Abstract
Background
Little prospectively collected data are available comparing the dietary intake of urea cycle disorder (UCD) patients to UCD treatment guidelines or to healthy individuals.
Objective
To examine the protein and calorie intakes of UCD subjects who participated in clinical trials of glycerol phenylbutyrate (GPB) and compare these data to published UCD dietary guidelines and nutritional surveys.
Design
Dietary data were recorded for 45 adult and 49 pediatric UCD subjects in metabolic control during participation in clinical trials of GPB. Protein and calorie intakes were compared to UCD treatment guidelines, average nutrient intakes of a healthy US population based on the National Health and Nutrition Examination Survey (NHANES) and Recommended Daily Allowances (RDA).
Results
In adults, mean protein intake was higher than UCD recommendations but lower than RDA and NHANES values, while calorie intake was lower than UCD recommendations, RDA and NHANES. In pediatric subjects, prescribed protein intake was higher than UCD guidelines, similar to RDA, and lower than NHANES data for all age groups, while calorie intake was at the lower end of the recommended UCD range and close to RDA and NHANES data. In pediatric subjects height, weight, and body mass index (BMI) Z-scores were within normal range (− 2 to 2).
Conclusions
Pediatric patients treated with phenylbutyrate derivatives exhibited normal height and weight. Protein and calorie intakes in adult and pediatric UCD subjects differed from UCD dietary guidelines, suggesting that these guidelines may need to be reconsidered.
Abbreviations: UCD, urea cycle disorder; GPB, glycerol phenylbutyrate; NaPBA, sodium phenylbutyrate; NHANESs, National Health and Nutrition Examination Surveys; RDA, Recommended Daily Allowances; FAO, Food and Agriculture Organization; WHO, World Health Organization; OTC, ornithine transcarbamylase
Keywords: Urea cycle disorders, Protein intake, Inherited metabolic disorders, Dietary management, Phenylbutyrate
1. Introduction
Urea cycle disorders (UCDs) are categorized as inborn errors of metabolism involving one of the six enzymes or two transporters that convert excess nitrogen through the urea cycle and excrete the waste nitrogen as urea in urine. Deficiency of any of these enzymes or transporters results in the accumulation of toxic levels of ammonia in the blood and brain of affected patients [1]. UCDs can present in the neonatal period or later in life depending on the severity and type of defect [1], [2], [3]. Dietary management and restriction of protein intake are the cornerstones of long-term disease management of UCDs in conjunction with therapeutic agents such as glycerol phenylbutyrate (GPB) or sodium phenylbutyrate (NaPBA), which remove excess nitrogen via alternative pathways to the urea cycle [4].
A key element of nutritional management of UCDs is to restrict protein intake to reduce nitrogen flow into the urea cycle and hence ammonia products. Nitrogen needs of the body can vary as a result of tissue remodeling, growth rate, activity level, type and category of enzymatic deficiency, as well as the patient's age, catabolic state, and developmental status [5]. Acute nutritional therapy includes modification of dietary protein, addition of hypercaloric solutions, supplementation with l-arginine or l-citrulline, followed by reinstitution of usual protein and oral nutrition. During chronic therapy, the overall goals are to maintain a positive nitrogen balance in pediatric patients, provide adequate essential amino acids, and manage fluid intake. The objectives of long-term nutritional disease management are to control blood ammonia and achieve normal growth and development and prevent protein deficiency by supplementing the diet with essential and conditional essential amino acids as well as prevent vitamin and mineral deficiencies [5], [6].
UCD nutrient guidelines vary with age and gender and have been primarily developed based on professional consensus rather than empirically collected data. UCD energy requirements for adults are derived from the Recommended Dietary Allowance (RDA), while protein intake requirements for pediatric patients are based on the Food and Agriculture Organization and World Health Organization (FAO/WHO) requirements [7].
There is little systematically or prospectively collected data on the prescribed or actual nutrient intake of UCD patients in relation to recommended UCD treatment guidelines or nutrient intake in healthy subjects. Indeed, current guidelines graded the strength of the evidence upon which the dietary recommendations were based as ‘C’ (mainly non-analytical studies such as case reports and case series) or ‘D’ (mainly expert opinion) [5]. This report summarizes the protein and calorie intakes of adult and pediatric UCD patients who participated as subjects in clinical trials of GPB in relation to published guidelines for UCD patients [6], [8] and the average nutrient intake levels of a healthy US population as described in the National Health and Nutrition Examination Surveys (NHANESs) [9], [10], [11], and RDA values [12].
