Abstract
Background
Approaches to prostate cancer (PCa) care have changed in recent years out of concern for overdiagnosis and overtreatment. Despite these changes, many patients continue to undergo some form of curative treatment and with a growing perception among multidisciplinary clinicians that more aggressive treatments are being favored. This study examines patterns of PCa care in Australia, focusing on current rates of screening and aggressive interventions that consist of high-dose-rate (HDR) brachytherapy and pelvic lymph node dissection (PLND).
Methods
Health services data were used to assess Australian men undergoing PCa screening and treatment from 2001 to 2014. Age-specific rates of prostate-specific antigen (PSA) screening were calculated. Ratios of radical prostatectomy (RP) with PLND to RP without PLND, and HDR brachytherapy to low-dose-rate (LDR) brachytherapy were determined by state jurisdictions.
Results
From 2008, the rate of PSA screening trended downward significantly with year for all age ranges (P < 0.02) except men aged ≥ 85 (P = 0.56). PLND rates for 2008–2014 were lower than rates for 2001–2007 across all states and territories. From 2008 to 2014, PLND was performed ≥ 2.7 times more frequently in New South Wales and the Australian Capital Territory than in other jurisdictions. Since 2007, brachytherapy practice across Australia has evolved towards a relatively low use of HDR brachytherapy (ratio of HDR to LDR brachytherapy < 0.5 for all jurisdictions except the Australian Capital Territory).
Conclusion
Rates of PLND and HDR brachytherapy for PCa have declined in Australia, providing evidence for the effect of stage migration due to widespread PSA screening. Currently, PSA screening rates remain high among older men, which may expose them to unnecessary investigations and treatment-related morbidity.
Keywords: Brachytherapy, Lymph node excision, Mass screening, Prostatectomy, Prostatic neoplasms
1. Introduction
The prostate cancer (PCa) landscape has been transformed over the past decade with a focus on limiting overdiagnosis and reducing overtreatment. In particular, the use of mainstay curative treatments, such as radical prostatectomy (RP) and radiotherapy, for low-risk men has been questioned because of the significant morbidity,1 quality-of-life impact,2 and economic burden3 associated with these treatments. PCa care has been further transformed by the emergence of active surveillance, which aims to delay or prevent treatment by closely monitoring disease progression. Active surveillance is an effective option for men with localized PCa1 and, encouragingly, active surveillance uptake from a state-based registry of Australian PCa patients has been shown to be higher than in many international registries.4 Widespread prostate-specific antigen (PSA) testing has resulted in increased PCa detection, as well as a ‘stage migration’ towards an earlier diagnosis and a reduced incidence of metastatic disease.5, 6 This has led to growing concern for the overdiagnosis in younger men at low risk of clinically significant disease7 and older men at low prostate-specific mortality risk.8
Despite these transformations in PCa care, a majority of men with newly diagnosed PCa undergo some form of curative treatment, regardless of their disease risk.5 Additionally, studies examining the impact of uro-oncology multidisciplinary meetings over the past decade have suggested a favoring of aggressive interventions for PCa management.9, 10 This includes a greater volume of high-dose-rate (HDR) brachytherapy,10 which is used primarily as ‘boost’ therapy [with either external beam radiotherapy (EBRT) or surgery] for higher-risk PCa than what is typically treated with low-dose-rate (LDR) brachytherapy.5 Furthermore, there is the potential for an increased uptake of pelvic lymph node dissection (PLND), the most reliable and accurate staging method for prostate cancer metastases.11 Therefore, the objective of our current study was to analyze for temporal trends in these key indicators of PCa care, in particular, the rates of screening and aggressive interventions for PCa.
2. Materials and methods
2.1. Study population
The study population consisted of all Australian men who underwent a screening PSA test, RP, or brachytherapy for PCa between 2001 and 2014. The study period commenced from when a dedicated PSA screening test was first incorporated into the Australian Government's Medicare Benefits Schedule (MBS) in 2001.
