Abstract
Type 1 diabetes is an autoimmune disorder that leads to destruction of pancreatic β islet cells and is a growing global health issue. While insulin replacement remains the standard therapy for type 1 diabetes, exogenous insulin does not mimic the physiology of insulin secretion. Transplantation of pancreatic islets has the potential to cure this disease; however, there are several major limitations to widespread implementation of islet transplants. The use of mesenchymal stromal cells (MSCs) in the treatment of type 1 diabetes has been investigated as an adjunct therapy during islet graft administration to prevent initial islet loss and promote engraftment and revascularization of islets. In this review we will discuss the results of recent MSC studies in animal models of diabetes with a focus on islet transplantation and explore the potential for these findings to be extended to clinical use for the treatment of type 1 diabetes.
Key words: Type 1 diabetes, Islet transplantation, Bone marrow, Mesenchymal stromal cell (MSCs), Immunomodulation
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is a T-cell-mediated, autoimmune disorder that leads to destruction of pancreatic β islet cells, and is characterized by the presence of anti-islet cell antibodies and severe insulitis (72). There are 41/100,000 people per year in Europe and 25/100,000 people per year in North America diagnosed with TIDM, and the incidence is increasing. This disease is associated with severe long-term vascular complications that are largely responsible for diabetes-related morbidity and mortality. While insulin replacement represents a current therapy for T1DM, exogenous insulin alone cannot exactly mimic the physiology of insulin secretion, and therefore metabolic control remains difficult. It is thought that pancreatic islet transplantation has the potential to cure this disease; however, there are currently major limitations to widespread implementation of these transplants (37,41,64). While the first report of insulin independence after islet transplantation occurred nearly 20 years ago, insulin treatment had to be reinstated after only 22 days (63).
More recent developments by a research group in Edmonton have demonstrated that more islets than can be isolated from a single pancreas are necessary to achieve long term insulin independence, and this strategy has been developed alongside a specific regimen of immunosuppressive therapy (41). According to the Edmonton protocol, approximately 12,000 islet equivalents/kg are needed to normalize hypoglycemia, and thus achieve insulin independence in humans. Insulin independence is sometimes achieved with a graft from one pancreas, but more often requires a second infusion of islets from a different donor (27,65). According a study report in 2005, only 50% of patients were still insulin free at 3 years, and insulin independence continues to wane over time, with approximately 10% of patients insulin independent at 5 years (61,64,66). However, persistent islet function can be seen in 83% of patients at 5 years as measured by C-peptide secretion. This continued islet function can prevent recurrent hypoglycemia and severe lability combined with correction in glycated hemoglobin (HbA1c) to a level far beyond what is possible with insulin therapy. Additionally, islet transplantation has been reported to positively influence diabetic complications and improve quality of life (25). Patients must weigh this potentially substantial improvement in glycemic control with the risks associated with life-long immunosuppression. Combinatorial immunosuppressive therapy with sirolimus and low-dose tacrolimus made initiation of these therapies possible, but their continued use is far from ideal (64). Sirolimus (rapamycin) inhibits the response to interleukin-2 (IL-2), thereby blocking the activation of B and T cells. Its mechanism of action is formation of an immunosuppressive complex with the intracellular protein, FKBP12, which blocks the activation of the cell cycle-specific kinase, target of rapamycin (TOR). Inactivation of TOR results in blockage of cell cycle progression at the juncture of G1 and S phase. Tacrolimus is a calcineurin inhibitor that directly inhibits IL-2 production, similar to cyclosporine, which is another immunosuppressant that has been used in the field of islet transplantation. Even though the mechanisms of action are different, these drugs have near-ubiquitous distribution, and lead to mouth ulcerations, peripheral edema, high rates of ovarian cysts in females, increased proteinuria in patients with preexisting kidney damage, hypertension, and hypercholesterolemia.
One of the most significant obstacles to widespread application of pancreatic islet transplantation is that there is a considerable loss of islets immediately following transplantation (39), due to a lack of blood supply and inflammation associated with transplantation (20). It is expected that 50–70% of the transplanted islets will be lost in the immediate posttransplantation period (41). This loss is due in part to a thrombotic/inflammatory reaction elicited when islets come into contact with ABO-compatible blood, and is characterized by binding and activation of platelets on the islet surface, as well as activation of complement systems. Leukocytes infiltrate islets rapidly, and within an hour both monocytes and granulocytes are present. This reaction is known as the instant blood-mediated inflammatory reaction (IBMIR) and is induced by monocyte chemotactic protein-1 (MCP-1) and tissue factor (TF). Despite the fact that MCP-1 and TF are produced by islets (62), recent data suggest that islet MCP-1 release does not have a direct role in graft failure (51). Instead, recipient MCP-1 results in the development of negative proinflammatory conditions. These detrimental effects of the IBMIR provide an explanation for the fact that 2–4 donor pancreases are needed to obtain normoglycemia in the Edmonton protocol. In addition to the direct damage by the IBMIR to the infused islets, it also provokes a powerful cascade, leading to accelerated T and B cell-mediated responses. The original Edmonton protocol has been modified in several ways in that most centers now culture isolated islets to decrease tissue factor expression, administer anti-inflammatory tumor necrosis factor-α (TNF-α) monoclonal antibody therapy peritransplant, and treat with heparin postislet infusion (33).
The ideal site for islet transplantation must enable successful and sustained engraftment of islets from a single donor, effective use of produced insulin, and maximum patient safety (71). Infusion of islets into the liver via the portal vein has been demonstrated to have the most success in large animal models (33,59). This site is used in the Edmonton protocol and is advantageous because the pancreas normally secretes insulin into the portal vein, intrahepatic islets avoid the systemic hyperinsulinemia observed in some pancreas allograft recipients, the portal blood is oxygenated, and the portal vein can be accessed with a minimally invasive procedure. Disadvantages of infusing islets into the portal vein include the risk of IBMIR, higher levels of exposure to immune suppressive drugs in the portal circulation that could impair engraftment, vascularization or function, and periportal steatosis. Alternative sites currently being considered in an attempt to enhance outcomes include: omentum, kidney capsule, intramuscular, subcutaneous, the gastric submucosa, and the anterior eye chamber (33,58).
POTENTIAL USES OF ADULT STEM CELLS IN DIABETES
Mesenchymal stromal cells (MSCs) are self-renewing, multipotent progenitor cells that can be isolated from many different tissues and have the capacity to differentiate into various lineages (16,19,56,72). It is well established that MSCs can exert immunosuppressive effects on T cells, inhibiting T-cell proliferation, cytotoxic T-lymphocyte activity, and decreasing interferon-γ production (1,16). MSCs have been shown to suppress auto-reactive T-cell responses in other models of autoimmunity, such as experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis, and autoimmune enteropathy (16), making them an ideal candidate for use in T1DM. The use of MSCs in the treatment of T1DM has been tested or proposed at several time points of potential intervention 1) as a preventative treatment or as a means to delay the full development of the disease, 2) as a therapeutic agent to treat complications arising from T1DM, 3) as a supportive treatment for increasing the yield of islets harvested per pancreas during the pre-transplant islet isolation and culture period, 4) as an adjunct therapy with islet administration, and 5) as a rescue therapy upon onset of islet graft failure. Several studies have attempted to drive MSCs to differentiate into insulin-secreting cells, with mixed results (72). However, differentiation as the mechanism of action of MSCs in vivo in various disease models has received far less attention in recent years, so in this review we have chosen to focus on the immunomodulatory and regeneration-promoting properties of MSCs.
