Abstract
Background
We evaluated ultrasonography variables associated with the improvement of nocturia after administration of alpha adrenoceptor antagonist (alpha blocker) monotherapy.
Methods
From February to October 2014, 679 men with lower urinary tract symptoms (LUTS) underwent ultrasonography including prostate volume, transitional zone volume, prostatic urethral length, the ratio between prostatic urethral length and prostate volume (RPUL), intravesical prostatic protrusion (IPP), and prostatic urethral angle (PUA). Among them, 108 men who had pre-treatment nocturia without nocturnal polyuria (nocturnal polyuria index < 33%) and were treated with alpha blocker monotherapy over 3 months were enrolled. Patients were divided into the improved (< 2 times of nocturia) and non-improved group (more than 2 times) after administration of alpha blockers. Along with ultrasonography, international prostate symptom score (IPSS) and uroflowmetry was assessed.
Results
After alpha blocker treatment, 25.0% of patients (27/108) showed improvement of nocturia. These patients were significantly younger (59.6 vs 68.0 years, P = < 0.001) with lower PUA (31.8 vs. 39.4°, P = 0.009) compared with the non-improved group. In ROC analysis, the area under the curve using the PUA was 0.653 (95% CI = 0.532–0.774, P = 0.018). Using 33.5° as a cut-off level, the sensitivity and specificity for predicting the improvement of nocturia after medication reached 67.9% and 55.6%, respectively. Patients with lower PUA (PUA < 33.5°) had more improvement of nocturia (36.6 vs. 17.9%, P = 0.030), lower IPSS score (14.2 vs. 18.3, P = 0.005), and better quality of life index (3.1 vs 3.8, P = 0.021).
Conclusions
In the patients with lower PUA (particularly lower than 33.5°), nocturia was improved by administration of alpha blocker monotherapy.
Keywords: Adrenergic alpha-antagonists, Benign prostatic hyperplasia, Nocturia, Prostate
1. Introduction
Nocturia, which is usually included as a lower urinary tract symptom (LUTS), is a common cause of an adult sleep disorder (e.g., obstructive sleep apnea, enuresis). A recent meta-analysis suggests that the prevalence rate of nocturia was 11–43.9% in younger persons (i.e., 20–40 years) and 68.9–93% in older persons (i.e., > 70 years).1 Nocturia is not a simple LUTS; it is a multifactorial condition with many contributing etiological factors. It has four major underlying causes: global polyuria, nocturnal polyuria, bladder storage disorders, or mixed etiology.2 Nocturia is often associated with men with benign prostatic hyperplasia (BPH).3 The effect of an alpha adrenoceptor antagonist (i.e., an alpha blocker) on nocturia was demonstrated in patients with BPH. It may reduce residual urine and thus increase the room for nocturnal urine storage.4 However, the improvement in nocturia is clinically marginal, poorly sustained, and depends on the patients.5
We hypothesized that individual differences in the effect of an alpha blocker can be attributed to structural differences of the prostate. To provide an integral description of individual differences in the prostate, we evaluated ultrasonography variables associated with the improvement of nocturia after the administration of alpha blocker monotherapy.
2. Materials and methods
2.1. Patient enrollment
This multicenter cross-sectional study was conducted using the same protocol in five tertiary care hospitals in the Daegu area (Dongguk University Kyeongju Hospital, Keimyung University Dongsan Medical Center, Kyungpook National University Hospital, Kyungpook National University Medical Center, Yeungnam University Medical Center, Daegu, Korea), after the approval of the local Institutional Review Board (approval number, 13-0496-O82). Six hundred and seventy-nine men were examined from February 2014 to October 2014. Of these, 108 men were included in this study who had pretreatment nocturia (defined as ≥ 2 awakenings at night to void) and were treated with alpha blockers monotherapy (i.e., tamsulocin, doxazocin, alfuzocin, terazocin, naftopidil, or silodocin) for > 3 months (average, 11.5 months; range, 3–102 months). However, patients with nocturnal polyuria (i.e., nocturnal polyuria index > 33%) were excluded from this study. Other exclusion criteria were as follows: presence of an indwelling urinary catheter, previous prostate surgery or pelvic radiation, urethral stricture, inflammation of urinary tract, prostate or bladder cancer, and neurogenic bladder disease. Patients were divided by the improved group (i.e., < 2 episodes of nocturia) and the nonimproved group (i.e., ≥ 2 episodes of nocturia) after the administration of alpha blockers.
