU-F) were larger and more numerous when compared to FBS cultures. This was mainly due to the increase in DNA replication with the suppression of differentiation during expansion and it was attributed to the effects of platelet-derived growth factor (PDGF), fibroblast growth factor 2 (FGF2), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF) (35,42,43,68). Tsutsumi and others noted that the addition of FGF2 boosted the life span of MSCs during expansion due to an early telomere extension (5,85). When Chung et al. injected chemotactic PDGF into a growth plate defect, he noted an increased MSC influx into the site (10). EGF was reported to regulate bone homeostasis by suppressing MSC differentiation (88). The advantage of platelet lysate arises from the presence of these cytokines but it is countered by constitutional variations between platelet donors, which leads to an inconsistent outcome in MSC cultures (69). This could be partially resolved with an optimal platelet concentration of 1.5 × 109/ml that was pooled from at least 10 donors (35). But this approach would strain the limited blood banking resources (35). Hence, there is an impetus to develop an “off the shelf” serum-free media consisting of cytokines.
Cancedda et al. submitted a patent on an MSC serum-free formulation that contained EGF, PDGF, FGF2, leukemia inhibitory factor (LIF), stem cell factor (SCF), and dexamethasone (8). But poor cell attachment has prompted the inclusion of FBS for initial plating (8). Pal et al. (59) and Shetty et al. (75) encountered the same limitation with serum-free media and cord blood serum, respectively. Hence Pal et al. concluded that these alternatives could only maintain ongoing cultures that were already isolated using FBS (59). However, this could be largely circumvented by surface coating, which enhanced MSC adhesion. Qian et al. tested poly lysine, collagen, fibronectin, and Matrigel coatings (64). He noted optimal expansion on fibronectin and collagen substrates. Fibronectin contains the arginine-glycine-aspartic acid (RGD) peptide required for cellular interactions while collagen is an ubiquitous extracellular matrix (ECM) protein. Invitrogen (Carlsbad, CA) recognized the importance of such interactions and it marketed Cellstart™ substrate with StemPro®. StemPro® is an MSC serum-free media that facilitated better MSC proliferation than allogenic human serum and FBS (40,55). Similar enhancements were reported for Mesencult® XF (Stemcell Technologies, Vancouver), Cellgro® (Cellgenix, Freiburg), and Ultroser™ (Pall Corporation, Washington) (45,63). The capacity for mesenchymal differentiation was maintained with most of these products, but a modulation in lineages may occur. During osteogenic induction, Agata et al. noted a lower alkaline phosphatase activity in StemPro® cultures compared to FBS controls (1). Lindroos et al. reported simultaneous adipogenesis during osteogenic induction (40). The understanding of these phenomena was hindered by the undisclosed composition of the commercial product, hence troubleshooting was difficult. This inevitably becomes an obstacle in the translational efforts for specific applications.
The present study was proposed to evaluate the efficacy of MSCs expanded in serum-free media towards osteogenic, chondrogenic, and adipogenic differentiations. This would gauge the effect of the serum-free medium on downstream musculoskeletal applications. The medium of interest consisted of Knockout™ Serum Replacement (KSR, Invitrogen, Carlsbad, CA), FGF-2, EGF, PDGF-AB, and dexamethasone. KSR was originally developed for embryonic stem cells (ESCs) (92). The serum-free alternative was coupled with either human recombinant fibronectin (Hfn) or collagen I (Hcol). MSC attachment, proliferation, surface antigens, and basal gene expressions were scrutinized during the expansion phase. Subsequently, osteogenic, chondrogenic, and adipogenic differentiations were assessed relative to FBS controls.
MATERIALS AND METHODS
Reagents and Chemical
Unless otherwise stated, all reagents were purchased from Sigma Aldrich (St. Louis, MO). High and low glucose Dulbecco’s modified Eagle’s medium (DMEM) was from Gibco BRL (Grand Islands, NY). All labware consumables were purchased from Becton-Dickinson (Franklin Lakes, NJ).
MSC Culture and Differentiation
Bone marrow was aspirated from the posterior iliac crest of patients who were undergoing elective orthopedic procedures. Patient consent was granted and the work was approved by the Hospital Institutional Review Board. Equal volumes of bone marrow were plated either in FBS-supplemented or serum-free medium. The FBS culture consisted of low glucose DMEM, 10% FBS (Invitrogen, Carlsbad, CA), and 100 U/ml penicillin-streptomycin (Invitrogen). The serum-free alternative contained low glucose DMEM, 10% KSR (Invitrogen), 2 ng/ml FGF2, 2 ng/ml EGF, 2 ng/ml PDGF-AB (Peprotech, Rocky Hill, NJ), 10 nM dexamethasone, and 100 U/ml penicillin-streptomycin (Invitrogen). The concentrations of these components had been optimized from prior studies. Cell plating in the serum-free medium was assisted either with Hcol (100 μg/cm2, Fibrogen, South San Francisco, CA) or Hfn (1 μg/cm2, Invitrogen) coatings. Solubilized Hcol and Hfn were coated onto the plates at 100 and 1 μg/cm2, respectively, overnight at 4°C. The coating solutions were aspirated and the plates were rinsed with sterile phosphate-buffered saline (PBS). Hcol and Hfn serum-free cultures were evaluated against FBS controls and cell adhesion was monitored via microscopy. Confluent cultures were trypsinized with 0.25% trypsin, which was removed via extensive PBS washings. After hemocytometer counts, the cells were plated at 10,000/cm2 and this was repeated every week until passage 5 (P5). Fold increase was calculated by dividing the cell counts of each passage by the number of seeded cells. P2 and P3 cultures were subjected to flow cytometry while basal gene expression during expansion was scrutinized. Moreover, P3 cells were exposed to chondrogenic, osteogenic, and adipogenic inductions.
Chondrogenic cultures were initiated by centrifuging aliquots of 0.25 million cells at 150 × g for 10 min in 15-ml polypropylene conical tubes. These pellets were maintained for 28 days with 100 nM dexamethasone, 1% ITS+ premix (Biomedical Diagnostics, Ann Arbor, MI), 50 μg/ml ascorbic acid, 1 mM sodium pyruvate (Invitrogen), 4 mM proline, 2 mM l-glutamine (Invitrogen), 100 U/ml penicillin-streptomycin (Invitrogen), and high glucose DMEM. Chondrogenic induction was achieved with 10 ng/ml of transforming growth factor-β3 (TGF-β3, R&D systems, Minneapolis, MN), which was omitted from the uninduced controls. Osteogenic potential was evaluated in cultures plated at 3000/cm2, maintained for 21 days in 10 nM dexamethasone, 50 μM ascorbic acid, 10 mM β-glycerophospate, 10% FBS, 100 U/ml penicillin-streptomycin, and high glucose DMEM. Uninduced controls were kept in the FBS expansion media. Adipogenic induction was achieved by seeding MSC at 30,000/cm2 and maintained for 21 days in high glucose DMEM, 10% FBS, 100 U/ml penicillin-streptomycin (Invitrogen), 2 mM l-glutamine (Invitrogen), 0.01 mg/ml insulin (Invitrogen), 0.02 mM indomethacin, 1 μM dexamethasome, and 0.5 mM 3-isobutyl-1-methylxanthine.
Flow Cytometry
Saturating concentrations of fluorescein osothiocyanate (FITC), phycoerythrin (PE), or phycoerythrin Cy5 (PE-Cy5) conjugated monoclonal mouse antibodies were incubated with 100,000 P2–P3 cells in the dark at room temperature for 30 min. Appropriate isotype-matched controls were included. Washing was performed with a buffer containing 0.1% sodium azide, 4% FBS, and PBS. The cells were suspended in fresh buffer prior to flow cytometry (Cyan LX, Beckman Coulter, Brea, CA) and identified by light scatter for 10,000 gated events. Analysis was performed with Summit v4.2 (Beckman Coulter) for the following markers: CD90-PE-Cy5, CD73-PE, CD45-PE-Cy5, CD44-FITC, and CD29-PE (BD Biosciences, San Jose, CA).