MSCs PREVENT OR DELAY ONSET OF DIABETES
Recently, a number of studies have examined the potential uses of MSCs in delaying the onset of T1DM. Bone marrow-derived BALB/c-MSCs have been shown to significantly delay disease onset in nonobese diabetic (NOD) mice, but MSCs from the NOD mice themselves were nearly ineffective (24). These results correlated with the finding that BALB/c-MSCs were significantly more effective in inhibiting T-cell proliferation, and trafficking of BALB/c-MSCs to pancreatic lymph nodes was greater than that of autologous MSCs in the NOD mouse model. The authors further investigated the cause of the disparity between the effects of the BALB/c-MSCs and NOD-MSCs and found that programmed death ligand 1 (PD-L1), a suppressor of autoreactive T cells, is more highly expressed in BALB/c-MSCs. Furthermore, NOD-MSCs produced a more proinflammatory or diabetogenic cytokine profile compared with BALB/c-MSCs. Therefore, the lack of efficacy of the NOD-MSCs is not simply a result of the autologous nature of the cells per se, but rather specific differences in the biology of the NOD-MSCs themselves. Future studies comparing autologous MSCs derived from either nondiabetic or diabetic mice and allogeneic MSCs are necessary to determine the source of cells that has the potential for greatest efficacy in support of islet transplantation in the clinical setting.
Using a streptozotocin (STZ)-induced model of diabetes in nonobese diabetic severe combined immunodeficient (NOD/scid) mice, Hess et al. (34) examined the effects of green fluorescent protein positive (GFP+) donor bone marrow-derived cells transplanted 10 days after the initiation of STZ treatment and found that these cells were able to significantly reduce blood glucose levels over 30 days compared to controls. Transplantation of bone marrow resulted in an increase in insulin-positive islets, but no GFP+/insulin+ cells were observed, suggesting that the results were due to proliferation of host islet cells. Hamamoto et al. (31) recently demonstrated that upon islet transplantation, recipient precursors to islet cells do not divide and contribute to graft function, so understanding the MSCs-induced stimulation of host cell division or insulin production may be particularly important.
Madec et al. (48) demonstrated that MSCs are able to prevent spontaneous diabetes following coinjection with diabetogenic T cells in irradiated NOD recipients. These results were dose dependent, and optimal reductions in onset of diabetes were achieved with 106 MSCs. Additionally, MSCs reduced the ability of diabetogenic T cells to infiltrate islets and this observation correlated with a preferential homing of carboxyfluorescein succinimidyl ester-labeled (CFSE) MSCs to pancreatic lymph nodes as determined by flow cytometry. Diabetogenic T cells decreased levels of IL-10-secreting forkhead box P3 positive (FoxP3+) regulatory T cells, but this was prevented with coinjection of MSCs. MSCs were able to completely suppress alloreactive T-cell proliferation as well as T-cell responses to insulin, which likely play an initiating role in autoimmune β-cell destruction.
Other studies have also reported that GFP+-labeled bone marrow-derived cells do not become insulin-producing cells in the pancreas of recipient mice (44,69), but these studies did not exclude the possibility that the GFP gene was inactivated. Work from Darwin Prockop’s laboratory examined the effects of human MSC-treated mice in a NOD/scid, low-dose STZ model of diabetes (45). They found higher blood insulin levels in treated mice, but no human insulin was detected. In the pancreas, islet number increased, and human Alu sequence in the pancreas and kidney was detected by PCR. Approximately 3% of infused hMSC engrafted into the pancreas, and up to 11% engrafted in the kidney, whereas no cells were detected in lung, liver, or spleen on day 17. The repeated dosing of STZ leads to glomerular changes similar to diabetic nephropathy, and the homing of these cells to the kidney was suggested to be another potential beneficial effect of MSCs. These studies have led to the working hypothesis that MSCs exert their beneficial effects through indirect mechanisms via protection of remaining β-cells or stimulation of endogenous β-cell replacement (12).
Another potential therapeutic use of MSCs is in the treatment of complications of T1DM (25,74). MSC transplantation into the myocardium has been shown to improve heart function in a model of diabetic cardiomyopathy via induction of angiogenesis and attenuation of remodeling, likely as a result of increasing concentrations of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2) among others (80). Additionally, MSC-derived factors may also support recovery of blood flow and angiogenesis in a model of diabetic limb ischemia (3). Similarly, both systemic and local administration of MSCs improved wound healing in the diabetic rat (42). MSCs also have the potential to treat or prevent diabetic nephropathy via prevention of damage to the glomeruli or enhanced angiogenesis (45,49). The ability of MSCs to home to the site of injury is of great benefit for the treatment of diabetic complications, but treatment via local versus system administration of these cells will have to be examined by indication.
MSCs PROMOTE ISLET SURVIVAL IN THE PRETRANSPLANT PERIOD
In addition to inflammation and inadequate revascularization upon transplantation, a significant loss in donor islet mass occurs before islet engraftment because of the innate immune reaction in brain-dead donors, cold storage, and warm collagenase digestion of the pancreas (37). It is widely accepted that prevention of islet loss in these processes would greatly enhance outcome (37,41). In an attempt to prevent loss of islets as a result of hypoxic stress and lack of nutrients during the isolation process, Park et al. (55) cultured islets with umbilical cord-derived MSC-conditioned media (MSC-CM). Islets cocultured with MSC-CM remained more viable, were found to have higher levels of antiapoptotic signaling molecules, increased VEGF receptor, and increased glucose-stimulated insulin secretion. STZ-induced diabetic mice that received islets cultured in MSC-CM for 48 h demonstrated significantly lower blood glucose levels and enhanced blood vessel formation. These results were attributed broadly to the prevalence of IL-6, IL-8, VEGF-A, hepatocyte growth factor (HGF), and transforming growth factor-β (TGF-β) in MSC-CM.
Although many islet preservation strategies and improved culture methods have been identified (15), the shortage of donors has stimulated research into the use of alternative means of obtaining islets for transplantation. The use of immortal β-cells, xenogeneic islets, embryonic stem cells, mesenchymal stromal cells, or induced pluripotent cells to guarantee a sufficient islet mass have been proposed in the literature for some time but the implementation of these techniques into clinical practice has not yet become a reality (2,7,76,77).