2.2. Parameter measurements
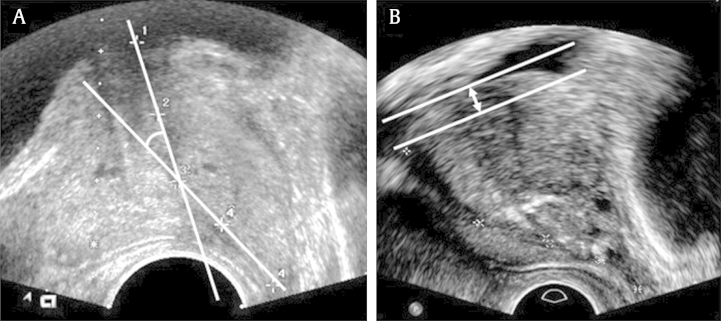
Detailed previous medical history, particularly BPH medication (e.g., use of alpha blockers, 5-α reductase inhibitors (5ARIS), phosphodiesterase inhibitors, antimuscarinic agents, and desmopressin) at the time of evaluation and previous admission or BPH-related surgical history was obtained from all enrolled patients. We reviewed the degree of LUTS at pretreatment by using clinical records of the international prostate symptom score (IPSS) and voiding diary. At post-treatment, routine subjective assessment of the degree of LUTS was performed using the IPSS and voiding diary, and objective assessment was performed using uroflowmetry and transrectal ultrasonography examination. Along with total IPSS, scores divided according to obstructive symptoms (Questionnaires 1, 3, 5, and 6), irritative symptoms (Questionnaires 2, 4, and 7), and quality of life (Questionnaire 8) were also utilized as a separate clinical indicator. Bothersome voiding symptoms, particularly the presence of nocturia were also evaluated. After treatment with alpha blocker monotherapy, uroflowmetry was performed in the usual manner. The amount of post-voiding residual urine was measured using ultrasonography. Patients also underwent transrectal ultrasonography (TRUS) for the evaluation of LUTS at post-treatment. During TRUS, the prostate volume, transitional zone volume, prostatic urethral length, the ratio between prostatic urethral length and prostate volume (RPUL), intravesical prostatic protrusion (IPP), and prostatic urethral angle (PUA) were determined during a single session. The prostate and transitional zone volume were measured by calculating the maximal height, width, and length on ultrasonography (i.e., 0.52 × transverse diameter × anteroposterior diameter × cephalocaudal diameter). The prostatic urethral length was measured by the continuous tracing of the route of the urethra, which runs within the apex to the base of the prostate via the midsagittal image of ultrasonography. The measured prostatic urethral length was then utilized to describe the individual morphologic variation of the prostate [i.e., RPUL (mm/mL)]. The IPP was measured by TRUS when the bladder volume was 100–200 mL. The degree of IPP was graded by measuring from the tip of the protruding gland perpendicular to the bladder circumference at the prostate base in the midsagittal plane. The PUA is the angle formed by two rays of the proximal and distal prostatic urethra on the midsagittal plane image, and was taken with the posterior wall of the prostate positioned as flat as possible to minimize the influence of pressure from the rectal probe (Fig. 1).
Fig. 1.
Ultrasonographic measurement of prostatic urethral angle and Intravesical prostatic protrusion. (A) The prostatic urethral angle measured on the ultrasound image. (B) Intravesical prostatic protrusion measured on the ultrasound image.
2.3. Data and statistical analysis
The correlation between the improvement in nocturia after alpha blocker monotherapy and clinical parameters such as age, prostate-specific antigen (PSA), post-treatment IPSS, post-treatment uroflowmetry, and post-treatment ultrasonography variables were analyzed. Patients were divided into two groups, according to improvement in nocturia after the administration of alpha blockers. Differences in parameters between groups were assessed using the Mann–Whitney U test. A receiver operating characteristic (ROC) curve was drawn and the sensitivity and specificity of the different cut-off points for PUA were determined. The best cut-off point was chosen according to the ROC curve. The area under the curve was also calculated. The results were expressed with a 95% confidence interval (CI). Univariate and multivariate analyses performed to determine the improvement in nocturia were assessed using logistic regression analysis. Statistical analysis was performed using SPSS 21.0 for Windows software (SPSS Inc., Chicago, IL, USA). The significance level for all analyses was set at P < 0.05.
