Abstract
Although intravenous administration of mesenchymal stem cells (MSCs) can enhance functional recovery after spinal cord injury (SCI), the underlying mechanisms have to be elucidated. In this study, we explored the mechanisms for functional recovery in SCI rats after intravenous transplantation of MSCs derived from human umbilical cord blood. Sprague-Dawley rats were randomly assigned to receive either MSCs (1 × 106 cells/0.5 ml) or PBS into the tail vein immediately after SCI. They were then evaluated by the Basso-Beattie-Bresnahan (BBB) locomotor rating scale weekly for 8 weeks and by somatosensory evoked potentials (SSEPs) 8 weeks after transplantation. MSC-treated rats showed a modest but significant improvement in BBB scores and latencies of SSEPs, compared with PBS controls. When human-specific Alu element was measured in the spinal cord, it was detected only 1 h after transplantation, suggesting transient engraftment of MSCs. Inflammatory cytokines were also determined using RT-PCR or Western blot in spinal cord extracts. In MSC-treated rats, the level of proinflammatory cytokine IL-1β was decreased, but that of anti-inflammatory cytokine IL-10 was increased. MSCs also immediately suppressed IL-6 at 1 h posttransplantation. However, the response of IL-6, which has an immunoregulatory role, was increased 1–3 days after transplantation. In addition, we quantified microglia/macrophage stained with Iba-1 around the damaged spinal cord using immunohistochemistry. A proportion of activated microglia and macrophages in total Iba-1+ cells was significantly decreased in MSC-treated rats, compared with PBS controls. These results suggest that early immunomodulation by intravenously transplanted MSCs is a potential underlying mechanism for functional recovery after SCI.
Key words: Spinal cord injury, Transplantation, Mesenchymal stem cells (MSCs), Immunomodulation, Functional recovery
INTRODUCTION
Currently, there is no effective treatment for spinal cord injury (SCI), although it can lead to permanent neurological deficits because of poor regenerative potential and the spread of secondary tissue damages. Secondary damages, which can occur following SCI, include ischemia, edema, glutamate excitotoxicity, free radical injury, and inflammatory reactions (13,16,34,47). While the inflammation eliminates damaged cells, excessive inflammation could cause further damage and exacerbate the disease process (13). An in vitro experiment shows that proinflammatory cytokines are produced by activated microglia (35). In vivo conditions also show that interleukin (IL)-1 and tumor necrosis factor-α (TNF-α) are well correlated with the severity of immune-mediated diseases (14,36). The inflammatory cytokines or chemokines are often increased in serum and spinal cord after SCI (9,13).
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into a variety of cell types and have a large capacity for self-renewal. However, the therapeutic mechanisms after transplantation of MSCs seem to have been derived from paracrine effects rather than cell replacement in a previous study (6). The paracrine effects from transplanted MSCs can be divided into trophic factor secretion and immunomodulation by cytokines (33). Recent investigations have shown that MSCs can act as an immune modulator to prevent the activation of microglia (22) and the proliferation of lymphocytes (8,24,38). MSCs also play an inhibitory role in the differentiation, maturation, and phenotype maintenance of dendritic cells (20,31,38,40,41). As a result, immunomodulation can have beneficial effects on functional outcomes of locomotor behavior by suppressing secondary inflammatory reactions (32).
Therefore, we investigated whether intravenous transplantation of MSCs derived from human umbilical cord blood (hUCB) can enhance functional recovery in a rat model of SCI, and explored the underlying mechanisms such as neurotrophic effects and immunomodulation by MSCs. We found that MSCs expressed immunomodulatory effects during the acute phase after SCI, which contributes to functional recovery in chronic phase.
MATERIALS AND METHODS
Isolation, Culture, and Characterization of MSCs
This study was approved by the Institutional Review Board (IRB), and all samples were obtained with the informed consent. MSCs were isolated and cultured as previously described (7,25,50). Briefly, mononuclear cells were isolated from hUCB using Ficoll-Hypaque density gradient centrifugation (Histopaque-1077; Sigma-Aldrich, St. Louis, MO). Cells were seeded in T25 culture flasks (Nalge Nunc, Naperville, IL) at a density of 3 × 105 cells/cm2 in low glucose Dulbecco’s modified Eagle’s medium (LG-DMEM; Invitrogen-Gibco, Rockville, MD) containing 10% fetal bovine serum (FBS; Invitrogen-Gibco) and 100 U/ml penicillin/streptomycin (Invitrogen-Gibco). The cells were incubated in a humidified atmosphere at 37°C with 5% CO2. Adherent cells were then resuspended with 0.05% trypsin-EDTA (Invitrogen-Gibco) and reseeded at 2 × 103 cells/cm2 and expanded under the same culture conditions. The MSCs were harvested at passages 3 to 4 for transplantation. For flow cytometry, a total of 5 × 105 cells were resuspended in 0.2 ml PBS and incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies for 20 min at room temperature. The flurorescence intensity of the cells was evaluated by flow cytometry (FACScan; Becton Dickinson, Franklin Lakes, NJ).
Animals
A total of 98 male Sprague-Dawley (SD) rats weighing 300–350 g were used. For behavioral testing, 25 animals were randomly grouped into SCI rats treated with MSCs (n = 12) or PBS (n = 13) to investigate the functional outcomes after intravenous transplantation of MSCs. In other set of genomic DNA PCR study, SCI rats treated with MSCs (n = 15) or PBS (n = 15) were used to evaluate the presence of human-specific element 1 h, 1 day, 2 days, 3 days, and 7 days (n = 3 each) after transplantation. Another two SCI rats were used 7 weeks after transplantation to evaluate the long-term presence of human Alu, and two rats not subjected to SCI were also used as negative controls. In additional set of reverse transcriptase (RT)-PCR or Western blot analysis, 30 SCI rats were also used to evaluate the expression of neurotrophic factors and inflammatory cytokines at serial time points (n = 3 each). Among the rats for behavioral testing, 18 SCI rats were used for immunohistochemistry (n = 3 / group) or electrophysiological study (n = 6 / group) 8 weeks after transplantation. Three SCI rats were used for additional immunohistochemistry to investigate survival and differentiation of grafted cells 4 weeks after transplantation. Another six SCI rats were used to compare the proportion of activated microglia between groups 1 day after administration of MSCs or PBS (n = 3 each). All rats were given food and water ad libitum with the 12/12-h light/dark cycle, and housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAA LAC). The Institutional Animal Care and Use Committee (IACUC) approved the experimental design.
Spinal Cord Injury
As previously described (6,7), all rats were anesthetized with pentobarbital sodium (50 mg/kg, IP) and administrated prophylactic atropine sulfate (0.8 mg/kg, IP) to reduce tracheal secretions. Laminectomies were performed at the T9 level, leaving the dura mater intact. SCI was induced by dropping a 10-g impact rod from a 25-mm height onto the exposed dorsal surface of the spinal cord of the rats using the NYU impactor (New York University, NY). Postoperative care included bladder expression once or twice daily until the animals recovered bladder function. Prophylactic kanamycin (1 mg/kg) was administered to all rats for 1 week after surgery or until they were sacrificed.
