Abstract
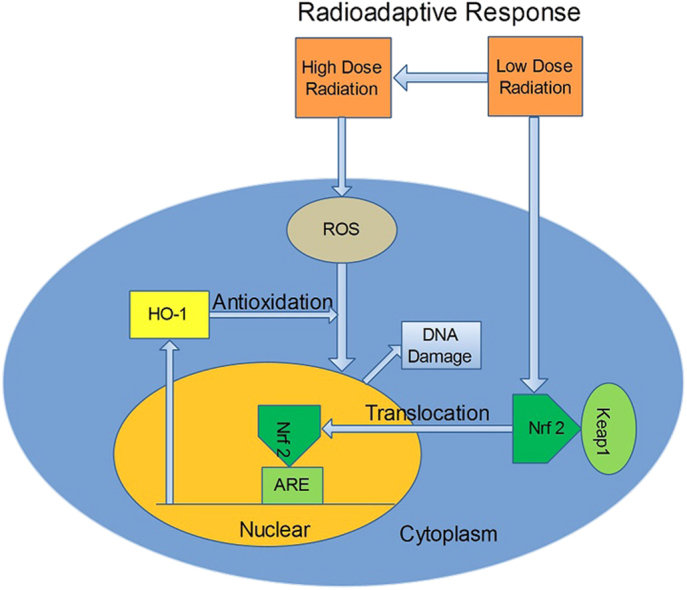
Radioadaptive response (RAR) is an important phenomenon induced by low dose radiation. However, the molecular mechanism of RAR is obscure. In this study, we focused on the possible role of heme oxygenase 1 (HO-1) in RAR. Consistent with previous studies, priming dose of X-ray radiation (1–10 cGy) induced significant RAR in normal human skin fibroblasts (AG 1522 cells). Transcription and translation of HO-1 was up-regulated more than two fold by a priming dose of radiation (5 cGy). Zinc protoporphyrin Ⅸ, a specific competitive inhibitor of HO-1, efficiently inhibited RAR whereas hemin, an inducer of HO-1, could mimic priming dose of X-rays to induce RAR. Knocking down of HO-1 by transfection of HO-1 siRNA significantly attenuated RAR. Furthermore, the expression of HO-1 gene was modulated by the nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which translocated from cytoplasm to nucleus after priming dose radiation and enhance the antioxidant level of cells.
Keywords: Heme Oxygenase 1, Radioadaptive response, Hemin, Znpp, Nrf2, Reactive oxidative species
Graphical abstract
Highlights
-
•
The critical role of HO-1 in low dose Radioadaptive response is proposed.
-
•
Low dose irradiation activates Nrf2 Translocation and HO-1 expression.
-
•
Nrf2/HO-1 pathway mediates Radioadaptive response via regulating ROS production.
1. Introduction
Radioadaptive response (RAR) is characterized by a reduction of radiobiological response in cells which have been pretreated with a low-dose radiation followed by a subsequent higher challenging dose [1]. Olivieri et al. were the first to demonstrate that when human lymphocytes were pre-cultured with [3H]thymidine, which acted as a source of low-level chronic radiation, and were then exposed to 1.5 Gy of x-rays at 5, 7, 9, or 11 h before fixation, the yield of chromatid aberrations was less than the sum of the individual yields of aberrations induced by [3H]thymidine and x-rays alone [2]. In the past three decades, accumulated experimental data have established the existence of such a response using a variety of endpoints [3], such as sister chromatid exchanges, micronuclei (MN) induction and clonogenic survival [4], [5], [6], [7]. Furthermore, RAR has been observed in many different organisms: bacteria, yeast, higher plants, insect cells, mammalian and human cells in vitro, and animal models in vivo [8], [9], [10].
Up to now, however, the molecular basis for RAR remains not clear. It is possible that RAR depends on the activation of DNA repair and cell cycle regulation, or activation of antioxidant enzymes due to the oxidative stress caused by ionizing radiation. Bravard et al. reported the activation of antioxidant enzymes such as manganese superoxide dismutase, glutathione peroxidase and catalase after administration of an initial low-dose radiation followed by a subsequent high-dose radiation [11]. The increased activities of these antioxidant enzymes led to rapid scavenging of reactive oxygen species (ROS) and consequently less cell damage in the adapted cells [11].
Nuclear factor erythroid 2-related factor 2 (NFE2L2 or Nrf2)/heme Oxygenase-1 (HO-1) pathway is an important antioxidative and protective pathway for cells [12], [13]. Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein (Keap1) under unstimulated conditions [14], [15], [16]. When stimulated, Nrf2 is translocated into the nucleus and activates the antioxidant response element (ARE) followed by the induction of HO-1, a downstream target of Nrf2 [17].
In the present study, we focused on the possible role of Nrf2/HO-1 pathway in RAR. Our results indicated that the priming radiation dose (5 cGy) activated Nrf2 translocation from cytoplasm to nucleus followed by the upregulation of the HO-1 gene, which enhanced the antioxidative level of cells to protect cells from a subsequent exposure to a 2 Gy dose of X-rays.
2. Materials and methods
2.1. Cell culture and radiation
Normal human skin fibroblasts AG 1522, which were used widely in RAR studies [18], [19], were maintained in α-Eagle's minimum essential medium (Gibco, Carlsbad, CA, USA) supplemented with 20% fetal bovine serum (Thermo Scientific Hyclone, Logan, UT, USA) and 2.0 mM l-glutamine plus 100 μg/ml streptomycin and 100 U/ml penicillin (Gibco, Carlsbad, CA, USA) at 37 °C in a humidified 5% CO2 incubator. The culture medium was replaced every 2 days until the cells were under full confluence before irradiation. At that time, ~92% of the cells were in the G0–G1 phases for contact inhibition [20].
The culture medium was replaced with fresh medium before delivery of the priming dose (1–10 cGy) using an irradiator of X-ray (SHINVA 600D, Zibo, Shandong, China) at a dose rate of 0.2 Gy/min. The cells were then further cultured for a chosen pre-defined period before the challenging dose (2 Gy) was applied at a dose rate of 2.0 Gy/min using the same X-ray irradiator.