3. Results
After alpha blocker monotherapy treatment, 25.0% (27/108) of patients showed improvement in nocturia. In comparison with the nonimproved group, these patients were significantly younger (59.6 years vs. 68.0 years, P ≤ 0.001) with a lower IPSS (13.4 vs. 17.9, P = 0.008), lower storage symptom score (4.5 vs. 7.4, P ≤ 0.001), better quality of life index (3.0 vs. 3.7, P = 0.030), and higher maximum flow rate (Qmax; 16.9 mL/s vs. 11.9 mL/s; P = 0.002) at post-treatment. On TRUS, the nocturia group had a lower PUA (31.8° vs. 39.4°, P = 0.009; Table 1).
Table 1.
Clinical parameters based on the improvement in nocturia after the use of alpha blockers.
| Nonimproved group |
Improved group |
P | |
|---|---|---|---|
| (n = 81) | (n = 27) | ||
| Age (y) | 68.0 ± 9.4 | 59.6 ± 9.9 | < 0.001 |
| Prostate-specific antigen (ng/mL) | 1.8 ± 2.1 | 2.2 ± 2.7 | 0.416 |
| Transrectal ultrasonography | |||
| Total prostate volume (mL) | 29.8 ± 15.4 | 33.1 ± 18.1 | 0.368 |
| Transition zone volume (mL) | 13.4 ± 12.2 | 14.0 ± 13.4 | 0.831 |
| Urethral length (cm) | 4.0 ± 0.6 | 4.0 ± 0.7 | 0.764 |
| RPUL (mm/mL) | 1.5 ± 0.5 | 1.4 ± 0.4 | 0.102 |
| PUA (°) | 39.4 ± 12.6 | 31.8 ± 12.3 | 0.009 |
| IPP (cm) | 0.17 ± 0.4 | 0.21 ± 0.5 | 0.665 |
| Post-treatment international prostate symptom score | |||
| Total score | 17.9 ± 7.9 | 13.4 ± 5.2 | 0.008 |
| Voiding symptom score | 10.5 ± 5.7 | 9.0 ± 4.0 | 0.133 |
| Storage symptom score | 7.4 ± 3.3 | 4.5 ± 2.2 | < 0.001 |
| Quality of life index | 3.7 ± 1.4 | 3.0 ± 1.4 | 0.030 |
| Post-treatment uroflowmetry | |||
| Qmax. (mL/s) | 11.9 ± 6.5 | 16.9 ± 6.6 | 0.002 |
| Voiding volume (mL) | 205.8 ± 126.8 | 254.5 ± 185.1 | 0.129 |
| Postvoid residual volume (mL) | 45.4 ± 54.7 | 30.3 ± 42.6 | 0.195 |
IPP, intravesical prostatic protrusion; PUA, prostatic urethral angle; Qmax, maximum flow rate; RPUL, the ratio between prostatic urethral length and prostate volume.
In univariate logistic analysis, age and the PUA were significantly associated with presence of nocturia (P ≤ 0.001 and P ≤ 0.010, respectively). In multivariate analysis, age and the PUA were also significantly associated with nocturia (P = 0.001 and P = 0.021, respectively; Table 2).
Table 2.
Logistic regression analysis used to determine the factors that predict improvement in nocturia.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age (y) | 1.095 (1.041–1.095) | < 0.001 | 1.094 (1.035–1.155) | 0.001 |
| Transrectal ultrasonography | ||||
| Total prostate volume (mL) | 0.989 (0.964–1.014) | 0.372 | 0.999 (0.882–1.132) | 0.989 |
| Transition zone volume (mL) | 0.996 (0.963–1.031) | 0.829 | 1.019 (0.902–1.152) | 0.762 |
| Urethral length (cm) | 1.116 (0.567–2.197) | 0.750 | 0.889 (0.218–3.632) | 0.870 |
| RPUL (mm/mL) | 2.348 (0.839–6.576) | 0.287 | 3.955 (0.419–37.343) | 0.230 |
| PUA (°) | 1.055 (1.013–1.099) | 0.010 | 1.059 (1.008–1.111) | 0.021 |
| IPP (cm) | 0.784 (0.263–2.334) | 0.662 | 0.674 (0.159–2.860) | 0.593 |
CI, confidence interval; IPP, intravesical prostatic protrusion; PUA, prostatic urethral angle; RPUL, the ratio between prostatic urethral length and prostate volume.