Cell Transplantation
The SCI rats were randomly assigned to two groups without bias right after inducing SCI: MSC- and PBS-treated groups. Thereafter, a total of 500 μl of cultured MSCs (1 × 106 cells) or PBS was injected into the tail vein immediately after SCI. During the recovery period, their body temperature was maintained at 37°C in the heating chamber. As previously described (6,7), all rats received cyclosporine A (10 mg/kg, IP) daily from 2 days before the transplantation until the completion of this experiment in order to prevent rejection of the transplanted cells.
Behavioral Assessment
The Basso, Beattie, and Bresnahan (BBB) locomotor rating scale was used to measure motor recovery of the SCI rats. This scale measures hindlimb movements with scores ranging from 0 (no observable movement) to 21 (normal movement) with a higher score given for improved movement of individual joints, limb coordination, and weight-supported stepping. Two independent investigators, blinded to the animal groups, observed the hindlimb movements in an open field for 5 min after the rats were gently adapted to the field. Locomotor functions were scored once a week from 1 day to 8 weeks after cell transplantation. The final score was obtained by averaging those of both sides of the limbs.
Electrophysiological Study
As previously described (6,7), somatosensory evoked potentials (SSEPs) were measured 8 weeks after transplantation. To record SSEPs, the rats were randomly assigned without any indication of the extent of the lesion. The animals were anesthetized with urethane (1.25 gm/ kg, IP). They were also treated with atropine sulfate (0.8 mg/kg, IP) to reduce tracheal secretions, and pancuronium bromide (1.0 mg/kg, IP) to induce muscle relaxation. The rats were then intubated with a tracheostomy and artificially respired using an animal respirator (Harvard Apparatus, South Natik, MA). The left sciatic nerve was exposed, and a pair of electrodes was hooked around the nerve. A single square pulse of electrical stimulation was delivered with a stimulus isolator (A365, World Precision Instruments, New Haven, CT), which was driven by a pulse generator (Pulsemaster A300, World Precision Instruments, New Haven, CT) with a 0.1-ms pulse duration of stimuli and a 6-mA intensity at 1–4 Hz. For the SSEP recording, a 4 × 4-mm-sized craniectomy was performed in the contralateral frontoparietal area. A recording electrode (NE-120, Rhodes Medical Instruments, Tujunga, CA) was fixed on the sensorimotor cortex at a point 2 mm posterior to bregma and 2 mm lateral to the sagittal suture after craniectomy. Each evoked potential consisted of an average of 100 single sweep epochs.
Tissue Preparation
A separate set of animals was anesthetized with pentobarbital sodium (50 mg/kg, IP) and perfused transcardially with ice-cold PBS for DNA, RNA, and protein extraction. The spinal cords were immediately removed, 20-mm-sized transverse segments of the injured region of the thoraco-lumbar spinal cord were dissected and stored in a freezer at −70°C for extraction. All procedures were performed according to animal use protocol approved by the IACUC.
Genomic DNA PCR of Human Alu Element
Genomic DNA was extracted from the spinal cords, lungs, and spleen using a commercial genomic DNA kit (Promega, Madison, WI) according to the manufacturer’s instructions. The human Alu gene, the most abundant repetitive element in the human genome but not present in rats, was amplified by PCR using specific primer pairs for the Alu element: forward 5′-GGCGCGGTGGCTCACG-3′ and reverse 5′-TTTTTTGAGACGGAGTCTCGCTC-3′. PCR was performed using Taq polymerase (BioQuest, Korea) in conditions as follows: 35 cycles consisting of a presoak for 4 min at 94°C, denaturation for 30 s at 94°C, annealing for 30 s at 63°C, and extension for 1 min at 72°C, with an additional 7 min of incubation at 72°C after completion of the cycle. Amplified DNA fragments were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and photographed under an ultraviolet light transilluminator (Bio-Rad, Hercules, CA).
Analysis of Cytokines Under In Vitro Condition
MSCs were seeded at 1 × 104 cells/cm2 in six-well plates in 2 ml LG-DMEM. The supernatants were collected 48 h later, and frozen at −70°C. Multiplex human cytokine detection kit (Upstate, Waltham, MA) was utilized to measure the production of inflammation-related cytokines such as TNF-α, IL-1β, IL-6, and IL-10 in the supernatant of MSCs (n = 3). This was performed on a chemiluminescence detector (Luminex Corp., Austin, TX) according to the manufacturer’s instructions.
Reverse Transcriptase-PCR Analysis
Total RNA was extracted from spinal cords using TRIzol Reagent (Gibco-BRL, Maryland, MD), and 2 μg of RNA was reverse-transcribed with avian myeloblastosis virus (AMV) reverse transcriptase XL (Takara, Shiga, Japan) for 90 min at 42°C. PCR was performed with Taq polymerase for 30 cycles using the specific RT-PCR probe sequences to analyze the expression of rat- or human-specific nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell-line derived neurotrophic factor (GDNF), neurotrophin-3 (NT-3), TNF-α, IL-1β, IL-6, and IL-10 (Table 1). The amplified cDNA fragments were electrophoresed on a 1% agarose gel, stained with ethidium bromide, and photographed under an ultraviolet light transilluminator (Bio-Rad, Hercules, CA).
Table 1.
Primer Sequences Used for Reverse Transcriptase-PCR Analysis
| Species | Target | Primer Sequence |
|---|---|---|
| Neurotrophic factors | ||
| Human | ||
| NGF | forward | 5′-ATACAGGCGGAACCACACTCAG-3′ |
| reverse | 5′-GTCCACAGTAATGTTGCGGGTC-3′ | |
| BDNF | forward | 5′-AGAGGCTTGACATCATTGGCTG-3′ |
| reverse | 5′-CAAAGGCACTTGACTACTGAGCATC-3′ | |
| GDNF | forward | 5′-CACCAGATAAACAAATGGCAGTGC-3′ |
| reverse | 5′-CGACAGGTCATCATCAAAGGCG-3′ | |
| NT-3 | forward | 5′-GGGAGATCAAAACGGGCAAC-3′ |
| reverse | 5′-ACAAGGCACACACACAGGAC-3′ | |
| Rat | ||
| NGF | forward | 5′-ATCCACCCACCCAGTCTTCCACAT-3′ |
| reverse | 5′-GGCAGCCTGTTTGTCGTCTGTTGT-3′ | |
| BDNF | forward | 5′-AGCCTCCTCTGCTCTTTCTGCTGGA-3′ |
| reverse | 5′-CTTTTGTCTATGCCCCTGCAGCCTT-3′ | |
| GDNF | forward | 5′-ACTCCAATATGCCCGAAGATTATCCTG-3′ |
| reverse | 5′-CCAAACCCAAGTCAGTGACATTTAAGTG-3′ | |
| NT-3 | forward | 5′-TTTCTTGCTTATCTCCGTGGCATCC-3′ |
| reverse | 5′-GGCAGGGTGCTCTGGTAATTTTCCT-3′ | |
| Pro-/anti-inflammatory cytokines | ||
| Human | ||
| TNF-α | forward | 5′-ATCTACTCCCAGGTCCTCTTCAA-3′ |
| reverse | 5′-GCAATGATCCCAAAGTAGACCT-3′ | |
| IL-1β | forward | 5′-TTGACGGACCCCAAAAGATG-3′ |
| reverse | 5′-AGAAGGTGCTCATGTCCTCA-3′ | |
| IL-6 | forward | 5′-GTAGCCGCCCCACACAGACAGCC-3′ |
| reverse | 5′-GCCATCTTTGGAAGGTTC-3′ | |
| IL-10 | forward | 5′-ATCCAAGACAACACTACTAA-3′ |
| reverse | 5′-TAAATATCCTCAAAGTTCC-3′ | |
| Rat | ||
| TNF-α | forward | 5′-GTAGCCCACGTCGTAGCAAAC-3′ |
| reverse | 5′-TGTGGGTGAGGAGCACATAGTC-3′ | |
| IL-1β | forward | 5′-CACCTTCTTTTCCTTCATCTTTG-3′ |
| reverse | 5′-GTCGTTGCTTGTCTCTCCTTGTA-3′ | |
| IL-6 | forward | 5′-AAGTTTCTCTCCGCAAGAGACTTCCAG-3′ |
| reverse | 5′-AGGCAAATTTCCTGGTTATATCCAGTT-3′ | |
| IL-10 | forward | 5′-CTGCTATGTTGCCTGCTCTTAC-3′ |
| reverse | 5′-TCATTCTTCACCTGCTCCACT-3′ | |
NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; GDNF, glial cell line-derived neurotrophic factor; NT-3, neurotrophin-3; TNF, tumor necrosis factor; IL, interleukin.