2.2. Antibodies
HO-1 primary antibody, β-tubulin primary antibody, lamin B primary antibody and HPR-conjugated secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), Nrf2 primary antibody was purchased from Abcam (Cambridge, MA, USA).
2.3. MN test
The frequency of MN formation was determined through the cytokinesis block technique [21]. The cells were trypsinized after irradiation, and ~3×104 cells were seeded in each 35 mm culture dish [20], [22]. Cytochalasin B (Sigma, St. Louis, MO, USA) was added into the culture medium at the final concentration of 2.5 μg/ml at 4–6 h post cell seeding. After 48 h incubation, the cells were fixed with 4% paraformaldehyde (Sigma, St. Louis, MO, USA), stained with 0.1% acridine orange (Sigma, St. Louis, MO, USA) for 5 min, and then viewed under a fluorescence microscope (Leica DMI 4000B, Wetzlar, German). At least 1000 binucleate cells were examined and the frequency of MN formation (r°) was calculated as: r0=a/b, where a was the total number of micronucleated cells scored, and b was the total number of binucleated cells examined.
2.4. Western blot
After irradiation, total protein was extracted with RIPA (Beyotime Biotechnology, Shanghai, China) and the concentration was determined by a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). The nuclear and cytosolic protein was extracted separately with a nuclear and cytoplasmic protein extraction kit (Shanghaishenggong Biotechnology, Shanghai, China) according to manufacturer’s protocols. Equal amounts of protein (20 μg) were resolved by SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blotted with the primary antibodies and developed after secondary antibody incubation using the ECL kit (Kangweishiji Biotechnology, Beijing, China) according to the manufacturer’s protocols. For statistical analysis, a box plot analysis was applied.
2.5. RT-PCR
RT-PCR was performed with Thermo Scientific Verso 1-step RT-qPCR Kits (Logan, UT, USA). Gene expression levels were normalized to the level of β-actin. The primers used for PCR amplification are shown as follows: 5′-ATGGATGATGATATCGCCGCG-3′, 5′-TCTCCATGTCGTCCCAGTTG-3′ (human β-actin) [23], as well as 5′-AAGATTGCCCAGAAAGCCCTGGAC-3′, 5′-AACTGTCGCCACCAGAAAGCTGAG-3′ (human HO-1) [24].
2.6. RNA interference
Specific siRNAs for HO-1 (sequence: 5′ UGCUCAACAUCCAGCUCUUtt 3′ and 5′ AAGAGCUGGAUGUUGAGCAtt 3′), Nrf2 (sequence: 5′ GCAUGCUACGUGAUGAAGAtt 3′ and 5′ UCUUCAUCACGUAGCAUGCtt 3′) and the control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Transfection medium and transfection reagent were also purchased from Santa Cruz Biotechnology. Cells were transfected with double-stranded siRNAs for 24 h with the transfection reagent according to manufacturer’s protocols and recovered in fresh media for 24 h. The cells were then irradiated and proteins were collected at 12 h after irradiation for further experiments.
2.7. Measurement of ROS
After irradiation, the cells were stained with 5 μM CellROX® Green Reagent (Invitrogen, Grand Island, NY, USA) dissolved in media and then incubated at 37 °C for 30 min. The cells were then washed with PBS, and the images were captured under a fluorescence microscope with a 40× objective (Leica DMI 4000B, Wetzlar, German). A semi-quantitative analysis of ROS-associated fluorescent signals was performed with the NIH Image J software. More than 100 individual cells were randomly selected in each sample and quantified. The relative intensities were expressed in arbitrary units per cell.
2.8. Statistical analysis
Statistical analysis was performed on the data obtained from at least three independent experiments. The data were presented as means±SD. The significance of variance was determined by ANOVA analysis. A p-value smaller than 0.05 between two independent groups was considered to correspond a statistically significant difference.
3. Results
3.1. Time interval and dose effect of RAR
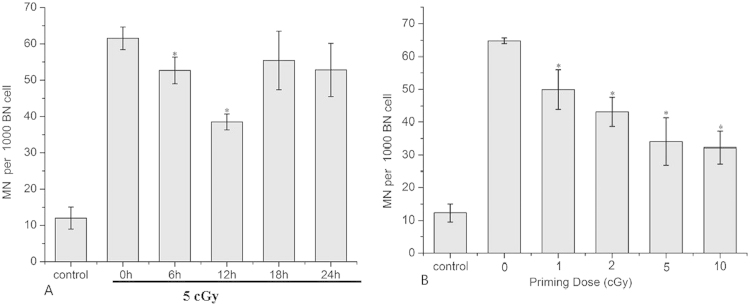
For assessing RAR, the frequency of MN formation of AG 1522 cells were determined. An X-ray dose of 5 cGy was used as a priming dose as described [25]. AG 1522 cells were primed and exposed to a 2 Gy challenging dose after the indicated time interval. As shown in Fig. 1, RAR was significantly induced after the application of a priming dose of 5 cGy and the level of RAR demonstrated a manner dependent on the time interval between the initial and challenging radiation doses (Fig. 1A). When the interval time was 12 h, the amount of RAR reached a peak value when the MN incidence decreased from 61.5±3.10 to 38.5±2.18 per 1000 binucleated cells (BN) (Fig. 1A).
Fig. 1.
Time interval and dose effect of RAR. Effects of time interval and priming dose on RAR. (A) MN test results showing the effect of time interval between the priming dose and the challenging dose on RAR. Cells were irradiated with 5 cGy of priming dose and then 2 Gy of challenging dose with time intervals of 0, 6, 12, 18 and 24 h.*: p<0.05 compared to 0 h. (B) MN test results showing the effect of priming dose on RAR. Cells were irradiated with 0, 1, 2, 5 and 10 cGy of priming dose and then 2 Gy of challenging dose with a time interval of 12 h between the priming dose and the challenging dose. *: p<0.05 compared to 0 cGy.