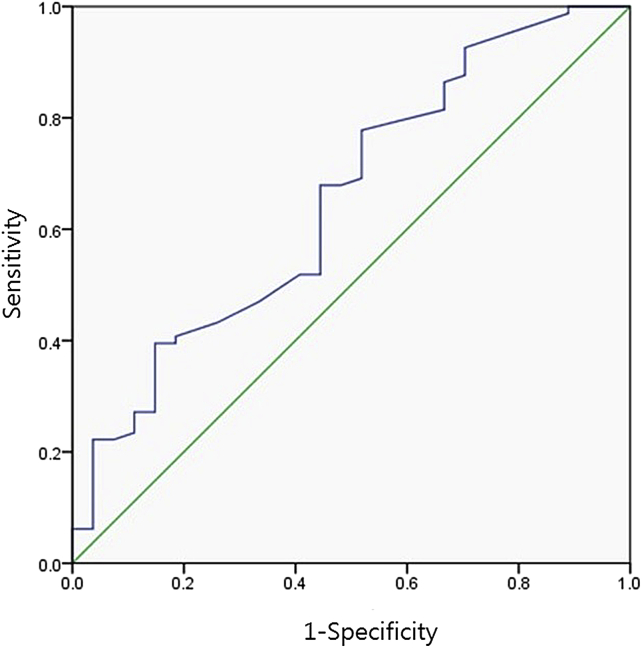
In ROC analysis, the area under the curve using the PUA was 0.653 [95% CI, 0.532–0.774; P = 0.018; Fig. 2]. Using 33.5° as the cut-off level, the sensitivity and specificity for predicting the improvement of nocturia after medication reached 67.9% and 55.6%, respectively.
Fig. 2.
The graph shows the receiver operating characteristic curves for prostatic urethral angle (AUC = 0.653, P = 0.018). AUC, area under the curve.
Patients with lower PUA (i.e., < 33.5°) had more improvement in nocturia [36.6% vs. 17.9%, P = 0.030], compared to patients with a higher PUA (i.e., ≥ 33.5°). On the post-treatment IPSS, patients with a lower PUA had a lower total IPSS score (14.2 vs. 18.3, P = 0.005), lower voiding symptom score (8.6 vs. 11.0, P = 0.025), lower storage symptom score (5.6 vs. 7.3, P = 0.006), and better quality of life index (3.1 vs. 3.8, P = 0.021) (Table 3).
Table 3.
Clinical parameters based on a prostatic urethral angle of 33.5°.
| PUA < 33.5° |
PUA ≥ 33.5° |
P | |
|---|---|---|---|
| (n = 41) | (n = 67) | ||
| Improvement in nocturia (%) | 36.6 | 17.9 | 0.030 |
| Age (y) | 63.8 ± 11.0 | 67.2 ± 9.6 | 0.094 |
| Prostate-specific antigen (ng/mL) | 1.6 ± 2.0 | 2.2 ± 2.3 | 0.167 |
| Transrectal ultrasonography | |||
| Total prostate volume (mL) | 29.8 ± 15.4 | 31.2 ± 16.6 | 0.667 |
| Transition zone volume (mL) | 12.7 ± 12.5 | 14.0 ± 12.5 | 0.599 |
| Urethral length (cm) | 3.8 ± 0.5 | 4.1 ± 0.7 | 0.004 |
| IPP (cm) | 0.1 ± 0.3 | 0.2 ± 0.4 | 0.082 |
| Post-treatment international prostate symptom score | |||
| Total score | 14.2 ± 7.6 | 18.3 ± 7.1 | 0.005 |
| Voiding symptom score | 8.6 ± 5.4 | 11.0 ± 5.2 | 0.025 |
| Storage symptom score | 5.6 ± 3.2 | 7.3 ± 3.1 | 0.006 |
| Quality of life index | 3.1 ± 1.5 | 3.8 ± 1.3 | 0.021 |
| Post-treatment uroflowmetry | |||
| Qmax. (mL/s) | 14.1 ± 6.1 | 12.6 ± 7.2 | 0.296 |
| Voiding volume (mL) | 223.1 ± 146.4 | 214.9 ± 143.8 | 0.776 |
| Postvoid residual volume (mL) | 31.4 ± 45.6 | 47.9 ± 55.2 | 0.111 |
PUA, prostatic urethral angle; Qmax, maximum flow rate.