MSCs SUPPORT ISLET TRANSPLANTATION
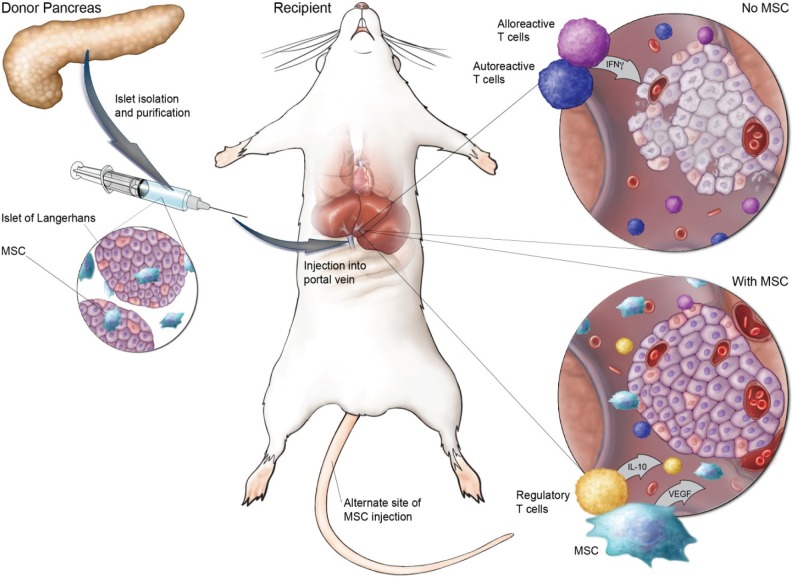
The early success of MSCs in delaying onset of diabetes, and the significant loss of islets upon transplantation led to studies examining the ability of MSCs to prevent initial loss and promote engraftment and re-vascularization of islets (Table 1). A schematic of the use of MSCs in support of islet transplantation is shown in Figure 1. The process of isolating islets destroys the external vasculature and may also compromise the internal islet vascular network (37). Angiogenesis and revascularization begin 2–4 days after islet transplantation and are generally complete by 10–14 days (10,38), but the vascular density and oxygen tension is still less in revascularized islets than islets in the native pancreas (11). Ito et al. (37) demonstrated that cotransplantation of MSCs and islets into the liver of STZ-induced diabetic rats resulted in significantly improved glucose tolerance tests, and more than double the number of capillaries formed at the graft site. Additionally, MSCs were shown to increase endothelial cell migration and upregulate local VEGF production.
Table 1.
An Overview of Recent Preclinical Studies Examining the Effects of MSCs on Islet Transplantation
| Model | Source of MSCs | Experimental Group Treatment | Timing | Result | Reference |
|---|---|---|---|---|---|
| STZ-induced diabetic rats | Lewis rat bone marrow-derived MSCs | Single injection of 1200–1600 islets and 3 × 104 BMC and 1 × 107 MSCs into portal vein | 2 weeks post-STZ, after irradiation, co-transplant of MSCs, BMCs and islets, half animals received more islets at 35 days | Induced stable mixed chimerism, MSCs enabled islet allograft tolerance without GVHD, and established immune tolerance in that after second islet transplant all rats reversed diabetes permanently | Itakura et al., 2007 (36) |
| STZ-induced diabetic Lewis rats | Lewis rat bone marrow-derived MSCs | Single injections of 3 × 6 allogeneic or syngeneic MSCs with allogeneic or syngeneic islets (600 or 1200) into the omentum | Cotransplant of MSCs and islets post onset of hyperglycemia | Allogeneic islets and syngeneic MSCs with short-term immunosuppression best, enhanced long-term graft survival, sustained normoglycemia and promoted IL-10 secreting T-cell generation | Solari et al., 2009 (67) |
| STZ-induced diabetic Lewis rats | Lewis rat bone marrow-derived MSCs | Single injection of 106 MSCs and 2000 islets into the kidney capsule | Cotransplant of MSCs and islets post onset of hyperglycemia | Animals receiving 2000 islets + MSCs maintained normoglycemia, showed increased vascularization and insulin levels | Figliuzzi et al., 2009 (23) |
| Diabetic BALB/c Rag−/− γ−/− mice reconstituted with CD4+CD25− BALB/c T cells | BALB/c mouse bone marrow-derived MSCs | Single injection of 1 × 105 syngeneic MSCs and 500 islets into the kidney capsule | Cotransplant of MSCs and islets post-onset of hyperglycemia | MSCs prolonged survival of allogeneic islet grafts in a process mediated at least in part by MMP-2 and MMP-9 | Ding et al., 2009 (17) |
| STZ-induced diabetic Lewis rats/NOD/scid mice | Lewis rat bone marrow-derived MSCs | Islets (500 or 600) and 107 MSCs into rat portal vein | Cotransplant of MSCs and islets post-onset of hyperglycemia | Reversal of diabetes with MSC s and islets, MSCs promoted vascularization | Ito et al., 2010 (37) |
| STZ-induced diabetic Sprague-Dawley and Wistar rats | Rat bone marrow-derived MSCs | Islets (700 or 1400) and single or multiple doses of syngeneic or allogeneic MSCs +/− immunosuppression into portal or tail vein | Islets 5 days post-STZ, MSCs at 0, 2, and 4 days post-transplant | Triple dose was most effective regardless of syngeneic or allogeneic MSCs, MSCs were comparable to immunosuppression but MSCs + immunosuppression were not more effective | Longoni et al., 2010 (47) |
| STZ-induced diabetic C57BL/6 mice | C57BL/6 bone marrow-derived MSCs | Allogeneic islets and 3 × 106 MSCs cotransplanted into the kidney capsule, and 1 × 106 MSCs via tail vein | MSCs delivered via tail vein injection at days 3, 2, and 0 preislet transplant and coinjected with islets | MSC treatment suppressed T-cell proliferation, promoted a shift to a T helper type 2 response, and inhibited maturation and function of dendritic cells | Li et al., 2010 (46) |
| STZ-induced diabetic cynomolgus monkeys | Cynomolgus monkey bone-marrow derived MSCs | Islets (3,000–14,000 IEQ/kg) and single or multiple doses allogeneic MSCs (1 to 6.5 × 106/kg) codelivered intraportally or via tail vein posttransplant | Islets and MSCs injection intraportally 4 weeks post STZ/IV MSCs used to treat rejection | MSC treatment enhanced islet engraftment and function at 1 month posttransplant and additional infusions of MSCs resulted in reversal of rejection episodes in 2 animals | Berman et al., 2010 (5) |
MSCs, mesenchymal stromal cells; STZ, streptozotocin; BMC, bone marrow cells; GVHD, graft versus host disease; IL-10, interleukin-10; MMP, matrix metalloproteinases; IEQ, islet equivalents; NOD/scid, nonobese diabetic/severe combined immunodeficient.
Figure 1.