The effect of priming dose was also studied, with the time interval between the application of priming and challenging doses set as 12 h and using a 2 Gy challenging dose all across. The results showed a dependence of RAR on the initial priming dose used (Fig. 1B). Based on these results, in the subsequent experiments on studying the underlying mechanisms, 5 cGy was chosen as a representative priming dose, while 12 h was chosen as a representative time interval between the priming and the challenging exposures.
3.2. Priming dose of radiation promoted HO-1 expression
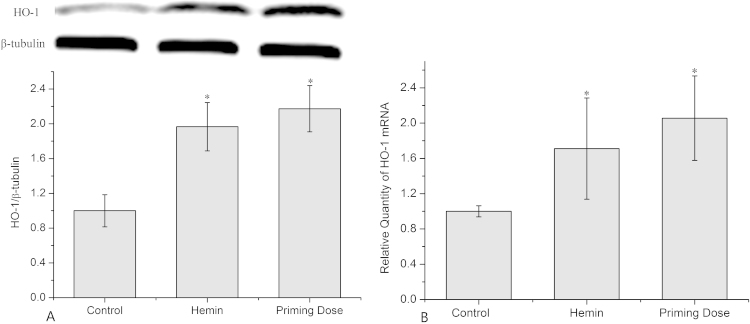
To determine the effects of priming dose radiation on HO-1 expression, the protein and mRNA levels were detected after 12 h of priming dose irradiation. The results of western blot and RT-PCR data showed that HO-1 was significantly upregulated by 2.17±0.27 and 2.06±0.48 fold, respectively, relative to controls (Fig. 2A and B). AG 1522 cells treated with hemin (Sigma, St. Louis, MO, USA), which was used as a positive control, also showed a significant up-regulated HO-1 expression at both the protein and mRNA levels.
Fig. 2.
Expression and transcription of HO-1 in cells exposed to priming dose of X-ray. (A) Relative expression of HO-1 protein in cells irradiated by 5 cGy of priming dose. (B) Relative mRNA abundance of HO-1 in cells irradiated by 5 cGy of priming dose. Data represent the means±SD of samples from three independent experiments. *: p<0.05 compared to control.
3.3. Time-course of HO-1 and Nrf2 expression in cells exposed to priming dose of radiation
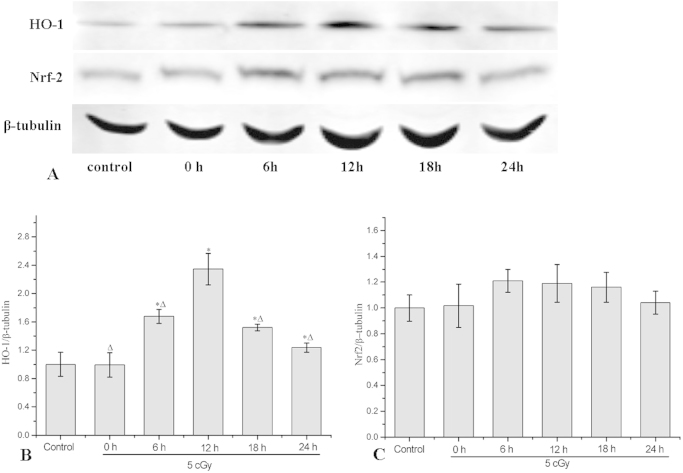
At 0, 6, 12, 18 and 24 h after the priming radiation dose, total protein of AG 1522 cell was collected and the expressions of HO-1 and Nrf2 were detected with western blot. The results in Fig. 3B showed that the priming dose induced significant increase of HO-1 in a time-dependent manner. The HO-1 expressions were 0.99±0.17, 1.68±0.1, 2.35±0.22, 1.52±0.05 and 1.24±0.06 folds of the control (non-irradiated cells), respectively, at 0, 6, 12, 18 and 24 h after the priming radiation dose. This result was consistent with that for RAR, which was also dependent on the time interval between priming and challenging radiation dose. The Nrf2 expression was up-regulated a little bit at 6, 12 and 18 h after the priming radiation dose, but there were no significant differences (Fig. 3C).
Fig. 3.
Time-course of HO-1 and Nrf2 expression in cells exposed to a priming dose of X-ray. (A) Typical western blot images of HO-1, Nrf2 and β-tubulin. (B) Relative expression of HO-1 at various time points post priming dose irradiation (5 cGy). (C) Relative expression of Nrf2 at various time points post priming dose irradiation (5 cGy). Data represent the means±SD of samples from three independent experiments. *: p<0.05 compared to control data. Δ: p<0.05 compared to 12 h data.
3.4. Effects of Hemin and Znpp on RAR
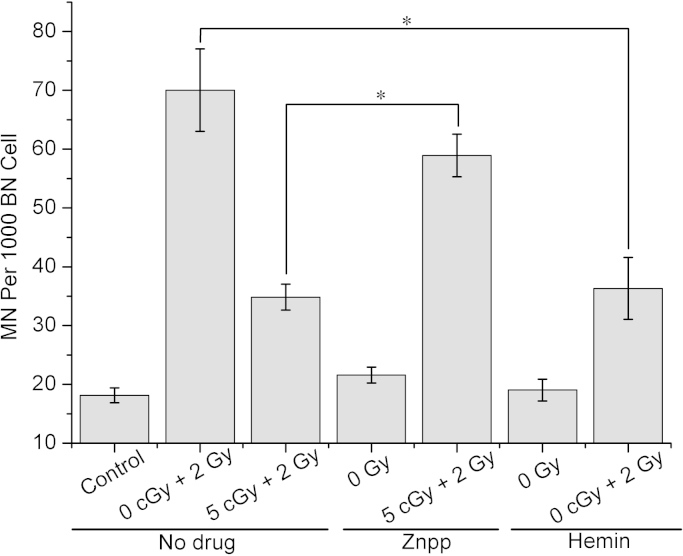
An HO-1 specific, competitive inhibitor Zinc protoporphyrin IX (Znpp, Sigma, St. Louis, MO, USA) [26] and HO-1 inducer hemin [27] were used to determine the role of HO-1 in RAR. Znpp (5 μM) was added 2 h before irradiation. Hemin (10 μM) was added 24 h before irradiation. As shown in Fig. 4, pretreatment with Znpp led to an increase in the yield of MN from 34.8±2.2 to 58.9±3.6 per 1000 BN cells in the adapted cells. This result indicated that the inhibitor of HO-1 could significantly attenuate the effect of RAR. Hemin, was used to mimic the HO-1 upregulation induced by the priming-dose radiation. The results showed that the hemin pretreatment effectively decreased the MN yield caused by a single 2 Gy irradiation from 70.0±7 to 36.3±5.3 per 1000 BN cells. These results implied that the induced HO-1 played an important role in the induction of RAR.