4. Discussion
Nocturia (i.e., nocturnal waking to void) occurs in up to 58.90% of people older than 50 years.6, 7 Its prevalence increases with age.8, 9 This condition can significantly impair a patient's perception of his or her well-being.10, 11 The International Continence Society definition of nocturia is a complaint of having to awaken once or more at night to void.2 However, the traditional definition of nocturia is a complaint of having to awaken twice or more at night to void. In a cross-sectional, community-based epidemiologic survey conducted in Korea, the mean number of nocturia episodes was 2.05 times for men with BPH and 1.04 times for men without BPH.12 Many studies on nocturia only consider patients with two or more voids per night, based on the observation that a nocturnal frequency of one void per night does not appear to be harmful or bothersome.13, 14 Therefore, in this study, nocturia was defined as awakening twice during sleep to void.
The etiology of nocturia recently included four major underlying causes: global polyuria, nocturnal polyuria, bladder storage disorders, or mixed etiology. Men with benign prostatic enlargement (BPE) often have nocturia and nocturnal polyuria.3 Benign prostatic enlargement leading to bladder outlet obstruction (BOO) clearly results in the obstructive type of voiding symptoms which comprises poor flow, hesitancy, prolonged stream, and terminal dribbling. In addition, storage symptoms are common in males in these age groups. However, as demonstrated in a cohort of 324 trial participants, urological problems were the only cause of nocturia in just 16% of patients.15 Patients with nocturia who do not have polyuria or nocturnal polyuria based on the aforementioned criteria will most likely have a bladder storage disorder that reduces their nighttime voided volume or a sleep disorder.16
One of the most pertinent aspects of the relationship between nocturia and BPE is whether successful treatment of BPE resolves nocturia. Margel et al17 report that nocturia appears to improve after transurethral resection of the prostate. Medical treatment with alpha blockers could similarly be indicated for male patients with nocturia when BPE is suspected. In a study17 with terazosin, 27% of patients reported that nocturia was reduced by more than half, and 14% reported that it was reduced by 25–49% on the frequency-volume chart. On the IPSS, 31% of patients reported that the treatment reduced their nocturia by more than half and 27% reported a reduction of 25–49%.18 However, in another trial,19, 20 the clinical significance of alpha blockers was doubted because the difference in nocturia episodes was too small between patients receiving treatment with alpha blockers and a placebo. A study with alfuzosin also reported a numerical improvement of −1.1 voids per night versus −0.8 with placebo (P = 0.04).19 In the Veterans Administration Cooperative Study, nocturia decreased from a baseline mean of 2.5 episodes to 1.8 episodes, 2.1 episodes, 2.0 episodes, and 2.1 episodes in the terazosin group, finasteride, combination group, and placebo group, respectively.20
With regard to the medical treatment of BPH, 5ARI (e.g., dutasteride and finasteride) effectively reduced the prostate volume.21 However, alpha blockers provide rapid relief of LUTS, presumably by relaxing the smooth muscle tone in the prostate and bladder neck.22 In addition, the effect of alpha blockers on the structure of the prostate remains unknown. In our study, we did not have pretreatment TRUS data.
However, we believed that alpha blocker monotherapy would not change the structure of the prostate. We also hypothesized that structural features of the prostate could predict improvement in nocturia after alpha blocker monotherapy. Therefore, we investigated ultrasonography variables to provide an integral description of the individual structural differences of the prostate. As a result of this study, the PUA was the only structural variable that could predict improvement of nocturia after treatment by alpha blockers.
The PUA is a well-known structural feature of the prostate. In a study by Ku et al,23 higher PSA levels, larger prostate volume, higher maximal urethral closure pressure, higher detrusor pressure at maximum flow rate, and higher BOO index were reported with a larger PUA. Using multivariate analysis, Bang et al24 reported that PUA has an independent association with the IPSS. Hou et al25 reported a change in symptoms after treatment with alpha blocker. In this study, the PUA had an independent association with the IPSS (P = 0.001) and Qmax (P = 0.004). After tamsulosin therapy, the PUA was associated with post-treatment IPSS change (P = 0.032) and post-treatment Qmax change (P = 0.001).