2. Subjects and methods
2.1. Clinical study design
Data from 4 clinical studies were pooled and analyzed. Subjects with a UCD treated with NaPBA were eligible for enrollment in the studies. Major exclusion criteria were liver transplant, hypersensitivity to PBA, PAA, or PAGN, clinically significant laboratory abnormalities or ECG findings, or any condition such as infection or medications that could affect ammonia levels. Full details of these studies have been published [13], [14], [15], [16]. In study 1, 45 adult UCD subjects participated in a randomized, double-blind, double dummy, 4-week, cross-over study, during which ammonia control, assessed as 24-hour area under the curve, was assessed during treatment with GPB (RAVICTI®; Horizon Pharma) or NaPBA for 2 weeks and during which weekly diet diaries were collected. In studies 2, 3 and 4, 51 adult UCD subjects (40 of whom had participated in study 1) and 49 pediatric UCD subjects received GPB for up to 12 months during which fasting ammonia, blood urea nitrogen (BUN) levels, height and weight, and prescribed dietary intake were periodically recorded. The protocol for each study was reviewed and approved by the Investigational Review Boards of each participating institution prior to the initiation of any study procedures.
2.2. Study population
The mean age of adult subjects was 33 years (range 18–75); 68.9% of subjects were female, 77.8% were white; 88.9% had ornithine transcarbamylase (OTC) deficiency, and 77.7% reported onset of their UCD at age 2 years or older (Table 1). The mean age of pediatric subjects was 7.1 years (range 2 months to 17 years); 69.4% of subjects were female, 79.6% were white, and 57.1% had OTC deficiency (Table 1).
Table 1.
Subject demographics.
| Adults (N = 45) | Pediatric (N = 49) | ||
|---|---|---|---|
| Age: (years) | Mean (SD) | 32.7 (13.53) | 7.1 (4.7) |
| Range | 18.0, 75.0 | 0.17, 17.0 | |
| Age group: N (%) | < 2 | – | 7 (14.3) |
| 2–5 | – | 16 (32.6) | |
| 6–11 | – | 17 (34.3) | |
| 12–17 | – | 9 (18.4) | |
| Sex: N (%) | Male | 14 (31.1) | 15 (30.6) |
| Female | 31 (68.9) | 34 (69.4) | |
| Race: N (%) | White | 35 (77.8) | 39 (79.6) |
| Non-white | 10 (22.2) | 10 (20.4) | |
| UCD deficiency: N (%) | OTC | 40 (88.9) | 28 (57.1) |
| ASS | 3 (6.7) | 9 (18.4) | |
| CPS 1 | 2 (4.4) | 0 | |
| ASL | 0 | 11 (22.4) | |
| ARG | 0 | 1 (2.0) | |
| Onset of UCD: N (%) | ≤ 2 years | 10 (22.2) | 36 (73.5) |
| > 2 years | 35 (77.7) | 13 (26.5) | |
Abbreviations: ASS —argininosuccinate synthetase deficiency, ASL —argininosuccinate lyase deficiency, ARG — arginase deficiency, CPS 1 — carbamoyl phosphate synthetase deficiency, OTC — ornithine transcarbamylase deficiency, UCD — urea cycle disorder.
During treatment with NaPBA (before enrollment in studies) and GPB, the branched-chain amino acid (BCAA) supplements, valine, isoleucine and leucine, were used by 6%, 6%, and 4% of pediatric subjects, respectively. Medical food was used by 45% of pediatric subjects compared with 20% of adult subjects and amino acid supplements other than BCAA, by 53% and 34% of pediatric and adult subjects respectively.