Fig. 4.
Effects of hemin (10 μM) or Znpp (5 μM) on RAR. Data represent the means±SD of samples from three independent experiments. *: p<0.05.
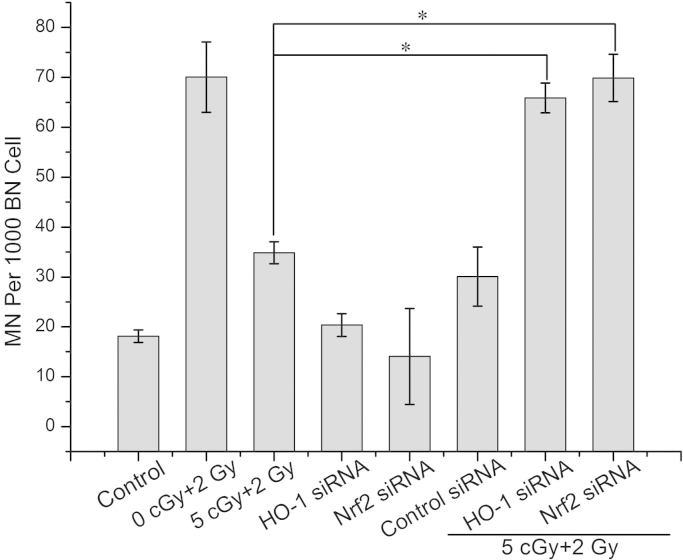
3.5. Knocking down of HO-1 or Nrf2 by siRNA attenuated RAR
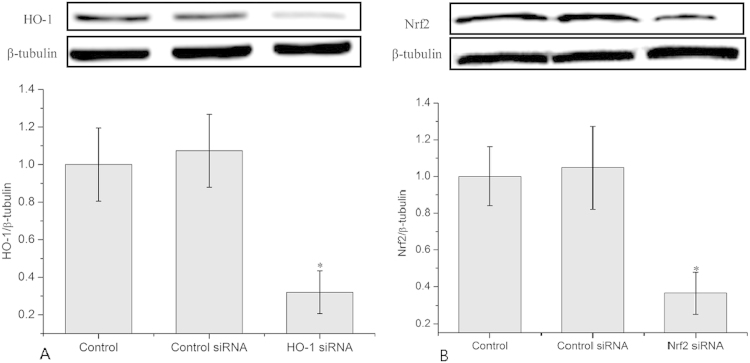
With transfection of cells with HO-1 siRNA or Nrf2 siRNA, HO-1 or Nrf2 was knocked down significantly (shown in supplemental Fig. 1). Consequently, radiation-induced MN formation was detected. Fig. 5 shows that knocking down of HO-1 with its siRNA results in nearly complete elimination of RAR, as evidence by an increase in the MN yield from 34.8±2.2 to 65.9±3 per 1000 BN cells. Nrf2, a transcription factor which upregulates HO-1, was also knocked down with its siRNA. The results showed that knocking down Nrf2 also increased the MN yield from 34.8±2.2 to 69.9±4.7 per 1000 BN cells, which suggested nearly complete elimination of RAR. It should be noted that control siRNA of HO-1 or Nrf-2 had no significant effect on the MN yield. These results strongly supported that RAR was mediated by HO-1 and Nrf2.
Fig. 5.
Effects of knocking down of HO-1 or Nrf2 on RAR. Data represent the means±SD of samples from three independent experiments. *: p<0.05.
3.6. Priming dose of radiation activated the translocation of Nrf2 from cytoplasm to nucleus
We next determined whether the priming radiation dose induced translocation of Nrf2 from the cytoplasm to the nucleus. Cytoplasm and nuclear proteins were extracted at 12 h after the priming-dose irradiation. Western blot was conducted to reveal the Nrf2 protein levels in both the cytoplasmic and the nuclear fractions. Fig. 6A shows that a priming dose significantly reduces the Nrf2 protein level to 0.49±0.1 folds of the control in the cytoplasm. In contrast, the Nrf2 protein level in the nucleus increased to 1.86±0.17 folds of the control (Fig. 6B). This result clearly confirmed that the priming radiation dose activated translocation of Nrf2 from the cytoplasm to the nucleus. Furthermore, it also demonstrated that transfection of Nrf2 siRNA reduced the protein expression of Nrf2 in both the cytoplasm and the nucleus.
Fig. 6.
Translocation of Nrf2 from cytoplasm to nucleus. (A) Nrf2 protein level in cytoplasm. (B) Nrf2 protein level in nucleus. Data represent the means±SD of samples from three independent experiments. *: p<0.05.