Real-Time Polymerase Chain Reaction (PCR)
Gene expressions of collagen I, sex-determining region Y Box-9 (Sox9), runt-related transcription factor 2 (Runx2), and peroxisome proliferator-activated receptor gamma (PPARG) were evaluated during MSC expansion, osteogenic and adipogenic inductions at day 14. RNA was extracted using Trizol (Invitrogen) and RNeasy Mini kit (Qiagen, Chatsworth, CA). Total RNA was measured via NanoDrop (Nanodrop Technologies, Wilmington, DE). Reverse transcription was achieved with 100 ng of RNA via the iScript™ cDNA synthesis kit (Biorad, Hercules, CA). Real-time PCR was performed using the SYBR green system (7500 real-time PCR system, ABI, Foster city, CA). Amplifications for cDNA were carried out at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for a min. Primer sequences are as shown in Table 1. Fold change in gene expression was calculated using the 2−ΔΔCt method. The gene expression was first normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) within each sample group. Subsequently, the values were normalized against the passage 1 FBS controls.
Table 1.
Real-Time PCR Primer Sequences
| Target Gene | Ascension No. | Forward | Reverse | Size |
|---|---|---|---|---|
| Collagen I | NM_000088 | 5′-CAG CCG CTT CAC CTA CAG C-3′ | 5′-TTT TGT ATT CAA TCA CTG TCT TGC C-3′ | 83 bp |
| Sox9 | NM_000346 | 5′-CAG TAC CCG CAC TTG CAC AA-3′ | 5′-CTC GTT CAG AAG TCT CCA GAG CTT-3′ | 69 bp |
| Runx2 | NM_004348 | 5′-AAC CCA CGA ATG CAC TAT CCA-3′ | 5′-CGG ACA TAC CGA GGG CAA TG-3′ | 76 bp |
| PPARG | NM_138711.3 | 5′-TGT CTC ATA ATG CCA TCA GGT TTG-3′ | 5′-CGC CAA CAG CTT CTC CTT CT-3′ | 62 bp |
| GAPDH | NM_002046 | 5′-ATG GGG AAG GTG AAG GTC G-3′ | 5′-TAA AAG CAG CCC TGG TGA CC-3′ | 70 bp |
Sox9: sex-determining region Y box-9; Runx2: Runt-related transcription factor 2; PPARG: peroxisome proliferator-activated receptor gamma; GAPH: glyceraldehyde 3-phosphate dehydrogenase.
Histology and Immunohistochemistry
Day 28 chondrogenic pellets were evaluated via histology and immunohistochemistry. The samples were fixed overnight in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Sections (5 μm) were taken from the center, deparaffinized, and hydrated. Glycosaminoglycans (GAGs) were stained with 0.5% Alcian blue. Immunohistology was conducted using collagen I (dilution factor 1:500), collagen II (Chemicon, Temecula, CA; dilution factor 1:500), collagen X (Quartett, Berlin, Germany; dilution factor 1:25), and aggrecan (Abcam, Cambridge, MA; dilution factor 1:100) primary antibodies. Isotype controls (Dakocytomation, Glostrup, Denmark) were included. Endogenous peroxidase was blocked with hydrogen peroxide. Antigen retrieval was performed via a 20-min pepsin treatment (Labvision, Fremont, CA). Biotinylated goat anti-mouse secondary antibodies (Labvision, Fremont, CA) were added at room temperature for 30 min. Streptavidin peroxidase was administered for 45 min with the use of 3,3′-diaminobenzidine as a chromogenic agent. Counterstaining was done with Gill’s hematoxylin, after which the sections were dehydrated before being cover slipped.
Mineralization in the osteogenic cultures was detected with 2% Alizarin red. The staining was quantified through 10% hexadecylpyridinium chloride elution with absorbance measured at 562 nm. Oil red stained the lipid deposits in the adipose induced samples. Dye extraction was performed using 100% isopropanol and absorbance was measured at 510 nm.
Quantitative Assay for Chondrogenesis
Cellularity, GAGs, collagen I, and collagen II in the chondrogenic pellet cultures were measured using PicoGreen® (Invitrogen), dimethymethylene blue (DMMB), enzyme-linked immunosorbent assay against collagen I and II (ELISA, Chondrex, Redmond, WA), respectively. Chondrogenic pellets were washed in PBS and digested in 1 mg/ml pepsin and 0.1 mg/ml pancreatic elastase. Once solubilized, the samples were centrifuged and the supernatant was kept aside. DNA content was measured via the PicoGreen® DNA quantification kit with the aid of a Tecan microplate reader (Männedorf, Switzerland). GAG measurements were derived from DMMB absorbance readouts, which were compared against that of chondroitin-6-sulfate standards. The amounts of collagen I and collagen II presented in each sample were measured in accordance to the Chondrex ELISA kit protocol.
Statistical Analysis
All quantitative data garnered from the samples taken from four patients were analyzed using Student t-test with p < 0.05 being considered for significant difference. Replications were conducted for each experimental group.
RESULTS
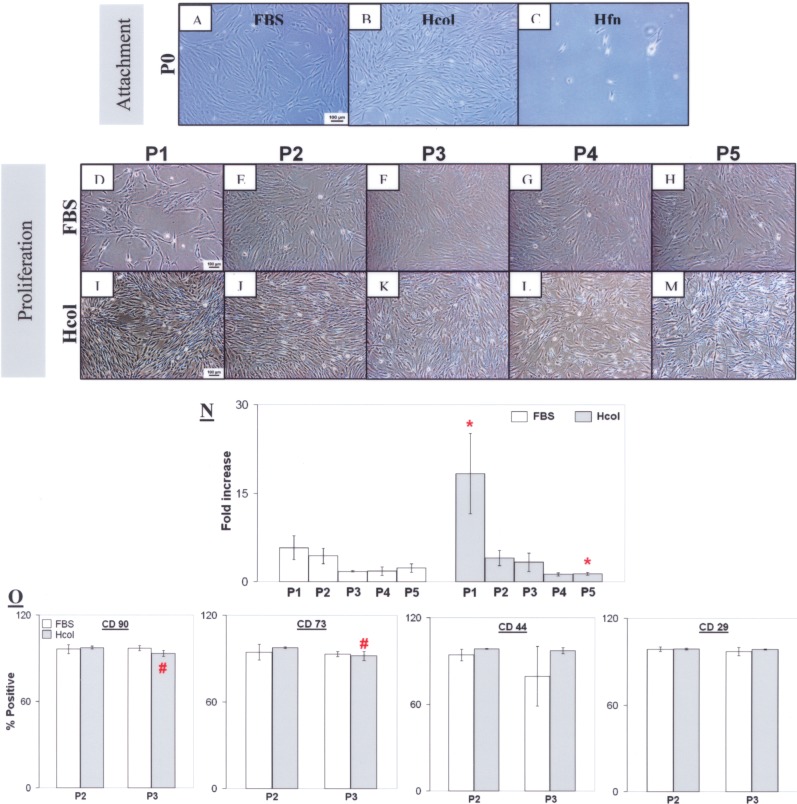
Bone marrow was plated equally in FBS, Hcol, and Hfn serum-free media. Cell attachment was observed on Hcol and it was comparable to the FBS control (Fig. 1A, B). Unfortunately, this was not the case for Hfn even after 14 days of seeding (Fig. 1C); hence, the coating was omitted from further investigations. Hcol and FBS cells were fibroblastic as observed in P1 to P5 (Fig. 1D–M). Cell proliferation was significantly promoted in the Hcol group at P1, but this did not arrest the general decline in proliferation as the fold increase dropped after each passage (Fig. 1N). However, there was a net increase in cell numbers as the cell counts derived at the end of each passage exceeded the initial number of seeded cells. MSC surface markers CD90, CD73, CD44, and CD29 were detected in both groups at P2 and P3 (Fig. 1O). But CD45, which is a leukocyte marker, was absent from the isolated cells. Hence, the MSC progenitor status was maintained under serum free conditions and it was comparable to the FBS group. But there was a significant reduction (p < 0.05) in the number of CD90- and CD73-positive cells when the Hcol culture transited from P2 to P3. This might be attributed to the effects of the serum-free culturing and the cytokines presented in the media.
Figure 1.
Cell attachment, proliferation, and surface marker profile. At P0, similar cell attachment was observed in the human collagen 1 (Hcol) and fetal bovine serum (FBS) groups (A, B). However, it was inconsistent on human recombinant fibronectin (Hfn) (C) even after 14 days of plating; hence, it was omitted from the following assessments. During proliferation, a fibroblastic morphology was noted in FBS and Hcol from P1 to P5 (D–M). Cell number qualification showed a significantly higher fold increase at P1 in the serum-free media with Hcol (N). *Significant difference between FBS and Hcol (p < 0.05, N = 4). A decline in cell multiplication was subsequently observed beyond P1 in both cultures. The cells stained positively for CD90, CD73, CD44, and CD29 (O). But there was negative staining for CD45 (not shown). The numbers of CD90- and CD73-positive Hcol cells decreased from P2 to P3, but they were not significantly different from the FBS group at P3 (p > 0.05, N = 4). #Significant difference between P2 and P3 within the same group (p < 0.05, N = 4).