Schematic of the preclinical rodent studies on MSC support of islet transplantation. Mesenchymal stromal cells (MSCs) can be coadministered with islets into the portal vein, or alternatively, injected intravenously posttransplant. After islet transplantation alone (top right inset), alloreactive T cells secrete inflammatory cytokines such as interferon-γ (IFN-γ), and a lack of revascularization can lead to poor engraftment and death of islets. In the presence of MSCs (bottom right, inset), alloreactive and autoreactive T-cell proliferation is suppressed, while interleukin 10 (IL-10)-secreting regulatory T-cell expansion is supported, and islets are able to sustain engraftment via revascularization in part through production of vascular endothelial growth factor (VEGF) by MSCs.
Others have reported success with administration of islets and autologous MSCs to the omentum of STZ-induced diabetic rats (67), avoiding potential complications of intraportal islet transplantation (68). The omentum enables islets to reestablish vascularization and provides structure, an aspect important to the hepatic-portal delivery of the secreted insulin. Cotransplantation of islets and autologous MSCs to this site resulted in long-term islet survival and normoglycemia, and promoted the generation of IL-10-secreting CD4+ T cells. T cells from recipients of islets and MSCs produced low levels of interferon-γ (IFN-γ) and TNF-α upon ex vivo activation. Interestingly, in this model, allogeneic MSCs had only a modest effect and did not promote long-term islet allograft survival.
Figliuzzi et al. (23) examined the effects of cotransplantation of MSCs and islets into the kidney capsule of diabetic Lewis rats. Animals receiving 3,000 islets, but not 2,000 islets, were able to achieve sustained normoglycemia. Animals receiving 2,000 islets along with 1 million MSCs into the kidney capsule were able to sustain normoglycemia out to 36 days posttransplantation, the end point of the study. MSC-treated animals promoted increased vascularization of islets as determined by the staining of capillaries using the anti-endothelial cell antibody RECA. This enhancement of angiogenesis was attributed to expression of VEGF165 and, to a lesser extent, VEGF189. Similarly, Ding et al. demonstrated that MSCs can prevent islet allograft rejection after transplantation into the kidney capsule, and further explored a possible mechanism by which MSCs exerted their effects (17). They found that inhibition of MMP-2 and MMP-9 abolished the protective effects of MSCs, resulting in rejection of the transplanted islets. The critical role of these MMPs was also confirmed in vitro as blocking the activity of MMP-2 and -9 prevented MSC-mediated suppression of T-cell proliferation. MSCs have also been shown to improve maintenance of islet morphology, organization, and revascularization after transplantation, providing additional support for cotransplantation of MSCs with islets (57).
In a recent well-designed study, the effects of multiple doses of MSCs in combination with immunosuppressive therapy on islet graft rejection were examined in STZ-induced diabetic rats (47). The results demonstrated that both intraportal and IV-administered MSCs prolonged graft function through prevention of acute rejection in a dose-dependent fashion, and no difference was seen between syngeneic or allogeneic MSCs. The ability of MSC transplants to prevent rejection was similar to immunosuppressive therapy, but the combination of MSCs and immunosuppressive therapy together was not more efficacious. Ten days after islet transplant, there was a large increase of IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) in the blood, which was prevented by MSC administration, immunosuppression, or combination therapy. A second study examining multiple dosing of MSCs found that treatment with MSCs suppressed T-cell proliferation, promoted a shift to a T helper type 2 response, and inhibited maturation and function of dendritic cells (46).
Bone marrow transplantation has been proposed as a means to establish mixed hematopoietic chimerism and induce robust donor-specific immune tolerance of islet allografts. However, conditioning treatment-related toxicity and the potential for graft-versus-host disease has made this approach not suitable for patients undergoing islet transplantation. A recent study demonstrated that diabetic rats receiving a nonmyeloablative conditioning regimen followed by co-infusion of MSCs, bone marrow cells, and islets into the portal vein developed allograft tolerance without incidence of graft-versus-host disease (36). Both experimental and clinical studies have demonstrated that MSCs have an anti-graft-versus-host effect (21), which is likely supported by the strong sup-pressive effects of MSCs on activated T cells.
The efficacy of islet and MSC cotransplantation has also been examined in a nonhuman primate STZ-induced model of diabetes (5). MSC administration significantly enhanced islet engraftment and function at 1 month posttransplant. Additional infusions of donor or allogeneic MSCs resulted in reversal of rejection episodes and prolonged islet function in 2 animals. Islet function was correlated with increased numbers of T regulatory cells in peripheral blood. Expression of IL-6, IL-10, VEGF, TGF-β, HGF, and galectin-1 varied widely over several passages of MSCs as well as between donors, a critical point when considering clinical implementation. Importantly, islet architecture and intraislet resident immune cell populations have recently been characterized in a nonhuman primate (13). Further insight into these populations will enable the development of tailored modulation strategies that can decrease islet immunogenicity, promote engraftment and prevent rejection in human islet transplantation.
MSCs AS A RESCUE THERAPY UPON GRAFT REJECTION
Islet grafts can deteriorate over time due to chronic allograft rejection, local islet toxicity as a result of the drug regimen, recurrent autoimmunity, and/or failure of islet regeneration (16,33). Drugs used for prevention of islet allograft loss adversely affect β-cell function and glycemic control (60). Sirolimus impairs islet engraftment (81), interferes with angiogenesis (9), induces insulin resistance (26), inhibits β-cell replication (79), and, along with corticosteroids, tacrolimus, and mycofenolate motefil (MMF), decreases insulin transcription and translation (54). MMF also inhibits β-cell neogenesis (28). While most centers now try to minimize the use of sirolimus, MSC therapy may have the potential to eliminate maintenance of some systemic immunosuppressive drug therapies, thus relieving negative effects of these drugs on the graft itself in addition to relieving the aforementioned risks of continued immunosuppression (47). The failure of the islet graft and the loss of insulin independence can potentially involve rejection resulting from the activation of alloreactive T cells and a reoccurrence of the original autoimmune disease. The breakdown of immunologic tolerance may result in a cross-reactive memory response against the transplanted islets, resulting in loss of β-cell mass. Leukopenia can result from immunosuppressive therapy and favors the generation of islet-reactive T cells, leading to islet destruction (52). It is thought that MSCs may protect transplanted allogeneic islets by negatively regulating persistent T-cell autoimmunity and control the activation and effector function of alloreactive T cells. MSCs may also suppress activation and proliferation of B cells, and prevent the differentiation and maturation of dendritic cells, effectively preventing islet destruction (16). Further studies are necessary to determine the ability of MSCs or other cell therapies to prolong graft function or reverse graft failure.