Western Blot Analysis
Spinal cords were lysed in 500 μl of cold RIPA buffer [50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), and 1% sodium deoxycholate] with a protease inhibitor cocktail (Sigma). Tissue lysate was centrifuged at 13,709 × g for 10 min at 4°C. The supernatant was harvested, and protein concentration was analyzed using a protein assay kit (Bio-Rad, Hercules, CA). For electrophoresis, 40 μg protein was dissolved in sample buffer (60 mM Tris-HCl, pH 6.8, 14.4 mM β-mercaptoethanol, 25% glycerol, 2% SDS, and 0.1% bromophenol blue), boiled for 5 min, and separated on a 10% SDS reducing gel. Separated proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Amersham Pharmacia Biotech, UK) using a trans-blot system (Bio-Rad). Blots were blocked for 1 h in Tris-buffered saline (TBS) (10 mM Tris-HCl, pH 7.5, 150 mM NaCl) containing 5% nonfat dry milk (Bio-Rad) at room temperature, washed three times with TBS, and incubated at 4°C overnight with an anti-IL-1β (1:1000, Chemicon), anti-TNF-α (1:1000, Chemicon), anti-IL-6 (1:500, SantaCruz Biotech), anti-IL-10 (1:500, SantaCruz Biotech), or anti-β-actin (1:1000, SantaCruz Biotech) antibody in TBST (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.02% Tween 20) containing 3% nonfat dry milk. On the next day, blots were washed three times with TBST, and incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies (1:2000, SantaCruz Biotech) in TBST containing 3% nonfat dry milk at room temperature. After washing three times with TBST, protein was visualized with an ECL detection system (Amersham Pharmacia Biotech).
Immunohistochemistry
Animals were transcardially perfusion fixed with ice-cold PBS and 4% paraformaldehyde. The spinal cords were immediately removed, 20-mm-sized transverse segments of the injured region of the thoraco-lumbar spinal cord were dissected and stored in the same fixative overnight, and tissues were serially immersed in 6%, 15%, and 30% sucrose until they sank down. The tissues were frozen and cryosectioned longitudinally into slices 12 μm thick using a cryomicrotome (Microm/HM500V, Walldorf, Germany). Immunostaining was performed on 10 sections over a range of 192 μm. Individual sections were stained overnight with the human nuclear protein HuNu (mAb 1281, 1:200, Chemicon, CA, USA) and one of the following markers: 1) βIII-tubulin (1:400, Covance, NJ, USA), 2) microtubule-associated protein2 (MAP2, Chemicon), 3) glial fibrillary acidic protein (GFAP, Chemicon), 4) myelin basic protein (MBP, Chemicon). Double-labeled cells were assessed by confocal imaging. Sections were then stained for ionized calcium binding adaptor molecule-1 (Iba-1, 1:600, Biocare) overnight to evaluate the microglia/macrophage. The sections were incubated with Alexa 563 secondary antibodies at 1:400 for 1 h, then washed, mounted on glass slides with fluorescent mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vectorshield, Vector). The sections were examined under a fluorescence microscope (BX51, Olympus, Tokyo, Japan) or an argon and krypton laser scanning confocal imaging system (LSM 510, Zeiss, Gottingen, Germany) to visualize double-labeled cells at a magnification of 600×, demonstrating colocalization of Iba-1 and DAPI. The colabeled cells were investigated 5 mm rostrally and caudally from the epicenter of the necrotic cavity derived from contusional injury. The evaluating area was obtained using the MetaMorph Imaging System (Molecular Device, Sunnyvale, CA), and converted to the volume (area × 12 μm); the number of colabeled cells was quantified as the density (/mm3). The Iba-1+ cells were divided into ameboid form representing activated microglia and macrophages (26), and ramified form representing resting microglia. The proportion of activated microglia and macrophages in total Iba-1+ cells was calculated one day after transplantation when the inflammatory cascades reached a peak (13).
Statistical Analysis
An independent t-test was used to compare BBB locomotor rating scores between MSCs- and PBS-treated groups at each time point of 1-week interval using SPSS version 18.0. The latencies and amplitudes of SSEPs and the proportion of activated microglia and macrophages were also compared between the groups using an independent t-test. Values of p < 0.05 were accepted as significant.
RESULTS
Characterization and Differentiation of MSCs Into Mesenchymal Lineage Cells
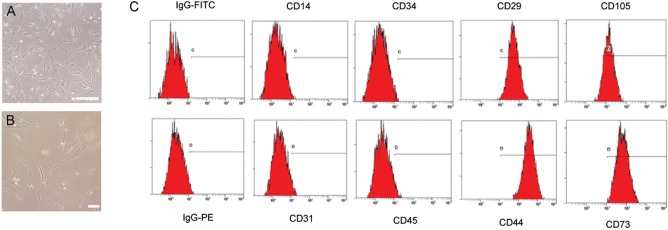
We characterized spindle-shaped, fibroblastic cells obtained from hUCB (25,50) (Fig. 1A, B). The cells could differentiate into mesenchymal lineage cells such as osteoblasts stained by alkaline phosphatase, adipocytes stained by oil red O, and chondrocytes stained by toluidine blue as previously described (25,50). In addition, these cells were strongly positive for MSC-specific markers such as CD105, CD73, CD44, and CD29, but negative for CD14, CD31, CD34, and CD45 by flow cytometry (Fig. 1C).
Figure 1.