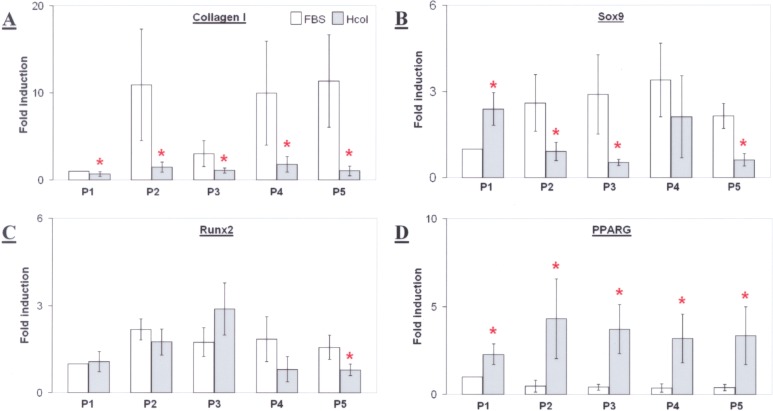
Mesenchymal differentiation might be affected by the preceding serum-free conditions. This could be elucidated from the basal gene expression of Sox9, Runx2, and PPARG, which were crucial markers for chondrogenesis, osteogenesis, and adipogenesis, respectively. The expression of collagen I was also assessed and it was found to be significantly lower in the Hcol cells from P1 to P5 (Fig. 2A). This trend was similarly observed for Sox9 except at P1 (Fig. 2B). Runx2 gene expression was not significantly different for both groups but it seemed to peak at P3 in the serum-free culture (Fig. 2C). Conversely, there was higher PPARG expression in the Hcol group across all passages (Fig. 2D). Such trends were observed even though the variations between patient samples were high, as shown by the large standard deviations in Figure 2. Therefore, the data suggested a potentially different response from the two cultures during chondrogenic and adipogenic inductions. Moreover, the secretion of collagen I might also differ. Such discrepancies were attributed to the cytokines presented in the serum-free media, which triggered signaling cascades, modulating the differentiation pathways.
Figure 2.
Collagen I, sex-determining region Y box-9 (Sox9), Runt-related transcription factor 2 (Runx2), and peroxisome proliferator-activated receptor gamma (PPARG) gene expression in P1–P5 mesenchymal stem cells (MSCs) cultured with FBS or serum-free Hcol coating (A–D). *Significant difference between FBS and Hcol at the same passage (p < 0.05, N = 4).
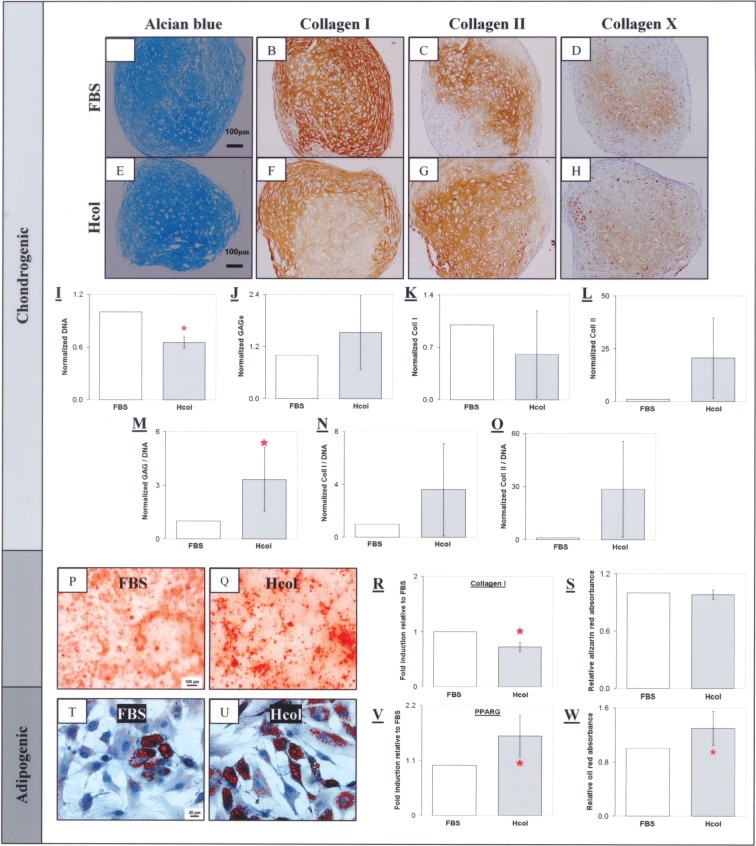
During chondrogenic induction, GAGs was synthesized in the MSC pellets as indicated by the positive Alcian blue staining (Fig. 3A, E). The FBS pellets stained strongly for collagen I but weakly for collagen II (Fig. 3B, C, F, G). Cellularity, GAGs, collagen I, and collagen II in the chondrogenic pellets were quantified by PicoGreen®, DMMB assay, and ELISA, respectively. It was observed that the Hcol pellet was less cellular with a lower total collagen I, but higher total GAGs and collagen II levels. When these measurements were normalized to DNA, it was discovered that GAGs and collagen II deposition per cell was reduced in the FBS control (Fig. 3M, O). This was not the case for collagen I as the normalized values were equivalent for both groups (Fig. 3N). In addition, chondrogenic, osteogenic, and adipogenic potentials were also evaluated. After 21 days of osteogenic induction, mineralization was observed in the two cultures as indicated by Alizarin red staining and it was comparable given the similar absorbance of the extracted dye (Fig. 3S). However, collagen I gene expression was significantly suppressed in the Hcol group during osteogenic induction (Fig. 3R). Conversely, PPARG was upregulated in the Hcol sample and this adipogenic enhancement was demonstrated by the increased lipid accumulation as shown by oil red staining and quantification.
Figure 3.
Chondrogenic, osteogenic, and adipogenic differentiation of P3 MSCs. Chondrocytic lacunaes were found in the induced MSC pellets as indicated by the presence of glycoaminoglycans (GAGs) (A, E) and collagen II (C, G). Collagen I staining was stronger in the FBS pellet (B) compared to the Hcol group (F). A mild collagen X staining was found in both groups (D, H). Quantitative analysis indicated high cellularity in the FBS pellet (I) with lower total GAGs (J) and collagen II (L) compared to the serum free group. This was similarly observed with the DNA normalized values (M, O). There was enhanced GAGs and collagen II synthesis per cell maintained under the serum-free conditions. Conversely, the FBS group has a higher total collagen I (K) but the DNA normalized value was equivalent for both groups (N). The Hcol MSC exhibited enhanced chondrogenic differentiation compared to the FBS culture. Mineralization was observed in the FBS and Hcol groups with Alizarin red (P, Q). There was a lower collagen I gene expression in the Hcol culture (R) but calcification levels were equivalent in both groups (S). Lipid accumulation was observed in the cells under adipogenic induction as shown by oil red (T, U). However, adipogenesis was superior in the Hcol culture with higher PPARG gene expression (V) and higher lipid content (W). All controls stained negatively. *Significant difference between FBS and Hcol (p < 0.05, N = 4).
There was considerable MSC donor variation, which led to large deviations in the numerical data. This was particularly so for chondrogenic differentiation. Despite that, trends were noted. The Hcol samples displayed higher levels of chondrogenic markers than the FBS group. This was supported by the histological staining, which indicated positive collagen II staining for all four Hcol pellets. Conversely, three out of four FBS samples failed to stain for this critical marker (Fig. 4A, C). Furthermore, the Hcol pellets registered higher levels of aggrecan as shown by immunostaining (Fig. 4B, D).
Figure 4.