2.2. Data selection
A number of studies have analyzed the impact of uro-oncology multidisciplinary meetings on PCa management decisions over the past decade. Notable trends have been: (1) a shift towards greater active surveillance of lower risk disease;10 and (2) the utilization of aggressive interventions, such as HDR brachytherapy.9, 10 The present study uses health services data to analyze trends in PSA screening and aggressive interventions. Future registries will be able to account for the number and characteristics of men on active surveillance.12
2.3. Data extraction
Data on services defined by specific item numbers were sourced from the MBS website.13 This captures all tests and treatments performed in the community. Delivery of HDR brachytherapy to the prostate uses the MBS billing code of ‘37227’ and was first introduced to the MBS in 2007. HDR brachytherapy must be performed ‘by a urologist or radiation oncologist’ in ‘association with a radiation oncologist’, with no limitations on tumor staging or grade. Counts of HDR brachytherapy include both the insertion and removal of catheters for radiation delivery. Seed implantation of low-dose-rate brachytherapy has been billed under code ‘37220’ since 2001, and must also be performed in ‘association with a radiation oncologist’. LDR brachytherapy is restricted to patients with localized PCa, Gleason ≤ 7 (Gleason ≤ 6 prior to 2008) and PSA ≤ 10 ng/mL at the time of diagnosis. Only LDR brachytherapy data from 2007 were analyzed to enable comparisons with HDR brachytherapy over the same time period.
A dedicated item code (‘66655’) for an initial ‘screening’ PSA test was introduced in 2001 and is defined as a single PSA test performed during a 12-month period in previously undiagnosed prostatic disease. Radical prostatectomy with ‘sparing of nerves around the bladder and bladder neck reconstruction’ is billed under code ‘37211’ when PLND is performed or ‘37210’ when PLND omitted. Both RP item codes were introduced prior to 2001. EBRT was excluded from this study because of different radiation field settings and dosimetry, along with poor capture of the actual treatment received when using MBS item codes.14 All population data were obtained from the Australian Bureau of Statistics.15
2.4. Statistical analysis
Age-specific rates of PSA-based screening were calculated from 2001 to 2014. Trends of annual change in PSA screening rates from 2008 were analyzed using Cuzick's nonparametric test for trend. Mean 7-year ratios (2001–2007 and 2008–2014) of RP with PLND compared to RP without PLND were determined for state jurisdictions. Annual ratios of HDR brachytherapy to LDR brachytherapy were determined for state jurisdictions from 2007.
3. Results
3.1. Radical prostatectomy
The average annual Australian male population was 10,529,409 between 2001 and 2014. A total of 64,824 RPs were performed over this period, of which 36,829 involved a PLND. The mean ratio of RP with PLND to RP without PLND from 2001 to 2007 and 2008 to 2014 for each state jurisdiction is shown in Fig. 1. Overall, PLND was performed more often than not, however, there was a considerable decrease between 2001 and 2007 (when the mean ratio of RP with PLND to RP without PLND was 1.9) and between 2008 and 2014 (when the same ratio was 1.1). This reduction in rate of PLND occurred for all state jurisdictions. From 2008 to 2014, relatively high rates of PLND were recorded in the Australian Capital Territory (ACT) (mean ratio of 5.6) and New South Wales (NSW) (mean ratio of 2.3), whereas rates were relatively low (< 0.9) across all other jurisdictions.
Fig. 1.
The graph shows the ratio of radical prostatectomy (RP) with pelvic lymph node dissection (PLND) to RP without PLND across state jurisdictions between 2001–2007 and 2008–2014 (all years inclusive). The ratio of the Australian Capital Territory for 2001–2007 (black) exceeds the maximum y-axis.