2.3. Diet and dietary data collection
All subjects were prescribed a diet outlining the recommended protein and calorie intakes by their treating physicians/dieticians. For all subjects, prescribed diets were individualized based on the subjects' age, developmental needs, protein tolerance, and estimated residual enzyme activity. For 49 pediatric subjects, only prescribed protein and calorie intakes (g/day or kcal/day) by physician/dieticians were recorded and no diet diaries were collected. However, for 45 adult subjects, 3-day diet histories (listing actual foods consumed and serving sizes) were collected weekly before each study visit during the double-blind study (a total of 4 weeks). Diet histories were checked for completeness using the Multiple-Pass Method for dietary collection [17] and daily actual protein and calorie intakes including medical foods were calculated using Nutritionist Pro Diet Analysis Software™ (Axxya Systems, Stafford, TX).
2.4. Protein and calorie intakes by UCD subtype
Because female patients with X-linked OTC deficiency tend to have milder disease than either males with OTC deficiency or patients with other UCD genetic subtypes [1], [2], [3], [4], prescribed dietary protein and calories for female OTC patients were compared with that for other subtypes, both overall and also separately for adult and pediatric patients.
2.5. Comparison to UCD treatment guidelines and a healthy US population
Protein and calorie intakes, derived from weekly diet diaries for adult subjects and from the physician-prescribed protein and calorie intakes for pediatric and adult subjects, were compared with published UCD nutritional guidelines [8], RDA values [12], and the actual intakes observed in a healthy US population using NHANES data for protein and calorie intakes [9], [10], [11]. The NHANES surveys used two days of dietary intake data, collected 3 to 10 days apart, for each participant. Adult NHANES and RDA protein and calorie data are presented separately for males and females [9], [10], [12]. When the NHANES and RDA data included different age groups, the data were averaged over all adult age groups (19 + years old) separately for males and females. Pediatric data were summarized by age group (< 2, 2 to 5, 6 to 11, and 12 to 17 years) and compared to the closest available age group for published UCD, RDA, and NHANES data. Where published pediatric data included separate values for males and females, the average of the male and female data was used for comparison.
2.6. Correlations of dietary intake with growth and BMI
Body mass index (BMI) was calculated at baseline and at month 12 for all subjects. Standardized Z-scores of height, weight, and BMI were calculated at baseline and at month 12 for pediatric subjects. Weight and height Z-scores were correlated with protein and calorie intakes at baseline and at month 12 for pediatric subjects using Spearman correlations. BMI was correlated with protein and calorie intakes at baseline and at month 12 for adult and pediatric subjects.
3. Results
3.1. Prescribed protein and calorie intakes relative to UCD subtype
Among 52 female adult and pediatric patients with OTC deficiency, prescribed mean [SD] total dietary protein (35.8 [12.7] g/day) and calories (1868 [415] kcal/day) were both very similar to the prescribed dietary protein (36.5 [18.9] g/day) and calories (1814 [648] kcal/day) for the remaining 47 patients, including both male patients with OTC deficiency or patients with other UCD subtypes. Similarly, mean [SD] prescribed protein was similar among the 24 pediatric female OTC vs. the remaining 25 pediatric patients (28.0 [11.5] vs. 24.2 [10.0] g/day), as was prescribed calories (1802 [439] vs. 1500 [613] kcal/day). The same was true among the 28 adult female OTC patients vs. remaining 22 adult patients both for prescribed protein (42.4 [9.6] vs. 50.4 [17.0] g/day) and total calories (1908 [403] vs. 2177 [486] kcal/day). Therefore, further analyses focused on adult and pediatric patients of all UCD subtypes.