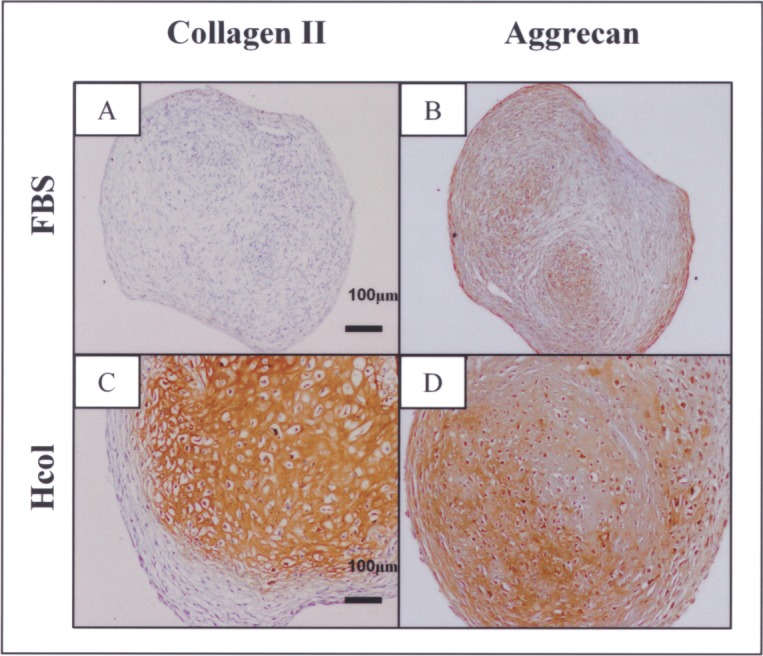
Collagen II and aggrecan immunostaining of chondrogenic-induced pellets derived from P3 FBS or serum-free cultures. Collagen II was not deposited in three out of four FBS samples, while this key chondrogenic extracellular matrix (ECM) was found in all four Hcol samples (A, C). This was accompanied by a stronger presence of aggrecan in the Hcol group (B, D).
Basal gene expression analyzed during the expansion suggested differences in mesenchymal differentiation between the FBS and Hcol cultures. Although Sox9 was lower in the later group, Hcol MSC chondrogenesis was enhanced, as shown by the superior collagen II and GAGs secretions. Furthermore, only one out of the four FBS samples expressed collagen II during chondrogenic differentiation. But all these donor cells were capable of doing so if maintained under serum-free conditions. Cytokines presented in the serum alternative might have enhanced proliferation while suppressing chondrogenic pathways during expansion. Sox9, a key molecular switch in the chondrogenic signaling cascade, was implicated as it was lower in the Hcol group. But this was only temporary. During induction, a reversal happened with the upregulation of chondrogenic pathways and the curtailment of proliferation. Hence, the Hcol pellets were less cellular than the FBS specimens and they contained higher levels of GAGs and collagen II. In contrast, adipogenic potential was upregulated from the very onset of serum-free expansion. This was maintained during induction with superior MSC lipid accumulation. There were no differences between the two osteogenic cultures as there was similar calcification. The findings would suggest the presence of chondrogenic inhibitors or inadequate supportive cues in FBS. Conventionally, MSC cultured with FBS could differentiate into collagen II synthesizing chondrocytes. The paucity of this key ECM marker in the FBS pellets was due to the compositional inconsistency between FBS batches. This could be resolved via serum-free expansion, which was supportive of cartilage and adipose regenerative applications. However, there were reservations pertaining to the use of the formulation in osseous therapies given the deficient expression of collagen I, which is an essential bone ECM protein.
DISCUSSION
The serum-free media supported cellular growth. Cells adhered on the Hcol coating but deficiently on Hfn. Hence, the latter was deemed unsuitable and omitted from further studies. Cell proliferation in the Hcol group was three times that of the FBS culture at P1 and this would mean that a larger number of serum-free expanded cells could be obtained at an earlier stage, thus avoiding the risk of malignant transformation and senescence, which are prevalent at the late passages (38,94). This proliferation enhancement was only observed at P1, probably due to the receptivity of the early cultures to the mitogenic effects of the serum-free environment. Surface antigen profiling identified the Hcol cells as MSCs because they were positive for CD90, CD73, CD44, and CD29 but negative for CD45. However, it should be noted as there was a decline in the expression of CD90 and CD73 when the cells were passaged. This was attributed to the biochemical effects of the serum-free media as it was not observed in the FBS control. Interestingly, the decline was not associated with a down-regulation of the differentiation potentials of the serum-free cultures. This was similarly observed when Shih et al. (76) and Sanchez-Guijo et al. (67) isolated MSCs from the parathyroid gland and trabecular bone, respectively, and they noted differing CD90 expressions with respect to bone marrow-derived MSCs. Despite that, the stem cells maintained the ability to undergo chondrogenic, adipogenic, and osteogenic differentiations. Thus, cell surface marker profiling may not be adequate to elucidate the differentiation capability of MSCs; therefore, differentiation studies are required.
Surface coating assisted MSC serum-free expansion, as cell isolation was poor in the absence of the substrate (results not shown). Biomaterials such as gelatin, fibronectin, and collagen promote cellular adhesion by mimicking certain aspects of the natural ECM. The primitive progenitor status of MSCs could be maintained on a gelatin substrate in a serum-free environment (4). Fibronectin is found in the provisional matrix of wounds and it encourages cell migration (89). Hence, it has been frequently used as a cell adhesion coating. Interestingly, this effectiveness was not demonstrated under serum-free conditions and it was likely that fibronectin must be complemented with other bioactive components for it to facilitate cell attachment effectively. In addition, the necessity of fibronectin for cell attachment was doubted by Steele et al. (80) when they observed a mere 20% reduction in epithelial cell attachment in fibronectin-depleted FBS supplemented cultures. They suspected that there were other more critical prerequisites for cellular adhesion. The current findings reinforced this suspicion as MSCs attached poorly on Hfn, probably because the essential signaling cascades were not triggered by the ligand in the serum-free environment. This was circumvented with collagen I, which possesses an integrin-binding domain and this substrate was employed in the conservation of chondrocytic phenotype in an earlier study (23). Barbero et al. (3) were able to improve the expression of GAGs and collagen II when they seeded chondrocytes on collagen coatings in a cytokine-enriched media. They reasoned that the enhancement was due to the interactions between the natural substrate and cell surface receptors, which promoted the chondrogenic pathway. Similarly, the collagen coating might have stimulated the chondrogenic potential of the serum free group as shown in the data.There were various attempts in developing an MSC serum-free formulation with cell proliferation as the prime focus. Although these cells were capable of chondrogenic, osteogenic, and adipogenic differentiations, the efficacies of each lineage might be modulated by the constituents of the serum-free media. This was witnessed in the present study. During expansion, basal gene expression revealed differences in the chondrogenic and adipogenic potentials of FBS and Hcol cultures. Sox9 and PPARG mRNA levels differed between the two cultures, but Runx2 was not significantly different, thus hinting of an equivalent osteogenic capacity. When the basal gene expressions were examined against actual differentiations, chondrogenic, osteogenic, and adipogenic capacities were inverted, maintained, or enhanced, respectively, during serum-free expansion and induction. Chondrogenic differentiation appeared to be suppressed in the Hcol serum-free cultures (P2–P5) given the lower Sox9 gene expression. However, the contrary happened as the chondrogenic-induced Hcol pellets registered higher levels of chondrocytic ECM markers such as GAGs and collagen II. Despite using the same donors, collagen II was prevalent in all the Hcol samples but only one FBS pellet. Hence, chondrogenesis was enhanced in the Hcol group even though the basal Sox9 gene expression suggested otherwise. It was probable that the chondrogenic pathway was suppressed during expansion with the upregulation of proliferative genes. But a reversal occurred upon induction as there was a lower cell proliferation in the Hcol pellet, as shown by the lower DNA content. This was compensated by the enhanced deposition of chondrocytic ECM. The deficient chondrogenesis in the FBS group mirrors the studies of Bilgen et al. (6) and Zaky et al. (93), who were unable to solicit GAGs and collagen II productions in their FBS-expanded MSCs. But one must be mindful that such reports and the present findings were contrary to the established protocol, whereby FBS expanded MSCs were shown to be able to undergo chondrogenesis upon induction. An example would be Yokogama et al.’s report, which indicated that high FBS content stimulated chondrogenic differentiation (91). However, they cautioned that the observations might be subjective given the varying cytokine concentrations in the different FBS lots (91). Therefore, it was likely such inconsistency was predominant in this study as the present batch of FBS used in the experiment was unable to maintain the chondrogenic status of the stem cell. This could be circumvented with a combination of growth factors in the serum-free media. MSC expansion with dexamethasone and FGF2 has been shown to promote in vitro chondrogenesis with increasing GAGs and collagen II expressions (9,28,78,81). When administered into rabbit cartilage defects, FGF2 stimulated chondroprogenitor expansion, thereby assisting the regeneration of hyaline cartilage (21). Fibrous repair was discovered when neutralizing monoclonal antibodies were used against FGF2. Although the cytokine is mitogenic, Yanada et al. suggested a reversal during MSC chondrogenesis as there was extensive telomere shortening with FGF2 pretreatment (90). This might explain the poor cellularity of the Hcol pellets compared to the FBS controls.