Characterization of MSCs. (A) MSCs were isolated from hUCB and cultured under LG-DMEM containing 10% FBS and 100 U/ml penicillin/streptomycin. Scale bar: 100 μm. (B) Spindle-shaped fibroblastic cells were characterized in high magnification. Scale bar: 100 μm. (C) Cells were labeled with FITC- and PE-conjugated antibodies and examined by flow cytometry. hUCB, human umbilical cord blood; MSCs, mesenchymal stem cells; LG-DMEM, low glucose Dulbecco’s modified Eagle’s medium; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Functional Recovery of Hindlimb Locomotion
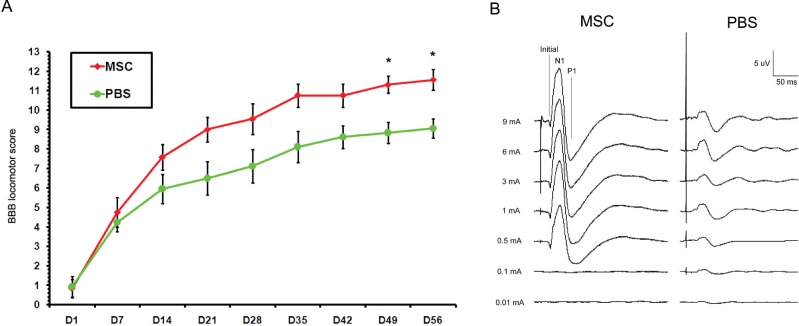
The SCI rats treated with MSCs exhibited a gradual improvement over time (Fig. 2A). They showed a modest but significant improvement in locomotor function 7 weeks (day 49) after transplantation, compared with PBS controls (p < 0.05). The BBB score of MSC-treated rats continued to increase to a final score of 11.56 ± 0.53, while the score of PBS-injected animals was maintained to 9.06 ± 0.50 at 8 weeks (day 56) posttransplantation (p < 0.05). The score of MSC-treated rats indicated a gait pattern characterized by frequent weight-supported plantar steps and occasional forelimb and hindlimb coordination, while that of PBS controls indicated a hind-limb dysfunction characterized by plantar placement of the paw with weight support in only stance phase but no plantar stepping.
Figure 2.
Locomotor and somatosensory assessment after intravenous transplantation of MSCs. (A) When locomotor performance was evaluated using the BBB locomotor rating scale, SCI rats treated with MSCs exhibited a gradual improvement over time. Namely, MSC-treated rats showed a modest but significant improvement in locomotor function 7–8 weeks (D49–D56) after transplantation, compared with PBS controls (*p < 0.05). (B) In addition, MSC-treated rats showed a representative finding of the shortened N1 latency and P1 latency of SSEPs compared to PBS controls. Values are mean ± SE. BBB, Basso-Beattie-Bresnahan; D, day; MSCs, mesenchymal stem cells; PBS, phosphate-buffered saline; SSEPs, somatosensory evoked potentials.
Improvement in Electrophysiological Findings
Eight weeks after transplantation, MSC-treated rats showed a significantly shortened N1 latency (15.88 ± 4.04 ms) compared with PBS controls (35.55 ± 8.60 ms) (p < 0.05). In addition, P1 latency (31.73 ± 4.99 ms) was significantly shorter in rats treated with MSCs than those treated with PBS controls (68.53 ± 10.63 ms) (p < 0.05) (Table 2). Although MSC-treated animals exhibited no significant improvement in amplitude of SSEPs, they had a strong tendency to show representative findings of greater amplitudes compared to the PBS controls (Fig. 2B).
Table 2.
Electrophysiological Findings in Rats Treated With MSCs or PBS 8 Weeks After Transplantation
| tSSEP | MSC (n = 6) | PBS (n = 6) |
|---|---|---|
| Latency | ||
| Initial (ms) | 8.90 ± 2.36 | 11.23 ± 2.79 |
| N1 (ms) | 15.88 ± 4.04* | 35.55 ± 8.60 |
| P1 (ms) | 31.73 ± 4.99* | 68.53 ± 10.63 |
| Amplitude | ||
| Negative peak (µV) | 4.54 ± 2.99 | 3.96 ± 1.18 |
| Positive peak (µV) | 7.51 ± 4.53 | 6.54 ± 1.63 |
| Peak to peak (µV) | 12.05 ± 7.52 | 10.51 ± 2.80 |
Values are mean − SE. SSEP, somatosensory evoked potential; MSC, mesenchymal stem cell; PBS, phosphate-buffered saline.
p < 0.05 compared with PBS by independent t-test.
Identification of Human-Specific Element
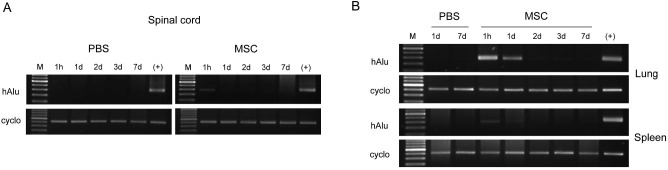
When evaluated with genomic DNA PCR from injured spinal cord extractions after intravenous transplantation of MSCs derived from hUCB (Fig. 3A), the human Alu gene, which is the most abundant repetitive element in the human genome but not present in rats, was detected only 1 h after transplantation. However, human Alu was not detected 1 day, 2 days, 3 days, and 7 days after transplantation, suggesting transient engraftment of MSCs disappeared within 1 day. We could not find the human Alu 7 weeks after transplantation either (data not shown). In negative controls, human Alu was never detected at any serial time points after administration of PBS, supporting that Alu expression is specific to transplanted hUCB-derived MSCs (Fig. 3A).
Figure 3.
Genomic DNA PCR of human Alu element after intravenous transplantation of MSCs. (A) To determine the engraftment of intravenously injected MSCs in the spinal cord, expression of human Alu gene was examined at the indicated time points after MSC transplantation. The human-specific Alu was detected in injured spinal cord extractions only 1 h after transplantation but not detected thereafter, suggesting that MSCs disappeared after transient engraftment. (B) On the contrary, the human Alu gene was clearly detected in the lungs and spleen 1 h and 1 day after transplantation of MSCs, but not 7 days after transplantation. hUCB, human umbilical cord blood; MSCs, mesenchymal stem cells; PBS, phosphate-buffered saline; hAlu, human Alu; Cyclo, cyclophilin; M, marker. (+): positive control, the human hepatoma Hep3B cells.
On the contrary, the human-specific Alu was clearly detected in the lung and spleen 1 h and 1 day, but not thereafter, after intravenous transplantation of MSCs (Fig. 3B). The expression of the Alu element in the lung was relatively higher than in the spleen, demonstrating that transplanted cells were largely infiltrated into the lungs (Fig. 3B).
Expression of Neurotrophic Factors
When neurotrophic factors released from transplanted MSCs derived from hUCB were evaluated 1 h, 1 day, 2 days, 3 days, and 7 days after transplantation by RT-PCR, human NGF, human BDNF, human GDNF, and human NT-3 were not detected in all SCI rats treated with MSCs or PBS (data not shown). On the other hand, when endogenous neurotrophic factors after intravenous transplantation of MSCs were evaluated by RT-PCR, rat NGF and rat BDNF were detected. However, there were no significant differences between the groups (data not shown).
Expression of Pro-/Anti-inflammatory Cytokines
For in vitro experiment, the culture supernatants were analyzed using a luminex multiplex detection system to determine the level of inflammation-related cytokines secreted by MSCs. As a result, IL-6 (153.93 ± 12.87 pg/ml) was highly secreted by the MSCs, compared with TNF-α (5.10 ± 5.10 pg/ml), IL-1β (0.97 ± 0.61 pg/ml), and IL-10 (0.57 ± 0.43 pg/ml) (Table 3).