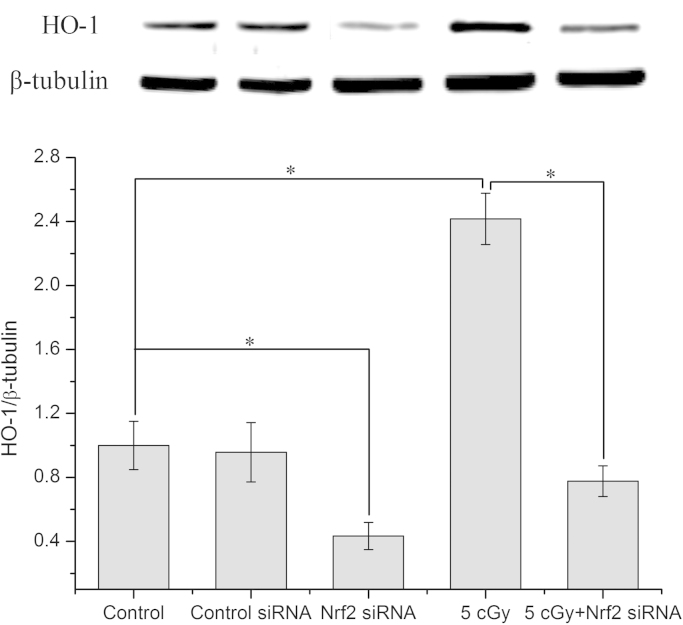
3.7. Knock down of Nrf2 down-regulated HO-1 protein level in cell
The effects of knocking down Nrf2 with Nrf2 siRNA on the HO-1 protein level were examined in both control cells and cells irradiated with a priming dose. The results in Fig. 7 showed that, with transfection of cells with Nrf2 siRNA, the HO-1 protein levels were dramatically reduced in both the control cells (from 1 to 0.43±0.1 folds of control) and the cells irradiated with the priming dose (from 2.42±0.16 to 0.78±0.09 folds of control).
Fig. 7.
Reduced HO-1 expression in cells transfected by Nrf2 siRNA. Data represent the means±SD of samples from three independent experiments. *: p<0.05.
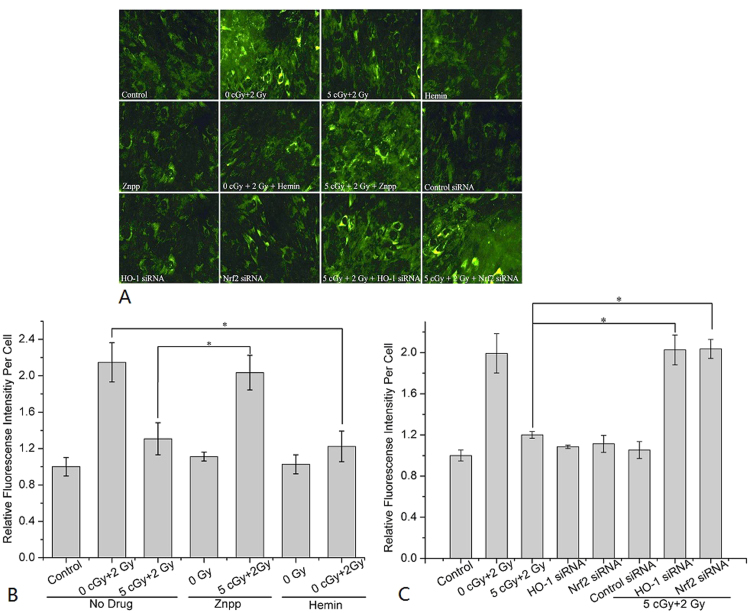
3.8. Nrf2/HO-1 pathway mediated RAR Via regulating ROS production
To determine the effects of Nrf2 and HO-1 on radiation-induced ROS production, CellROX® Green Reagent was used to detect ROS. Fig. 8A shows typical images of ROS under various conditions. As shown in Fig. 8B, a single irradiation with the challenging dose (2 Gy) significantly induced ROS production to 2.15±0.22 folds of the control. However, adaptation of cells through priming-dose irradiation effectively reduced the ROS production caused by the challenging dose, from 2.15±0.22 folds of the control down to 1.31±0.18 folds. Treatment with hemin showed a similar attenuated effect on ROS production (reduced from 2.15±0.22 fold to 1.03±0.1 folds of the control) after the challenging-dose irradiation. However, with the treatment of Znpp, ROS production in adapted cells was increased from 1.31±0.18 to 2.03±0.19 folds of the control. As shown in Fig. 8C, when the cells were transfected with HO-1 siRNA or Nrf2 siRNA, the ROS production induced by the challenging dose in the adapted cells reverted back to higher levels (2.03±0.15 and 2.04±0.1 folds of the control, respectively). These results clearly illustrated that the priming-dose irradiation could attenuate the production of ROS induced by the challenging-dose irradiation via the Nrf2/HO-1 pathway.
Fig. 8.
Photomicrographs and quantitative evaluating of ROS in cells. (A) Typical images of ROS results. (B) Effects of hemin or Znpp on ROS production. (C) Effects of transfection of HO-1 siRNA or Nrf2 siRNA on ROS production. Data represent the means±SD of samples from three independent experiments. *p<0.05.
4. Discussion
RAR, a phenomenon induced by low-dose radiation, has a potential “protective” effect against a subsequent high-dose irradiation [28]. Previous studies elucidated some distinct features of the protective reaction of human cells against high-dose exposures. Generally speaking, adaptation is triggered by a very low dose (2~5 cGy) and acts in several hours after stimulation. RAR is dependent not only on the rate of priming-dose irradiation but also on the time interval between priming and challenging dose irradiation [25], [29]. RAR has also been found to be effective for a relatively long time, approximately for three cell cycles [25], [30]. Similar to previous studies [25], [29], we showed that a prior exposure to 5 cGy X rays could reduce the MN yield in AG1522 cells induced by a subsequent higher radiation dose (2 Gy). In addition, we demonstrated that the optimum time interval between the application of priming and challenging doses was 12 h in AG 1522 cells. However, the extent of RAR was attenuated if the time interval was prolonged.
Evidences have suggested various possible pathways mediating RAR. For example, it was reported that the expression and transcription of some specific genes participating in DNA repair and cell cycle regulation were required for RAR in human lymphocytes [31], [32], [33]. It was also shown that RAR was inhibited by 3-aminobenzamide and cycloheximide and there was de novo synthesis of several proteins in response to a low-dose priming dose [34], [35]. Although the initiating signal of RAR was not elucidated, it was demonstrated that on receiving this unidentified signal, a subset of components including various protein kinases and early response genes regulating transcription machinery of the cell were involved [36]. Sasaki et al. reported that activation of protein kinase C (PKC) was required for RAR in murine m5S cells [37]. The intracellular signal transduction pathway activated by protein phosphorylation by PKC was a key step induced by low-dose irradiation [38]. A critical role of the p53 protein in channeling radiation-induced DNA double-strand breaks (DSBs) into adaptive repair pathways was also proposed [39].