3.2. Brachytherapy
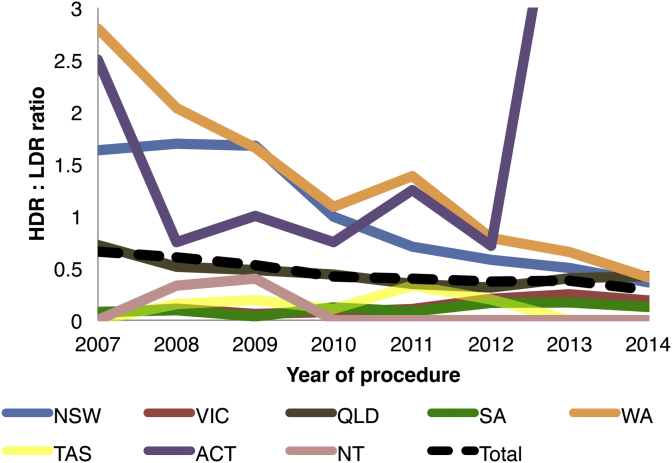
Between 2007 and 2014, 2,326 HDR brachytherapy procedures (either implant insertion or removal) were performed across Australia. Most cases arose from NSW (1,123), Queensland (512), Western Australia (357), Victoria (191), and South Australia (77). Between 2007 and 2014, 5,060 LDR brachytherapy implantation procedures were performed, with most occurring in Victoria (1,555), NSW (1,163), Queensland (1,111), South Australia (673), Western Australia (317) and Tasmania (201). The ratio of HDR to LDR brachytherapy across state jurisdictions is shown in Fig. 2.
Fig. 2.
The graph shows the ratio of high-dose-rate (HDR) brachytherapy to low-dose-rate (LDR) brachytherapy from 2007 to 2014. The ratio of the Australian Capital Territory for 2013–2014 exceeds the maximum y-axis.
3.3. PSA screening tests
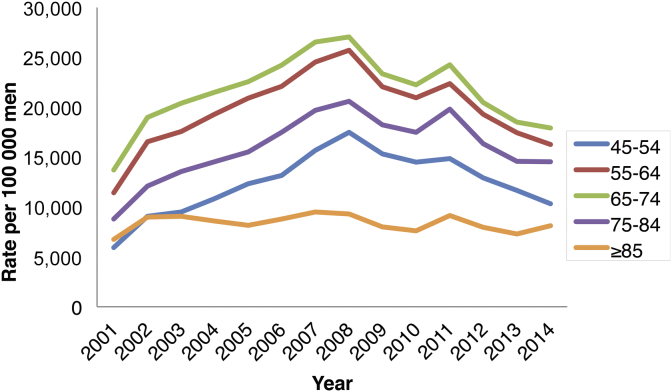
PSA screening rates have been highest for patients aged 65–74 years (an annual average of 21,453 per 100,000 men) and 55–64 years (19,575 per 100,000 men) from 2001 to 2014, which together accounted for 56% of all screening tests over this period (Fig. 3). The next highest screening rates were for patients aged 75–84 years (15,793 per 100,000 men), 45–55 years (12,315 per 100,000 men) and ≥ 85 years (8,064 per 100,000 men). From 2008, PSA screening rates trended downward significantly year-to-year for all ranges (P < 0.02) except for men aged ≥ 85 years (P = 0.56), and excluding a small aberrant rise in 2011.
Fig. 3.
The graph shows age-specific rates of PSA screening tests per 100,000 men for combined states and territories since 2001. PSA, prostate-specific antigen.