Anti-β-cell autoimmunity after islet transplantation correlates with progressively deteriorating β-cell function, whereas the absence of both auto- and alloreactivity is associated with successful outcome (32,33). Unfortunately, while autoantibodies have proven useful for predicting onset of T1DM, their predictive power after islet transplantation is controversial (33). There have been reports of earlier islet graft failure in autoantibody-positive recipients, while others have found no association. These differences could be due in part to different immunosuppressive regimens, graft composition, or procedures. Cytokine profiles also correlate with islet graft maintenance, in that T cells skewed towards a regulatory phenotype were found in insulin-independent recipients, but not insulin-requiring recipients (35). Production of IL-10 inversely correlated with proliferation in alloreactive mixed lymphocyte cultures and with cytotoxic T-cell precursor frequency and associated significantly with insulin independence.
Deteriorating islet allograft function can now be predicted through monitoring of cellular-mediated immune reactivity using parameters such as granzyme B, perforin, and Fas ligand, with granzyme B most reliably, indicating ongoing graft loss (33). Unfortunately, this correlation does not identify if the type of immune reaction against the islet transplant represents recurrent autoimmunity and/or alloimmunity. Significant improvements in validated assays to monitor graft function as well as antigraft immune responses are necessary for forward movement in this field.
POTENTIAL SAFETY ISSUES ASSOCIATED WITH MSCs
Despite the success of MSCs in preclinical models, several potential safety issues are associated with the use of MSCs, particularly in immunosuppressed patients. Given the strong capacity of MSCs for immunomodulation, concerns have arisen that MSCs might interfere with immune responses against pathogens and therefore increase the risks of infection (53). Clinical data from graft-versus-host disease trials suggests that antiviral immune reactions may occur following systemic administration of MSCs (43). However, in models in which a bacterial infection triggers systemic inflammation, as seen in sepsis, MSCs are stimulated by proinflammatory cytokines and acquire an immunosuppressive phenotype, allowing for the control of sepsis-associated complications (30). Recently, Meisel et al. demonstrated that stimulated human MSCs have potent antimicrobial effector function against bacteria, protozoal parasites, and viruses (50). The fact that significant differences have been observed in the functional capacities of MSCs in response to stimulation warrants further investigation and attention to the microenvironment present in the particular disease or injury to which MSCs will be exposed.
In preclinical models it has been shown that infused MSCs can home to the stroma bed of preexisting tumors, with the implication that through trophic support or immunomodulation these cells can promote tumor growth or block tumor clearance (18,40,78). Conflicting data exist in the literature for this hypothesis without clarification for whether exogenously provided MSCs impact the endogenous endothelial and stromal populations involved in tumor initiation or growth; however, the majority of reports do not reflect increased tumorigenic risk (8). It is important to contrast those studies in which coadministration of MSCs with tumor cells report on increased tumor initiation with models of tumor metastases, as the coadministration model does not have clinical relevance and it is not surprising that MSCs would acutely modulate the local inflammatory environment permitting tumor establishment. Two publications reporting tumorigenic potential of human MSCs linked to cytogenetic abnormalities in long-term passage have recently been retracted (73). Original observations from two independent laboratories have subsequently been shown to have been erroneously based on contaminating tumor cell lines (29,70). Although human MSCs appear to be less susceptible to chromosomal aberration in culture (53), expanded MSCs should be tested for karyotypical stability and purity prior to clinical administration. No other preclinical or clinical reports for MSC donor-based tumorigenicity have been made. A recent review article (4) reports on completed clinical studies covering more than 5,000 patients treated in over 100 clinical studies bracketing 15 therapeutic areas including both acute and chronic diseases. To date, with studies initiated over 16 years ago, no reports of tumorigenicity by donor product, or increased frequency of host tumorigenesis have been reported. While it is clear that only long-term patient follow-up will provide a statistically valid evaluation of this association, near-term therapeutic decisions should be driven by a careful analysis of patient risk/benefit considerations in specific disease settings.
CONCLUSIONS
MSCs have immunomodulatory properties that make them uniquely suited to treat autoimmune disease and inflammation. Multiple clinical studies have examined the ability of bone marrow-derived cells to improve clinical outcome in both type 1 and type 2 diabetes (6,14,22,75) and have established a safety profile for the use of these cells in diabetic patients. Recently, a Phase II, multicenter, randomized, double-blind, placebo-controlled study has been initiated to evaluate the safety and efficacy of adult human MSCs for the treatment of recently diagnosed T1DM (Clinicaltrials.gov Identifier: NCT006 90066). Based on the preclinical studies presented in this review, we believe that the use of MSCs for the treatment of diabetes, particularly as an adjunct therapy for islet transplantation, warrants further exploration and advancement towards clinical use. Optimization of several parameters regarding MSC support for islet transplantation is still necessary, including the ideal time of administration, dosing regimen, route of administration, and identification of biomarkers for graft failure that will identify the optimal time for intervention. Despite these and other challenges, MSCs and related cell types have the potential to act as effective therapeutic agents in the treatment of type 1 diabetes.
ACKNOWLEDGMENTS
This work was supported in part by the UK Technology Strategy Board’s Regenerative Medicine, Developing Therapeutics Programme. We extend our thanks to Amanda Mendelsohn for the graphic design work and to Alexander Veenstra and Michael Mendicino for helpful comments on the manuscript. S.B., R.D., and A.T. are employees of Athersys, Inc. S.vC. is an employee of ReGenesys.