However, the relationship between the PUA and nocturia has not been reported. In the current study, patients with improved nocturia were significantly younger and had a lower PUA.
Age is a well-known factor in progression of nocturia.3 The incidence and prevalence of nocturia showed a clear increase with age. Häkkinen et al26 estimated the incidence and natural course of nocturia in an unselected Finnish male population. Every year, 10% more males older than 50 years start to void during the night. The incidence of mild nocturia increases, particularly in men aged 50–60 years. Older men with mild symptoms are more stable, but the incidence of severe nocturia increases significantly after the age of 75 years. Thus, in younger patients, a higher improvement rate of nocturia after alpha blocker monotherapy is very reasonable.
However, the relationship between the PUA and nocturia is meaningful. The value of the PUA is also confirmed by univariate analysis and multivariate analysis. In this report, we used a PUA of 33.5° as the cut-off level. We then determined that patients with a lower PUA had lower incidences of nocturia, lower total IPSS score, and better quality of life index. The cut-off level was slightly different according to the paper23, 27 (34°–35°); however, a lower PUA has generally been associated with improvement in symptoms and quality of life.23, 27 In this report, a lower PUA was also associated with an improvement in nocturia. In our opinion, a lower PUA indicates that the urethra is straighter. Relaxing smooth muscle tone by alpha blockers provides more rapid relief of LUTS in a straight urethra. Therefore, it seems that nocturia also improved more in patients with a straight urethra.
In this study, we attempted to find other ultrasonography variables for predicting improvement of nocturia after alpha blocker monotherapy. Benign prostatic enlargement, which is a well-recognized feature of male aging, is commonly associated with LUTS. However, opinions are divided on whether prostatic enlargement has a causal relationship with storage symptoms such as nocturia.28 In this paper, the total prostate volume and transition zone volume did not show a statistical difference between the improved group and the nonimproved group (P = 0.368 and P = 0.831, respectively). The RPUL is the relationship between the whole prostate and the prostate urethra. There are multitudinous patterns that consequently produce distinctive structural variation. The structural variation of the prostatic urethra within the prostate, as reflected by the ratio between prostate volume and prostatic urethral length, showed a correlation with the degree of LUTS.29 We believe that the change in the urethra within the prostate due to the enlargement of the gland—instead of the prostate volume itself—may cause the development of nocturia. However no statistical difference was observed between the two groups (P = 0.102).
Intravesical prostatic protrusion is a useful predictor of infravesical obstruction, Qmax, acute urinary retention, and the outcomes of a trial without a catheter after acute urinary retention.30, 31, 32 In our study, IPP also showed no statistical difference between the improved group and the nonimproved group (P = 0.665).
The authors recognize several limitations of this series. First, this trial was a multicenter prospective study conducted in five clinics. We tried to minimize deviation, although there was some technical difficulty. Second, many kinds of alpha blockers were used in this trial. A few recent studies reporting the effect of a selective alpha-1D blocker, naftopidil, on nocturia concluded that naftopidil was better than tamsulosin for treating nocturia.33 However, differences between alpha blockers were not reflected in this study. Third, we only investigated patients who had nocturia without a decrease in the number of episodes after alpha blocker monotherapy. Fourth, most importantly, we did not report the change in TRUS findings before and after treatment. Further trials with a proper study design will be required to overcome these limitations and to obtain a solid answer for nocturia and anatomical variations of the prostate.
In patients who had a lower PUA (particularly lower than 33.5°), nocturia was improved by administration of alpha blocker monotherapy. These findings suggest an individualized approach in the treatment of nocturia, based on anatomical characteristics illustrated by ultrasonography.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by research fund from the Hannam Urological Association (Seoul, Korea) in 2014.