3.2. Protein and calorie intakes — adults
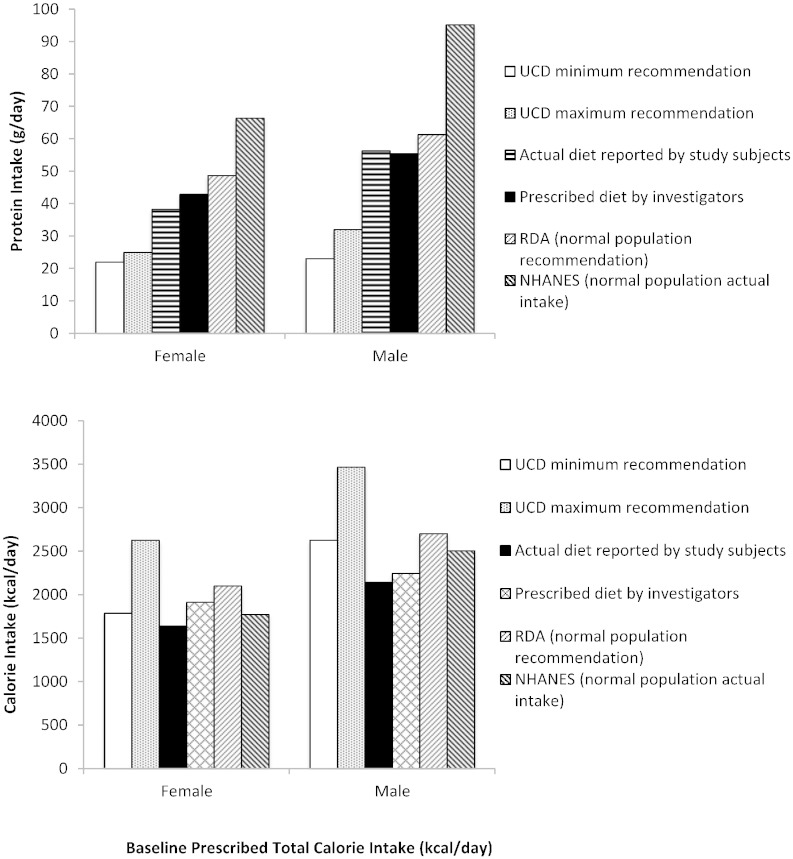
Mean actual protein intake in adult male and female UCD patients as calculated from diet diaries was similar to the mean prescribed protein as summarized in Table 2. Actual and prescribed protein intakes in study subjects were greater than the recommended protein intake of 22 to 25 g/day for female UCD subjects and 23 to 32 g/day for male UCD subjects per UCD guidelines [6], [8]and slightly lower than healthy subjects as compared to RDA values [12] and NHANES data [10] (Fig. 1). Approximately 10% of total calorie intake was derived from dietary protein compared with 15% for the healthy population [10].
Table 2.
Protein and calorie intakes in the study population.
| Pediatric patients |
Adult patients (≥ 18 years old) |
|||||
|---|---|---|---|---|---|---|
| ≤ 2 years | 2–5 years | 6–11 years | ≥ 12–17 years | Males | Females | |
| Protein prescribed | 1.76 g/kg | 1.41 g/kg | 0.98 g/kg | 0.64 g/kg | 55.4 g/day | 42.9 g/day |
| Protein consumed | – | – | – | – | 56.3 g/day | 38.3 g/day |
| Calories prescribed | 100.7 kcal/kg/day | 88.1 kcal/kg/day | 67.8 kcal/kg/day | 42.3 kcal/kg/day | 1913 kcal/day | 2245 kcal/day |
| Calories consumed | – | – | – | – | 2143 kcal/day | 2245 kcal/day |
Fig. 1.
Actual and recommended protein (upper panel) and calorie (lower panel) intakes for adult UCD and healthy subjects. Actual and prescribed protein intake (upper panel) and calorie intake (lower panel) for adult UCD subjects compared with the recommended minimum and maximum intakes per UCD guidelines [5], Recommended Dietary Allowance (RDA) guidelines [11], and National Health and Nutrition Examination Survey (NHANES) data (NHANES 2003–2004 for protein [9] and NHANES 2007–2008 for calories [8]).
Mean actual caloric intake in adult male and female UCD patients calculated from diet diaries was similar to the mean prescribed calorie intake (Table 2). Actual calorie intake was below the recommended range for female and male UCD patients (1785–2625 and 2625–3465 kcal/day, respectively, per UCD guidelines (8), was 78% and 79% of the RDA for females and males, respectively, and was 92% and 86% of that in the NHANES (1771 and 2504 kcal/day) for females and males, respectively [9], [12] (Fig. 1).