REFERENCES
- 1. Abdi R.; Fiorina P.; Adra C. N.; Atkinson M.; Sayegh M. H. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes 57(7):1759–1767; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguayo-Mazzucato C.; Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat. Rev. Endocrinol. 6(3):139–148; 2010. [DOI] [PubMed] [Google Scholar]
- 3. Amin A. H.; Abd Elmageed Z. Y.; Nair D.; Partyka M. I.; Kadowitz P. J.; Belmadani S.; Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab. Invest. 90(7):985–996; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ankrum J.; Karp J. M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 16(5):203–209; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berman D. M.; Willman M. A.; Han D.; Kleiner G.; Kenyon N. M.; Cabrera O.; Karl J. A.; Wiseman R. W.; O’Connor D. H.; Bartholomew A. M.; Kenyon N. S. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 59(10):2558–2568; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhansali A.; Upreti V.; Khandelwal N.; Marwaha N.; Gupta V.; Sachdeva N.; Sharma R. R.; Saluja K.; Dutta P.; Walia R.; Minz R.; Bhadada S.; Das S.; Ramakrishnan S. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 18(10):1407–1416; 2009. [DOI] [PubMed] [Google Scholar]
- 7. Biancone L.; Ricordi C. Pancreatic islet transplantation: An update. Cell Transplant. 11(4):309–311; 2002. [PubMed] [Google Scholar]
- 8. Burt R. K.; Loh Y.; Pearce W.; Beohar N.; Barr W. G.; Craig R.; Wen Y.; Rapp J. A.; Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA 299(8):925–936; 2008. [DOI] [PubMed] [Google Scholar]
- 9. Cantaluppi V.; Biancone L.; Romanazzi G. M.; Figliolini F.; Beltramo S.; Ninniri M. S.; Galimi F.; Romagnoli R.; Franchello A.; Salizzoni M.; Perin P. C.; Ricordi C.; Segoloni G. P.; Camussi G. Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: Relevance for islet transplantation. Am. J. Transplant. 6(11):2601–2611; 2006. [DOI] [PubMed] [Google Scholar]
- 10. Carlsson P. O.; Andersson A.; Carlsson C.; Hellerstrom C.; Hoglund E.; King A.; Kallskog O.; Liss P.; Mattsson G.; Olsson R.; Palm F.; Sandler S.; Tyrberg B.; Jansson L. Engraftment and growth of transplanted pancreatic islets. Ups. J. Med. Sci. 105(2):107–123; 2000. [DOI] [PubMed] [Google Scholar]
- 11. Carlsson P. O.; Mattsson G. Oxygen tension and blood flow in relation to revascularization in transplanted adult and fetal rat pancreatic islets. Cell Transplant. 11(8):813–820; 2002. [PubMed] [Google Scholar]
- 12. Ciceri F.; Piemonti L. Bone marrow and pancreatic islets: An old story with new perspectives. Cell Transplant. 19(12):1511–1522; 2010. [DOI] [PubMed] [Google Scholar]
- 13. Coffey L. C.; Berman D. M.; Willman M. A.; Kenyon N. S. Immune cell populations in nonhuman primate islets. Cell Transplant. 18(10):1213–1222; 2009. [DOI] [PubMed] [Google Scholar]
- 14. Couri C. E.; Oliveira M. C.; Stracieri A. B.; Moraes D. A.; Pieroni F.; Barros G. M.; Madeira M. I.; Malmegrim K. C.; Foss-Freitas M. C.; Simoes B. P.; Martinez E. Z.; Foss M. C.; Burt R. K.; Voltarelli J. C. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 301(15):1573–1579; 2009. [DOI] [PubMed] [Google Scholar]
- 15. Daoud J.; Rosenberg L.; Tabrizian M. Pancreatic islet culture and preservation strategies: Advances, challenges, and future outlook. Cell Transplant. 19(12):1523–1535; 2010. [DOI] [PubMed] [Google Scholar]
- 16. Ding Y.; Bushell A.; Wood K. J. Mesenchymal stem-cell immunosuppressive capabilities: Therapeutic implications in islet transplantation. Transplantation 89(3):270–273; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding Y.; Xu D.; Feng G.; Bushell A.; Muschel R. J.; Wood K. J. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58(8):1797–1806; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Djouad F.; Plence P.; Bony C.; Tropel P.; Apparailly F.; Sany J.; Noel D.; Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102(10):3837–3844; 2003. [DOI] [PubMed] [Google Scholar]
- 19. Dominici M.; Le Blanc K.; Mueller I.; Slaper-Cortenbach I.; Marini F.; Krause D.; Deans R.; Keating A.; Prockop D.; Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317; 2006. [DOI] [PubMed] [Google Scholar]
- 20. Emamaullee J. A.; Shapiro A. M. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 16(1):1–8; 2007. [DOI] [PubMed] [Google Scholar]
- 21. English K.; French A.; Wood K. J. Mesenchymal stromal cells: Facilitators of successful transplantation? Cell Stem Cell 7(4):431–442; 2010. [DOI] [PubMed] [Google Scholar]
- 22. Estrada E. J.; Valacchi F.; Nicora E.; Brieva S.; Esteve C.; Echevarria L.; Froud T.; Bernetti K.; Cayetano S. M.; Velazquez O.; Alejandro R.; Ricordi C. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 17(12):1295–1304; 2008. [DOI] [PubMed] [Google Scholar]
- 23. Figliuzzi M.; Cornolti R.; Perico N.; Rota C.; Morigi M.; Remuzzi G.; Remuzzi A.; Benigni A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant. Proc. 41(5):1797–1800; 2009. [DOI] [PubMed] [Google Scholar]
- 24. Fiorina P.; Jurewicz M.; Augello A.; Vergani A.; Dada S.; La Rosa S.; Selig M.; Godwin J.; Law K.; Placidi C.; Smith R. N.; Capella C.; Rodig S.; Adra C. N.; Atkinson M.; Sayegh M. H.; Abdi R. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J. Immunol. 183(2):993–1004; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fotino C.; Ricordi C.; Lauriola V.; Alejandro R.; Pileggi A. Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev. Diabet. Stud. 7(2):144–157; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fraenkel M.; Ketzinel-Gilad M.; Ariav Y.; Pappo O.; Karaca M.; Castel J.; Berthault M. F.; Magnan C.; Cerasi E.; Kaiser N.; Leibowitz G. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 57(4):945–957; 2008. [DOI] [PubMed] [Google Scholar]
- 27. Froud T.; Ricordi C.; Baidal D. A.; Hafiz M. M.; Ponte G.; Cure P.; Pileggi A.; Poggioli R.; Ichii H.; Khan A.; Ferreira J. V.; Pugliese A.; Esquenazi V. V.; Kenyon N. S.; Alejandro R. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am. J. Transplant. 5(8):2037–2046; 2005. [DOI] [PubMed] [Google Scholar]
- 28. Gao R.; Ustinov J.; Korsgren O.; Otonkoski T. Effects of immunosuppressive drugs on in vitro neogenesis of human islets: Mycophenolate mofetil inhibits the proliferation of ductal cells. Am. J. Transplant. 7(4):1021–1026; 2007. [DOI] [PubMed] [Google Scholar]