References
- 1.Bosch J.L., Weiss J.P. The prevalence and causes of nocturia. J Urol. 2010;184:440–446. doi: 10.1016/j.juro.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 2.van Kerrebroeck P., Abrams P., Chaikin D., Donovan J., Fonda D., Jackson S. The standardization of terminology in nocturia: report from the standardization subcommittee of the International Continence Society. BJU Int. 2002;90(Suppl 3):11–15. doi: 10.1046/j.1464-410x.90.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Blanker M.H., Bohnen A.M., Groeneveld F.P., Bernsen R.M., Prins A., Ruud Bosch J.L. Normal voiding patterns and determinants of increased diurnal and nocturnal voiding frequency in elderly men. J Urol. 2000;164:1201–1205. [PubMed] [Google Scholar]
- 4.Park H.K., Kim H.G. Current evaluation and treatment of nocturia. Korean J Urol. 2013;54:492–498. doi: 10.4111/kju.2013.54.8.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koseoglu H., Aslan G., Ozdemir I., Esen A. Nocturnal polyuria in patients with lower urinary tract symptoms and response to alpha-blocker therapy. Urology. 2006;67:1188–1192. doi: 10.1016/j.urology.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Homma Y., Yamaguchi O., Hayashi K. Neurogenic Bladder Society C. Epidemiologic survey of lower urinary tract symptoms in Japan. Urology. 2006;68:560–564. doi: 10.1016/j.urology.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 7.van Kerrebroeck P., Abrams P., Chaikin D., Donovan J., Fonda D., Jackson S. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:179–183. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
- 8.Britton J.P., Dowell A.C., Whelan P. Prevalence of urinary symptoms in men aged over 60. British J Urol. 1990;66:175–176. doi: 10.1111/j.1464-410x.1990.tb14898.x. [DOI] [PubMed] [Google Scholar]
- 9.Homma Y. Classification of nocturia in the adult and elderly patient: a review of clinical criteria and selected literature. BJU Int. 2005;96(Suppl 1):8–14. doi: 10.1111/j.1464-410X.2005.05655.x. [DOI] [PubMed] [Google Scholar]
- 10.Asplund R., Aberg H. Health of the elderly with regard to sleep and nocturnal micturition. Scand J Prim Health Care. 1992;10:98–104. doi: 10.3109/02813439209014044. [DOI] [PubMed] [Google Scholar]
- 11.Miranda Ede P., Gomes C.M., Torricelli F.C., de Bessa J., Jr., de Castro J.E., Ferreira B.R. Nocturia is the lower urinary tract symptom with greatest impact on quality of life of men from a community setting. Int Neurourol J. 2014;18:86–90. doi: 10.5213/inj.2014.18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh H.J., Kim S.A., Nam J.W., Choi B.Y., Moon H.S. Community-based research on the benign prostatic hyperplasia prevalence rate in Korean rural area. Korean J Urol. 2015;56:68–75. doi: 10.4111/kju.2015.56.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jolleys J.V., Donovan J.L., Nanchahal K., Peters T.J., Abrams P. Urinary symptoms in the community: how bothersome are they? British J Urol. 1994;74:551–555. doi: 10.1111/j.1464-410x.1994.tb09182.x. [DOI] [PubMed] [Google Scholar]
- 14.Weiss J.P., Blaivas J.G., Stember D.S., Brooks M.M. Nocturia in adults: etiology and classification. Neurourol Urodyn. 1998;17:467–472. doi: 10.1002/(sici)1520-6777(1998)17:5<467::aid-nau2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Klingler H.C., Heidler H., Madersbacher H., Primus G. Nocturia: an Austrian study on the multifactorial etiology of this symptom. Neurourol Urodyn. 2009;28:427–431. doi: 10.1002/nau.20665. [DOI] [PubMed] [Google Scholar]
- 16.Franche C., Bogusz D., Schopke C., Fauquet C., Beachy R.N. Transient gene expression in cassava using high-velocity microprojectiles. Plant Mol Biol. 1991;17:493–498. doi: 10.1007/BF00040643. [DOI] [PubMed] [Google Scholar]
- 17.Margel D., Lifshitz D., Brown N., Lask D., Livne P.M., Tal R. Predictors of nocturia quality of life before and shortly after prostatectomy. Urology. 2007;70:493–497. doi: 10.1016/j.urology.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Paick J.S., Ku J.H., Shin J.W., Yang J.H., Kim S.W. alpha-Blocker monotherapy in the treatment of nocturia in men with lower urinary tract symptoms: a prospective study of response prediction. BJU International. 2006;97:1017–1023. doi: 10.1111/j.1464-410X.2006.06075.x. [DOI] [PubMed] [Google Scholar]