Osteogenesis was similar in the FBS and Hcol cultures as shown by the equivalent mineralization. Mizuno et al. commented that the osteogenic differentiation of MSC was dependent on the quality of the FBS used during expansion (49). Dexamethasone supplementation could resolve such inconsistencies as it activates Runx2, hence stimulating osteogenesis (47). When Oshina et al. pretreated MSC cultures with 100 nm of dexamethasone, there was an increase expression of alkaline phosphatase and osteocalcin (58). However, Ito et al. commented that dexamethasone (1–1000 nM) was unable to sustain terminal differentiation given the absence of osterix, which is a late osseous marker (27). This could be overcome by the complementary use of 2.5 ng/ml of FGF2 (19). But FGF2 on its own seems to inhibit osteogenesis in a reversible, dose-dependent manner (65). Therefore, in an enriched cytokine environment, complex biochemical interactions arise, leading to outcomes that are contrary to that expected of single growth factors. The osteogenic potential of the Hcol culture was unchanged despite the presence of dexamethasone and FGF2. This might be due to the inclusion of EGF and PDGF. Krampera et al. observed that EGF signaling led to self-renewal while suppressing differentiation during MSC expansion (33). PDGF had a similar effect, but it was expressed in osteoblasts during bone fracture repair (30,83).
Adipogenic potential was increased in the serum-free environment with improved responsiveness towards inductive agents as shown by the high PPARG expression. Consequently, there was a higher level of lipid accumulation in the Hcol adipocytes compared to the FBS control. Neubauer et al. observed similar outcomes when he pretreated MSCs with FGF2 (52). The combination of FGF2 and dexamethasone stimulated adipogenic differentiation through a sarcoma tyrosine kinase (Src) mechanism (37). Studies have shown that dexamethasone primes the progenitor cells for this lineage (27,58). Moreover, adipogenic differentiation was reported to be induced with 100 nM of the glucocorticoid in the absence of other reagents (25).
The upregulation of one signaling pathway can occur at the expense of another, especially so when there is cross-talk. This intricate balance was maintained between adipogenesis and osteogenesis in the bone marrow and it might be disrupted when specific MSC lineages are augmented artificially. Bone formation involves the activation of MSC osteogenic pathways, concomitant with an active suppression of adipogenesis (51). PPARG is the regulator of interest and it is part of a complex signaling circuitry encompassing other factors such as bone morphogenic proteins (BMPs), wingless type MMTV integration site (Wnts), Runx2, and FGF (51). Adipogenesis was suppressed in PPARG knockout mice while bone mass increased due to enhanced osteoblastogenesis (2,32). Conversely, PPARG expression was upregulated in premature aging animals with a decline in Runx2, thus resulting in fatty bone marrow (50). This compensatory mechanism was not observed in the Hcol group despite its disposition towards adipogenesis, as the osteogenic potential was similar to that of the FBS culture. This conservation might be due to the various supplements in the serum-free media. FGF2 was a probable candidate as it was recognized to be a strong stimulator of both adipogenesis and osteogenesis. This cytokine promoted adipose generation on collagen scaffolds seeded with preadipocytes; it also stimulated bone formation in osteopenic ovariectomized rats (22,39). Conversely, the exposure to FGF2 triggered a reduction of osteogenic markers in osteoblastic MC3T3-E1 cells while upregulating adipogenic genes (14). Muruganandan et al. stressed that the effects of FGF was determined by other stimuli (51).
Improved MSC proliferation and differentiation could arise from population selection. Previous studies were able to derive a subpopulation with high telomerase activity via serum deprivation, and dexamethasone was thought to induce the apoptosis of cells with impaired differentiation capacities (58,62). Esposito et al. tested this selection theory by plating bone marrow MSCs in supernatants extracted from bone fragments, stroma, and embryonic fibroblast cell cultures (15). Different subpopulations were isolated in each group and adipoprogenitors were enriched in the last supernatant. Similarly, chondroprogenitors and adipoprogenitors might be selected in the Hcol group under serum-free conditions. But this suspicion could only be confirmed if a larger donor population was used as the authors acknowledged that a donor size of four was rather limited.
Interestingly, collagen I mRNA was significantly lower in the serum-free group (P1–P5) and this was also observed during osteogenic induction. This could be attributed to the addition of FGF2 in the serum-free formulation, as Hurley et al. (26) and Shimko et al. (77) observed collagen I inhibition in FGF2-pretreated cultures during expansion and osteogenic differentiation. However, this was not the case in the chondrogenic specimens as collagen I expression per cell was similar in both the FBS and Hcol pellets. It was likely that the in vivo factors necessary for cartilage development were not fully recapitulated in the pellet cultures; hence, collagen I was secreted in the samples.
KSR has been used as a serum substitute in ESC and MSC cultures. Horii et al. were able to maintain the undifferentiated status of embryonic germ cells by using KSR (24). This advantage was exploited by Battula et al. in MSC cultivation as the cells had highly primitive markers such as the wnt receptor frizzled-9, octamer binding transcription protein 4 (Oct-4), and Nanog3 when treated with KSR (4). However, Lund et al. warned that the resultant cultures exhibited poor mesenchymal differentiations (41). Such constraints were not encountered in the current work as KSR was supplemented with a cocktail of growth factors. This strategy has been used to boost the performance of human allogenous serum. Pytlik et al. encountered growth arrest and senescence when he tried to cultivate MSCs in pooled human serum (63). However, a 40-fold increase in cell yield was achieved when EGF, PDGF, FGF2, dexamethasone, insulin, and macrophage colony stimulating factor (M-CSF) were added to the serum. Roche et al. used a similar approach to promote the differentiation efficiency of MSCs towards osteogenic and adipogenic phenotypes under reduced serum conditions (66). Zaky et al. (93) and Lindroos et al. (40) managed to coax their cultures towards chondrogenesis by using platelet lysates and StemPro® media, both containing cytokines. Hence, it was possible to tailor MSC expansion in a serum-free manner towards specific clinical applications. This serum-free approach was similarly applied to other cell types in liver and pancreas tissue regeneration (48,57).
CONCLUSION
MSCs were successfully isolated and expanded in a novel serum-free media. Differentiation studies revealed a disposition of the cells towards chondrogenesis and adipogenesis. These findings demonstrate that it is possible to cultivate MSCs in a serum-free environment while enhancing the specific differentiation capacities. This dualistic approach not only eliminates the safety concerns with regard to the use of FBS but it also tailors MSCs to suit applications in cartilage repair and adipose regeneration. This finding has not been reported conclusively before. The results represent a significant breakthrough for potential clinical use and warrants future in vivo studies.
ACKNOWLEDGMENTS
This study was supported by the Singapore National Medical Research Council, NMRC grant R175000074213. The authors would like to thank Tan Sing Chik, Liew Yun Rou, and Chong Sue Wee from the Department of Orthopaedic Surgery, National University Hospital and also Dr. Yang Zheng from National University Tissue Engineering Program, National University of Singapore for their invaluable contributions.