3.3. Protein and calorie intakes and growth — pediatric subjects
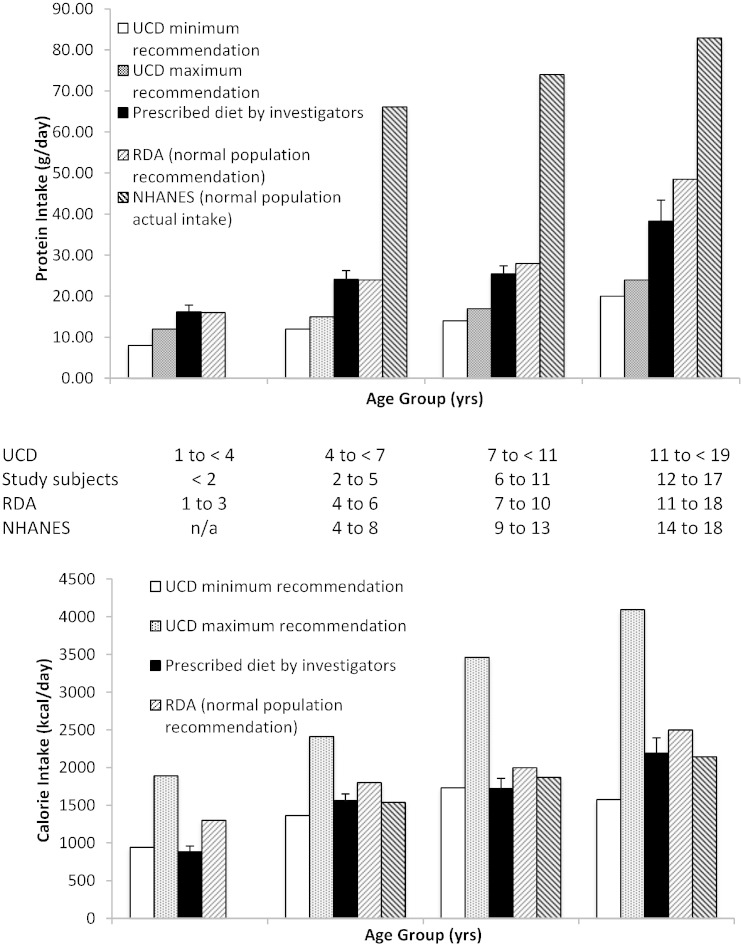
For pediatric subjects, mean prescribed protein normalized to body weight decreased with increasing age (Table 2), although absolute protein intake increased with age as expected (Fig. 2). In general, prescribed protein was slightly higher than recommended by UCD guidelines, similar to the RDA recommendations, and substantially lower than the actual intake as reported in the NHANES for all age groups (Fig. 2).
Fig. 2.
Recommended protein (upper panel) and calorie (lower panel) intakes for pediatric UCD and healthy subjects. Protein (upper panel) and calorie (lower panel) intakes for pediatric UCD subjects by age group compared with closest available age groups from the recommended minimum and maximum intakes per UCD guidelines [5], RDA guidelines [11] and NHANES data (NHANES 2003–2004 for protein [9] and NHANES 2009–2010 for calories [10]). Age groups used for each data set are shown beneath the X axes. Data for the prescribed diet are mean (SE). UCD, RDA, and NHANES data for older age groups are the average of male and female data. NHANES data were not available for the youngest age group.
Mean prescribed calories normalized to body weight also decreased with increasing age (Table 2), although older subjects were prescribed higher total daily calories (Fig. 3). Prescribed calorie intakes for each age group were generally at the lower end of recommended UCD guidelines, and close to both the RDA values and NHANES data (Fig. 2).
