Abstract
Phenylketonuria (PKU) is a rare metabolic disorder characterized by impaired conversion of phenylalanine (Phe) to tyrosine. If left untreated, the resultant accumulation of excess blood Phe can cause physiological, neurological, and intellectual disabilities. The National PKU Alliance (NPKUA) conducted a survey of its membership to assess current health status and interest in new treatments for PKU. Of the 625 survey respondents, less than half (46.7%) reported blood Phe within (120–360 μmol/L) — the range recommended by the American College of Medical Genetics and Genomics (ACMG). The survey results also showed that younger (≤ 18 years) individuals were about 3-times as successful in keeping their blood Phe concentrations within the recommended clinical range compared with adults. Blood Phe over 360 μmol/L was reported in one-quarter (25.5%) of ≤ 18 year old individuals and almost two-thirds (61.5%) of adults. A little more than half (51.7%) of respondents reported having difficulty in managing their PKU, including the maintenance of a Phe-restricted diet. Individuals with PKU desire new treatments that would allow them to increase their intake of natural protein, discontinue or reduce their intake of medical foods (medical formula and foods modified to be low in protein), improve their mental health (including a reduction in depression and anxiety), and a reduction of their blood Phe concentrations. Respondents preferred oral administration of any newly developed therapies and, in general, disliked therapeutic injections. Injections at home were preferred over injections at a clinic. Payers, government agencies, clinicians, and industry partners should consider patient input when developing and approving new therapies and treatments for PKU.
Abbreviations: ACMG, American College of Medical Genetics and Genomics; NPKUA, National PKU Alliance; PAH, phenylalanine hydroxylase; Phe, phenylalanine; PKU, phenylketonuria
Keywords: Phenylalanine, Phenylketonuria, Phenylalanine hydroxylase deficiency
1. Introduction
Phenylketonuria [PKU, MIM 261600, also referred to as phenylalanine hydroxylase (PAH; EC 1.14.16.1) deficiency] is a rare (prevalence < 1/10,000–1/15,000 births) autosomal recessive disorder characterized by an impairment of the body's ability to metabolize phenylalanine (Phe) [1]. Without a fully functional PAH enzyme, Phe accumulates in the blood, brain, and body tissues. High concentrations of blood Phe are toxic to the central nervous system and can cause severe neurological complications and intellectual disability [2]. The new treatment guidelines of the American College of Medical Genetics and Genomics (ACMG) state that the clinical treatment goal for individuals with PKU are to maintain blood Phe within the range of (120–360 μmol/L) for individuals of all ages and that treatment should be lifelong [2].
Newborn screening for PKU was instituted in the 1960s to prevent the most serious consequences of the disease [2], [3]. Dietary treatment [including the use of Phe-free medical foods and the avoidance of high protein foods] successfully lowers blood Phe in most individuals with PKU [4]. Pharmaceutical treatments include the use of sapropterin dihydrochloride (a synthetic form of tetrahydrobiopterin (BH4) — the natural co-factor for the PAH enzyme). Studies have shown that sapropterin dihydrochloride is well tolerated, lowers blood Phe, and improves Phe tolerance in approximately 25–50% of individuals with PKU [2], [5].
There are substantial unmet needs of individuals with PKU [6]. Lifetime adherence to a Phe-restricted diet is challenging and blood Phe is difficult to maintain within the recommended range. Even individuals who successfully manage their blood Phe from birth can exhibit subtle deficits in neuropsychological functioning [7], anxiety, depression, and executive function deficits can occur even in those individuals who are well controlled [2]. Therefore, new therapies are needed to improve the quality of life of individuals with PKU across their lifespans [8].
The National PKU Alliance (NPKUA) is a patient advocacy nonprofit organization dedicated to improving the lives of individuals with PKU and assisting in the development of a cure. Formed in 2008 by 17 state and regional support groups across the country, the NPKUA focuses on critical and unmet needs of the PKU patient community. Patient advocacy organizations (like NPKUA) are important because they disseminate reliable information about new treatments to affected individuals and their families [9]. The NPKUA provides information and support to adults and families of children with PKU, advocates for the reimbursement of Phe-free and modified low-protein foods, and invests in targeted and peer reviewed research for the development of new therapies and a future cure for PKU.