HO-1, one of the important components of the anti-oxidant defense system [40], [41], is identified as the 32-kDa stress (heat shock) protein (HSP32) [42], [43]. HO-1 is a microsomal enzyme to catalyze oxidative breakdown of free heme (a pro-oxidant) molecule to carbon monoxide (CO), Fe2+, and biliverdin, which is subsequently reduced to bilirubin by biliverdin reductase [44]. Accumulating evidence had shown the importance of HO-1 expression in mediating antioxidant, antiinflammatory and antiapoptotic effects [26], [44], [45], [46]. It was reported that HO-1 could decrease tissue damages induced by lethal-dose irradiation through modulation of DNA repair [47]. The upregulation of HO-1 was also reported to mediate adaptive response induced by UVA [48], [49]. The membrane damage in human skin fibroblasts induced by UVA was reduced through pre-irradiation with a low-dose UVA exposure. On the other hand, pretreating cells with HO-1 antisense oligonucleotide inhibited the UVA-dependent induction of both the heme oxygenase I enzyme and ferritin, and eliminated the protective effect of UVA pre-irradiation [48]. In the present work, we also found that both the levels of HO-1 mRNA and HO-1 protein were significantly increased at 12 h after the priming-dose exposure.
The cytoprotective role of HO-1 was related to the removal of heme and the production of bilirubin, CO and Fe2+ [50], [51]. Bilirubin generated by HO-1 is an antioxidant capable of scavenging peroxy radicals and inhibiting lipid peroxidation [52]. CO at a low concentration has been shown to exert biological functions as diverse as protection against cell death, anti-inflammatory effects, protection against oxidative injury, inhibition of cell proliferation, neurotransmission and tolerance of organ transplantation [53], [54], [55]. The cytoprotective effects of Fe2+ released by HO-1 have been explained by the fact that Fe2+ promotes gene expression of ferritin, a protein which gives additional cytoprotection against oxidative stress [48]. Furthermore, Fe2+ itself has recently been reported to provide cytoprotection via NF-κB activation [56].
Nrf2 is translocated into the nucleus and activates the antioxidant response element (ARE) under oxidative stress [17]. Nuclear Nrf2 can bind to ARE and regulate ARE-mediated antioxidant enzyme gene expression and induction in response to a variety of stimuli including antioxidants, xenobiotics, metals, and UV irradiation [57]. It is also reported that ionizing radiation activated the Nrf2-mediated ARE antioxidant response [58]. Activated ARE mediates expression of a host of antioxidant genes including quinone oxidoreductase 1, glutathione S-transferase, γ-glutamylcysteine synthetase and HO-1 [59], [60]. In this study, we observed an increase in the nuclear fraction while a decrease in the cytosolic fraction of the Nrf2 protein, indicating a translocation of Nrf2 from the cytosol to the nucleus. Our results also showed that RAR was abolished by the administration of Znpp, HO-1 siRNA or Nrf2 siRNA. The increased expression of antioxidant enzymes after radiation resulted in rapid scavenging of ROS and consequently less cell damage. We found that ROS production induced by the challenging radiation dose was significantly increased by Znpp or transfection of HO-1 siRNA or Nrf2 siRNA. These results suggested that the priming radiation dose might activate the Nrf2/HO-1 pathway and the cellular antioxidant response.
In summary, our study supported that Nrf2-activated HO-1 up-regulation played a critical role in RAR. This contributes to the understanding of the mechanisms underlying RAR.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Dr. Gongming Xu (Institute of Nuclear Energy Safety Technology, Chinese Academy of Science) for operation of X-ray irradiation. This work was funded by the National Natural Science Foundation of China under Grant nos. 81573093, 81172602, the Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology no. 2014FXCX008, and project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Jiangsu Provincial Key Laboratory of Radiation Medicine and Protection.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.03.002.
Appendix A. Supplementary material
Fig. S1.
Transfection efficiency of HO-1 and Nrf2 siRNA. (A) Transfection efficiency of HO-1 siRNA. (B) Transfection efficiency of Nrf2 siRNA. Data represent the means±SD of samples from three independent experiments. *p<0.05 compared to control.