4. Discussion
The key findings of this population-based analysis are that a considerable decline in PLND and HDR brachytherapy rates occurred over the study period. This trend was observed across all Australian states and territories, excluding brachytherapy rates in the Australian Capital Territory. Together these results indicate that fewer Australian men are undergoing aggressive interventions for PCa. Previous United States (US) studies16, 17 have observed a similar decline in PLND rates and this was attributed to stage migration and an improved ability to risk-stratify patients before surgery. Stage migration of PCa that has been observed in a number of Australian studies5, 6 is a highly feasible explanation for our result. We suspect that more patients are being diagnosed with organ-confined and lower risk disease as a result of PSA testing, which has reduced the need for aggressive interventions. Risk-stratifying tools, or nomograms, that are based on routinely available preoperative variables have been shown to better identify men at risk of nodal disease18 and are currently used in Australian institutions.19 Notably, many of these risk-stratifying tools are based on a limited PLND, which may underestimate the incidence of positive nodes, and none have had oncologic efficacy verified with a prospective randomized clinical trial.11
In the past few decades, PCa treatment planning has become more complicated and contentious owing to the proliferation of treatment options and an absence of high-quality evidence supporting one method over another.2 Consequently, multidisciplinary teams often decide on and coordinate clinical treatments,9 which may involve a number of modalities for an individual patient. Technical advances and the publication of trial results are two major reasons multidisciplinary clinicians will choose to treat pelvic nodes and to recommend particular therapies.20 Brachytherapy has been an established technique since the commencement of our study period. Conversely, prostate surgery has seen the rapid adoption of a minimally invasive, robot-assisted technique.3 Currently it is unclear to what effect a shift from an open to a minimally invasive approach has had on the use of PLND.21, 22 Surgeon experience is one important determinant for the likelihood of performing PLND, irrespective of the surgical approach, and this likely reflects the increased risk of complications with PLND, such as lymphedema, lymphocele, deep vein thrombosis, and pulmonary emboli.21
Although local registry and health services data have shown that RP incidence is rising for Australian populations,5, 23 to our knowledge, patterns of PLND have not previously been explored. We found that PLND rates during prostate surgery greatly varied amongst Australian state jurisdictions despite overall widespread reductions. For example, between 2008 and 2014, PLND rates in NSW and the ACT were nearly three times higher than in other jurisdictions. A subanalysis has shown that PSA screening rates in NSW and ACT over this same period were within 10% of the annual national average. Hence, the elevated PLND rates in NSW and ACT compared to other jurisdictions were not simply due to these states performing less screening and therefore treating higher-risk PCa. More plausible explanations include the dissemination of robot-assisted technology, as well as variable involvement of multidisciplinary teams and the adequacy and/or access to radiotherapy centers.24
HDR brachytherapy uses a high dose of radioactive seeds (≥ 12 Gy/h) that can only be placed temporarily, compared with permanent seed placement in LDR brachytherapy. Since 2007, we have found a shift towards much lower use of HDR brachytherapy relative to LDR brachytherapy. By 2014, all state jurisdictions, except ACT, had a ratio of HDR to LDR brachytherapy of < 0.5. Previous Australian registries have shown that HDR brachytherapy is a minor contributor to overall PCa care2, 5 and is used as either adjunct therapy to androgen deprivation therapy or as boost therapy with EBRT or surgery.5 Currently, HDR brachytherapy has not been tested in sufficient numbers to be recommended as monotherapy.11 Importantly, dosing and fractionation for HDR brachytherapy is inconsistently delivered across Australian radiotherapy departments,25 which is unsurprising since no Australian guidelines exist and there is no clear consensus amongst international brachytherapy societies.26, 27
Another important finding of our study was that PSA-based screening rates have significantly declined since 2008 in all age groups except for men aged ≥ 85 years (excluding a small aberrant rise in 2011). Two large randomized trials have reported that mass PSA-based screening offers limited benefit or no benefit over usual care.28, 29 Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) showed screening did not affect all-cause mortality after 11 years of follow-up but did show a reduced incidence of advanced disease and prostate-specific mortality in men aged 55–69.29 Thus far, urological societies have discouraged widespread population screening but have endorsed a role for opportunistic screening of men aged 55–69.11, 30, 31 We found that the majority of screening tests are conducted in men aged 55–74 and this potentially reflects the uptake of opportunistic screening.
It is possible that the publication of the two major trials questioning the benefits of screening and the resultant publicity afforded to these studies32 have contributed to the reduction in screening rates. Negative media reporting conceivably has alerted patients to the limitations of screening and thereby influenced their willingness to participate.32 Men aged ≥ 85 years may not have the same access to contemporary online media sources, which potentially explains the unchanged and unnecessarily high screening rates amongst this age group. Another feasible explanation for the reduction in screening rates in most age groups is the penetrance of regional30 and key international guidelines32 such as the US Preventive Services Task Force. It will be interesting to see in time whether the decline in PSA-based screening rates we recorded for most age groups leads to a reversal of stage migration, such that rates of advanced disease return towards pre-PSA era levels.