The NPKUA conducted a survey of adults and children with PKU in the United States to better understand the current and future needs of the PKU community. The goal of the survey was to help inform the NPKUA's research priorities, provide feedback from individuals to medical providers, payers, and other stakeholders, and to serve as a resource to industry to ensure the patient perspective is central to the development of new therapies.
2. Methods
The NPKUA conducted a self-selected, non-randomized patient survey during May–June 2015 that focused on current therapy status, treatment preferences, and risk tolerance for new treatments. The survey consisted of 28 questions, 21 primary questions and 7 supplemental questions that asked for either categorical, free form, or Likert-scale responses (Table 1). A link to an online survey was distributed to NPKUA members and families via e-mail, posted on the PKU listserv, posted on the NPKUA's Facebook page, and sent out via e-mail to addresses collected through the PKU.com website. If more than one child had PKU in a household, parents and caregivers were asked to respond to the survey using their experience of the oldest child with PKU.
Table 1.
National PKU Alliance (NPKUA) survey questions and question type.
| Number | Question | Response type |
|---|---|---|
| Q01 | What is your relationship to PKU? | Categorical |
| Q02 | How old are you when completing this form? | Categorical |
| Q03 | Select the statements below that reflect how you currently treat your/your loved one's PKU | Categorical |
| Q04 | When was the last time you drew blood to monitor Phe levels? | Categorical |
| Q05 | What has been the average blood Phe level in the last years? | Categorical |
| Q06 | Select preferred blood Phe level | Categorical |
| Q07 | When was the last visit to a metabolic clinic that focuses on PKU patients? | Categorical |
| Q08 | Select the statement below that best reflects your current PKU treatment status. | Categorical |
| Q08x | Explain ‘Other’ current PKU treatment status | Categorical |
| Q09 | Indicate difficulty managing PKU | Likert |
| Q09x | Explain ‘Other’ difficulty managing PKU | Free form |
| Q10 | Indicate how PKU restricts lifestyle | Likert |
| Q10x | Indicate how PKU restricts lifestyle | Free form |
| Q11 | Select statement that best reflects current PKU treatment plan | Categorical |
| Q12 | Indicate importance of having new PKU treatments | Likert |
| Q13 | What benefits do you hope to see with a new PKU treatment? | Free form |
| Q14 | What symptoms or results would you like to see with a new PKU treatment? | Categorical |
| Q14x | Explain ‘Other’ symptoms or results with new treatment | Free form |
| Q15 | Rank the symptoms or results from Question 14 | Likert |
| Q15x | Force Rank the symptoms or results from Question 14 | Likert |
| Q16 | What change in lifestyle would you like to see having a new PKU treatment? | Categorical |
| Q16x | Explain ‘Other’ change in lifestyle | Free form |
| Q17 | Rank the two top changes in lifestyle from Question 16 | Likert |
| Q17x | Force Rank the two top changes in lifestyle from Question 16 | Likert |
| Q18 | Indicate willingness to new PKU treatments | Likert |
| Q19 | Indicate willingness to tolerate side effects from a new treatment | Likert |
| Q20 | Indicate willingness to take immunosuppressant each time period | Likert |
| Q21 | Please tell us anything else you would consider in deciding to choose a new therapy for PKU | Free form |
x: Questions with an ‘x’ relate to the immediately preceding question and denote either a corollary question or a request for more information.
2.1. Statistical analysis
Response rates were computed using the number of respondents to each survey question. Some responses required the responder to self-report their data according to interval ranges, other responses used Likert-Scales, and some responses permitted free-form entries. Similar Likert-Scale responses were combined to simplify the statistics (e.g., ‘very easy’, ‘easy’, and ‘somewhat easy’ responses were combined into a single group). Information regarding gender, BMI, and highest lifetime Phe was not requested. An ANOVA test was used to compare group means using 95% confidence intervals.
3. Results
3.1. Survey response
From a pool of 6312 potential respondents, at total of 625 (9.9%) responded to the survey. Respondents included individuals with PKU (N = 220), parents of children with PKU (N = 362), other caregivers (N = 39), and others (N = 4). Of the 21 primary questions, 19 had a response rate of 74% (N = 465) or higher and 13 questions had a response rate of 96% (N = 606) or higher. Respondents were almost evenly divided by age. About half of respondents (53%, 332/622) were ≤ 18 and half (47%, 290/622 were > 18 years of age.