References
- 1.Tyrrell R.M. Modulation of gene expression by the oxidative stress generated in human skin cells by UVA radiation and the restoration of redox homeostasis. Photochem. Photobiol. Sci. 2012;11:135–147. doi: 10.1039/c1pp05222e. [DOI] [PubMed] [Google Scholar]
- 2.Olivieri G., Bodycote J., Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 3.Zhong J.L., Raval C., Edwards G.P., Tyrrell R.M. A role for Bach1 and HO-2 in suppression of basal and UVA-induced HO-1 expression in human Keratinocytes. Free Radic. Biol. Med. 2010;48:196–206. doi: 10.1016/j.freeradbiomed.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 4.Ikushima T. Radio-adaptive response: characterization of a cytogenetic repair induced by low-level ionizing radiation in cultured Chinese hamster cells. Mutat. Res. 1989;227:241–246. doi: 10.1016/0165-7992(89)90104-8. [DOI] [PubMed] [Google Scholar]
- 5.Reeve V.E., Tyrrell R.M., Allanson M., Domanski D., Blyth L. The role of interleukin-6 in UVA protection against UVB-induced immunosuppression. J. Invest. Dermatol. 2009;129:1539–1546. doi: 10.1038/jid.2008.377. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z.Q., Saigusa S., Sasaki M.S. Adaptive response to chromosome damage in cultured human lymphocytes primed with low doses of X-rays. Mutat. Res. 1991;246:179–186. doi: 10.1016/0027-5107(91)90120-d. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez I., Panneerselvam N., Escalza P., Natarajan A.T., Cortes F. Adaptive response to radiation damage in human lymphocytes conditioned with hydrogen peroxide as measured by the cytokinesis-block micronucleus technique. Mutat. Res. 1993;301:135–141. doi: 10.1016/0165-7992(93)90036-u. [DOI] [PubMed] [Google Scholar]
- 8.Dimowa E.G., Bryant P.E., Stephka G. “Adaptive response”-some underlying mechanisms and open questions. Genet. Mol. Biol. 2008;31:396–408. [Google Scholar]
- 9.Choi V.W., Cheng S.H., Yu K.N. Radioadaptive response induced by alpha-particle-induced stress communicated in vivo between Zebrafish embryos. Environ. Sci. Technol. 2010;44:8829–8834. doi: 10.1021/es101535f. [DOI] [PubMed] [Google Scholar]
- 10.Choi V.W., Yu K.N. Embryos of the Zebrafish danio Rerio in studies of non-targeted effects of ionizing radiation. Cancer Lett. 2015;356:91–104. doi: 10.1016/j.canlet.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Bravard A., Luccioni C., Moustacchi E., Rigaud O. Contribution of antioxidant enzymes to the adaptive response to ionizing radiation of human lymphoblasts. Int. J. Radiat. Biol. 1999;75:639–645. doi: 10.1080/095530099140285. [DOI] [PubMed] [Google Scholar]
- 12.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.H., Yu S., Chen J.D., Kong A.N. The nuclear cofactor RAC3/AIB1/Src-3 enhances Nrf2 signaling by interacting with Transactivation domains. Oncogene. 2013;32:514–527. doi: 10.1038/onc.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinkova-Kostova A.T., Holtzclaw W.D., Cole, Itoh R.N., Wakabayashi K.;N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh K., Wakabayashi N., Katoh Y., Ishii T., O’connor T., Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 16.Kang M.I., Kobayashi A., Wakabayashi N., Kim S.G., Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Numazawa S., Ishikawa M., Yoshida A., Tanaka S., Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am. J. Physiol. Cell Physiol. 2003;285:334–342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 18.de Toledo S.M., Asaad N., Venkatachalam P., Li L., Howell R.W., Spitz D.R., Azzam E.I. Adaptive responses to low-dose/low-dose-rate gamma rays in normal human fibroblasts: the role of growth architecture and oxidative metabolism. Radiat. Res. 2006;166:849–857. doi: 10.1667/RR0640.1. [DOI] [PubMed] [Google Scholar]
- 19.Broome E.J., Brown D.L., Mitchel R.E. Dose responses for adaption to low doses of (60)Co gamma rays and (3)H beta particles in normal human fibroblasts. Radiat. Res. 2002;158:181–186. doi: 10.1667/0033-7587(2002)158[0181:drfatl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Azzam E.I., de Toledo S.M., Spitz D.R., Little J.B. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 21.Fenech M. The in vitro micronucleus technique. Mutat. Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 22.Azzam E.I., de Toledo S.M., Little J.B. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc. Natl. Acad. Sci. USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang D., Dohle F., Terstesse M., Bangen P., August C., Pauels H.G., Heidenreich S. Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a Caspase-9, Caspase-3, and heat shock protein 70-dependent pathway. J. Immunol. 2002;168:6152–6158. doi: 10.4049/jimmunol.168.12.6152. [DOI] [PubMed] [Google Scholar]
- 24.Barber A., Robson S.C., Lyall F. Hemoxygenase and nitric oxide synthase do not maintain human uterine quiescence during pregnancy. Am. J. Pathol. 1999;155:831–840. doi: 10.1016/S0002-9440(10)65182-6. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shadley J.D., Afzal V., Wolff S. Characterization of the adaptive response to ionizing radiation induced by low doses of X rays to human lymphocytes. Radiat. Res. 1987;111:511–517. [PubMed] [Google Scholar]
- 26.Lee T.S., Chau L.Y. Heme Oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 27.Chen M., Bao W.J., Aizman R., Huang P., Aspevall O., Gustafsson L.E., Ceccatelli S., Celsi G. Activation of extracellular signal-regulated kinase mediates apoptosis induced by Uropathogenic Escherichia Coli toxins via nitric oxide synthase: protective role of heme Oxygenase-1. J. Infect. Dis. 2004;190:127–135. doi: 10.1086/421243. [DOI] [PubMed] [Google Scholar]
- 28.Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ. Health Perspect. 1998;106(Suppl. 1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shadley J.D. Chromosomal adaptive response in human lymphocytes. Radiat. Res. 1994;138 S9-12. [PubMed] [Google Scholar]
- 30.Youngblom J.H., Wiencke J.K., Wolff S. Inhibition of the adaptive response of human lymphocytes to very low doses of ionizing radiation by the protein synthesis inhibitor cycloheximide. Mutat. Res. 1989;227:257–261. doi: 10.1016/0165-7992(89)90107-3. [DOI] [PubMed] [Google Scholar]