We could not be certain from the data available to us whether patients undergoing PSA-based screening were asymptomatic or at high suspicion for PCa (such as suspect digital rectal exam or family history). A previous study of Australian men undergoing PSA-based screening reported minimal prior symptoms for the majority of men, suggesting that these may be true screening tests.33 It is possible that incorrect item numbers were used for brachytherapy (HDR or LDR) and prostate surgery (with or without PLND) in some proportion of our data. It is also possible that PSA screening tests were misclassified as monitoring or follow-up tests, each of which have different item numbers. Medicare Australia performs ‘coning’ of multiple pathology items, whereby only the three most costly items are reimbursed and recorded.8 Due to this approach, we may have underestimated the true number of PSA screening tests.
Combination therapy is not currently recorded through health services data and we suspect that androgen deprivation therapy with HDR brachytherapy is widely used but not reported. Since Medicare data does not presently capture the type of prostatectomy approach, further studies are needed to determine if PLND rates differ with a minimally invasive approach compared to a conventional open prostatectomy. Additionally, we are unable to exclude data for RPs (with or without PLND) performed in conjunction with radical cystectomy for an invasive bladder tumor. A separate analysis of radical cystectomy (MBS billing code ‘37014’) has revealed 3,190 procedures were recorded for male patients between 2001 and 2014, which would account for < 5% of all RPs over this period. We did not examine rates of focal therapy (not presently reimbursed by Medicare) or EBRT, which is poorly captured with Medicare data.14 Notably, this study does not account for all men undergoing aggressive interventions over the study period because both androgen deprivation therapy (ADT) and EBRT can also be used for higher risk disease. Our study has biases inherent with observational data.
Although Medicare data have been validated to provide a true account of prostate surgery and brachytherapy, accuracy can be improved when combined with local registries.14 Local registries also enable patient characteristics and cancer staging to be linked to the treatment outcomes of PLND and HDR brachytherapy. An up-to-date analysis of clinical-decision making for aggressive interventions, including the recommendations from multidisciplinary clinicians and use of risk-stratifying tools, as well as a patient's beliefs and anxieties, would make a useful adjunct to our study. Future studies should examine why PSA-based screening rates remains relatively high among older men in Australia, particularly because no specific guidelines currently exist for managing a positive screening result in this age group.34
In conclusion, rates of PLND and HDR brachytherapy have declined in Australia, which provides evidence for the effect of stage migration from widespread PSA screening. Currently, PSA screening rates among older men remain proportionately high, which potentially exposes them to unnecessary investigations and treatment. Our results reveal that contemporary approaches to prostate surgery vary among jurisdictions and further investigation is warranted to understand the clinical decision-making process behind overall and regional trends.
Conflicts of interest
No potential conflicts of interest relevant to this article are reported.