3.2. Current PKU clinical status
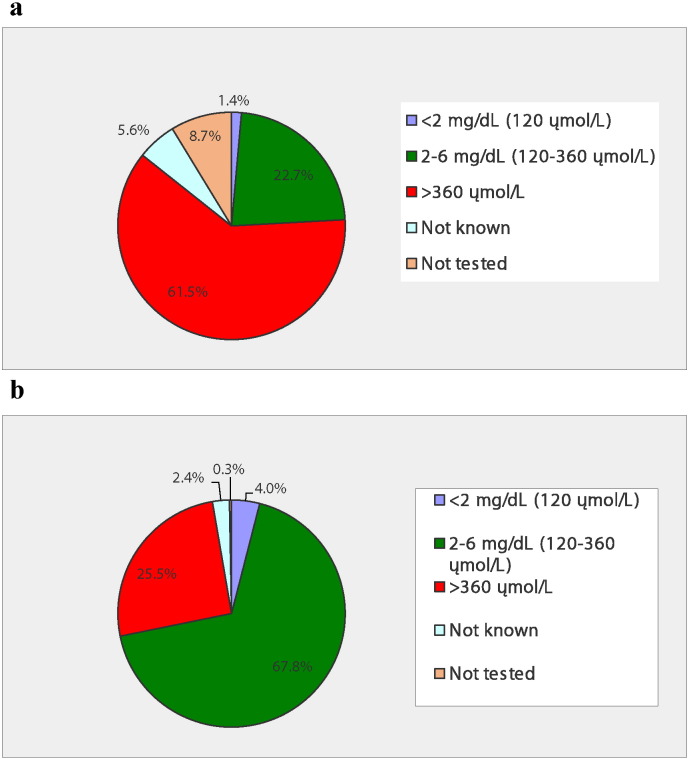
Over half (61.8%, 383/620) of those surveyed said that they had their blood drawn to monitor blood Phe concentrations in the last month. More than two-thirds (68.4%, N = 423) stated that they desired blood Phe within the ACMG range of 120–360 μmol/L. However, less than half (46.7%; 288/617) managed to attain Phe within this range. There was a difference in Phe control by age. Fig. 1 shows that individuals with PKU ≤ 18 years were 3-times as likely to have blood Phe within the 120–360 μmol/L range compared to adults > 18 years. Fig. 1 also shows that Phe concentrations > 360 μmol/L were reported for one-quarter of ≤ 18 year old individuals and almost two-thirds of > 18 year old individuals. For individuals with blood Phe concentrations > 360 μmol/L, the mean for ≤ 18 year old individuals (447.6 ± 116.5 μmol/L, N = 63) was not significantly different compared to > 18 year old individuals (477.0 ± 120.3 μmol/L, N = 160).
Fig. 1.
a, b. Percentage of blood Phe values within the range of < 120, 120–360, and > 360 μmol/L for individuals with PKU > 18 years (Fig. 1a, N = 286) and ≤ 18 years (Fig. 1b, N = 329) during the past year.
3.3. Current PKU therapy
Overall, 70% of survey respondents reported consumption of medical formula, 54% consumed modified low-protein foods, 76% consumed foods that are naturally low in protein, 40% used sapropterin dihydrochloride, 3% were prescribed large neutral amino acids (LNAA), while 6% stated they are currently not treating PKU with medical foods or pharmacotherapy. Younger individuals reported consuming medical foods more than adults. Sapropterin dihydrochloride use was not associated with subject age.
Fifty-eight percent of individuals with blood Phe < 360 μmol/L reported clinic visits within the past year compared to 38.6% of individuals with blood Phe > 360 μmol/L. Eight-percent of respondents reported that it had been more than two years since their last clinic visit. Individuals on sapropterin dihydrochloride therapy generally had more recent metabolic clinic visits; however, most individuals (86.3%) reported visiting a metabolic clinic within the past year. About two-thirds (68%) of respondents said that they had followed their PKU treatment plan for all or most of their life.