Table 3.
The Levels of Cytokines Released by hUCB-Derived MSCs In Vitro
| Cytokines | MSC (n = 3) |
|---|---|
| TNF-α (pg/ml) | 5.10 ± 5.10 |
| IL-1β (pg/ml) | 0.97 ± 0.61 |
| IL-6 (pg/ml) | 153.93 ± 12.87 |
| IL-10 (pg/ml) | 0.57 ± 0.43 |
Values are mean ± SE. hUCB, human umbilical cord blood; MSC, mesenchymal stem cell; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10.
For in vivo experiment, inflammatory mediators released from hUCB-MSCs were evaluated by RT-PCR, and human TNF-α, human IL-1β, human IL-6, and human IL-10 were not detected in the spinal cord extractions of SCI rats treated with MSCs or PBS. On the other hand, an endogenous anti-inflammatory cytokine, rat IL-10, was increased in MSC-treated rats especially 1 day after transplantation (Fig. 4A). Endogenous inflammatory cytokines were also confirmed by Western blot, and rat IL-10 was highly expressed in MSC-treated rats until at least 2 days after transplantation (Fig. 4B). In addition, an endogenous proinflammatory cytokine, rat IL-1β, was modestly decreased after transplantation of MSCs, and endogenous TNF-α seemed to be downregulated 3 days after transplantation (Fig. 4B). Expression of proinflammatory cytokine, IL-6, was also suppressed 1 h after transplantation. However, both RT-PCR and Western blot assay showed increased response of rat IL-6, which also has an immunoregulatory role, 1–3 days after intravenous administration of MSCs (Fig. 4A, B).
Figure 4.
Expression of inflammatory cytokines after intravenous transplantation of MSCs. (A) RT-PCR study showed an increase of anti-inflammatory cytokine IL-10 in MSCs-treated rats especially 1 day after transplantation. (B) In Western blot results, IL-10 was highly expressed in MSC-treated rats until at least 2 days after transplantation. In addition, proinflammatory cytokine IL-1β was modestly decreased after transplantation of MSCs. TNF-α seems to be downregulated 3 days after transplantation. MSCs also immediately suppressed proinflammatory cytokine IL-6 at 1 h after transplantation. However, the response of rat IL-6, which has an immunoregulatory role as well, was increased 1-3 days after intravenous administration of MSCs. MSCs, mesenchymal stem cells; PBS, phosphate-buffered saline; TNF, tumor necrosis factor; IL, interleukin; N, normal spinal cord.
Immunohistochemistry
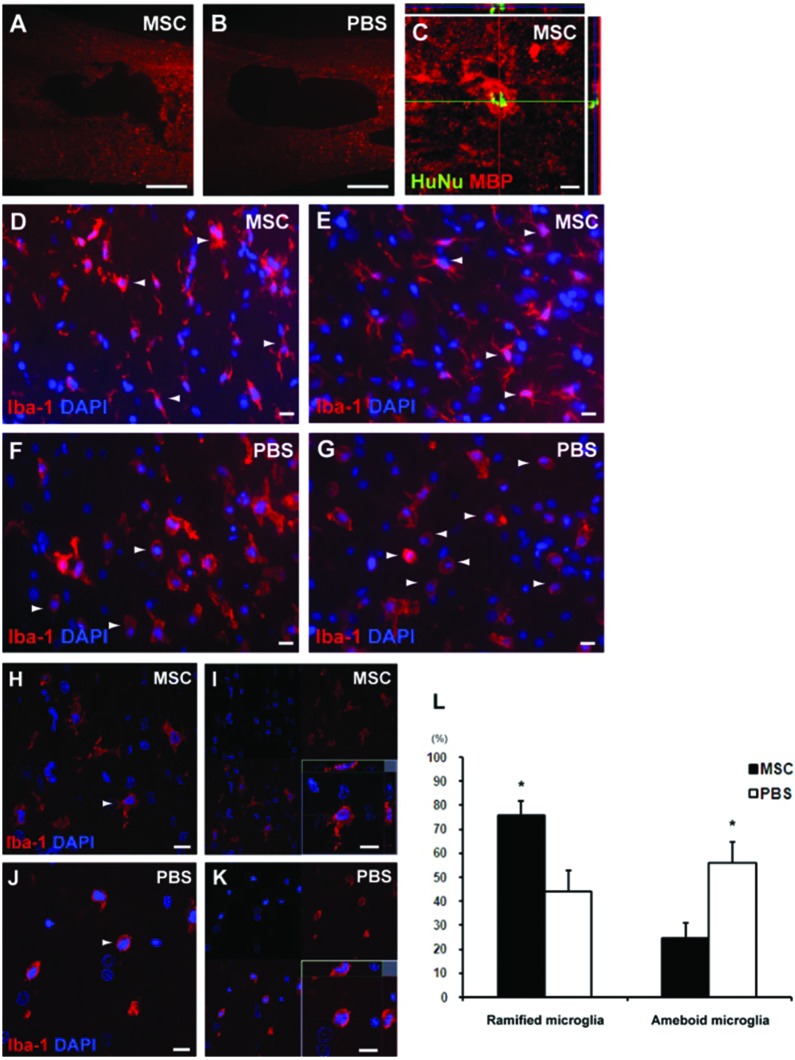
Eight weeks after the transplantation, injured tissues showed no significant difference in the mean area of cavitary lesion per section between MSC-treated rats and PBS controls, although MSC-treated rats (1.41 ± 0.19 mm2) had a tendency to show a smaller lesion cavity than PBS control (1.60 ± 0.09 mm2) (Fig. 5A, B). When we also performed immunostaining with human-specific HuNu and neural-lineage markers in animals treated with MSCs, only a few cells were suspected to be double-labeled with MBP 4 weeks after transplantation (Fig. 5C). Additionally, we did not find grafted cells differentiated into neurons, astrocytes, or myelin-forming oligodendrocytes colabeled with βIII-tubulin, MAP2, GFAP, or MBP 8 weeks after transplantation (data not shown).
Figure 5.
Immunohistochemistry after intravenous transplantation of MSCs. (A, B) When we investigated the injured tissues in MSC-treated rats (A) and PBS controls (B) 8 weeks after transplantation, there was no significant difference in the mean area of cavitary lesion per section between them. Scale bars: 500 μm. (C) When we also performed immunostaining with human-specific HuNu and neural-lineage markers in animals treated with MSCs, only a few cells were suspected to be double-labeled with MBP at posttransplantation 4 weeks. Scale bar: 10 μm. (D–G) The sections of SCI rats treated with MSCs (D, E) and PBS (F, G) were costained for DAPI and Iba-1, which are highly expressed in microglia/macrophage. Scale bars: 10 μm. (H–K) The Iba-1+ cells were divided into two groups: ramified form representing resting microglia (H, I) and ameboid form representing activated microglia and macrophage (J, K). Scale bars: 10 μm. (L) When we compared the proportion of activated microglia/macrophage among total Iba-1+ cells between the groups 1 day after transplantation when the inflammatory cascades temporally peaked, a proportion of the activated microglia/macrophage was significantly suppressed in MSC-treated rats, compared with PBS-treated controls (*p < 0.05). MBP, myelin basic protein; Iba-1, ionized calcium binding adaptor molecule-1; DAPI, 4′,6-diamidino-2-phenylindole; MSCs, mesenchymal stem cells; PBS, phosphate-buffered saline.