- 29. Garcia S.; Bernad A.; Martin M. C.; Cigudosa J. C.; Garcia-Castro J.; de la Fuente R. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp. Cell Res. 316(9):1648–1650; 2010. [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez-Rey E.; Anderson P.; Gonzalez M. A.; Rico L.; Buscher D.; Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 58(7):929–939; 2009. [DOI] [PubMed] [Google Scholar]
- 31. Hamamoto Y.; Akashi T.; Inada A.; Bonner-Weir S.; Weir G. C. Lack of evidence for recipient precursor cells replenishing β-cells in transplanted islets. Cell Transplant. 19(12):1563–1572; 2010. [DOI] [PubMed] [Google Scholar]
- 32. Han D.; Xu X.; Baidal D.; Leith J.; Ricordi C.; Alejandro R.; Kenyon N. S. Assessment of cytotoxic lymphocyte gene expression in the peripheral blood of human islet allograft recipients: Elevation precedes clinical evidence of rejection. Diabetes 53(9):2281–2290; 2004. [DOI] [PubMed] [Google Scholar]
- 33. Harlan D. M.; Kenyon N. S.; Korsgren O.; Roep B. O. Current advances and travails in islet transplantation. Diabetes 58(10):2175–2184; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hess D.; Li L.; Martin M.; Sakano S.; Hill D.; Strutt B.; Thyssen S.; Gray D. A.; Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat. Biotechnol. 21(7):763–770; 2003. [DOI] [PubMed] [Google Scholar]
- 35. Huurman V. A.; Velthuis J. H.; Hilbrands R.; Tree T. I.; Gillard P.; van der Meer-Prins P. M.; Duinkerken G.; Pinkse G. G.; Keymeulen B.; Roelen D. L.; Claas F. H.; Pipeleers D. G.; Roep B. O. Allograft-specific cytokine profiles associate with clinical outcome after islet cell transplantation. Am. J. Transplant. 9(2):382–388; 2009. [DOI] [PubMed] [Google Scholar]
- 36. Itakura S.; Asari S.; Rawson J.; Ito T.; Todorov I.; Liu C. P.; Sasaki N.; Kandeel F.; Mullen Y. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am. J. Transplant. 7(2):336–346; 2007. [DOI] [PubMed] [Google Scholar]
- 37. Ito T.; Itakura S.; Todorov I.; Rawson J.; Asari S.; Shintaku J.; Nair I.; Ferreri K.; Kandeel F.; Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 89(12):1438–1445; 2010. [DOI] [PubMed] [Google Scholar]
- 38. Jansson L.; Carlsson P. O. Graft vascular function after transplantation of pancreatic islets. Diabetologia 45(6):749–763; 2002. [DOI] [PubMed] [Google Scholar]
- 39. Jirak D.; Kriz J.; Strzelecki M.; Yang J.; Hasilo C.; White D. J.; Foster P. J. Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. MAGMA 22(4):257–265; 2009. [DOI] [PubMed] [Google Scholar]
- 40. Karnoub A. E.; Dash A. B.; Vo A. P.; Sullivan A.; Brooks M. W.; Bell G. W.; Richardson A. L.; Polyak K.; Tubo R.; Weinberg R. A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449(7162):557–563; 2007. [DOI] [PubMed] [Google Scholar]
- 41. Korsgren O.; Nilsson B.; Berne C.; Felldin M.; Foss A.; Kallen R.; Lundgren T.; Salmela K.; Tibell A.; Tufveson G. Current status of clinical islet transplantation. Transplantation 79(10):1289–1293; 2005. [DOI] [PubMed] [Google Scholar]
- 42. Kwon D. S.; Gao X.; Liu Y. B.; Dulchavsky D. S.; Danyluk A. L.; Bansal M.; Chopp M.; McIntosh K.; Arbab A. S.; Dulchavsky S. A.; Gautam S. C. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int. Wound J. 5(3):453–463; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Le Blanc K.; Frassoni F.; Ball L.; Locatelli F.; Roelofs H.; Lewis I.; Lanino E.; Sundberg B.; Bernardo M. E.; Remberger M.; Dini G.; Egeler R. M.; Bacigalupo A.; Fibbe W.; Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 371(9624):1579–1586; 2008. [DOI] [PubMed] [Google Scholar]
- 44. Lechner A.; Yang Y. G.; Blacken R. A.; Wang L.; Nolan A. L.; Habener J. F. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes 53(3):616–623; 2004. [DOI] [PubMed] [Google Scholar]
- 45. Lee R. H.; Seo M. J.; Reger R. L.; Spees J. L.; Pulin A. A.; Olson S. D.; Prockop D. J. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. USA 103(46):17438–17443; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li F. R.; Wang X. G.; Deng C. Y.; Qi H.; Ren L. L.; Zhou H. X. Immune modulation of co-transplantation mesenchymal stem cells with islet on T and dendritic cells. Clin. Exp. Immunol. 161(2):357–363; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Longoni B.; Szilagyi E.; Quaranta P.; Paoli G. T.; Tripodi S.; Urbani S.; Mazzanti B.; Rossi B.; Fanci R.; Demontis G. C.; Marzola P.; Saccardi R.; Cintorino M.; Mosca F. Mesenchymal stem cells prevent acute rejection and prolong graft function in pancreatic islet transplantation. Diabetes Technol. Ther. 12(6):435–446; 2010. [DOI] [PubMed] [Google Scholar]
- 48. Madec A. M.; Mallone R.; Afonso G.; Abou Mrad E.; Mesnier A.; Eljaafari A.; Thivolet C. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 52(7):1391–1399; 2009. [DOI] [PubMed] [Google Scholar]
- 49. Magnasco A.; Corselli M.; Bertelli R.; Ibatici A.; Peresi M.; Gaggero G.; Cappiello V.; Chiavarina B.; Mattioli G.; Gusmano R.; Ravetti J. L.; Frassoni F.; Ghiggeri G. M. Mesenchymal stem cells protective effect in adriamycin model of nephropathy. Cell Transplant. 17(10–11):1157–1167; 2008. [DOI] [PubMed] [Google Scholar]
- 50. Meisel R.; Brockers S.; Heseler K.; Degistirici O.; Bulle H.; Woite C.; Stuhlsatz S.; Schwippert W.; Jager M.; Sorg R.; Henschler R.; Seissler J.; Dilloo D.; Daubener W. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia 25(4):648–654; 2011. [DOI] [PubMed] [Google Scholar]
- 51. Melzi R.; Mercalli A.; Sordi V.; Cantarelli E.; Nano R.; Maffi P.; Sitia G.; Guidotti L. G.; Secchi A.; Bonifacio E.; Piemonti L. Role of CCL2/MCP-1 in islet transplantation. Cell Transplant. 19(8):1031–1046; 2010. [DOI] [PubMed] [Google Scholar]
- 52. Monti P.; Scirpoli M.; Maffi P.; Ghidoli N.; De Taddeo F.; Bertuzzi F.; Piemonti L.; Falcone M.; Secchi A.; Bonifacio E. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J. Clin. Invest. 118(5):1806–1814; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nauta A. J.; Fibbe W. E. Immunomodulatory properties of mesenchymal stromal cells. Blood 110(10):3499–3506; 2007. [DOI] [PubMed] [Google Scholar]
- 54. Nir T.; Melton D. A.; Dor Y. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 117(9):2553–2561; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park K. S.; Kim Y. S.; Kim J. H.; Choi B.; Kim S. H.; Tan A. H.; Lee M. S.; Lee M. K.; Kwon C. H.; Joh J. W.; Kim S. J.; Kim K. W. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation 89(5):509–517; 2010. [DOI] [PubMed] [Google Scholar]