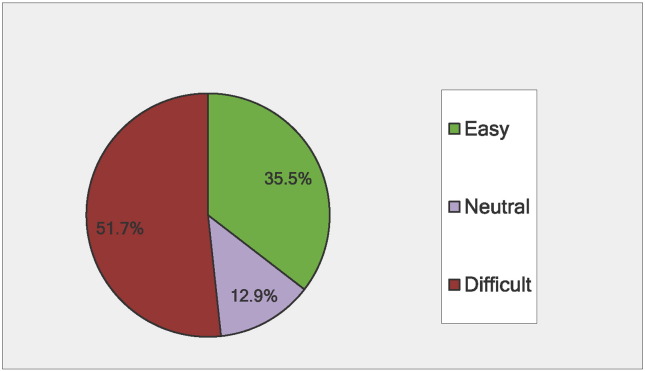
Fig. 2 shows that about a third of respondents reported that managing their PKU was easy, while half reported that PKU management was difficult. Similarly, two-thirds reported that PKU restricted their lifestyle. More adults reported struggling with their treatment plan than children. Almost half (49.5%) of individuals with blood Phe within the recommended range reported that their PKU was easy to manage compared to 20.7% of individuals with blood Phe > 360 μmol/L. More individuals on sapropterin dihydrochloride reported that their PKU was easy to manage when compared to individuals on other treatments (p < .0001) and sapropterin dihydrochloride use was associated with lower blood Phe (p < .0001).
Fig. 2.
Percentage of respondents reporting that managing PKU treatment was: Easy1, Neutral, or Difficult2 (N = 615).
1: Combines Likert scale responses for: ‘very easy’, ‘easy’, and ‘somewhat easy’
2: Combines Likert scale responses for: ‘somewhat difficult’, ‘difficult’, and ‘very difficult’.
3.4. Future therapy and treatments
When asked about the importance of new treatments, 474 individuals responded. A majority (91.4%, N = 433) of those responding stated that it was ‘somewhat important’, ‘important’, or ‘very important’ to develop new treatments for PKU, 4.2% (N = 20) were neutral on the topic, and 4.5% (N = 21) stated that new treatments were not important. When the responses were subdivided by subject blood Phe the desire for new treatment did not depend on the concentration of blood Phe. The majority (92.7%, 202/218) of individuals with blood Phe between 120 and 360 μmol/L stated that new treatments were important as did 89.9% (187/208) of individuals with blood Phe > 360 μmol/L.
Respondents were asked to select a statement that best reflected their feeling about their current PKU treatment plan and their hopes for the future. Over half (57%) responded that they were happy with their current treatment plan but hoped that new treatments would be available in the future. About a third (35%) stated that they struggle with the current treatment plan or have no current treatment plan but also hope for new treatments.
Table 2 shows the survey results when respondents were asked which symptoms of PKU they would like to improve as a benefit of new treatments. A total of 463 individuals responded and reported that blood Phe reduction, improved attention, and improved executive function were the most important potential benefits of new treatments. Results were consistent when the responses were subdivided by subject blood Phe. Eighty-two (82%, 169/206) percent of individuals with blood Phe 120–360 μmol/L desired treatments that had the potential to lower blood Phe whereas 95% (200/211) of individuals with blood Phe > 360 μmol/L desired this same benefit.
Table 2.
Ranked responses for most desired outcomes when considering new treatments.
| Desired individual preferences | Response percent | Response count |
|---|---|---|
| Drop in blood Phe concentrations | 87.5% | 405 |
| Improved attention span and ability to focus | 65.7% | 304 |
| Improved executive function skills, such as the ability to plan, organize and prioritize | 61.6% | 285 |
| Reduced depression, anxiety and/or ups and downs in overall mood | 55.1% | 255 |
| Improved processing speed — the ability to start and complete tasks | 52.1% | 241 |
| Increase in energy | 51.0% | 236 |
| Improved memory | 49.5% | 229 |
| Lifting of “the fog” | 43.0% | 199 |
| Reduced bone loss | 30.0% | 139 |
| Reduced tremors | 19.2% | 89 |
| Reduced other damage such as muscle weakness, and gait disorders | 18.8% | 87 |
Table 3 shows respondent preferences regarding which lifestyle improvements they considered most desirable in the context of future treatments — and over three-fourths (77.7%) desire a treatment that allows for an increase in natural protein intake without an increase in symptoms.
Table 3.