Modulation of Microglial Activation
In order to evaluate a proportion of the activated microglia and macrophages among total Iba-1+ cells in the injured spinal cords, the tissue sections of SCI rats treated with MSCs and PBS were costained for DAPI and Iba-1, which are largely expressed in micoglia/macrophage. Iba-1+ cells were divided into ramified form as resting microglia (Fig. 5D, E) and ameboid form as activated microglia and macrophages (Fig. 5F, G). One day after transplantation when the inflammatory cascades temporally peaked, we compared the proportion of ameboid cells in total Iba-1 + cells between the groups (Fig. 5H–K). We found significantly lower expression of activated microglia and macrophages in MSC-treated rats (24.45 ± 6.23%) compared with PBS controls (56.00 ± 8.72%) (p < 0.05) (Fig. 5L) (Table 4).
Table 4.
Histological Findings in Rats Treated With MSCs or PBS 1 Day After Transplantation
| MSC (n = 3) | PBS (n = 3) | |
|---|---|---|
| Number | ||
| Total Iba-1+ cells (/mm3) | 9233.09 ± 787.58 | 11713.78 ± 1326.04 |
| Ramified cells (/mm3) | 6602.62 ± 683.58* | 3117.09 ± 627.06 |
| Ameboid cells (/mm3) | 2630.47 ± 851.50 | 8596.69 ± 1743.22* |
| Proportion | ||
| Ramified cells (%) | 75.55 ± 6.23* | 44.00 ± 8.72 |
| Ameboid cells (%) | 24.45 ± 6.23 | 56.00 ± 8.72* |
Values are mean ± SE. MSC, mesenchymal stem cell; PBS, phosphate-buffered saline; Iba-1, ionized calcium binding adaptor molecule-1.
p < 0.05 between groups by independent t-test.
DISCUSSION
The most common method to deliver cells to SCI in animal models is direct transplantation into the injured lesion, which allows a delivery of a larger number of cells (17,21,30). However, the method of intralesional injection harbors a risk of further injuring the spinal cord (31). Noninvasive or less invasive methods of cell delivery, therefore, should be developed for clinical application. The intravenous administration method can decrease the risk and perform serial transplantation over a planned design (31). Recently, intravenous transplantation of MSCs has been shown to home to the injured site and enhance functional recovery in SCI (28,42), cerebral ischemia (18,48), and Parkinson’s disease (33,46). As a beneficial source, MSC is able to increase neurotrophic factors such as BDNF and NT-3 (2,28) and has an immunomodulatory effect that blocks a secondary injury cascade (27). Intravenous administration will be an ideal and preferable minimally invasive method to deliver cell transplants for clinical translation if the therapeutic effect is superior or at least similar to direct intralesional injection.
Previous studies demonstrated that grafted cells even survived for at least several weeks following intravenous transplantation of MSCs in animal models of SCI (19,38). In clinical trials, intravenous transplantation of autologous bone marrow stem cells has been performed in SCI patients (5,37). However, published data show a discrepancy in detection of bone marrow stromal cells or neural stem cells after intravenous administration. In contrast to the effects seen following the intravenous administration of MSCs (19,38), few grafted cells were detected at the site of injury after the intravenous transplantation in other studies (3,10,31,39,45). Grafted cells were primarily trapped in the lung, and then secondarily in the spleen, liver, and kidney (11,15,39). In this study, we detected the human Alu gene in the lung and spleen after intravenous administration of MSCs, which was similar to previous results. The presence of foreign cells can also induce a systemic immune response, resulting in elevated levels of inflammatory cytokines and immune cells (31). Since intravenously delivered MSCs are vulnerable to immune cells circulating in the blood, elimination of MSCs by the host immune system might explain the markedly lower efficiency of engraftment at the site of SCI compared with direct intralesional administration (10). In our study, only a few cells differentiated into oligodendrocytes 4 weeks after transplantation, but they did not survive thereafter. These results suggest that MSCs that immediately homed to the injured site on the spinal cord did not survive for a long term in the hostile environment of damaged host tissue (13,31,39).
In behavioral and electrophysiological tests of this study, MSC-treated rats showed a significant improvement of Δ 2.5 score in BBB locomotor function, and Δ 36.8 ms in P1 latency of SSEPs relative to those of the PBS controls. In respect to the previous study using the same type of MSCs derived from hUCB (7), functional recovery after intravenous delivery was found to be similar or slightly better than intralesional injection in SCI rats. It suggests that the method of direct injection into the parenchyma may further damage already compromised tissue, even though it allows a delivery of a large number of stem cells (31). In addition, the human-specific element was present only for a short while, and it was not detected at the time when the locomotor function was significantly improved compared to the PBS controls. Thus, the positive effect derived from intravenous transplantation cannot be ascribed to cell replacement and substitution of damaged tissue (4). MSC-treated rats also showed a comparative motor recovery in a relatively early stage, 2 weeks after transplantation, demonstrating that treatment during acute phase after SCI has a major influence on the final functional recovery. Therefore, we investigated exogenous neurotrophic factors released by transplanted MSCs and endogenous neurotrophic factors stimulated by MSCs as a possible therapeutic mechanism based on a previous study (29). However, we did not detect any expression of human-specific neurotrophins in both groups. Neither did we find any difference in the expression of rat-derived neurotrophic factors between the groups. It seems that there were no viable MSCs releasing a variety of neurotrophic factors or stimulating adjacent cells to produce neurotrophic factors in the injured spinal cord, although human Alu was detected 1 h after transplantation.
Finally, we evaluated exogenous inflammation-related cytokines released from hUCB-MSCs, and endogenous cytokines released from the microglia stimulated by MSCs in the injured spinal cord. Whereas IL-6 was largely secreted by MSCs under in vitro condition, human-specific inflammatory cytokines released from grafted MSCs were not detected after intravenous transplantation, suggesting that temporary presence of MSCs in the damaged tissue could not change the in vivo level of anti-inflammatory cytokines. On the other hand, an endogenous anti-inflammatory cytokine, rat IL-10, increased in MSC-treated rats, especially until 2 days after transplantation. On the contrary, proinflammatory cytokine, IL-1β, levels were modestly decreased after transplantation of MSCs during the same period.
While IL-6 is originally considered as a proinflammatory cytokine and a B cell differentiation factor (44), it also has anti-inflammatory characteristics as well (43). Namely, IL-6 inhibits activation of T cells and differentiation of monocytes and dendritic cells (12,23). It also regulates growth and differentiation of immune cells by inhibiting IL-1β and TNF-α (43). In our study, intravenous administration of MSCs immediately suppresses IL-6. The MSC-induced IL-6 expression, thereafter, increased. Delayed response of IL-6 may inhibit TNF-α expression 3 days after transplantation as described by Aderka et al. (1). These results suggest the existence of a balanced network of proinflammatory cytokines and anti-inflammatory cytokines after transplantation of MSCs, which was determined by the response of resident cells at a particular time point after SCI. In a previous in vitro experiment, we demonstrated that MSCs derived from hUCB effectively suppressed mitogen-induced T-cell proliferation and reduced the levels of interferon (IFN)-γ and TNF-α produced by activated T-cells (50).