References
- 1.Wilt T.J., Brawer M.K., Jones K.M., Barry M.J., Aronson W.J., Fox S. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith D.P., King M.T., Egger S., Berry M.P., Stricker P.D., Cozzi P. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ. 2009;339:b4817. doi: 10.1136/bmj.b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolenz C., Freedland S.J., Hollenbeck B.K., Lotan Y., Lowrance W.T., Nelson J.B. Costs of radical prostatectomy for prostate cancer: a systematic review. Eur Urol. 2014;65:316–324. doi: 10.1016/j.eururo.2012.08.059. [DOI] [PubMed] [Google Scholar]
- 4.Weerakoon M., Papa N., Lawrentschuk N., Evans S., Millar J., Frydenberg M. The current use of active surveillance in an Australian cohort of men: a pattern of care analysis from the Victorian Prostate Cancer Registry. BJU Int. 2015;115(Suppl 5):50–56. doi: 10.1111/bju.13049. [DOI] [PubMed] [Google Scholar]
- 5.Evans S.M., Millar J.L., Davis I.D., Murphy D.G., Bolton D.M., Giles G.G. Patterns of care for men diagnosed with prostate cancer in Victoria from 2008 to 2011. Med J Aust. 2013;198:540–545. doi: 10.5694/mja12.11241. [DOI] [PubMed] [Google Scholar]
- 6.Smith D.P., Supramaniam R., Marshall V.R., Armstrong B.K. Prostate cancer and prostate-specific antigen testing in New South Wales. Med J Aust. 2008;189:315–318. doi: 10.5694/j.1326-5377.2008.tb02048.x. [DOI] [PubMed] [Google Scholar]
- 7.Ranasinghe W.K., Kim S.P., Lawrentschuk N., Sengupta S., Hounsome L., Barber J. Population-based analysis of prostate-specific antigen (PSA) screening in younger men (< 55 years) in Australia. BJU Int. 2014;113:77–83. doi: 10.1111/bju.12354. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael L.K., Goldsbury D.E., O'Connell D.L. Prostate cancer screening for men aged 75–84 years in New South Wales. Aust N Z J Public Health. 2013;37:492–494. doi: 10.1111/1753-6405.12115. [DOI] [PubMed] [Google Scholar]
- 9.Rao K., Manya K., Azad A., Lawrentschuk N., Bolton D., Davis I.D. Uro-oncology multidisciplinary meetings at an Australian tertiary referral centre—impact on clinical decision-making and implications for patient inclusion. BJU Int. 2014;114(Suppl 1):50–54. doi: 10.1111/bju.12764. [DOI] [PubMed] [Google Scholar]
- 10.Korman H., Lanni T., Jr., Shah C., Parslow J., Tull J., Ghilezan M. Impact of a prostate multidisciplinary clinic program on patient treatment decisions and on adherence to NCCN guidelines: the William Beaumont Hospital experience. Am J Clin Oncol. 2013;36:121–125. doi: 10.1097/COC.0b013e318243708f. [DOI] [PubMed] [Google Scholar]
- 11.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Ta A.D., Papa N.P., Lawrentschuk N., Millar J.L., Syme R., Giles G.G. Increased prostate cancer specific mortality following radical prostatectomy in men presenting with voiding symptoms—a whole of population study. Prostate Int. 2015;3:75–79. doi: 10.1016/j.prnil.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Department of Health . Department of Health; Canberra, ACT, Australia: 2015. Medicare Benefits Schedule Online [Internet]http://www.mbsonline.gov.au [cited 2015 Apr 2]. Available from: [Google Scholar]
- 14.Goldsbury D.E., Smith D.P., Armstrong B.K., O'Connell D.L. Using linked routinely collected health data to describe prostate cancer treatment in New South Wales, Australia: a validation study. BMC Health Serv Res. 2011;11:253. doi: 10.1186/1472-6963-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Australian Bureau of Statistics . Australian Government; Canberra, ACT, Australia: 2015. Australian Bureau of Statistics [Internet]http://www.abs.gov.au/ [cited 2015 Apr 2]. Available from: [Google Scholar]
- 16.Joslyn S.A., Konety B.R. Impact of extent of lymphadenectomy on survival after radical prostatectomy for prostate cancer. Urology. 2006;68:121–125. doi: 10.1016/j.urology.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami J., Meng M.V., Sadetsky N., Latini D.M., Duchane J., Carroll P.R. Changing patterns of pelvic lymphadenectomy for prostate cancer: results from CaPSURE. J Urol. 2006;176:1382–1386. doi: 10.1016/j.juro.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Cagiannos I., Karakiewicz P., Eastham J.A., Ohori M., Rabbani F., Gerigk C. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–1803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 19.Sengupta S., Weerakoon M., Sethi K., Ischia J., Webb D.R. Algorithm for selecting men for pelvic lymph node dissection (PLND) during radical prostatectomy based on clinical risk factors in an Australian population. BJU Int. 2012;109:48–51. doi: 10.1111/j.1464-410X.2012.11047.x. [DOI] [PubMed] [Google Scholar]