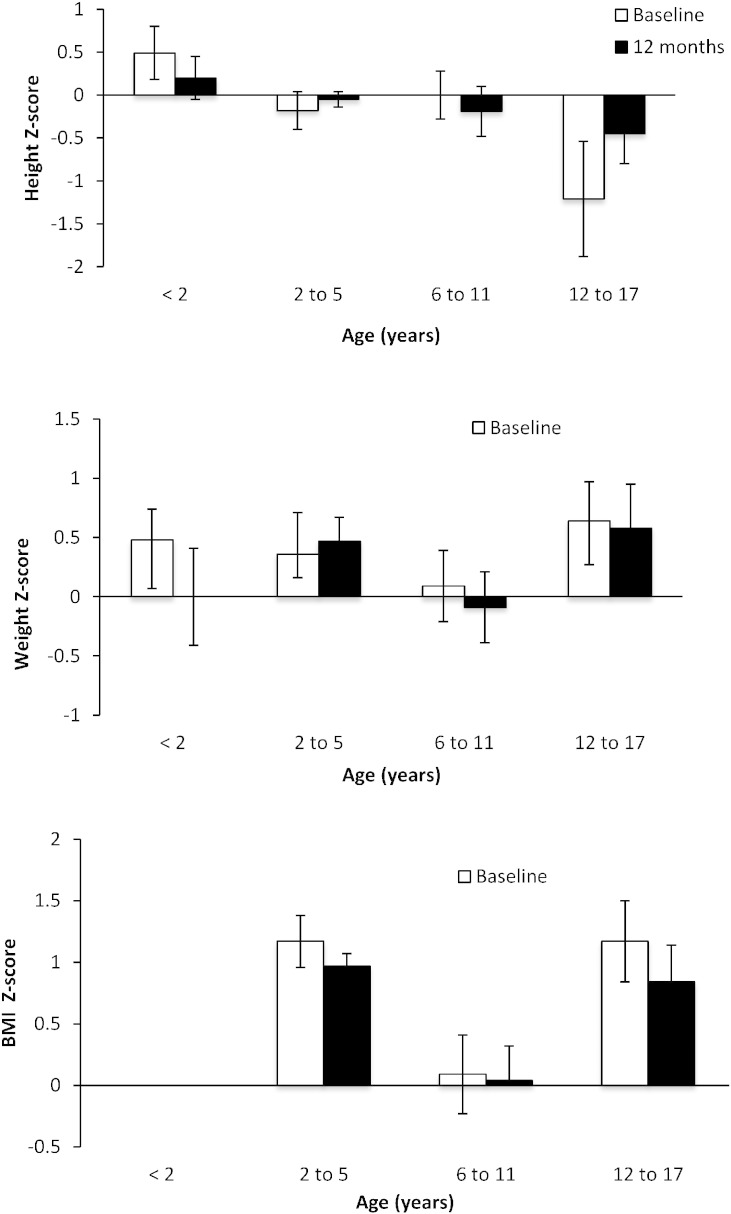
Fig. 3.
Z scores for height (upper panel), weight (center panel), and BMI (lower panel) for pediatric subjects. Height (upper panel), weight (center panel), and BMI (lower panel) Z-scores at baseline and 12 months by baseline age categories for pediatric subjects. BMI Z-scores were not available for subjects < 2 years of age. The Z scores are calculated based on mean of US population for that age group. The Z score indicates how many standard deviations the study participants deviate from the average age-corrected US population and a score between − 2 and 2 is typically considered within normal range.
All pediatric age groups showed an increase in absolute height and weight during the study. Mean height, weight, and BMI Z-scores were within normal range (− 2 to 2) at baseline and did not change significantly during 12 months of GPB treatment within any age group (Fig. 3).
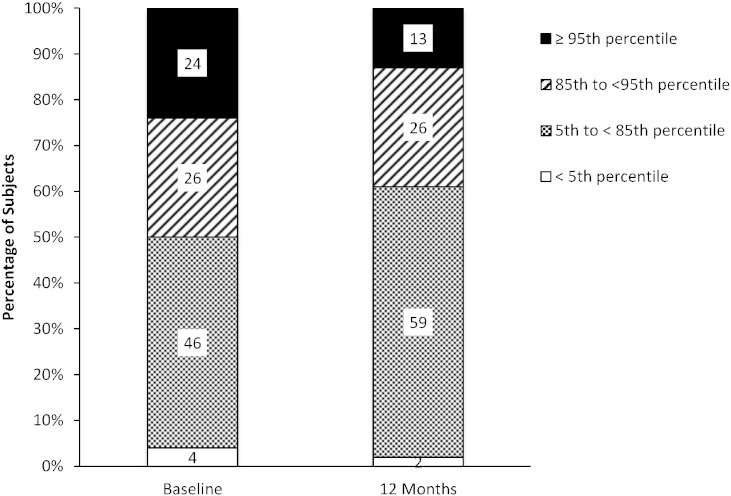
At baseline and after 12 months of GPB treatment, 46% and 59% of all pediatric subjects' BMI, respectively, were within normal range (> 5th and < 85th percentiles), and 50% and 39% of patients were either overweight or obese (≥ 85th percentile), respectively (Fig. 4). There was also a significant positive correlation between prescribed protein (g/day) and BMI at baseline (r = 0.314; p = 0.030) and at 12 months (r = 0.491; p < 0.001).