In this in vivo study, we found that intravenous transplantation of MSCs suppressed activated microglia and inflammatory cytokines, increased anti-inflammatory cytokines, and consequently promoted functional recovery in SCI rats, even though we could not detect any difference in human-specific inflammatory factors or neurotrophic factors between the MSCs-treated animals and controls. As underlying mechanisms of therapeutic effects, we suggest that grafted MSCs in acute phase can alter cytokines secreted from the host immune cells such as microglia and macrophages (22). Although this study did not demonstrate a significant difference in the area of cavitary lesion between MSC-treated rats and PBS controls, intravenous transplantation of MSCs might modulate local signals from these immune cells, reducing further prominent damage of the spinal cord in the animals (49).
In a future study, to overcome limitations of this study, intravenous transplantation of autologous MSCs rather than human MSCs should be tried, because intra-peritoneal injection of cyclosporine A might be insufficient to suppress undesirable immune response. Delayed transplantation of MSCs 1 week after SCI rather than immediate transplantation should be also tried, because the hostile environment immediately after injury has a lot of secondary tissue damages and no clinical relevance as sufficient cells would be available for treatment so soon.
Taken together, our data confirmed the established link between microglial activation and inflammatory cytokines, and demonstrated that functional recovery might be attributed to immunomodulatory effects rather than cell replacement itself. In conclusion, early attenuation and modulation of excessive inflammatory reactions by intravenously transplanted MSCs could mediate functional recovery in chronic phase after SCI.
ACKNOWLEDGMENTS
We give thanks to Bae Hwan Lee for electrophysiological study. This study was supported by grants from Yonsei University College of Medicine (6-2011-0078), Stem Cell Research Center of the 21st Century Frontier Research Program (SC-4160) and National Research Foundation (NRF-2010-0020408; 2010-0024334) funded by the Ministry of Science and Technology, Republic of Korea. The authors declare no conflicts of interest.
REFERENCES
- 1. Aderka D.; Le J. M.; Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J. Immunol. 143:3517–3523; 1989. [PubMed] [Google Scholar]
- 2. Bao X.; Wei J.; Feng M.; Lu S.; Li G.; Dou W.; Ma M.; Ma S.; An Y.; Qin C.; Zhao R. C.; Wang R. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 1367:103–113; 2011. [DOI] [PubMed] [Google Scholar]
- 3. Barbash I. M.; Chouraqui P.; Baron J.; Feinberg M. S.; Etzion S.; Tessone A.; Miller L.; Guetta E.; Zipori D.; Kedes L. H.; Kloner R. A.; Leor J. Systemic delivery of bone marrow derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation 108(7):863–868; 2003. [DOI] [PubMed] [Google Scholar]
- 4. Bottai D.; Madaschi L.; Di Giulio A. M.; Gorio A. Viability-dependent promoting action of adult neural precursors in spinal cord injury. Mol. Med. 14(9–10):634–644; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chernykh E. R.; Stupak V. V.; Muradov G. M.; Sizikov M. Y.; Shevela E. Y.; Leplina O. Y.; Tikhonova M. A.; Kulagin A. D.; Lisukov I. A.; Ostanin A. A.; Kozlov V. A. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull. Exp. Biol. Med. 143(4):543–547; 2007. [DOI] [PubMed] [Google Scholar]
- 6. Cho S. R.; Kim Y. R.; Kang H. S.; Yim S. H.; Park C. I.; Min Y. H.; Lee B. H.; Shin J. C.; Lim J. B. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone barrow in a rat model of spinal cord injury. Cell Transplant. 18(12):1359–1368; 2009. [DOI] [PubMed] [Google Scholar]
- 7. Cho S. R.; Yang M. S.; Yim S. H.; Park J. H.; Lee J. E.; Eom Y. W.; Jang I. K.; Kim H. E.; Park J. S.; Kim H. O.; Lee B. H.; Park C. I.; Kim Y. J. Neurally induced umbilical cord blood cells modestly repair injured spinal cords. Neuroreport 19(13):1259–1263; 2008. [DOI] [PubMed] [Google Scholar]
- 8. Corcione A.; Benvenuto F.; Ferretti E.; Giunti D.; Cappiello V.; Cazzanti F.; Risso M.; Gualandi F.; Mancardi G. L.; Pistoia V.; Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood 107(1):367–372; 2006. [DOI] [PubMed] [Google Scholar]
- 9. Davies A. L.; Hayes K. C.; Dekaban G. A. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 88(11):1384–1393; 2007. [DOI] [PubMed] [Google Scholar]
- 10. De Haro J.; Zurita M.; Ayllón L.; Vaquero J. Detection of 111In-oxine-labeled bone marrow stromal cells after intravenous or intralesional administration in chronic paraplegic rats. Neurosci. Lett. 377(1):7–11; 2005. [DOI] [PubMed] [Google Scholar]
- 11. Detante O.; Moisan A.; Dimastromatteo J.; Richard M. J.; Riou L.; Grillon E.; Barbier E.; Desruet M. D.; De Fraipont F.; Segebarth C.; Jaillard A.; Hommel M.; Ghezzi C.; Remy C. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: In vivo imaging and biodistribution. Cell Transplant. 18(12):1369–1379; 2009. [DOI] [PubMed] [Google Scholar]
- 12. Djouad F.; Charbonnier L. M.; Bouffi C.; Louis-Plence P.; Bony C.; Apparailly F.; Cantos C.; Jorgensen C.; Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25(8):2025–2032; 2007. [DOI] [PubMed] [Google Scholar]
- 13. Donnelly D.; Popovich P. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 212:337–388; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feldmann M.; Brennan F. M.; Maini R. N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 14:397–440; 1996. [DOI] [PubMed] [Google Scholar]
- 15. Gao J.; Dennis J. E.; Muzic R. F.; Lundberg M.; Caplan A. I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169(1):12–20; 2001. [DOI] [PubMed] [Google Scholar]
- 16. Hamamoto Y.; Ogata T.; Morino T.; Hino M.; Yamamoto H. Real-time direct measurement of spinal cord blood flow at the site of compression: Relationship between blood flow recovery and motor deficiency in spinal cord injury. Spine 32:1955–1962; 2007. [DOI] [PubMed] [Google Scholar]
- 17. Himes B. T.; Neuhuber B.; Coleman C.; Kushner R.; Swanger S. A.; Kopen G. C.; Wagner J.; Shumsky J. S.; Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil. Neural Repair 20(2):278–296; 2006. [DOI] [PubMed] [Google Scholar]
- 18. Horita Y.; Honmou O.; Harada K.; Houkin K.; Hamada H.; Kocsis J. D. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J. Neurosci. Res. 84(7):1495–1504; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jendelova P.; Herynek V.; Urdzikova L.; Glogarova K.; Kroupova J.; Andersson B.; Bryja V.; Burian M.; Hajek M.; Sykova E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J. Neurosci. Res. 76:232–243; 2004. [DOI] [PubMed] [Google Scholar]