Ranked responses when asked which lifestyle improvements were most desired.
| Preferences | Response percent | Response count |
|---|---|---|
| I would like to be able to increase my protein intake without increasing my symptoms of PKU. | 77.7% | 365 |
| I would like to be able to eat any foods I choose regardless of their protein content. | 76.0% | 357 |
| I would like to be able to consume less of my medical foods (formula and low protein foods). | 57.7% | 271 |
| I would like to discontinue the use of my medical foods (formula and low protein foods). | 47.2% | 222 |
| I would like to have better mental health. | 45.7% | 215 |
| I would like to improve my social relationships. | 34.5% | 162 |
| I would like to decrease the frequency of blood tests. | 28.9% | 136 |
3.5. Benefit/risks and adverse events related to new treatments
Respondents were asked to describe their interest in therapeutic administration of new therapies using a 7-point Likert scale (from ‘not at all interested’ to ‘extremely interested’). Table 4 shows that respondents prefer oral ingestion for new therapies and dislike injections. Injections at home were preferred over injections at a medical facility — and half stated no interest in daily injections at a medical facility.
Table 4.
Ranked Responses when Asked their Degree of Interest in the Method of Administration of New Therapies.
| Methods of therapeutic administration | No interest (%) | Neutral (%) | Some or strong interest (%) |
|---|---|---|---|
| Weekly consumption of a probiotic | 1.7 | 7.2 | 91.1 |
| Daily oral pill(s) | 3.9 | 7.6 | 88.5 |
| Daily consumption of a probiotic | 2.8 | 9.5 | 87.7 |
| Monthly injection at home | 9.3 | 4.8 | 85.9 |
| Inserting a gene into your cells at a medical facility to correct PKU | 7.3 | 7.1 | 85.7 |
| Infusion of stem cells over several days at a medical facility to correct PKU | 8.1 | 9.2 | 82.7 |
| Weekly injection at home | 14.0 | 5.7 | 80.3 |
| Monthly injection at a medical facility | 16.0 | 7.0 | 76.9 |
| Daily injection at home | 21.7 | 8.1 | 70.2 |
| Weekly injection at a medical facility | 37.7 | 7.8 | 54.5 |
| Daily injection at a medical facility | 50.1 | 8.0 | 41.9 |
Table 5 shows the results of willingness to tolerate at least some temporary injection site pain (81%) or mild skin irritation (79%). However, internal bleeding and severe allergic reactions were generally considered intolerable side effects. Two-thirds (66.8%) were willing to tolerate immunosuppressant drugs for ≤ 6 months, 56.3% for ≤ 1 year, 29.7% for ≤ 5 years, and 22.6% for ≤ 10 years.
Table 5.
Ranked responses by tolerance for side effects from a new therapy or treatment.
| Possible treatment side effect | Some intolerance (%) | Neutral (%) | Some tolerance (%) |
|---|---|---|---|
| Temporary injection site pain | 11.9 | 7.1 | 81.0 |
| Mild skin irritation (slight redness) | 13.4 | 7.6 | 79.0 |
| Temporary allergic reaction at injection site | 24.7 | 10.8 | 64.5 |
| Moderate skin irritations (redness with swelling/itching) | 24.8 | 11.4 | 63.7 |
| Mild headache | 29.9 | 13.4 | 56.6 |
| Mild upset stomach | 34.8 | 14.3 | 51.0 |
| Mild nausea | 43.3 | 14.4 | 42.2 |
| Moderate headache | 47.1 | 13.7 | 39.3 |
| Mild joint pain | 50.5 | 11.1 | 38.3 |
| Moderate upset stomach | 51.5 | 12.8 | 35.7 |
| Mild chills/shaking chills | 52.2 | 12.7 | 35.2 |
| Moderate nausea | 56.7 | 12.3 | 31.1 |
| Moderate chills/shaking chills | 60.2 | 11.4 | 28.4 |
| Moderate joint pain | 64.0 | 9.1 | 26.9 |
| Severe allergic reaction that requires medical attention | 88.7 | 5.0 | 6.3 |
| Risk of internal bleeding | 92.2 | 3.3 | 4.6 |
4. Discussion
With the advent of newborn screening and the development of medical foods and dietary therapy, severe mental retardation due to PKU has abated. This progress has resulted in significant economic gains to society as well as improvements in quality of life and health benefits for affected individuals [2]. Many clinicians and health care organizations may hold the view that, for individuals with PKU, current strategies and therapies are therefore adequate and further healthcare investment is no longer needed.
In contrast to this view, these NPKUA survey data show that many individuals with PKU report difficultly managing their disease, have problems maintaining their blood Phe concentrations within the recommended range, and that the ability to control blood Phe worsens with age. Managing PKU is difficult, complex, and life-long. There are substantial challenges for individuals with PKU including compliance with a Phe-restricted diet, limited therapeutic options, side effects of existing treatments, lack of insurance coverage for medical foods, non-reimbursed costs, and a need for palatable alternatives [6], [10]. Adults with PKU face even more challenges and comorbidities [11].