Fig. 4.
BMI-for-age percentiles at baseline and after 12 months of treatment with GPB for pediatric subjects. BMI-for-age percentiles relative to a normal population at baseline and after 12 months of treatment with GPB for pediatric subjects. The percentiles depicted are in relation to a normal population as defined by the Center for Disease Control for underweight (< 5th percentile), healthy (5th–85th percentiles), overweight (> 85th percentile) and obese (> 95th percentile) populations.
4. Discussion
The present report summarizes actual and/or prescribed protein and calorie intakes among adult and pediatric UCD subjects, as well as growth in pediatric subjects, all of whom participated in the GPB clinical trials. These subjects, nearly all of who were taking NaPBA at the time of enrollment, were well controlled with respect to blood ammonia, were managed by experienced metabolic geneticists and dieticians and collectively comprise an estimated 20% of all UCD subjects in the US who are currently treated with NaPBA [15]. Because of the unexpected finding that prescribed protein and calories for adult and pediatric female OTC patients was similar to that prescribed to other UCD patients, the present analyses focused on adult and pediatric populations without respect to subtype.
For adult subjects, actual protein intake derived from diet diaries was similar to that prescribed by their physicians, exceeded the maximum recommended intake for UCD subjects [8] and was lower than RDA values [12] and NHANES data [10]. While direct comparisons of protein intake with UCD guidelines, RDA values and NHANES data were not possible for pediatric subjects due to different age cut-offs, prescribed protein was generally higher than recommendations for UCD subjects [8], similar to RDA guidelines [12], and lower than actual reported intake based on NHANES data [10]. The finding that prescribed protein was lower in both adult and pediatric UCD subjects compared to the healthy population is not surprising; however, the observation that protein intake was greater than recommendations for UCD patients was not anticipated in light of the dietary protein aversion reported for UCD patients [18].
Both prescribed and actual calorie intakes in adult subjects were lower than recommended for UCD subjects [8] and also lower than the RDA for males and females [12] and actual intake by the general population based on NHANES [9], in particular for male subjects. Prescribed calories in pediatric subjects were generally at the lower end of the recommended UCD range [8] and close to both the RDA [12] and NHANES data [11] for children and adolescents.
Dietary management of pediatric patients with UCDs should allow for normal growth while controlling blood ammonia. It is encouraging in this regard that, based on Z-scores which represent deviation from normal population, children of all ages in the study population exhibited normal parameters for height, weight, and BMI during treatment with either NaPBA or GPB. This finding further suggests that that patients with UCDs sufficiently severe as to require treatment with phenylbutyrate derivatives can achieve growth and development similar to normal population and that the growth of these children was not significantly affected by the dietary restriction as implemented by their physicians and dieticians.
Several limitations of the study merit emphasis. Actual dietary intake data based on dietary diaries was available for adult but not for pediatric subjects, in whom only prescribed dietary data by treating physicians were used. Therefore, the actual dietary intake may have been different than the prescribed intake in pediatric subjects. Additionally, comparison of prescribed and/or actual intake did not always align with the published age groups for UCD treatment guidelines, NHANES or RDA. Finally, these analyses, which are not categorized with respect to UCD subtypes beyond female OTC patients vs. all others, are derived from the population of UCD patients who participated in the GPB clinical trials, which consisted exclusively of North American patients, nearly all of whom were taking NaPBA and none of whom were taking sodium benzoate. The data and analyses may therefore not pertain to patients in other geographies, those taking sodium benzoate, or those not on drug therapy.
In summary, analysis of this comprehensive dietary dataset suggests that protein intake in UCD subjects may be higher and calorie intake may be lower than recommended by published guidelines for UCD management and that pediatric subjects exhibited age-appropriate growth in height and weight. The findings further suggest that future guidelines for dietary management, in addition to expert opinion and theoretical calculations, should take into account actual dietary data from UCD patients.
Acknowledgments
Conflict of interest
Masoud Mokhtarani and Bruce Scharschmidt were employees of Hyperion at the time of these studies. Masoud Mokhtarani and Marty Porter are current employees of Hyperion.