- 31.Wolff S., Afzal V., Jostes R.F., Wiencke J.K. Indications of repair of radon-induced chromosome damage in human lymphocytes: an adaptive response induced by low doses of X-rays. Environ. Health Perspect. 1993;101(Suppl. 3):73–77. doi: 10.1289/ehp.93101s373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikushima T., Aritomi H., Morisita J. Radioadaptive response: efficient repair of radiation-induced DNA damage in adapted cells. Mutat. Res. 1996;358:193–198. doi: 10.1016/s0027-5107(96)00120-0. [DOI] [PubMed] [Google Scholar]
- 33.Wolff S., Afzal V., Olivieri G. Inducible repair of cytogenetic damage to human lymphocytes: adaptation to low-level exposures to DNA-damaging agents. Prog. Clin. Biol. Res. 1990;340B:397–405. [PubMed] [Google Scholar]
- 34.Ikushima T. Chromosomal responses to ionizing radiation reminiscent of an adaptive response in cultured Chinese hamster cells. Mutat. Res. 1987;180:215–221. doi: 10.1016/0027-5107(87)90217-x. [DOI] [PubMed] [Google Scholar]
- 35.Wiencke J.K., Afzal V., Olivieri G., Wolff S. Evidence that the [3H]thymidine-induced adaptive response of human lymphocytes to subsequent doses of X-rays involves the induction of a chromosomal repair mechanism. Mutagenesis. 1986;1:375–380. doi: 10.1093/mutage/1.5.375. [DOI] [PubMed] [Google Scholar]
- 36.Stecca C., Gerber G.B. Adaptive response to DNA-damaging agents: a review of potential mechanisms. Biochem. Pharmacol. 1998;55:941–951. doi: 10.1016/s0006-2952(97)00448-6. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki M.S. On the reaction kinetics of the radioadaptive response in cultured mouse cells. Int. J. Radiat. Biol. 1995;68:281–291. doi: 10.1080/09553009514551211. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki M.S., Ejima Y., Tachibana A., Yamada T., Ishizaki K., Shimizu T., Nomura T. DNA damage response pathway in radioadaptive response. Mutat. Res. 2002;504:101–118. doi: 10.1016/s0027-5107(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 39.Ryter S.W., Tyrrell R.M. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme Oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 40.Otterbein L.E., Choi A.M. Heme Oxygenase: colors of defense against cellular stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 41.Taketani S., Kohno H., Yoshinaga T., Tokunaga R. The human 32-kDa stress protein induced by exposure to Arsenite and cadmium ions is heme Oxygenase. FEBS Lett. 1989;245:173–176. doi: 10.1016/0014-5793(89)80215-7. [DOI] [PubMed] [Google Scholar]
- 42.Keyse S.M., Tyrrell R.M. Heme Oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maines M.D. Heme Oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 44.Choi B.M., Kim H.J., Oh G.S., Pae H.O., Oh H., Jeong S., Kwon T.O., Kim Y.M., Chung H.T. 1,2,3,4,6-Penta-O-Galloyl-beta-d-glucose protects rat neuronal cells (Neuro 2A) from hydrogen peroxide-mediated cell death via the induction of heme Oxygenase-1. Neurosci. Lett. 2002;328:185–189. doi: 10.1016/s0304-3940(02)00513-x. [DOI] [PubMed] [Google Scholar]
- 45.Brouard S., Ottebein L.E., Anrather J., Tobiasch E., Bach F.H., Choi A.M.K., Soares M.P. Carbon monoxide generated by heme Oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z.M., Chen G.G., Ng E.K., Leung W.K., Sung J.J., Chung S.C. Upregulation of heme Oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23:503–513. doi: 10.1038/sj.onc.1207173. [DOI] [PubMed] [Google Scholar]
- 47.Otterbein L.E., Hedblom A., Harris C., Csizmadia E., Gallo D., Wegiel B. Heme Oxygenase-1 and carbon monoxide modulate DNA repair through ataxia-telangiectasia mutated (ATM) protein. Proc. Natl. Acad. Sci. USA. 2011;108:14491–14496. doi: 10.1073/pnas.1102295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vile G.F., Basu-Modak S., Waltner C., Tyrrell R.M. Heme Oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc. Natl. Acad. Sci. USA. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeve V.E., Tyrrell R.M. Heme Oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse. Proc. Natl. Acad. Sci. USA. 1999;96:9317–9321. doi: 10.1073/pnas.96.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi B.M., Pae H.O., Jeong Y.R., Oh G.S., Jun C.D., Kim B.R., Kim Y.M., Chung H.T. Overexpression of heme Oxygenase (HO)-1 renders Jurkat T cells resistant to fas-mediated apoptosis: involvement of iron released by HO-1. Free Radic. Biol. Med. 2004;36:858–871. doi: 10.1016/j.freeradbiomed.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Nowis D., Legat M., Grzela T., Niderla J., Wilczek E., Wilczynski G.M., Głodkowska E., Mrówka P., Issat T., Dulak J., Józkowicz A., Waś H., Adamek M., Wrzosek A., Nazarewski S., Makowski M., Stokłosa T., Jakóbisiak M., Gołab J. Heme Oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006;25:3365–3374. doi: 10.1038/sj.onc.1209378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stocker R., McDonagh A.F., Glazer A.N., Ames B.N. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol. 1990;186:301–309. doi: 10.1016/0076-6879(90)86123-d. [DOI] [PubMed] [Google Scholar]
- 53.Wu L., Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 54.Han W., Wu L., Chen S., Yu K.N. Exogenous carbon monoxide protects the bystander Chinese hamster ovary cells in mixed coculture system after alpha-particle irradiation. Carcinogenesis. 2010;31:275–280. doi: 10.1093/carcin/bgp301. [DOI] [PubMed] [Google Scholar]
- 55.Han W., Yu K.N., Wu L., Wu Y., Wang H. Mechanism of protection of bystander cells by exogenous carbon monoxide: impaired response to damage signal of radiation-induced bystander effect. Mutat. Res. 2011;709–710:1–6. doi: 10.1016/j.mrfmmm.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Xiong S., She H., Takeuchi H., Han B., Engelhardt J., Barton C.H., Zandi E., Giulivi C., Tsukamoto H. Signaling role of intracellular iron in NF-kappaB activation. J. Biol. Chem. 2003;278:17646–17654. doi: 10.1074/jbc.M210905200. [DOI] [PubMed] [Google Scholar]
- 57.Jaiswal A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 58.McDonald J.T., Kim K., Norris A.J., Vlashi E., Phillips T.M., Lagadec C., Della Donna L., Ratikan J., Szelag H., Hlatky L., McBride W.H. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70:8886–8895. doi: 10.1158/0008-5472.CAN-10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhakshinamoorthy S., Long D.J., 2nd, Jaiswal A.K. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr. Top. Cell Regul. 2000;36:201–216. doi: 10.1016/s0070-2137(01)80009-1. [DOI] [PubMed] [Google Scholar]
- 60.Jaiswal A.K. Regulation of genes encoding Nad(P)H:quinone Oxidoreductases. Free Radic. Biol. Med. 2000;29:254–262. doi: 10.1016/s0891-5849(00)00306-3. [DOI] [PubMed] [Google Scholar]