The survey data confirm that dietary therapy remains the mainstay of clinical treatment with three-quarters of the cohort consuming natural low-protein foods. More individuals on sapropterin dihydrochloride reported that their PKU was easier to manage when compared to those not receiving sapropterin dihydrochloride (p < .0001) and sapropterin dihydrochloride use was associated with lower blood Phe (p < .0001). However, responsiveness to sapropterin dihydrochloride varies from 25 to 50% of individuals with PKU [2]. The majority of surveyed individuals stated that development of new treatments and therapies is an important goal. Table 2 shows that the most desired outcomes for new treatments would reduce blood Phe concentrations, improve attention, and improve executive function. Respondents preferred oral administration of any newly developed therapies with desired outcomes of a lower blood Phe and improvement in neuropsychological performance. Injections at home were preferred over injections at a medical facility — half (50.1%) have no interest in daily injections at a medical facility. This information may be pertinent to current trials of rAvPAL-PEG, which is administered by injections with concomitant mild to moderate skin and injection site adverse events [6].
Blood Phe is a compliance measure for PKU clinical treatment as well as a biomarker of disease severity. Only about half of survey respondents reported blood Phe within 120–360 μmol/L – the ACMG recommended range – even though most were knowledgeable about this guidance. Fig. 1 shows that younger (≤ 18 years) individuals were 3-times as successful in keeping their blood Phe concentrations within the recommended clinical range compared with adults. High blood Phe was reported more often for adults and less often in children and adolescents. These data confirm previous studies that difficulty managing PKU increases with age [12], [13], [14]. In early childhood, PKU dietary compliance is often high due to high parental control and low peer pressure; however dietary compliance generally decreases in adolescence and adulthood [15]. Reinstatement of dietary therapy after discontinuation in adolescence or adulthood can be extremely difficult [16]. In adults, lack of insurance coverage for medical foods the temptation of eating high protein foods once the patient has developed a taste for them, insufficient knowledge of PKU, and socio-economic status are likely influences for adherence to the very difficult PKU diet.
These survey data have several limitations — most of which are typical of survey questionnaires. The survey data were weighted toward informant-report and had less representation of either self-report or caregiver report. While data were not clinically verified and medical histories were unknown, the blood Phe data are consistent with previous studies [12], [13], [14]. The NPKUA questionnaire was not assessed for reliability or bias (including social desirability bias). Many of the survey questions asked for opinions and not verifiable facts. However, none of the conclusions conflict with published evidence. Survey responders may have been more motivated and compliant than other individuals, which may explain the high rate (86%) of annual clinic visits in this cohort compared to a previous report [17] as well as the relatively low mean blood Phe even for the group of individuals with blood Phe above 360 μmol/L.
5. Conclusions
Individuals with PKU face a variety of challenges and unmet needs. Even individuals who are conscientious with self-care, and who have adequate Phe control as a result, report difficulties treating their condition and maintaining recommended treatment. The NPKUA survey data show that many individuals with PKU report difficultly managing their disease, have problems maintaining their blood Phe within the recommended range, and that the ability to control blood Phe worsens with age. In addition, many individuals with PKU desire new therapies and treatments with tolerable side effects. Payers, government agencies, clinicians, and industry partners should consider patient input when developing and approving new therapies and treatments for PKU.
Potential conflicts of interest & competing interests
Christine S. Brown is a parent of children with PKU. The National PKU Alliance received funding from BioMarin Pharmaceutical Inc. to conduct the statistical analysis and draft the manuscript. Uta Lichter-Konecki received travel support from BioMarin to serve as an advisor in a PKU/Phenylalanine Hydroxylase (PAH) Deficiency Treatment Guidelines Consultancy Meeting in July 2014; no other conflicts.
Acknowledgment
The authors wish to thank Katrina Swenson and John Gerlach for statistics and the data analysis and John Mahoney for his contribution to writing and editing this manuscript with funding provided by an educational grant from BioMarin Pharmaceutical Inc. (Grant # 1035).
Contributor Information
Christine S. Brown, Email: Christine.brown@npkua.org.
Uta Lichter-Konecki, Email: Uta.Lichter-Konecki@DrexelMed.edu.