REFERENCES
- 1. Agata H.; Watanabe N.; Ishii Y.; Kubo N.; Ohshima S.; Yamazaki M.; Tojo A.; Kagami H. Feasibility and efficacy of bone tissue engineering using human bone marrow stromal cells cultivated in serum-free conditions. Biochem. Biophys. Res. Commun. 382(2):353–358; 2009. [DOI] [PubMed] [Google Scholar]
- 2. Akune T.; Ohba S.; Kamekura S.; Yamaguchi M.; Chung U. I.; Kubota N.; Terauchi Y.; Harada Y.; Azuma Y.; Nakamura K.; Kadowaki T.; Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 113(6):846–855; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbero A.; Grogan S. P.; Mainil-Varlet P.; Martin I. Expansion on specific substrates regulates the phenotype and differentiation capacity of human articular chondrocytes. J. Cell. Biochem. 98(5):1140–1149; 2006. [DOI] [PubMed] [Google Scholar]
- 4. Battula V. L.; Bareiss P. M.; Treml S.; Conrad S.; Albert I.; Hojak S.; Abele H.; Schewe B.; Just L.; Skutella T.; Buhring H. J. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation 75(4):279–291; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Bianchi G.; Banfi A.; Mastrogiacomo M.; Notaro R.; Luzzatto L.; Cancedda R.; Quarto R. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp. Cell Res. 287(1):98–105; 2003. [DOI] [PubMed] [Google Scholar]
- 6. Bilgen B.; Orsini E.; Aaron R. K.; Ciombor D. M. FBS suppresses TGF-beta1-induced chondrogenesis in synoviocyte pellet cultures while dexamethasone and dynamic stimuli are beneficial. J. Tissue Eng. Regen. Med. 1(6):436–442; 2007. [DOI] [PubMed] [Google Scholar]
- 7. Bonfield T. L.; Koloze M.; Lennon D. P.; Zuchowski B.; Yang S. E.; Caplan A. I. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am. J. Physiol. Lung Cell Mol. Physiol. 299(6):L760–770; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cancedda R.; Dozin B. Serum-free medium for mesenchymal stem cells. Assigned to Consorzio, Per La Gestione Del Centro Di Biotechnologie Avanzate Istituto, Nazionale Per La Ricerca Sul Cancro, United states patent, US7109032B2, 2006.
- 9. Chiou M.; Xu Y.; Longaker M. T. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem. Biophys. Res. Commun. 343(2):644–652; 2006. [DOI] [PubMed] [Google Scholar]
- 10. Chung R.; Foster B. K.; Zannettino A. C.; Xian C. J. Potential roles of growth factor PDGF-BB in the bony repair of injured growth plate. Bone 44(5):878–885; 2009. [DOI] [PubMed] [Google Scholar]
- 11. Cui Q.; Wang G. J.; Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J. Bone Joint Surg. Am. 79(7):1054–1063; 1997. [DOI] [PubMed] [Google Scholar]
- 12. De Bruyn C.; Najar M.; Raicevic G.; Meuleman N.; Pieters K.; Stamatopoulos B.; Delforge A.; Bron D.; Lagneaux L. A rapid, simple, and reproducible method for the isolation of mesenchymal stromal cells from Wharton’s jelly without enzymatic treatment. Stem Cells Dev. 20(3):547–557; 2011. [DOI] [PubMed] [Google Scholar]
- 13. De Rosa A.; Tirino V.; Paino F.; Tartaglione A.; Mitsiadis T.; Feki A.; d’Aquino R.; Laino L.; Colacurci N.; Papaccio G. Amniotic fluid-derived mesenchymal stem cells lead to bone differentiation when cocultured with dental pulp stem cells. Tissue Eng. Part A 17(5–6):645–653; 2011. [DOI] [PubMed] [Google Scholar]
- 14. Eda H.; Aoki K.; Marumo K.; Fujii K.; Ohkawa K. FGF-2 signaling induces downregulation of TAZ protein in osteoblastic MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 366(2):471–475; 2008. [DOI] [PubMed] [Google Scholar]
- 15. Esposito M. T.; Di Noto R.; Mirabelli P.; Gorrese M.; Parisi S.; Montanaro D.; Del Vecchio L.; Pastore L. Culture conditions allow selection of different mesenchymal progenitors from adult mouse bone marrow. Tissue Eng. Part A 15(9):2525–2536; 2009. [DOI] [PubMed] [Google Scholar]
- 16. Fedorovich N. E.; Haverslag R. T.; Dhert W. J.; Alblas J. The role of endothelial progenitor cells in prevascularized bone tissue engineering: Development of heterogeneous constructs. Tissue Eng. Part A 16(7):2355–2367; 2010. [DOI] [PubMed] [Google Scholar]
- 17. Feng J.; Mantesso A.; Sharpe P. T. Perivascular cells as mesenchymal stem cells. Expert Opin. Biol. Ther. 10(10):1441–1451; 2010. [DOI] [PubMed] [Google Scholar]
- 18. Friedenstein A. J.; Deriglasova U. F.; Kulagina N. N.; Panasuk A. F.; Rudakowa S. F.; Luria E. A.; Ruadkow I. A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 2(2):83–92; 1974. [PubMed] [Google Scholar]
- 19. Hanada K.; Dennis J. E.; Caplan A. I. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J. Bone Miner. Res. 12(10):1606–1614; 1997. [DOI] [PubMed] [Google Scholar]
- 20. Higashino K.; Viggeswarapu M.; Bargouti M.; Liu H.; Titus L.; Boden S. D. Stromal cell-derived factor-1 potentiates bone morphogenetic protein-2 induced bone formation. Tissue Eng. Part A 17(3–4):523–530; 2011. [DOI] [PubMed] [Google Scholar]
- 21. Hiraki Y.; Shukunami C.; Iyama K.; Mizuta H. Differentiation of chondrogenic precursor cells during the regeneration of articular cartilage. Osteoarthritis Cartilage 9(Suppl. A):S102–108; 2001. [DOI] [PubMed] [Google Scholar]
- 22. Hiraoka Y.; Yamashiro H.; Yasuda K.; Kimura Y.; Inamoto T.; Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 12(6):1475–1487; 2006. [DOI] [PubMed] [Google Scholar]
- 23. Ho S. T.; Yang Z.; Hui H. P.; Oh K. W.; Choo B. H.; Lee E. H. A serum free approach towards the conservation of chondrogenic phenotype during in vitro cell expansion. Growth Factors 27(5):321–333; 2009. [DOI] [PubMed] [Google Scholar]
- 24. Horii T.; Nagao Y.; Tokunaga T.; Imai H. Serum-free culture of murine primordial germ cells and embryonic germ cells. Theriogenology 59(5–6):1257–1264; 2003. [DOI] [PubMed] [Google Scholar]
- 25. Hung S. H.; Yeh C. H.; Huang H. T.; Wu P.; Ho M. L.; Chen C. H.; Wang C.; Chao D.; Wang G. J. Pioglitazone and dexamethasone induce adipogenesis in D1 bone marrow stromal cell line, but not through the peroxisome proliferator-activated receptor-gamma pathway. Life Sci. 82(11–12):561–569; 2008. [DOI] [PubMed] [Google Scholar]
- 26. Hurley M. M.; Abreu C.; Harrison J. R.; Lichtler A. C.; Raisz L. G.; Kream B. E. Basic fibroblast growth factor inhibits type I collagen gene expression in osteoblastic MC3T3-E1 cells. J. Biol. Chem. 268(8):5588–5593; 1993. [PubMed] [Google Scholar]
- 27. Ito S.; Suzuki N.; Kato S.; Takahashi T.; Takagi M. Glucocorticoids induce the differentiation of a mesenchymal progenitor cell line, ROB-C26 into adipocytes and osteoblasts, but fail to induce terminal osteoblast differentiation. Bone 40(1):84–92; 2007. [DOI] [PubMed] [Google Scholar]
- 28. Ito T.; Sawada R.; Fujiwara Y.; Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology 56(1):1–7; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeon Y. K.; Jang Y. H.; Yoo D. R.; Kim S. N.; Lee S. K.; Nam M. J. Mesenchymal stem cells’ interaction with skin: Wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen. 18(6):655–661; 2010. [DOI] [PubMed] [Google Scholar]
- 30. Kang Y. J.; Jeon E. S.; Song H. Y.; Woo J. S.; Jung J. S.; Kim Y. K.; Kim J. H. Role of c-Jun N-terminal kinase in the PDGF-induced proliferation and migration of human adipose tissue-derived mesenchymal stem cells. J. Cell. Biochem. 95(6):1135–1145; 2005. [DOI] [PubMed] [Google Scholar]
- 31. Kassem M.; Kristiansen M.; Abdallah B. M. Mesenchymal stem cells: Cell biology and potential use in therapy. Basic Clin. Pharmacol. Toxicol. 95(5):209–214; 2004. [DOI] [PubMed] [Google Scholar]