Hyperion provided financial support to the following institutions for services provided in the conduct of the clinical studies upon which this report is based: Miller Children's Hospital/Long Beach Memorial Medical Center (DH, JB); Mount Sinai School of Medicine (GD); Baylor College of Medicine (BL); University of Utah (NL); Stanford University (WB); and the National Urea Cycle Disorders Foundation (CLM). These authors had no other conflicts of interest.
Medical writing assistance was provided by Jacqueline Wu, Castle Peak Medical Writing, with funding provided by Horizon Pharma.
Footnotes
ClinicalTrials.gov identifiers: NCT00947544, NCT00551200, NCT00992459, NCT01347073.
References
- 1.Brusilow S.W., Maestri N.E. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv. Pediatr. Infect. Dis. 1996;43:127–170. [PubMed] [Google Scholar]
- 2.Summar M.L., Dobbelaere D., Brusilow S., Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr. 2008;97(10):1420–1425. doi: 10.1111/j.1651-2227.2008.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuchman M., Lee B., Lichter-Konecki U. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol. Genet. Metab. 2008;94(4):397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanpher B.C., Gropman A., Chapman K.A., Lichter-Konecki U., Urea Cycle Disorders C., Summar M.L. Urea cycle disorders overview. In: Pagon R.A., Adam M.P., Ardinger H.H., editors. GeneReviews(R) 1993. (Seattle, WA) [Google Scholar]
- 5.Haberle J., Boddaert N., Burlina A. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J. Rare Dis. 2012;7:32. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R.H. Nutritional management of patients with urea cycle disorders. J. Inherit. Metab. Dis. 2007;30(6):880–887. doi: 10.1007/s10545-007-0718-4. [DOI] [PubMed] [Google Scholar]
- 7.Leonard J.V. The nutritional management of urea cycle disorders. J. Pediatr. 2001;138(1 Suppl.):S40–S44. doi: 10.1067/mpd.2001.111835. (discussion S4-5). [DOI] [PubMed] [Google Scholar]
- 8.Singh R.H., Rhead W.J., Smith W., Lee B., Sniderman King L., Summar M. Nutritional management of urea cycle disorders. Crit. Care Clin. 2005;21(4 Suppl):S27–S35. doi: 10.1016/j.ccc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Wright J., Wang C.-Y. In; National Center for Health Statistics: 2010. Trends in Energy and Macronutrients in Adults from 1999 to 2000 Through 2007–2008. [Google Scholar]
- 10.Fulgoni V.L., III Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008;87(5):1554S–1557S. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]
- 11.Slining M.M., Mathias K.C., Popkin B.M. Trends in food and beverage sources among US children and adolescents: 1989–2010. J. Acad. Nutr. Diet. 2013;113(12):1683–1694. doi: 10.1016/j.jand.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Council N.R. 10th ed. National Academic Press; Washington, DC: 1989. Recommended Dietary Allowances. [Google Scholar]
- 13.Lichter-Konecki U., Diaz G.A., Merritt J.L., II Ammonia control in children with urea cycle disorders (UCDs); phase 2 comparison of sodium phenylbutyrate and glycerol phenylbutyrate. Mol. Genet. Metab. 2011;103(4):323–329. doi: 10.1016/j.ymgme.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith W., Diaz G.A., Lichter-Konecki U. Ammonia control in children ages 2 months through 5 years with urea cycle disorders: comparison of sodium phenylbutyrate and glycerol phenylbutyrate. J. Pediatr. 2013;162(6):1228–1234. doi: 10.1016/j.jpeds.2012.11.084. (34 e1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz G.A., Krivitzky L.S., Mokhtarani M. Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology. 2013;57(6):2171–2179. doi: 10.1002/hep.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry S.A., Lichter-Konecki U., Diaz G.A. Glycerol phenylbutyrate treatment in children with urea cycle disorders: pooled analysis of short and long-term ammonia control and outcomes. Mol. Genet. Metab. 2014;112(1):17–24. doi: 10.1016/j.ymgme.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moshfegh A.J., Rhodes D.G., Baer D.J. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 18.Gardeitchik T., Humphrey M., Nation J., Boneh A. Early clinical manifestations and eating patterns in patients with urea cycle disorders. J. Pediatr. 2012;161(2):328–332. doi: 10.1016/j.jpeds.2012.02.006. [DOI] [PubMed] [Google Scholar]