- 20. Jiang X. X.; Zhang Y.; Liu B.; Zhang S. X.; Wu Y.; Yu X. D.; Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105(10):4120–4126; 2005. [DOI] [PubMed] [Google Scholar]
- 21. Karimi-Abdolrezaee S.; Eftekharpour E.; Wang J.; Morshead C. M.; Fehlings M. G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 26(13):3377–3389; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim Y. J.; Park H. J.; Lee G.; Bang O. Y.; Ahn Y. H.; Joe E.; Kim H. O.; Lee P. H. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 57(1):13–23; 2009. [DOI] [PubMed] [Google Scholar]
- 23. Kode J. A.; Mukherjee S.; Joglekar M. V.; Hardikar A. A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 11(4):377–391; 2009. [DOI] [PubMed] [Google Scholar]
- 24. Le Blanc K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy 8(6):559–561; 2006. [DOI] [PubMed] [Google Scholar]
- 25. Lee M. W.; Choi J.; Yang M. S.; Moon Y. J.; Park J. S.; Kim H. C.; Kim Y. J. Mensenchymal stem cells from cryopreserved human umbilical cord blood. Biochem. Biophys. Res. Commun. 320:273–278; 2004. [DOI] [PubMed] [Google Scholar]
- 26. Ling E. A.; Dahlström A.; Polinsky R. J.; Nee L. E.; McRae A. Studies of activated microglial cells and macrophages using Alzheimer’s disease cerebrospinal fluid in adult rats with experimentally induced lesions. Neuroscience 51(4):815–825; 1992. [DOI] [PubMed] [Google Scholar]
- 27. Ohtaki H.; Ylostalo J. H.; Foraker J. E.; Robinson A. P.; Reger R. L.; Shioda S.; Prockop D. J. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc. Natl. Acad. Sci. USA 105(38):14638–14643; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osaka M.; Honmou O.; Murakami T.; Nonaka T.; Houkin K.; Hamada H.; Kocsis J. D. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 1343:226–235; 2010. [DOI] [PubMed] [Google Scholar]
- 29. Park W. B.; Kim S. Y.; Lee S. H.; Kim H. W.; Park J. S.; Hyun J. K. The effect of mesenchymal stem cell transplantation on the recovery of bladder and hindlimb function after spinal cord contusion in rats. BMC Neurosci. 11:119; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parr A. M.; Kulbatski I.; Tator C. H. Transplantation of adult rat spinal cord stem/ progenitor cells for spinal cord injury. J. Neurotrauma 24(5):835–845; 2007. [DOI] [PubMed] [Google Scholar]
- 31. Paul C.; Samdani A. F.; Betz R. R.; Fischer I.; Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: A comparison of delivery methods. Spine 34(4):328–334; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Potas J. R.; Zheng Y.; Moussa C.; Venn M.; Gorrie C. A.; Deng C.; Waite P. M. Augmented locomotor recovery after spinal cord injury in the athymic nude rat. J. Neurotrauma 23(5):660–673; 2006. [DOI] [PubMed] [Google Scholar]
- 33. Sadan Q.; Melamed E.; Offen D. Bone marrow-derived mesenchymal stem cell therapy for neurodegenerative disease. Expert Opin. Biol. Ther. 9(12):1487–1497; 2009. [DOI] [PubMed] [Google Scholar]
- 34. Schwab M.; Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 76:319–370; 1996. [DOI] [PubMed] [Google Scholar]
- 35. Skaper S. D. Ion channels on microglia: Therapeutic targets for neuroprotection. CNS Neurol. Disord. Drug Targets 10(1):44–56; 2011. [DOI] [PubMed] [Google Scholar]
- 36. Steinman L. Multiple sclerosis: A coordinated immunological attack against myelin in the central nervous system. Cell 85(3):299–302; 1996. [DOI] [PubMed] [Google Scholar]
- 37. Syková E.; Homola A.; Mazanec R.; Lachmann H.; Konrádová S. L.; Kobylka P.; Pár R.; Neuwirth J.; Komrska V.; Vávra V.; Stulík J.; Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 15(8–9):675–687; 2006. [DOI] [PubMed] [Google Scholar]
- 38. Syková E.; Jendelova P. Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ. 14:1336–1342; 2007. [DOI] [PubMed] [Google Scholar]
- 39. Takahashi Y.; Tsuji O.; Kumagai G.; Hara C. M.; Okano H. J.; Miyawaki A.; Toyama Y.; Okano H.; Nakamura M. Comparative study of methods for administering neural stem/progenitor cells to treat spinal cord injury in mice. Cell Transplant. 20(5):727–739; 2011. [DOI] [PubMed] [Google Scholar]
- 40. Tasso R.; Pennesi G. When stem cells meet immunoregulation. Int. Immunopharmacol. 9(5):596–598; 2009. [DOI] [PubMed] [Google Scholar]
- 41. Uccelli A.; Moretta L.; Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8(9):726–736; 2008. [DOI] [PubMed] [Google Scholar]
- 42. Urdzíková L.; Jendelová P.; Glogarová K.; Burian M.; Hájek M.; Syková E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J. Neurotrauma 23(9):1379–1391; 2006. [DOI] [PubMed] [Google Scholar]
- 43. Vallejo R.; Tilley D. M.; Vogel L.; Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 10(3):167–184; 2010. [DOI] [PubMed] [Google Scholar]
- 44. Van Damme J.; Cayphas S.; Van Snick J.; Conings R.; Put W.; Lenaerts J. P.; Simpson R. J.; Billiau A. Purification and characterization of human fibroblast-derived hybridoma growth factor identical to T-cell-derived B-cell stimulatory factor-2 (interleukin-6). Eur. J. Biochem. 168(3):543–550; 1987. [DOI] [PubMed] [Google Scholar]
- 45. Vaquero J.; Zurita M.; Oya S.; Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: Systemic or local administration? Neurosci. Lett. 398(1–2):129–134; 2006. [DOI] [PubMed] [Google Scholar]
- 46. Wang F.; Yasuhara T.; Shingo T.; Kameda M.; Tajiri N.; Yuan W. J.; Kondo A.; Kadota T.; Baba T.; Tayra J. T.; Kikuchi Y.; Miyoshi Y.; Date I. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: Focusing on neuro-protective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 11:52; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wrathall J.; Teng Y.; Marriott R. Delayed antagosism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp. Neurol. 145:565–573; 1997. [DOI] [PubMed] [Google Scholar]
- 48. Wu J.; Sun Z.; Sun H. S.; Wu J.; Weisel R. D.; Keating A.; Li Z. H.; Feng Z. P.; Li R. K. Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant. 16(10):993–1005; 2007. [DOI] [PubMed] [Google Scholar]
- 49. Yagi H.; Soto-Gutierrez A.; Parekkadan B.; Kitagawa Y.; Tompkins R. G.; Kobayashi N.; Yarmush M. L. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 19(6):667–679; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoo K. H.; Jang I. K.; Lee M. W.; Kim H. E.; Yang M. S.; Eom Y.; Lee J. E.; Kim Y. J.; Yang S. K.; Jung H. L.; Sung K. W.; Kim C. W.; Koo H. H. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell. Immunol. 259(2):150–156; 2009. [DOI] [PubMed] [Google Scholar]