- 20.Chong C.C., Austen L., Kneebone A., Lalak A., Jalaludin B. Patterns of practice in the management of prostate cancer: results from multidisciplinary surveys of clinicians in Australia and New Zealand in 1995 and 2000. BJU Int. 2006;97:975–980. doi: 10.1111/j.1464-410X.2006.06065.x. [DOI] [PubMed] [Google Scholar]
- 21.Hu J.C., Prasad S.M., Gu X., Williams S.B., Lipsitz S.R., Nguyen P.L. Determinants of performing radical prostatectomy pelvic lymph node dissection and the number of lymph nodes removed in elderly men. Urology. 2011;77:402–406. doi: 10.1016/j.urology.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Koo K.C., Jung D.C., Lee S.H., Choi Y.D., Chung B.H., Hong S.J. Feasibility of robot-assisted radical prostatectomy for very-high risk prostate cancer: surgical and oncological outcomes in men aged ≥ 70 years. Prostate Int. 2014;2:127–132. doi: 10.12954/PI.14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baade P.D., Youlden D.R., Coory M.D., Gardiner R.A., Chambers S.K. Urban–rural differences in prostate cancer outcomes in Australia: what has changed? Med J Aust. 2011;194:293–296. doi: 10.5694/j.1326-5377.2011.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 24.Papa N., Lawrentschuk N., Muller D., MacInnis R., Ta A., Severi G. Rural residency and prostate cancer specific mortality: results from the Victorian Radical Prostatectomy Register. Aust N Z J Public Health. 2014;38:449–454. doi: 10.1111/1753-6405.12210. [DOI] [PubMed] [Google Scholar]
- 25.van Nieuwenhuysen J., Waterhouse D., Bydder S., Joseph D., Ebert M., Caswell N. Survey of high-dose-rate prostate brachytherapy practice in Australia and New Zealand, 2010–2011. J Med Imaging Radiat Oncol. 2014;58:101–108. doi: 10.1111/1754-9485.12101. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y., Rogers L., Demanes D.J., Morton G., Prestidge B.R., Pouliot J. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy. 2012;11:20–32. doi: 10.1016/j.brachy.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs G., Potter R., Loch T., Hammer J., Kolkman-Deurloo I.K., de la Rosette J.J. GEC/ESTRO-EAU recommendations on temporary brachytherapy using stepping sources for localised prostate cancer. Radiother Oncol. 2005;74:137–148. doi: 10.1016/j.radonc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Andriole G.L., Crawford E.D., Grubb R.L., 3rd, Buys S.S., Chia D., Church T.R. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder F.H., Hugosson J., Roobol M.J., Tammela T.L., Ciatto S., Nelen V. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urological Society of Australia and New Zealand . Urological Society of Australia and New Zealand; Edgecliff, NSW, Australia: 2009. Urological Society of Australia and New Zealand: PSA Testing Policy 2009 [Internet]www.usanz.org.au/uploads/29168/ufiles/USANZ_2009_PSA_Testing_Policy_Final1.pdf [cited 2015 May 22]. Available from: [Google Scholar]
- 31.Carter H.B., Albertsen P.C., Barry M.J., Etzioni R., Freedland S.J., Greene K.L. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrentschuk N., Daljeet N., Trottier G., Crawley P., Fleshner N.E. An analysis of world media reporting of two recent large randomized prospective trials investigating screening for prostate cancer. BJU Int. 2011;108:e190–e195. doi: 10.1111/j.1464-410X.2010.09983.x. [DOI] [PubMed] [Google Scholar]
- 33.Litchfield M.J., Cumming R.G., Smith D.P., Naganathan V., Le Couteur D.G., Waite L.M. Prostate-specific antigen levels in men aged 70 years and over: findings from the CHAMP study. Med J Aust. 2012;196:395–398. doi: 10.5694/j.1326-5377.2012.tb04214.x. [DOI] [PubMed] [Google Scholar]
- 34.Droz J.P., Balducci L., Bolla M., Emberton M., Fitzpatrick J.M., Joniau S. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106:462–469. doi: 10.1111/j.1464-410X.2010.09334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]