- 32. Kawaguchi H.; Akune T.; Yamaguchi M.; Ohba S.; Ogata N.; Chung U. I.; Kubota N.; Terauchi Y.; Kadowaki T.; Nakamura K. Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. J. Bone Miner. Metab. 23(4):275–279; 2005. [DOI] [PubMed] [Google Scholar]
- 33. Krampera M.; Pasini A.; Rigo A.; Scupoli M. T.; Tecchio C.; Malpeli G.; Scarpa A.; Dazzi F.; Pizzolo G.; Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: Inducing cell expansion and reversibly preventing multilineage differentiation. Blood 106(1):59–66; 2005. [DOI] [PubMed] [Google Scholar]
- 34. Kumar S.; Wan C.; Ramaswamy G.; Clemens T. L.; Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol. Ther. 18(5):1026–1034; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lange C.; Cakiroglu F.; Spiess A. N.; Cappallo-Obermann H.; Dierlamm J.; Zander A. R. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J. Cell. Physiol. 213(1):18–26; 2007. [DOI] [PubMed] [Google Scholar]
- 36. Lee K. B.; Hui J. H.; Song I. C.; Ardany L.; Lee E. H. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cells 25(11):2964–2971; 2007. [DOI] [PubMed] [Google Scholar]
- 37. Lee S. Y.; Lim J.; Khang G.; Son Y.; Choung P. H.; Kang S. S.; Chun S. Y.; Shin H. I.; Kim S. Y.; Park E. K. Enhanced ex vivo expansion of human adipose tissue-derived mesenchymal stromal cells by fibroblast growth factor-2 and dexamethasone. Tissue Eng. Part A 15(9):2491–2499; 2009. [DOI] [PubMed] [Google Scholar]
- 38. Lepperdinger G.; Brunauer R.; Jamnig A.; Laschober G.; Kassem M. Controversial issue: Is it safe to employ mesenchymal stem cells in cell-based therapies? Exp. Gerontol. 43(11):1018–1023; 2008. [DOI] [PubMed] [Google Scholar]
- 39. Liang H.; Pun S.; Wronski T. J. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology 140(12):5780–5788; 1999. [DOI] [PubMed] [Google Scholar]
- 40. Lindroos B.; Boucher S.; Chase L.; Kuokkanen H.; Huhtala H.; Haataja R.; Vemuri M.; Suuronen R.; Miettinen S. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 11(7):958–972; 2009. [DOI] [PubMed] [Google Scholar]
- 41. Lund P.; Pilgaard L.; Duroux M.; Fink T.; Zachar V. Effect of growth media and serum replacements on the proliferation and differentiation of adipose-derived stem cells. Cytotherapy 11(2):189–197; 2009. [DOI] [PubMed] [Google Scholar]
- 42. Marx R. E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 62(4):489–496; 2004. [DOI] [PubMed] [Google Scholar]
- 43. Marx R. E.; Carlson E. R.; Eichstaedt R. M.; Schimmele S. R.; Strauss J. E.; Georgeff K. R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 85(6):638–646; 1998. [DOI] [PubMed] [Google Scholar]
- 44. Mendez-Ferrer S.; Michurina T. V.; Ferraro F.; Mazloom A. R.; Macarthur B. D.; Lira S. A.; Scadden D. T.; Ma’ayan A.; Enikolopov G. N.; Frenette P. S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466(7308):829–834; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meuleman N.; Tondreau T.; Delforge A.; Dejeneffe M.; Massy M.; Libertalis M.; Bron D.; Lagneaux L. Human marrow mesenchymal stem cell culture: Serum-free medium allows better expansion than classical alpha-MEM medium. Eur. J. Haematol. 76(4):309–316; 2006. [DOI] [PubMed] [Google Scholar]
- 46. Meyerrose T.; Olson S.; Pontow S.; Kalomoiris S.; Jung Y.; Annett G.; Bauer G.; Nolta J. A. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv. Drug Deliv. Rev. 62(12):1167–1174; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mikami Y.; Takahashi T.; Kato S.; Takagi M. Dexamethasone promotes DMP1 mRNA expression by inhibiting negative regulation of Runx2 in multipotential mesenchymal progenitor, ROB-C26. Cell Biol. Int. 32(2):239–246; 2008. [DOI] [PubMed] [Google Scholar]
- 48. Miyamoto Y.; Teramoto N.; Hayashi S.; Enosawa S. An improvement in the attaching capability of cryopreserved human hepatocytes by a proteinaceous high molecule, sericin, in the serum-free solution. Cell Transplant. 19(6):701–706; 2010. [DOI] [PubMed] [Google Scholar]
- 49. Mizuno D.; Agata H.; Furue H.; Kimura A.; Narita Y.; Watanabe N.; Ishii Y.; Ueda M.; Tojo A.; Kagami H. Limited but heterogeneous osteogenic response of human bone marrow mesenchymal stem cells to bone morphogenetic protein-2 and serum. Growth Factors 28(1):34–43; 2010. [DOI] [PubMed] [Google Scholar]
- 50. Moerman E. J.; Teng K.; Lipschitz D. A.; Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 3(6):379–389; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muruganandan S.; Roman A. A.; Sinal C. J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 66(2):236–253; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Neubauer M.; Fischbach C.; Bauer-Kreisel P.; Lieb E.; Hacker M.; Tessmar J.; Schulz M. B.; Goepferich A.; Blunk T. Basic fibroblast growth factor enhances PPAR-gamma ligand-induced adipogenesis of mesenchymal stem cells. FEBS Lett. 577(1–2):277–283; 2004. [DOI] [PubMed] [Google Scholar]
- 53. Neubauer M.; Hacker M.; Bauer-Kreisel P.; Weiser B.; Fischbach C.; Schulz M. B.; Goepferich A.; Blunk T. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 11(11–12):1840–1851; 2005. [DOI] [PubMed] [Google Scholar]
- 54. Newman C. Serum-free cell culture—the ethical, scientist and economic choice. Biomedical Scientist 47:941–942; 2003. [Google Scholar]
- 55. Ng F.; Boucher S.; Koh S.; Sastry K. S.; Chase L.; Lakshmipathy U.; Choong C.; Yang Z.; Vemuri M. C.; Rao M. S.; Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112(2):295–307; 2008. [DOI] [PubMed] [Google Scholar]
- 56. Niemeyer P.; Szalay K.; Luginbuhl R.; Sudkamp N. P.; Kasten P. Transplantation of human mesenchymal stem cells in a non-autogenous setting for bone regeneration in a rabbit critical-size defect model. Acta Biomater. 6(3):900–908; 2010. [DOI] [PubMed] [Google Scholar]
- 57. Noguchi H.; Naziruddin B.; Jackson A.; Shimoda M.; Ikemoto T.; Fujita Y.; Chujo D.; Takita M.; Kobayashi N.; Onaca N.; Hayashi S.; Levy M. F.; Matsumoto S. Characterization of human pancreatic progenitor cells. Cell Transplant. 19(6):879–886; 2010. [DOI] [PubMed] [Google Scholar]
- 58. Oshina H.; Sotome S.; Yoshii T.; Torigoe I.; Sugata Y.; Maehara H.; Marukawa E.; Omura K.; Shinomiya K. Effects of continuous dexamethasone treatment on differentiation capabilities of bone marrow-derived mesenchymal cells. Bone 41(4):575–583; 2007. [DOI] [PubMed] [Google Scholar]
- 59. Pal R.; Hanwate M.; Jan M.; Totey S. Phenotypic and functional comparison of optimum culture conditions for upscaling of bone marrow-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 3(3):163–174; 2009. [DOI] [PubMed] [Google Scholar]
- 60. Park T. S.; Gavina M.; Chen C. W.; Sun B.; Teng P. N.; Huard J.; Deasy B. M.; Zimmerlin L.; Peault B. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 20(3):451–463; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pereira R. F.; O’Hara M. D.; Laptev A. V.; Halford K. W.; Pollard M. D.; Class R.; Simon D.; Livezey K.; Prockop D. J. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 95(3):1142–1147; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pochampally R. R.; Smith J. R.; Ylostalo J.; Prockop D. J. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood 103(5):1647–1652; 2004. [DOI] [PubMed] [Google Scholar]