- 19.Roehrborn C.G., Van Kerrebroeck P., Nordling J. Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies. BJU Int. 2003;92:257–261. doi: 10.1046/j.1464-410x.2003.04309.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson T.M., 2nd, Jones K., Williford W.O., Kutner M.H., Issa M.M., Lepor H. Changes in nocturia from medical treatment of benign prostatic hyperplasia: secondary analysis of the Department of Veterans Affairs Cooperative Study Trial. J Urol. 2003;170:145–148. doi: 10.1097/01.ju.0000069827.09120.79. [DOI] [PubMed] [Google Scholar]
- 21.Nickel J.C., Gilling P., Tammela T.L., Morrill B., Wilson T.H., Rittmaster R.S. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS) BJU Int. 2011;108:388–394. doi: 10.1111/j.1464-410X.2011.10195.x. [DOI] [PubMed] [Google Scholar]
- 22.Silva J., Silva C.M., Cruz F. Current medical treatment of lower urinary tract symptoms/BPH: do we have a standard? Curr Opin Urol. 2014;24:21–28. doi: 10.1097/MOU.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 23.Ku J.H., Ko D.W., Cho J.Y., Oh S.J. Correlation between prostatic urethral angle and bladder outlet obstruction index in patients with lower urinary tract symptoms. Urology. 2010;75:1467–1471. doi: 10.1016/j.urology.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 24.Bang W.J., Kim H.W., Lee J.Y., Lee D.H., Hah Y.S., Lee H.H. Prostatic urethral angulation associated with urinary flow rate and urinary symptom scores in men with lower urinary tract symptoms. Urology. 2012;80:1333–1337. doi: 10.1016/j.urology.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 25.Hou C.P., Chen C.L., Lin Y.H., Tsai Y.L., Chang P.L., Juang H.H. Prostatic urethral angle might be a predictor of treatment efficacy of alpha-blockers in men with lower urinary tract symptoms. Drug Des Devel Ther. 2014;8:937–943. doi: 10.2147/DDDT.S62428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakkinen J.T., Hakama M., Shiri R., Auvinen A., Tammela T.L., Koskimaki J. Incidence of nocturia in 50 to 80-year-old Finnish men. J Urol. 2006;176:2541–2545. doi: 10.1016/j.juro.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Park Y.J., Bae K.H., Jin B.S., Jung H.J., Park J.S. Is increased prostatic urethral angle related to lower urinary tract symptoms in males with benign prostatic hyperplasia/lower urinary tract symptoms? Korean J Urol. 2012;53:410–413. doi: 10.4111/kju.2012.53.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapple C.R., Roehrborn C.G. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006;49:651–658. doi: 10.1016/j.eururo.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Ko Y.H., Song P.H. Structural variation of prostate urethra reflected by the ratio between prostate volume and prostatic urethral length is associated with the degrees of lower urinary tract symptoms. Low Urin Tract Symptoms. 2014 doi: 10.1111/luts.12083. [DOI] [PubMed] [Google Scholar]
- 30.Keqin Z., Zhishun X., Jing Z., Haixin W., Dongqing Z., Benkang S. Clinical significance of intravesical prostatic protrusion in patients with benign prostatic enlargement. Urology. 2007;70:1096–1099. doi: 10.1016/j.urology.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Kojima M., Ochiai A., Naya Y., Ukimura O., Watanabe M., Watanabe H. Correlation of presumed circle area ratio with infravesical obstruction in men with lower urinary tract symptoms. Urology. 1997;50:548–555. doi: 10.1016/S0090-4295(97)00310-5. [DOI] [PubMed] [Google Scholar]
- 32.Tan Y.H., Foo K.T. Intravesical prostatic protrusion predicts the outcome of a trial without catheter following acute urine retention. J Urol. 2003;170:2339–2341. doi: 10.1097/01.ju.0000095474.86981.00. [DOI] [PubMed] [Google Scholar]
- 33.Nishino Y., Masue T., Miwa K., Takahashi Y., Ishihara S., Deguchi T. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU Int. 2006;97:747–751. doi: 10.1111/j.1464-410X.2006.06030.x. [DOI] [PubMed] [Google Scholar]