References
- 1.Phenylketonuria; PKU Online Mendelian Inheritance in Man® omim.org. http://www.omim.org/entry/261600 (last accessed 10-17-15)
- 2.Vockley J., Anderson H.C., Antshel K.M., Braverman N.E., Burton B.K., Frazier D.M. For the American College of Medical Genetics and Genomics Therapeutic Committee, phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet. Med. 2014;16:188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 3.Wappner R., Cho S., Kronmal R.A., Schuett V., Seashore M.R. Management of phenylketonuria for optimal outcome: a review of guidelines for phenylketonuria management and a report of surveys of parents, patients, and clinic directors. Pediatrics. 1999;104(6):1–9. doi: 10.1542/peds.104.6.e68. [DOI] [PubMed] [Google Scholar]
- 4.Singh R.H., Rohr F., Frazier D., Cunningham A., Mofidi S., Ogata B., Splett P.L., Moseley K., Huntington K., Acosta P.B., Vockley J., Van Calcar S.C. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet. Med. 2014;16(2):121–131. doi: 10.1038/gim.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longo N., Arnold G.L., Pridjian G., Enns G.M., Ficicioglu C., Parker S. Long-term safety and efficacy of sapropterin: the PKUDOS registry experience. Mol. Genet. Metab. 2015;114:557–563. doi: 10.1016/j.ymgme.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Blau N., Longo N. Alternative therapies to address the unmet medical needs of patients with phenylketonuria. Expert. Opin. Pharmacother. 2015;16:1–10. doi: 10.1517/14656566.2015.1013030. [DOI] [PubMed] [Google Scholar]
- 7.Gentile J.K., Ten Hoedt A.E., Bosch A.M. Psychosocial aspects of PKU: hidden disabilities—a review. Mol. Genet. Metab. 2010;99:S64–S67. doi: 10.1016/j.ymgme.2009.10.183. [DOI] [PubMed] [Google Scholar]
- 8.Camp K.M., Parisi M.A., Acosta P.B., Berry G.T., Bilder D.A., Blau N. Phenylketonuria Scientific Review Conference: State of the science and future research needs. Mol. Genet. Metab. 2014;112:87–122. doi: 10.1016/j.ymgme.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Hagedorn T.S., van Berkel P., Hammerschmidt G., Lhotáková M., Saludes R.P. Requirements for a minimum standard of care for phenylketonuria: the patients' perspective. Orphanet. J. Rare Dis. 2013;8:1–8. doi: 10.1186/1750-1172-8-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald A., Gokmen-Ozel H., van Rijn M., Burgard P. The reality of dietary compliance in the management of phenylketonuria. J. Inherit. Metab. Dis. 2010;33:665–670. doi: 10.1007/s10545-010-9073-y. [DOI] [PubMed] [Google Scholar]
- 11.Hoeks M.P.A., den Heijer M., Janssen M.C.H. Adult issues in phenylketonuria. Neth. J. Med. 2009;67:2–7. [PubMed] [Google Scholar]
- 12.Walter J.H., White F.J., Hall S.K., MacDonald A., Rylance G., Boneh A. How practical are recommendations for dietary control in phenylketonuria? Lancet. 2002;360:55–57. doi: 10.1016/s0140-6736(02)09334-0. [DOI] [PubMed] [Google Scholar]
- 13.Walter J.H., White F.J. Blood phenylalanine control in adolescents with phenylketonuria. Int. J. Adolesc. Med. Health. 2004;16:41–45. doi: 10.1515/ijamh.2004.16.1.41. [DOI] [PubMed] [Google Scholar]
- 14.Cazzorla C., Cegolon L., Burlina A.P., Celato A., Massa P., Giordano L. Quality of Life (QoL) assessment in a cohort of patients with phenylketonuria. BMC Public Health. 2014;14:1243. doi: 10.1186/1471-2458-14-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLeod E.L., Ney D.M. Nutritional management of phenylketonuria. Ann. Nestlé Eng. 2010;68:58–69. doi: 10.1159/000312813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuett V.E., Brown E.S., Michals K. Reinstitution of diet therapy in PKU patients from twenty-two US clinics. Am. J. Public Health. 1985;75:39–42. doi: 10.2105/ajph.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry S.A., Brown C., Grant M., Greene C.L., Jurecki E. Newborn screening 50 years later: access issues faced by adults with PKU. Genet. Med. 2013;15:591–599. doi: 10.1038/gim.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]