- 63. Pytlik R.; Stehlik D.; Soukup T.; Kalbacova M.; Rypacek F.; Trc T.; Mulinkova K.; Michnova P.; Kideryova L.; Zivny J.; Klener P. Jr.; Vesela R.; Trneny M.; Klener P. The cultivation of human multipotent mesenchymal stromal cells in clinical grade medium for bone tissue engineering. Biomaterials 30(20):3415–3427; 2009. [DOI] [PubMed] [Google Scholar]
- 64. Qian L.; Saltzman W. M. Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials 25(7–8):1331–1337; 2004. [DOI] [PubMed] [Google Scholar]
- 65. Quarto N.; Longaker M. T. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 12(6):1405–1418; 2006. [DOI] [PubMed] [Google Scholar]
- 66. Roche S.; Richard M. J.; Favrot M. C. Oct-4, Rex-1, and Gata-4 expression in human MSC increase the differentiation efficiency but not hTERT expression. J. Cell. Biochem. 101(2):271–280; 2007. [DOI] [PubMed] [Google Scholar]
- 67. Sanchez-Guijo F. M.; Blanco J. F.; Cruz G.; Muntion S.; Gomez M.; Carrancio S.; Lopez-Villar O.; Barbado M. V.; Sanchez-Abarca L. I.; Blanco B.; Brinon J. G.; del Canizo M. C. Multiparametric comparison of mesenchymal stromal cells obtained from trabecular bone by using a novel isolation method with those obtained by iliac crest aspiration from the same subjects. Cell Tissue Res. 336(3):501–507; 2009. [DOI] [PubMed] [Google Scholar]
- 68. Schallmoser K.; Bartmann C.; Rohde E.; Reinisch A.; Kashofer K.; Stadelmeyer E.; Drexler C.; Lanzer G.; Linkesch W.; Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 47(8):1436–1446; 2007. [DOI] [PubMed] [Google Scholar]
- 69. Schallmoser K.; Rohde E.; Reinisch A.; Bartmann C.; Thaler D.; Drexler C.; Obenauf A. C.; Lanzer G.; Linkesch W.; Strunk D. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng. Part C Methods 14(3):185–196; 2008. [DOI] [PubMed] [Google Scholar]
- 70. Schantz J. T.; Hutmacher D. W.; Lam C. X.; Brinkmann M.; Wong K. M.; Lim T. C.; Chou N.; Guldberg R. E.; Teoh S. H. Repair of calvarial defects with customised tissue-engineered bone grafts II. Evaluation of cellular efficiency and efficacy in vivo. Tissue Eng. 9(Suppl. 1):S127–139; 2003. [DOI] [PubMed] [Google Scholar]
- 71. Sellheyer K.; Krahl D. Skin mesenchymal stem cells: Prospects for clinical dermatology. J. Am. Acad. Dermatol. 63(5):859–865; 2010. [DOI] [PubMed] [Google Scholar]
- 72. Selvaggi T. A.; Walker R. E.; Fleisher T. A. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood 89(3):776–779; 1997. [PubMed] [Google Scholar]
- 73. Semon J. A.; Nagy L. H.; Llamas C. B.; Tucker H. A.; Lee R. H.; Prockop D. J. Integrin expression and integrin-mediated adhesion in vitro of human multipotent stromal cells (MSCs) to endothelial cells from various blood vessels. Cell Tissue Res. 341(1):147–158; 2010. [DOI] [PubMed] [Google Scholar]
- 74. Shahdadfar A.; Fronsdal K.; Haug T.; Reinholt F. P.; Brinchmann J. E. In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 23(9):1357–1366; 2005. [DOI] [PubMed] [Google Scholar]
- 75. Shetty P.; Bharucha K.; Tanavde V. Human umbilical cord blood serum can replace fetal bovine serum in the culture of mesenchymal stem cells. Cell Biol. Int. 31(3):293–298; 2007. [DOI] [PubMed] [Google Scholar]
- 76. Shih Y. R.; Kuo T. K.; Yang A. H.; Lee O. K.; Lee C. H. Isolation and characterization of stem cells from the human parathyroid gland. Cell Prolif. 42(4):461–470; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shimko D. A.; Burks C. A.; Dee K. C.; Nauman E. A. Comparison of in vitro mineralization by murine embryonic and adult stem cells cultured in an osteogenic medium. Tissue Eng. 10(9–10):1386–1398; 2004. [DOI] [PubMed] [Google Scholar]
- 78. Solchaga L. A.; Penick K.; Porter J. D.; Goldberg V. M.; Caplan A. I.; Welter J. F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell. Physiol. 203(2):398–409; 2005. [DOI] [PubMed] [Google Scholar]
- 79. Spees J. L.; Gregory C. A.; Singh H.; Tucker H. A.; Peister A.; Lynch P. J.; Hsu S. C.; Smith J.; Prockop D. J. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol. Ther. 9(5):747–756; 2004. [DOI] [PubMed] [Google Scholar]
- 80. Steele J. G.; Johnson G.; Griesser H. J.; Underwood P. A. Mechanism of initial attachment of corneal epithelial cells to polymeric surfaces. Biomaterials 18(23):1541–1551; 1997. [DOI] [PubMed] [Google Scholar]
- 81. Stewart A. A.; Byron C. R.; Pondenis H. C.; Stewart M. C. Effect of dexamethasone supplementation on chondrogenesis of equine mesenchymal stem cells. Am. J. Vet. Res. 69(8):1013–1021; 2008. [DOI] [PubMed] [Google Scholar]
- 82. Stute N.; Holtz K.; Bubenheim M.; Lange C.; Blake F.; Zander A. R. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp. Hematol. 32(12):1212–1225; 2004. [DOI] [PubMed] [Google Scholar]
- 83. Tamama K.; Fan V. H.; Griffith L. G.; Blair H. C.; Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 24(3):686–695; 2006. [DOI] [PubMed] [Google Scholar]
- 84. Toupadakis C. A.; Wong A.; Genetos D. C.; Cheung W. K.; Borjesson D. L.; Ferraro G. L.; Galuppo L. D.; Leach J. K.; Owens S. D.; Yellowley C. E. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Res. 71(10):1237–1245; 2010. [DOI] [PubMed] [Google Scholar]
- 85. Tsutsumi S.; Shimazu A.; Miyazaki K.; Pan H.; Koike C.; Yoshida E.; Takagishi K.; Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem. Biophys. Res. Commun. 288(2):413–419; 2001. [DOI] [PubMed] [Google Scholar]
- 86. Turnovcova K.; Ruzickova K.; Vanecek V.; Sykova E.; Jendelova P. Properties and growth of human bone marrow mesenchymal stromal cells cultivated in different media. Cytotherapy 11(7):874–885; 2009. [DOI] [PubMed] [Google Scholar]
- 87. Wakitani S.; Goto T.; Pineda S. J.; Young R. G.; Mansour J. M.; Caplan A. I.; Goldberg V. M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Joint Surg. Am. 76(4):579–592; 1994. [DOI] [PubMed] [Google Scholar]
- 88. Xian C. J. Roles of epidermal growth factor family in the regulation of postnatal somatic growth. Endocr. Rev. 28(3):284–296; 2007. [DOI] [PubMed] [Google Scholar]
- 89. Yamada K. M. Fibronectin peptides in cell migration and wound repair. J. Clin. Invest. 105(11):1507–1509; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yanada S.; Ochi M.; Kojima K.; Sharman P.; Yasunaga Y.; Hiyama E. Possibility of selection of chondrogenic progenitor cells by telomere length in FGF-2-expanded mesenchymal stromal cells. Cell Prolif. 39(6):575–584; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yokoyama M.; Miwa H.; Maeda S.; Wakitani S.; Takagi M. Influence of fetal calf serum on differentiation of mesenchymal stem cells to chondrocytes during expansion. J. Biosci. Bioeng. 106(1):46–50; 2008. [DOI] [PubMed] [Google Scholar]
- 92. Yu X.; Jin G.; Yin X.; Cho S.; Jeon J.; Lee S.; Kong I. Isolation and characterization of embryonic stem-like cells derived from in vivo-produced cat blastocysts. Mol. Reprod. Dev. 75(9):1426–1432; 2008. [DOI] [PubMed] [Google Scholar]
- 93. Zaky S. H.; Ottonello A.; Strada P.; Cancedda R.; Mastrogiacomo M. Platelet lysate favours in vitro expansion of human bone marrow stromal cells for bone and cartilage engineering. J. Tissue Eng. Regen. Med. 2(8):472–481; 2008. [DOI] [PubMed] [Google Scholar]
- 94. Zhou Y. F.; Bosch-Marce M.; Okuyama H.; Krishnamachary B.; Kimura H.; Zhang L.; Huso D. L.; Semenza G. L. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 66(22):10849–10854; 2006. [DOI] [PubMed] [Google Scholar]