- 56. Pittenger M. F.; Mackay A. M.; Beck S. C.; Jaiswal R. K.; Douglas R.; Mosca J. D.; Moorman M. A.; Simonetti D. W.; Craig S.; Marshak D. R. Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147; 1999. [DOI] [PubMed] [Google Scholar]
- 57. Rackham C. L.; Chagastelles P. C.; Nardi N. B.; Hauge-Evans A. C.; Jones P. M.; King A. J. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia 54(5):1127–1135; 2011. [DOI] [PubMed] [Google Scholar]
- 58. Rajab A. Islet transplantation: Alternative sites. Curr. Diab. Rep. 10(5):332–337; 2010. [DOI] [PubMed] [Google Scholar]
- 59. Rajab A.; Buss J.; Diakoff E.; Hadley G. A.; Osei K.; Ferguson R. M. Comparison of the portal vein and kidney subcapsule as sites for primate islet autotransplantation. Cell Transplant. 17(9):1015–1023; 2008. [DOI] [PubMed] [Google Scholar]
- 60. Robertson R. P. Islet transplantation as a treatment for diabetes—a work in progress. N. Engl. J. Med. 350(7):694–705; 2004. [DOI] [PubMed] [Google Scholar]
- 61. Ryan E. A.; Paty B. W.; Senior P. A.; Bigam D.; Alfadhli E.; Kneteman N. M.; Lakey J. R.; Shapiro A. M. Five-year follow-up after clinical islet transplantation. Diabetes 54(7):2060–2069; 2005. [DOI] [PubMed] [Google Scholar]
- 62. Saito Y.; Goto M.; Maya K.; Ogawa N.; Fujimori K.; Kurokawa Y.; Satomi S. Brain death in combination with warm ischemic stress during isolation procedures induces the expression of crucial inflammatory mediators in the isolated islets. Cell Transplant. 19(6):775–782; 2010. [DOI] [PubMed] [Google Scholar]
- 63. Scharp D. W.; Lacy P. E.; Santiago J. V.; McCullough C. S.; Weide L. G.; Falqui L.; Marchetti P.; Gingerich R. L.; Jaffe A. S.; Cryer P. E.; et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes 39(4):515–518; 1990. [DOI] [PubMed] [Google Scholar]
- 64. Shapiro A. M.; Lakey J. R.; Paty B. W.; Senior P. A.; Bigam D. L.; Ryan E. A. Strategic opportunities in clinical islet transplantation. Transplantation 79(10):1304–1307; 2005. [DOI] [PubMed] [Google Scholar]
- 65. Shapiro A. M.; Lakey J. R.; Ryan E. A.; Korbutt G. S.; Toth E.; Warnock G. L.; Kneteman N. M.; Rajotte R. V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343(4):230–238; 2000. [DOI] [PubMed] [Google Scholar]
- 66. Shapiro A. M.; Ricordi C.; Hering B. J.; Auchincloss H.; Lindblad R.; Robertson R. P.; Secchi A.; Brendel M. D.; Berney T.; Brennan D. C.; Cagliero E.; Alejandro R.; Ryan E. A.; DiMercurio B.; Morel P.; Polonsky K. S.; Reems J. A.; Bretzel R. G.; Bertuzzi F.; Froud T.; Kandaswamy R.; Sutherland D. E.; Eisenbarth G.; Segal M.; Preiksaitis J.; Korbutt G. S.; Barton F. B.; Viviano L.; Seyfert-Margolis V.; Bluestone J.; Lakey J. R. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355(13):1318–1330; 2006. [DOI] [PubMed] [Google Scholar]
- 67. Solari M. G.; Srinivasan S.; Boumaza I.; Unadkat J.; Harb G.; Garcia-Ocana A.; Feili-Hariri M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J. Autoimmun. 32(2):116–124; 2009. [DOI] [PubMed] [Google Scholar]
- 68. Suchak A. A.; O’Kelly K.; Al Saif F.; Shapiro A. M.; Owen R. J. Hepatic artery-portal venous fistula after percutaneous intraportal islet cell transplant. Transplantation 83(5):669–670; 2007. [DOI] [PubMed] [Google Scholar]
- 69. Taneera J.; Rosengren A.; Renstrom E.; Nygren J. M.; Serup P.; Rorsman P.; Jacobsen S. E. Failure of transplanted bone marrow cells to adopt a pancreatic beta-cell fate. Diabetes 55(2):290–296; 2006. [DOI] [PubMed] [Google Scholar]
- 70. Torsvik A.; Rosland G. V.; Svendsen A.; Molven A.; Immervoll H.; McCormack E.; Lonning P. E.; Primon M.; Sobala E.; Tonn J. C.; Goldbrunner R.; Schichor C.; Mysliwietz J.; Lah T. T.; Motaln H.; Knappskog S.; Bjerkvig R. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: Putting the research field on track—letter. Cancer Res. 70(15):6393–6396; 2010. [DOI] [PubMed] [Google Scholar]
- 71. van der Windt D. J.; Echeverri G. J.; Ijzermans J. N.; Cooper D. K. The choice of anatomical site for islet transplantation. Cell Transplant. 17(9):1005–1014; 2008. [PubMed] [Google Scholar]
- 72. Vija L.; Farge D.; Gautier J. F.; Vexiau P.; Dumitrache C.; Bourgarit A.; Verrecchia F.; Larghero J. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 35(2):85–93; 2009. [DOI] [PubMed] [Google Scholar]
- 73. Vogel G. Cell biology. To scientists’ dismay, mixed-up cell lines strike again. Science 329(5995):1004; 2010. [DOI] [PubMed] [Google Scholar]
- 74. Volarevic V.; Arsenijevic N.; Lukic M. L.; Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells 29(1):5–10; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Voltarelli J. C.; Couri C. E.; Stracieri A. B.; Oliveira M. C.; Moraes D. A.; Pieroni F.; Coutinho M.; Malmegrim K. C.; Foss-Freitas M. C.; Simoes B. P.; Foss M. C.; Squiers E.; Burt R. K. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 297(14):1568–1576; 2007. [DOI] [PubMed] [Google Scholar]
- 76. Wagner R. T.; Lewis J.; Cooney A.; Chan L. Stem cell approaches for the treatment of type 1 diabetes mellitus. Transl. Res. 156(3):169–179; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang H. S.; Shyu J. F.; Shen W. S.; Hsu H. C.; Chi T. C.; Chen C. P.; Huang S. W.; Shyr Y. M.; Tang K. T.; Chen T. H. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant. 20(3):455–466; 2011. [DOI] [PubMed] [Google Scholar]
- 78. Xu W. T.; Bian Z. Y.; Fan Q. M.; Li G.; Tang T. T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 281(1):32–41; 2009. [DOI] [PubMed] [Google Scholar]
- 79. Zahr E.; Molano R. D.; Pileggi A.; Ichii H.; Jose S. S.; Bocca N.; An W.; Gonzalez-Quintana J.; Fraker C.; Ricordi C.; Inverardi L. Rapamycin impairs in vivo proliferation of islet beta-cells. Transplantation 84(12):1576–1583; 2007. [DOI] [PubMed] [Google Scholar]
- 80. Zhang N.; Li J.; Luo R.; Jiang J.; Wang J. A. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp. Clin. Endocrinol. Diabetes 116(2):104–111; 2008. [DOI] [PubMed] [Google Scholar]
- 81. Zhang N.; Su D.; Qu S.; Tse T.; Bottino R.; Balamurugan A. N.; Xu J.; Bromberg J. S.; Dong H. H. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes 55(9):2429–2436; 2006. [DOI] [PubMed] [Google Scholar]